Abstract
Purpose
The purpose of this study was to investigate the prognostic implications of carcinoembryonic antigen (CEA) levels that are inconsistent with Response Evaluation Criteria in Solid Tumor (RECIST) responses in metastatic colorectal cancer patients.
Materials and Methods
We retrospectively evaluated 360 patients with at least one measurable lesion who received first-line palliative chemotherapy. CEA-response was defined as CEA-complete response (CR; CEA normalization), CEA-partial response (PR; ≥ 50% decrease in CEA levels), CEA-progressive disease (PD; ≥ 50% increase in CEA levels), and CEA-stable disease (SD; non-CR/PR/PD). Overall survival (OS) and progression-free survival (PFS) were evaluated according to CEA-response.
Results
In RECIST-PR patients, poorer CEA-response was associated with disease progression at the subsequent evaluation. In RECIST-SD patients, CEA-CR and -PR were associated with lower disease progression rates than CEA-PD at the subsequent evaluation. Correlations between survival outcome and CEA-response in same-category RECIST patients were assessed. In RECIST-PR patients, discordant CEA-response (CEA-PD/SD) was associated with poorer survival than CEA-CR/PR (median OS and PFS, 44.0 and 15.4 [CEA-CR], 28.9 and 12.5 [CEA-PR], 21.0 and 9.8 [CEA-SD], and 13.0 and 7.0 [CEA-PD] months, respectively; all p < 0.001). In RECIST-SD patients, favorable CEA-response produced better survival (median OS and PFS, 26.8 and 21.0 [CEA-CR], 21.0 and 11.0 [CEA-PR], 16.1 and 8.2 [CEA-SD], and 12.2 and 6.0 [CEA-PD] months, respectively; all p < 0.001). RECIST-PD patients with CEA-CR showed longer OS than those with CEA-PD. Multivariate analysis demonstrated that discordant CEA-response is a powerful prognostic factor for RECIST-PR and RECIST-SD patients.
Conclusion
Among patients of the same RECIST-response categories, CEA-response patterns are significantly prognostic and strongly predictive of subsequent evaluation outcomes.
Keywords: Carcinoembryonic antigen, Chemotherapy, Colorectal neoplasms, Prognosis, Survival
Introduction
Of patients diagnosed with colorectal cancer, approximately 30% will eventually die of metastatic disease [1]. Systemic combination chemotherapy is the mainstay treatment for patients with metastatic colorectal cancer (mCRC), and the introduction of targeted molecular agents has significantly improved patient prognoses [2-4].
In palliative settings, response to chemotherapy and subsequent treatment decisions are generally based on radiologic assessment, the Response Evaluation Criteria in Solid Tumors (RECIST), and clinical information such as symptoms and the results of physical examinations [5,6]. Carcinoembryonic antigen (CEA) is an important tumor marker in mCRC [7] that can be used to monitor patients with metastatic disease [8,9]. However, the relationship between the extent of CEA level change and chemotherapy response remains undefined. In clinical settings, response to chemotherapy is generally evaluated based on radiologic response together with CEA changes. The outcome of chemotherapy is usually clear if these two parameters are consistent. However, when the parameters are discordant, (e.g., partial response [PR] on radiology but rising CEA levels, or progressive disease [PD] on radiology concomitant with decreasing CEA levels), the tumor response to chemotherapy is uncertain. In cases with discordant responses, the radiological response usually takes precedence; however, the clinical significance of a change in CEA patterns while radiologic response remains unchanged has not been investigated. Therefore, we conducted a retrospective study of the prognostic impact of different CEA change patterns in patients with the same RECIST categories post-chemotherapy.
Materials and Methods
1. Study population
The study design was approved by the Institutional Review Board of Seoul St. Mary's Hospital. We evaluated 563 patients who received first-line palliative chemotherapy for mCRC during 2008-2014. Of these, 142 patients who had no measurable lesions, as well as nine who had other malignancies within the previous 5 years were excluded as they could have affected CEA levels. Moreover, 52 patients with initial CEA levels that were within the normal limit (< 5 ng/mL) were also excluded. All diagnoses were confirmed via biopsy or examination of the surgical specimen from the primary tumor. Histological types were classified as well/moderate differentiation or poor differentiation/mucinous adenocarcinoma/signet ring cell adenocarcinoma. Metastatic presentation was defined as metachronous or synchronous [10].
