Abstract
Background and Purpose
Despite the social, health, and economic burdens associated with cognitive impairment post-stroke, there is considerable uncertainty about the types of interventions that might preserve or restore cognitive abilities. The objective of this systematic review and meta-analysis was to evaluate the effects of physical activity (PA) training on cognitive function post-stroke and identify intervention and sample characteristics that may moderate treatment effects.
Methods
Randomized controlled trials examining the association between structured PA training and cognitive performance post-stroke were identified using electronic databases EMBASE and MEDLINE. Intervention effects were represented by Hedges' g and combined into pooled effect sizes using random- and mixed-effects models. Effect sizes were subjected to moderation analyses using the between-group heterogeneity test.
Results
Fourteen studies met inclusion criteria, representing data from 736 participants. The primary analysis yielded a positive overall effect of PA training on cognitive performance (Hedges' g [95% CI]=0.304[0.14-0.47]). Mixed-effects analyses demonstrated that combined aerobic and strength training programs generated the largest cognitive gains, and that improvements in cognitive performance were achieved even in the chronic stroke phase (mean=2.6 years post-stroke). Positive moderate treatment effects were found for attention/processing speed measures (Hedges' g [CI]=0.37[0.10-0.63]), while the executive function and working memory domains did not reach significance (p>0.05).
Conclusions
We found a significant positive effect of PA training on cognition post-stroke with small to moderate treatment effects that are apparent even in the chronic stroke phase. Our findings support the use of PA training as a treatment strategy to promote cognitive recovery in stroke survivors.
Keywords: stroke, exercise, physical activity, cognition, meta-analysis, systematic review
Introduction
Cognitive impairment occurs in up to 83% of stroke survivors, and is associated with reduced quality of life, accelerated functional decline, and increased risk of dependent living and mortality.1-3 Given the lack of successful pharmaceutical treatments for cognitive decline and dementia, identifying alternative treatment strategies that mitigate cognitive deficits after stroke is a public health imperative.
Physical activity (PA) holds promise as a widely accessible, low-cost treatment that may preserve or restore cognitive abilities post-stroke. Randomized controlled trials suggest that structured PA training improves cognitive function in other populations susceptible to cognitive decline, including neurologically healthy older adults4 and those with dementia.5 According to data from randomized controlled trials, stroke survivors may also achieve cognitive gains through PA training. One meta-analysis to date has synthesized data from randomized controlled trials to quantitatively evaluate the impact of PA training on cognitive outcomes post-stroke.6 Results revealed a small but significant benefit of PA training on a domain-general estimate of global cognition across the 9 included studies. However, since this publication in 2012, the number of PA intervention trials investigating this relationship in stroke survivors has nearly doubled.7-13 Furthermore, in contrast to earlier studies14-19, cognition has been the primary outcome of interest in recent trials and thus more comprehensive neuropsychological batteries have been employed.9-12,20 Given the increase in available data, an updated quantitative synthesis incorporating the latest studies and investigating the domain specificity of PA effects on cognition is warranted. In addition to characterizing the effects of PA training on cognition following a stroke, there is also a need to identify the population characteristics and particular training parameters that may generate the largest cognitive gains.
Here, we provide a meta-analytic review of the effects of PA training on cognition in stroke survivors. In addition to determining whether cognition is enhanced with PA training, we also examine whether PA has broad or selective effects on cognitive function by assessing 3 domains characteristically affected in stroke: executive function, attention and processing speed, and working memory. Finally, for the first time, we evaluate study and sample characteristics that might moderate the efficacy of PA training, including intervention length, the mode of PA, and time from stroke onset to initiation of the intervention. Determining the particular intervention and population characteristics that are most likely to maximize cognitive gains could have important implications for cognitive and functional recovery following a stroke.
Methods
Search Criteria
We conducted a systematic search of the literature using electronic databases MEDLINE and EMBASE for human studies published in English or Chinese in peer-reviewed journals from the earliest available record up to November 14, 2016. The keywords of the search were combined with the following terms: (physical activity OR physiotherapy OR fitness OR aerobic OR exercise OR resistance training) AND (cognitive function OR cognition OR attention OR memory OR executive function OR neuropsychological test) AND (stroke OR cerebrovascular accident OR brain ischemia OR poststroke OR post-stroke). Reference lists from included articles were manually examined to identify other potentially relevant manuscripts.