2. Chemotherapy protocol and tumor response evaluation
All patients received fluorouracil and leucovorin plus oxaliplatin (FOLFOX) or plus irinotecan (FOLFIRI) with or without target agents (bevacizumab or cetuximab) as first-line palliative chemotherapy. The initial CEA and imaging tests included abdominal and chest computed tomography or magnetic resonance imaging 1 week before initiation of chemotherapy. Patients were subsequently evaluated for chemotherapy response with CEA testing and imaging studies every 8±2 weeks. Radiological changes were evaluated using RECIST [11], and the first imaging response evaluation was defined as “RECIS-T-response.” In RECIST-complete response (CR)/PR/stable disease (SD) patients, the same chemotherapy regimen was maintained, and chemotherapy response was assessed using RECIST at the subsequent evaluation session, whereupon the response was defined as the “second RECIST-response.”
3. Definition of CEA-response
Serum CEA levels were measured using an electro-chemiluminescent immunoassay (normal, < 5 ng/mL). A previous study [12] showed that the CEA ratio was significantly correlated with RECIST-response (as determined by imaging) as long as changes in CEA levels were ±50%; therefore, the change patterns in CEA (baseline to first post-chemotherapy evaluation) were defined as CEA-CR (< 5 ng/mL), CEA-PR (≥ 50% decrease in CEA levels while maintaining absolute values ≥ 5 ng/mL), CEA-PD (≥ 50% increase in CEA levels), and CEA-SD (change in CEA levels that did not qualify as CEA-CR/-PR/-PD) at the time of the first response evaluation. These change patterns are collectively referred to as “CEA-response.”
4. Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were calculated from the date at which first-line palliative chemotherapy was started until the date of death and of disease progression, respectively. Objective response was defined as CR or PR, while disease control was defined as CR, PR, or SD. The cut-off for RECIST PR was 50% tumor shrinkage, whereas that for RECIST SD was 0% tumor shrinkage. For survival analyses, patients who were alive or had no disease progression were censored at the date of last contact. Univariate analyses for OS and PFS were conducted using the Kaplan-Meier method with the log-rank test. Multivariate Cox regression models were employed to verify the prognostic values of CEA-response, and were adjusted for age, sex, cancer location, histological type, metastatic presentation, number of metastatic organs, and first-line chemotherapy regimen. The correlations between CEA-response and first or second RECIST-response, as well as between CEA-response and extent of tumor shrinkage, were analyzed using the linear by linear association test. All analyses were conducted using the SPSS ver. 21 (IBM Corp., Armonk, NY), and a two-sided p-value of < 0.05 was considered statistically significant.
Results
1. Patient characteristics and survival according to clinicopathologic factors
We included 360 patients (64.7% men; 35.3% women) with a median age of 63 years (range, 23 to 88 years), among which 63.9% of patients had colon cancer and 36.1% had rectal cancer. The median follow-up time was 21.8 months. At the study end date, 285 patients (79.1%) had experienced disease progression and 261 (72.5%) had died. The patient characteristics are described in Table 1. Univariate analyses (S1 Table) revealed that poor survival was significantly associated with multiple metastatic organs (PFS hazard ratio [HR], 1.27; p=0.04). Chemotherapy with a target agent produced better survival than chemotherapy alone (OS HR, 0.68; p=0.01; PFS HR, 0.6; p=0.002). Poorer CEA-response was associated with poor survival. Moreover, multivariate analyses revealed that a poor prognosis was independently associated with synchronous metastasis (OS HR, 1.37; p=0.03) and multiple metastatic organs (PFS HR, 1.32; p=0.02). Chemotherapy with target agents was a significantly favorable prognostic factor for disease progression (PFS HR, 0.72; p=0.02). Poorer CEA-response was associated with poor OS and PFS.
Table 1.
Baseline characteristics
| Characteristic | No. (%) (n=360) |
|---|---|
| Age, median (range, yr) | 63 (23-88) |
| < 65 | 204 (56.7) |
| ≥ 65 | 156 (43.3) |
| Sex | |
| Female | 127 (35.3) |
| Male | 233 (64.7) |
| Location | |
| Colon/S-colon | 230 (63.9) |
| Rectum | 130 (36.1) |
| Histological type | |
| Well/Moderate differentiation | 304 (84.4) |
| Poor differentiation/Mucinous/Signet ring cell | 56 (15.6) |
| Metastatic presentation | |
| Metachronous | 128 (35.6) |
| Synchronous | 232 (64.4) |
| No. of metastatic organs | |
| Only one (1) | 204 (56.7) |
| More than one (≥ 2) | 156 (43.3) |
| First-line chemotherapy | |
| FOLFOX/FOLFIRI | 260 (72.2) |
| FOLFOX/FOLFIRI+targeting agent (bevacizumab or cetuximab) | 100 (27.8) |
| Initial CEA, median (range, ng/mL) | 24.62 (0.10-5,158.00) |
Values are presented as number (%). FOLFOX, oxaliplatin plus fluorouracil and leucovorin; FOLFIRI, cetuximab plus irinotecan, fluorouracil, and leucovorin; CEA, carcinoembryonic antigen.
2. Evaluation of tumor response using both CEA- and RECIST-response
Table 2 shows the correlation between CEA-response and RECIST-response. The CEA-CR and CEA-PR groups exhibited better objective response rates than the CEA-SD and CEA-PD groups (p < 0.001). Additionally, a more favorable CEA-response was significantly associated with a better disease control rate (p < 0.001). We assessed the prognostic implications of CEA-response as a second response evaluation method for patients in the same RECIST-response categories (Table 3). In RECIST-PR patients, poorer CEA-response was significantly associated with disease progression at the time of the second response evaluation (p < 0.001). Furthermore, an improved CEA-response in RECIST-SD patients was significantly associated with less disease progression than a poorer CEA-response at the second response evaluation (p < 0.001).
Table 2.
Correlation between CEA-response and RECIST-response
| RECIST-response |
||||||||
|---|---|---|---|---|---|---|---|---|
| CR (n=11) | PR (n=168) | SD (n=114) | PD (n=67) | ORR (n=179) | p-value | DCR (n=293) | p-value | |
| CEA-response | ||||||||
| CEA-CR (n=53) | 3 (27.3) | 36 (21.4) | 7 (6.1) | 7 (10.4) | 39 (73.6) | < 0.001 | 46 (86.8) | < 0.001 |
| CEA-PR (n=124) | 3 (27.3) | 77 (45.8) | 35 (30.7) | 9 (13.4) | 80 (64.5) | 115 (92.7) | ||
| CEA-SD (n=107) | 3 (27.3) | 35 (20.8) | 45 (39.5) | 24 (35.8) | 38 (35.5) | 83 (77.6) | ||
| CEA-PD (n=76) | 2 (18.1) | 20 (12.0) | 27 (23.7) | 27 (40.3) | 22 (28.9) | 49 (64.5) | ||
Values are presented as number (%). CEA, carcinoembryonic antigen; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Table 3.
Correlation between CEA-response and second RECIST-response
| Second RECIST-response | In RECIST-CR patients |
In RECIST-PR patients |
In RECIST-SD patients |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-PD (n=9) | PD (n=2) | p-value | Non-PD (n=140) | PD (n=28) | p-value | Non-PD (n=85) | PD (n=29) | p-value | |
| CEA-response | |||||||||
| CEA-CR | 3 (100) | 0 | 0.113 | 33 (91.7) | 3 (8.3) | < 0.001 | 7 (100) | 1 (10) | < 0.001 |
| CEA-PR | 3 (100) | 0 | 67 (87.0) | 10 (13.0) | 31 (88.6) | 4 (11.4) | |||
| CEA-SD | 2 (66.7) | 1 (33.3) | 29 (82.9) | 6 (17.1) | 33 (73.3) | 12 (26.7) | |||
| CEA-PD | 1 (50.0) | 1 (50.0) | 11 (55.5) | 9 (45.0) | 14 (51.9) | 13 (48.1) | |||
Values are presented as number (%). CEA, carcinoembryonic antigen; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
We investigated the correlation between CEA-response and tumor shrinkage at the first response evaluation among assessable RECIST-PR and RECIST-SD patients (136 of 168 and 92 of 114, respectively) (Table 4, Fig. 1). In RECIST-PR and RECIST-SD patients, the median tumor shrinkage was 50% and 2%, respectively; therefore, we set the cut-off values of tumor shrinkage at 50% and 0%, respectively. Improved CEA-response was correlated with marked tumor shrinkage (> 50%) in the RECIST-PR patients (p < 0.001), whereas CEA-response was not correlated with the extent of tumor shrinkage (> 0%) in RECIST-SD patients (p=0.105). We also investigated the correlation between CEA-response, RECIST-response, and tumor shrinkage according to target agents (S2 and S3 Tables). Among patients receiving cetuximab, a more favorable CEA-response was significantly associated with better objective response rates (p=0.046); however, this was not the case among patients receiving bevacizumab. Additionally, improved CEA-response was correlated with marked tumor shrinkage (> 50%) in RECIST-PR patients receiving cetuximab (p=0.043), but not in those receiving bevacizumab. However, CEA-response was not correlated with the extent of tumor shrinkage (> 0%) in RECIST-SD patients in either the cetuximab or bevacizumab groups.