Study Selection
Studies were selected for inclusion according to the following criteria: 1) recruitment of stroke survivors ≥ 18 years old (ischemic or hemorrhagic stroke, any location, number of strokes) 2) randomized controlled trials that included a clearly defined control condition and an experimental condition that included a component that aimed to increase PA (aerobic exercise, resistance training, or physiotherapy), 3) duration of training > 4 weeks to provide sufficient time for benefits to accrue and 4) included a validated neuropsychological test of cognition with data reported at baseline and post-intervention. Due to the limited number of studies, no restrictions were placed on the characteristics of control groups. Studies including subjects with other neurological conditions were excluded unless separate data were provided for the stroke subset. For studies in which training participation was not monitored (i.e., home-based), a change in physical function from pre- to post-intervention, represented by changes in performance on objective PA or fitness tests, must have been observed. This was to increase the likelihood that adherence to PA recommendations were followed. Two studies included in a prior meta-analysis6 did not meet these inclusion criteria and were excluded from the present study (please see http://stroke.ahajournals.org).
Data Collection and Extraction
One member of the research team (LEO) performed the initial search and removed titles and abstracts that were clearly outside of the scope of the review. Two members of the research team (LEO and AMW) independently assessed remaining titles and abstracts and obtained the full text for all abstracts that 1) did not provide enough data for exclusion or 2) appeared potentially eligible for inclusion. LEO and AMW then independently assessed full text articles and selected studies for inclusion based on information within the full-text. Any conflicts in article selection were resolved by consensus among the primary raters, and KE was consulted if further clarification was required. Data were extracted from the full texts by one member of the review team (LEO) using a standard template, and was independently verified by a second member of the research team (AMW). The extracted data included study, participant, and intervention characteristics, and cognitive outcome data (for more detail, see online supplement). To achieve a high standard of reporting, we followed the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses' (PRISMA) guidelines.21
Quality Assessment
The methodological quality of included trials was evaluated using the Cochrane risk-of-bias tool.22 The Cochrane collaboration specifies 6 potential sources of bias, including sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, and selective outcome reporting. Risk of bias and quality of evidence was independently assessed by two members of the research team (LEO, AMW), and was rated per domain based on the published study reports (or, if applicable, based on information from related protocols) as low, unclear (insufficient detail or not reported), or high risk of bias, according to Section 8.5 of the Cochrane handbook.22 This tool is commonly used to assess bias in PA intervention studies and conforms to the PRISMA guidelines.21
Calculation of Effect Sizes
Data were entered into an electronic database and analyzed using Comprehensive Meta-Analysis (CMA) software, version 2.23 Intervention effects of each study were represented by Hedges' g, a bias adjusted estimate of the standardized mean difference that applies an additional correction for small sample sizes. Hedges' g was calculated by subtracting the mean change in performance from baseline in the control group from the experimental group, and dividing the difference by the pooled standard deviation of the change from baseline. Using Cohen's criteria, effect sizes were interpreted as small (≤ 0.2), moderate (0.5) or large (≥ 0.8).24 Weighted mean effect size values, along with standard errors and 95% confidence intervals, were estimated using random-effects or mixed-effects models. Random effects models are considered more conservative than a fixed-effects approach, and assume that the true treatment effect may differ across studies due to expected variations in sample, intervention, and assessment characteristics. Mixed-effects models were used for all moderation analyses, as this approach assumes that variability between studies may be attributable to fixed and random components as well as subject-level sampling error.25 Data were analyzed using an intent-to-treat framework, and therefore sample sizes at baseline, rather than post-intervention, were considered in the calculation of effect size. The sign of the effect was calculated so that positive effect sizes were indicative of an improvement in cognitive performance among the experimental groups, relative to controls.
Quantitative Data Synthesis and Analysis
Using a random effects model, we first assessed whether participation in PA training resulted in significant cognitive gains relative to control samples. For trials reporting multiple cognitive outcomes, effect sizes were calculated separately for each test and averaged together to obtain an overall effect size estimate for each study. This approach ensured that each study was represented by no more than one effect size, and allowed us to assess the overall or domain-general effect of PA on cognition post-stroke. Heterogeneity was evaluated using the Chi-squared (Q) and I2 statistics, according to procedures outlined in the Cochrane handbook.22 Consistent with these guidelines, a p-value of < 0.10 indicated statistically significant heterogeneity between studies.
In addition, several a priori analyses were conducted to investigate whether sample and study characteristics moderate the effects of PA training on cognitive performance. Intervention characteristics included trial duration (< 3 months or ≥ 3 months), mode of exercise (aerobic, stretching and toning/physiotherapy, or combined aerobic and stretching and toning) and type of cognitive assessment (objective vs. subjective). We also examined whether the time from stroke to initiation of the intervention (≤ 3 months post-stroke, > 3 months post-stroke) affected the magnitude of the effect size. Moderation effects were analyzed in mixed-effects models using the between-group heterogeneity (QB) test, which provides an estimate of the between-groups variance. Significance was set at p < 0.05.