Table 4.
Correlation between CEA-response and extent of tumor shrinkage
| Tumor shrinkage in assessable patients |
||||||||
|---|---|---|---|---|---|---|---|---|
| In RECIST-PR patients |
In RECIST-SD patients |
|||||||
| Median (%) | ≤ 50% | > 50% | p-value | Median (%) | ≤ 0% | > 0% | p-value | |
| CEA-CR | 57 | 8 (27.6) | 21 (72.4) | < 0.001 | 22 | 2 (33.3) | 4 (66.7) | 0.105 |
| CEA-PR | 51 | 30 (48.4) | 32 (51.6) | 3 | 12 (38.7) | 19 (61.3) | ||
| CEA-SD | 36 | 19 (67.9) | 9 (32.1) | 3 | 14 (41.2) | 20 (58.8) | ||
| CEA-PD | 34 | 13 (76.5) | 4 (23.5) | –17 | 13 (61.9) | 8 (38.1) | ||
Values are presented as number (%) unless otherwise indicated. In RECIST-PR and RECIST-SD patients, the median percentages of tumor shrinkage were 50% and 2%, respectively. Therefore, the cut-off value for tumor shrinkage was set at 50% and 0% in RECIST-PR and RECIST-SD patients, respectively. CEA, carcinoembryonic antigen; RECIST, Response Evaluation Criteria in Solid Tumors; PR, partial response; SD, stable disease; CR, complete response; PD, progressive disease.
Fig. 1.
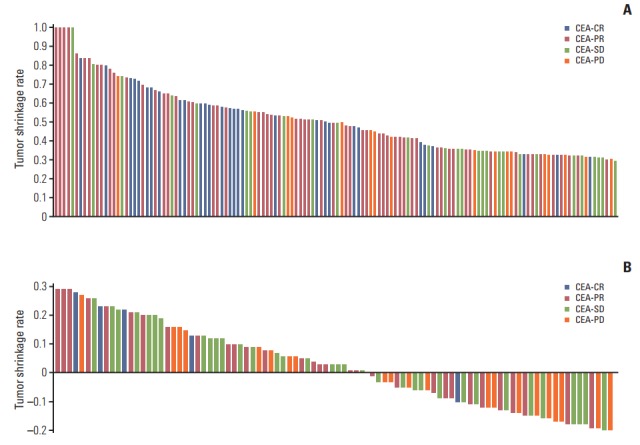
Distribution of tumor shrinkage rates according to CEA-response in RECIST-PR (A) and RECIST-SD (B) patients at the time of first response evaluation. CEA, carcinoembryonic antigen; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumors.
3. Prognostic implications of discordant CEA-responses in patients with the same RECIST-response
Among all patients, the CEA-CR group exhibited better OS (42.2±5.6 months) and PFS (15.4±2.0 months) than the CEA-PR (OS, 25.1±1.9 months; PFS, 11.5±0.8 months), CEA-SD (OS, 17.0±1.2 months; PFS, 7.6±0.7 months), and CEA-PD groups (OS, 12.2±1.0 months; PFS, 4.2±0.5 months) (p < 0.001). We evaluated survival outcomes according to CEA-response in RECIST-PR or -SD patients (Fig. 2). In RECIST-PR patients, better CEA-response showed longer OS and PFS (all p < 0.001), while in RECIST-SD patients, better CEA-response was correlated with a more favorable OS than a poorer CEA-response (p < 0.001). Additionally, CEA-CR and PR showed longer PFS in RECIST-SD patients (p < 0.001). Moreover, there was no significant difference in OS according to CEA-response in RECIST-PD patients (p=0.082). Evaluation of the prognostic impact of CEA-response in the same RECIST-response patients revealed discordant CEA-response (CR, SD, and PD) among RECIST-PR patients was associated with different prognoses upon univariate analysis (S4 Table). Multivariate analysis (Table 5) showed that CEA-SD and CEA-PD were poor prognostic factors for OS (CEA-SD: HR, 3.13; p < 0.001; CEA-PD: HR, 6.43; p < 0.001) and PFS (CEA-SD: HR, 2.40; p=0.004; CEA-PD: HR, 3.81; p < 0.001) in RECIST-PR patients. Among RECIST-SD patients, univariate analysis showed that discordant CEA-responses (PR and PD) have significantly different and opposing effects on survival (S4 Table). Furthermore, multivariate analysis (Table 5) indicated that discordant CEA-responses have varying prognostic impacts on OS (CEA-PR: HR, 0.53; p=0.03; CEA-PD: HR, 1.77; p=0.04). Additionally, discordant CEA-responses have varying prognostic effects on PFS (CEA-PR: HR, 0.48; p=0.03; CEA-PD: HR, 1.86; p=0.03). Among RECIST-PD patients (S5 Table), multivariate analysis showed that CEA-CR was associated with longer OS than CEA-PD (HR, 0.37; p=0.04).
Fig. 2.
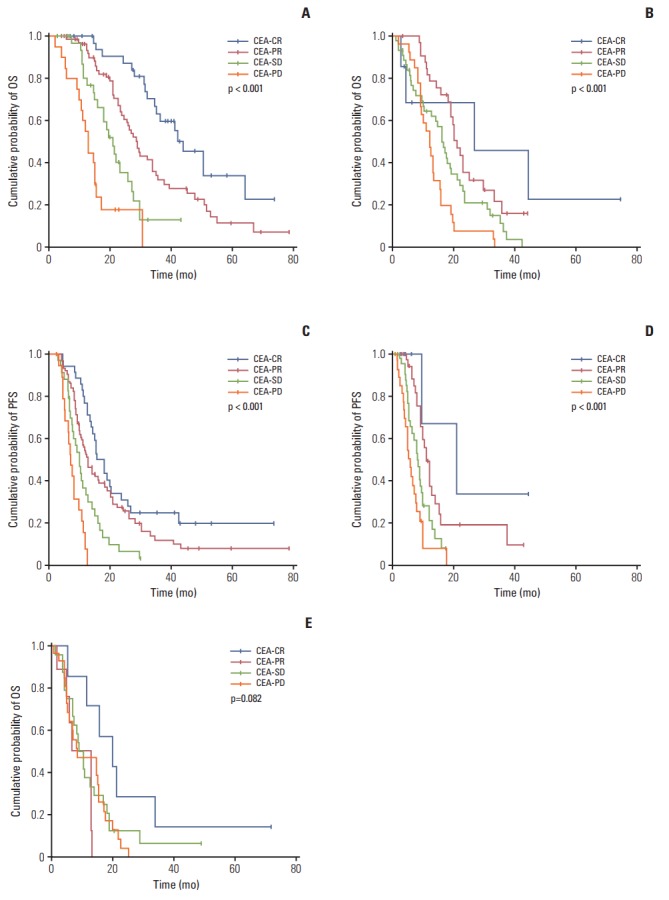
Cumulative survival rates according to CEA-response in patients with different RECIST evaluations. In patients with RECIST-PR (A and C), a discordant CEA-response (CEA-PD/SD) showed poorer survival than CEA-CR/PR (median OS and PFS: 44.0±5.9 and 15.4±1.9 months [CEA-CR], 28.9±1.8 and 12.5±1.3 [CEA-PR], 21.0±2.1 and 9.8±1.0 [CEA-SD], and 13.0±1.1 and 7.0±0.8 [CEA-PD], respectively; all p < 0.001). In patients with RECIST-SD (B and D), a more favorable CEA-response demonstrated better OS and PFS (median OS and PFS: 26.8±19.6 and 21.0±9.3 months [CEA-CR], 21.0±1.4 and 11.0±0.8 [CEA-PR], 16.1±1.4 and 8.2±0.8 [CEA-SD], and 12.2±1.1 and 6.0±0.7 [CEA-PD], respectively; all p < 0.001). In patients with RECIST-PD (E), there was no significant difference in OS according to CEA-response (median OS: 20.1±5.8 months [CEA-CR], 13.0±4.8 [CEA-PR], 9.0±1.4 [CEA-SD], and 8.7±4.6 [CEA-PD]; p=0.082). (A) OS in RECIST-PR patients, (B) OS in RECIST-SD patients, (C) PFS in RECIST-PR patients, (D) PFS in RECIST-SD patients, (E) OS in RECST-PD patients. CEA, carcinoembryonic antigen; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumors; OS, overall survival; PFS, progression-free survival.