We also evaluated whether there were domain-specific effects of PA training on cognitive performance. Of the 14 studies included in the meta-analysis, 5 employed multiple neuropsychological assessments that could be classified into different cognitive domains. Studies were not represented by a single effect size; instead, an effect size estimate was calculated for all cognitive outcomes within each study. The analysis included tests that could be classified into the following categories: attention and information processing speed, working memory, and executive function (supplementary materials: Table I, please see http://stroke.ahajournals.org).
Finally, a post hoc subgroup analysis assessed the overall effect of PA training on cognitive performance among studies that screened for and included participants with motor impairments (e.g., chronic hemiparesis including upper or lower limb paralysis, inability to walk without an assistive device (cane, walker)). This allowed us to assess whether stroke survivors with significant motor limitations were able to engage in PA training to the extent needed to generate cognitive gains.
Results
Characteristics of included studies
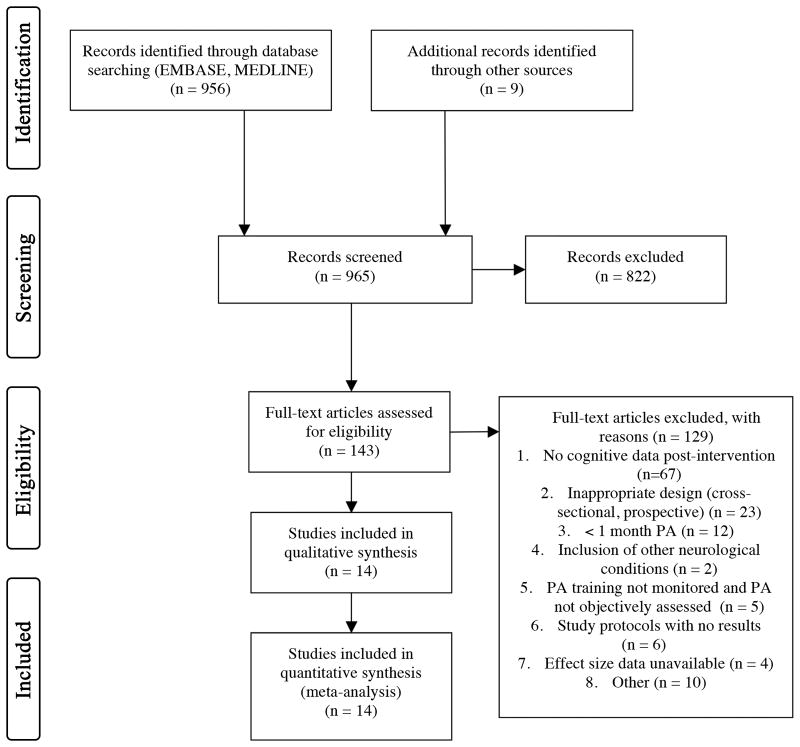
The initial search yielded a total of 956 potentially relevant studies, which was further refined to the retrieval of 143 full text articles. Of these, 14 randomized controlled trials met all inclusion criteria, representing data from 736 participants. The study selection process, including reasons for exclusion at the full-text level, is summarized in a PRISMA study flow diagram (Figure 1). All included studies had been published between November, 2000 and October, 2016. The studies varied in size, duration, and intervention type. Study sizes ranged from 14 to 156 and the average age was 62.5 (6.44), with a large range across studies (21-80 years). Mean age was similar across PA (61.93; SD 6.62) and control groups (63.11; SD 6.67). Men comprised approximately 59% of the sample across all studies. Among the PA training conditions, 5 studies involved stretching and toning/physiotherapy without a primary aerobic component, 3 trials consisted only of aerobic exercise training (e.g., treadmill, bicycle training), and 6 studies included a combined PA training program, involving a mixture of both aerobic exercise and stretching and toning activities. Six studies included control groups that received standard medical practice or waitlist control, 2 studies contained an active control condition that did not involve any form of PA (social communication, progressive muscle relaxation), and 6 studies included a control condition that involved PA without a primary aerobic component (stretching, toning, and balance). The effect size estimates stratified by control group can be found in the online supplement (please see http://stroke.ahajournals.org, Table II).
Figure 1.
PRISMA Study Selection Flow Diagram.