Table 5.
Multivariate analysis of OS and PFS according to the first same RECIST-response
| In RECIST-PR patients |
In RECIST-SD patients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS |
PFS |
OS |
PFS |
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | ||||||||||||
| < 65 | 1 | 1 | 1 | 1 | ||||||||
| ≥ 65 | 0.89 | 0.58-1.37 | 0.60 | 0.72 | 0.48-1.06 | 0.10 | 1.45 | 0.90-2.33 | 0.13 | 0.82 | 0.49-1.34 | 0.43 |
| Sex | ||||||||||||
| Female | 1 | 1 | 1 | 1 | ||||||||
| Male | 0.83 | 0.54-1.28 | 0.40 | 0.81 | 0.56-1.17 | 0.27 | 0.62 | 0.38-1.00 | 0.05 | 1.30 | 0.74-2.26 | 0.36 |
| Location | ||||||||||||
| Colon/S-colon | 1 | 1 | 1 | 1 | ||||||||
| Rectum | 1.09 | 0.71-1.65 | 0.70 | 1.01 | 0.68-1.48 | 0.96 | 0.93 | 0.58-1.49 | 0.78 | 1.10 | 0.65-1.86 | 0.72 |
| Histological type | ||||||||||||
| Well/Moderate differentiation | 1 | 1 | 1 | 1 | ||||||||
| Poor differentiation/Mucinous/Signet ring cell | 1.19 | 0.66-2.14 | 0.57 | 1.07 | 0.64-1.78 | 0.80 | 2.07 | 1.17-3.65 | 0.01 | 1.14 | 0.61-2.12 | 0.68 |
| Metastatic presentation | ||||||||||||
| Metachronous | 1 | 1 | 1 | 1 | ||||||||
| Synchronous | 1.69 | 0.96-2.99 | 0.07 | 1.24 | 0.76-2.01 | 0.39 | 1.62 | 1.02-2.54 | 0.04 | 1.50 | 0.92-2.44 | 0.10 |
| No. of metastatic organs | ||||||||||||
| Only one (1) | 1 | 1 | 1 | 1 | ||||||||
| More than one (≥ 2) | 1.30 | 0.86-1.96 | 0.21 | 1.39 | 0.96-2.01 | 0.08 | 0.72 | 0.46-1.11 | 0.14 | 1.43 | 0.89-2.29 | 0.14 |
| First-line chemotherapy | ||||||||||||
| FOLFOX/FOLFIRI | 1 | 1 | 1 | 1 | ||||||||
| FOLFOX/FOLFIRI+targeting agent | 0.74 | 0.47-1.16 | 0.19 | 0.75 | 0.50-1.11 | 0.15 | 1.07 | 0.59-1.92 | 0.81 | 1.19 | 0.65-2.16 | 0.56 |
| CEA-response | ||||||||||||
| CEA-CR | 0.52 | 0.29-0.90 | 0.02 | 0.74 | 0.45-1.20 | 0.23 | 0.38 | 0.10-1.37 | 0.14 | 0.22 | 0.04-1.05 | 0.06 |
| CEA-PR | 1 | 1 | 0.53 | 0.30-0.93 | 0.03 | 0.48 | 0.25-0.92 | 0.03 | ||||
| CEA-SD | 3.13 | 1.69-5.78 | < 0.001 | 2.40 | 1.456-3.944 | 0.004 | 1 | 1 | ||||
| CEA-PD | 6.43 | 3.31-12.49 | < 0.001 | 3.81 | 2.287-6.334 | < 0.01 | 1.77 | 1.01-3.09 | 0.04 | 1.86 | 1.04-3.31 | 0.03 |
OS, overall survival; PFS, progression free survival; RECIST, Response Evaluation Criteria in Solid Tumors; PR, partial response; SD, stable disease; HR, hazard ratio; CI, confidence interval; FOLFOX, oxaliplatin plus fluorouracil and leucovorin; FOLFIRI, cetuximab plus irinotecan, fluorouracil, and leucovorin; CEA, carcinoembryonic antigen; CR, complete response; PD, progressive disease.
Discussion
We found that the number of metastases and target agents used were associated with prognosis, which is consistent with the results of previous studies [13,14]. However, we also showed that synchronous metastasis was associated with poor prognosis, which is contrary to the findings of a previous study [10]; therefore, further investigations are required to clarify these findings.
Our study showed that CEA-response is an important prognostic factor in mCRC that is highly associated with RECIST-response. Huang et al. [12], whose data were consistent with ours, suggested that CEA change patterns are highly correlated with findings on images acquired for RECIST determination.
Discordance between CEA- and RECIST-responses can lead clinicians to question the actual effects of chemotherapy; however, the impact of such discordance in mCRCs has not been fully determined. To the best of our knowledge, this is the first study to investigate the clinical significance of the discordance between CEA-response and RECIST-response in mCRC patients. Our findings showed that different CEA-responses in the same RECIST-response patients have important, yet varying prognostic impacts on mCRC prognosis.
In the present study, poor CEA-response in RECIST-PR patients was associated with disease progression and shorter OS. This result can likely be explained by the early acquisition of tumor resistance to chemotherapy. Generally, response evaluation is performed after 3-4 cycles of chemotherapy. Even though tumor size may have decreased temporarily after 1-2 cycles, chemoresistance may have developed during the latter cycles. Hence, the tumor size is still smaller than shown on baseline imaging for RECIST evaluation, while the tumor marker levels are rapidly increasing because the cancer is again progressing, resulting in a discordance between CEA- and RECIST-responses.
In our study, 8% of patients with CEA-CR and 13% of those with CEA-PR showed disease progression at the time of the second response evaluation; however, approximately 50% of patients with CEA-PD had disease progression at that point. Based on these data, we suggest that an earlier second response evaluation would be beneficial for these patients, and that an individualized next response evaluation for patients with poor CEA-response/good RECIST-response status may be necessary. For example, Neki et al. [15] proposed that circulating tumor cell presence after chemotherapy might be useful for predicting the response to anticancer therapy. It is also necessary to identify host factors related to early chemoresistance, as suggested by previous studies of colon cancer [16,17]; however, suitable biomarkers of chemoresistance remain elusive despite several ongoing studies.
The discordant pattern of poor RECIST-response and favorable CEA-response is also worth investigating. Among patients with RECIST-PD, those who achieved CEA-CR showed longer OS than those with CEA-PD. Similarly, in RECIST-SD patients, those with better CEA-response showed better second response and survival outcomes than those with poorer CEA-response. This may be explained by a delayed chemotherapy response. It is also possible that tumor size does not decrease on imaging modalities, even though tumor viability has decreased [18]. Hence, it could be helpful to evaluate metabolic activity using positron emission tomography with fluorodeoxyglucose, which can reflect pathologic responses related to prognosis in mCRC [19-22]. These multimodal response evaluations could help prevent mistaking chemosensitivity for chemoresistance, since treatment options are still limited.
The results of our study showed that improved CEA-response was associated with greater tumor shrinkage in RECIST-PR patients, which clearly demonstrates the relevance of CEA-response to outcomes in the same RECIST-response patients. Our findings can support previous studies [23,24] which showed that the extent of tumor shrinkage is significantly associated with outcomes. In particular, our findings suggest that CEA-response may reflect better tumor shrinkage in patients receiving cetuximab compared to those treated with bevacizumab. These findings are consistent with those of a previous study that showed higher response rates and depth of responses following first-line treatment with anti–epidermal growth factor receptor than with anti–vascular endothelial growth factor agents in RAS wild-type cancer [25]. However, our results should be interpreted with caution because of the small number of targeting agents used.
It should be noted that this study had several limitations. First, baseline CEA and RECIST responses were measured on different days for some patients. Even though our study set the interval between the start of chemotherapy and CEA/radiology testing at less than 1 week, discordant patterns could be affected by differences in the time of measurement in patients with very rapid progression. Thus, minimizing the time gap between chemotherapy and baseline studies may be important for accurate response evaluation. Second, there is no established cut-off value for the CEA-response. We set a 50% increase or decrease rate for CEA level in our study, while other studies proposed different CEA-response cut-offs [5,25]; therefore, it is necessary to establish a more definitive threshold. Finally, our study was retrospective and therefore subject to the known limitations and biases of such investigations.
Among patients with same RECIST responses, discordant CEA-response patterns are strongly predictive of second response evaluation outcomes and are a significant prognostic marker. These patients would likely benefit from individualized and detailed multimodal chemotherapy response evaluation.
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
References
- 1.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375:1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 4.Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014;19:1156–68. doi: 10.1634/theoncologist.2014-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim G, Jung EJ, Ryu CG, Hwang DY. Usefulness of carcinoembryonic antigen for monitoring tumor progression during palliative chemotherapy in metastatic colorectal cancer. Yonsei Med J. 2013;54:116–22. doi: 10.3349/ymj.2013.54.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101:396–400. doi: 10.1002/jso.21495. [DOI] [PubMed] [Google Scholar]
- 8.Ward U, Primrose JN, Finan PJ, Perren TJ, Selby P, Purves DA, et al. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer. 1993;67:1132–5. doi: 10.1038/bjc.1993.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preketes AP, King J, Caplehorn JR, Clingan PR, Ross WB, Morris DL. CEA reduction after cryotherapy for liver metastases from colon cancer predicts survival. Aust N Z J Surg. 1994;64:612–4. doi: 10.1111/j.1445-2197.1994.tb02302.x. [DOI] [PubMed] [Google Scholar]
- 10.Mekenkamp LJ, Koopman M, Teerenstra S, van Krieken JH, Mol L, Nagtegaal ID, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103:159–64. doi: 10.1038/sj.bjc.6605737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Huang SC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, et al. Concordance of Carcinoembryonic Antigen Ratio and Response Evaluation Criteria in Solid Tumors as Prognostic Surrogate Indicators of Metastatic Colorectal Cancer Patients Treated with Chemotherapy. Ann Surg Oncol. 2015;22:2262–8. doi: 10.1245/s10434-014-4228-y. [DOI] [PubMed] [Google Scholar]
- 13.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–8. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 15.Neki K, Kawahara H, Watanabe K, Toyama Y, Akiba T, Yanaga K. Usefulness of circulating tumor cells after preliminary chemotherapy for prediction of response to further anticancer therapy in patients with initially unresectable metastatic colorectal cancer. Anticancer Res. 2013;33:1769–72. [PubMed] [Google Scholar]
- 16.Zhao J, Li W, Zhu D, Yu Q, Zhang Z, Sun M, et al. Association of single nucleotide polymorphisms in MTHFR and ABCG2 with the different efficacy of first-line chemotherapy in metastatic colorectal cancer. Med Oncol. 2014;31:802. doi: 10.1007/s12032-013-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Xu Y, Cai S. Specific microRNAs as novel biomarkers for combination chemotherapy resistance detection of colon adenocarcinoma. Eur J Med Res. 2015;20:95. doi: 10.1186/s40001-015-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 19.Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, et al. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer. 2013;108:2549–56. doi: 10.1038/bjc.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 22.Walker AS, Zwintscher NP, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, et al. Future directions for monitoring treatment response in colorectal cancer. J Cancer. 2014;5:44–57. doi: 10.7150/jca.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26:1188–94. doi: 10.1093/annonc/mdv112. [DOI] [PubMed] [Google Scholar]
- 24.Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764–75. doi: 10.1200/JCO.2012.42.8532. [DOI] [PubMed] [Google Scholar]
- 25.Michl M, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial) Ann Oncol. 2016;27:1565–72. doi: 10.1093/annonc/mdw222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