The average time since stroke was approximately 1.9 years (range 1 week – 5.1 years). Regarding stroke characteristics, all but 3 studies7,12,20 included subjects with ischemic and hemorrhagic stroke subtypes. Recurrent stroke was an exclusion criterion in 4 studies.7,10,15,20 In 7 studies, those with a history of dementia or probable clinically significant cognitive impairment were excluded 7,9,13,14,17,18,20 and in 3 studies individuals with mild cognitive impairment (MCI) or dementia were included.10,12,16 Baseline cognitive status was not reported in 4 studies.8,11,15,19 Attrition occurred in 8 studies and ranged from 3-18%. Further details regarding study characteristics can be found in the online supplement (please see http://stroke.ahajournals.org, Table III).
Quality Assessment
In total, 2 of the 14 studies were judged to be of high methodological quality, as they scored positively on all 6 of the quality criteria. The remainder of studies varied in the amount of methodological detail reported, with several quality indices not fully discussed in many trials. Information on allocation concealment was not reported in 50% and blinding of participants and study assessors was not reported in 57% and 21% of trials, respectively. The randomization method led to a high risk of bias in only 1 of the included studies. Although attrition occurred in 8 studies, only 2 met criteria for high risk of bias in outcome reporting according to the Cochrane guidelines. Details on the methodological quality of each study are presented in Table 1. Selective outcome reporting did not appear to occur in any of the included studies, and was therefore not included in Table 1.
Table 1.
Quality of intervention trials included in the meta-analysis, judged according to the Cochrane risk-of-bias tool.
| References | Sequence Generation | Allocation Concealment | Blinding (Participants) | Blinding (Assessors) | Incomplete Outcome Data |
|---|---|---|---|---|---|
| Chen, 2006 | Low | NR | NR | NR | Low |
| El-Tamawy, 2014 | NR | NR | NR | NR | Low |
| Fang, 2003 | Low | Low | NR | Low | High |
| Fernandez-Gonzalo, 2016 | Low | Low | NR | Low | Low |
| Immink, 2014 | Low | Low | NR | Low | Low |
| Liu-Ambrose, 2015 | Low | Low | Low* | Low | Low |
| Mead, 2007 | Low | Low | NR | Low | Low |
| Moore, 2014 | Low | NR | Low* | Low | Low |
| Nilsson, 2001 | Low | Low | Low | Low | High |
| Ozdemir, 2001 | High | NR | NR | High | Low |
| Quaney, 2009 | Low | NR | Low | Low | Low |
| Schachten & Jansen, 2015 | Low | NR | NR | NR | Low |
| Studenski, 2005 | Low | NR | Low* | Low | Low |
| Tang, 2016 | Low | Low | Low* | Low | Low |
Effects of PA training on cognition
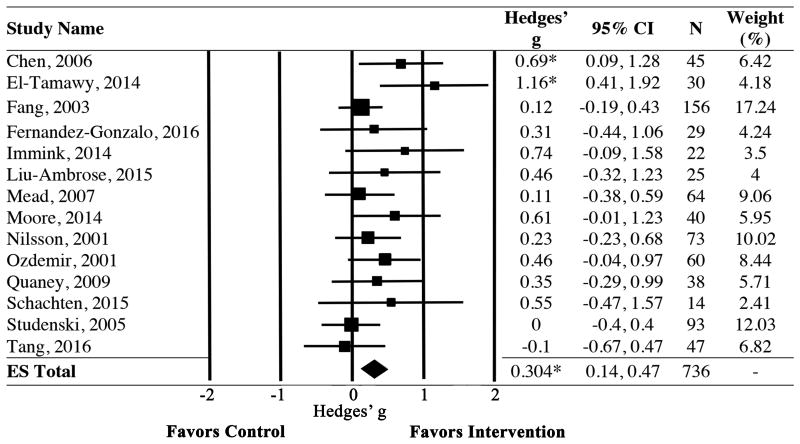
Using data from 14 randomized controlled trials, we first investigated whether stroke survivors experienced cognitive gains following structured PA training relative to controls. Results revealed a significant and positive effect of PA training on cognitive performance, with a small to moderate mean effect size (Hedges' g [CI] = 0.304 [0.14, 0.47], p < 0.001)(Figure 2). The significance remained when any 1 trial was removed from the analysis. The assumption of homogeneity was met for the full sample (Q (df) = 15.36 (13); p = 0.29).
Figure 2.
Forest plot showing individual study and pooled effects of PA training on cognitive function. Positive values of Hedges' g reflect improvements in cognitive performance among those in the PA group relative to controls. Random effects model used. ES=Effect Size; CI=Confidence Interval. *p<0.05
Moderation Analyses
Intervention and Outcome Characteristics
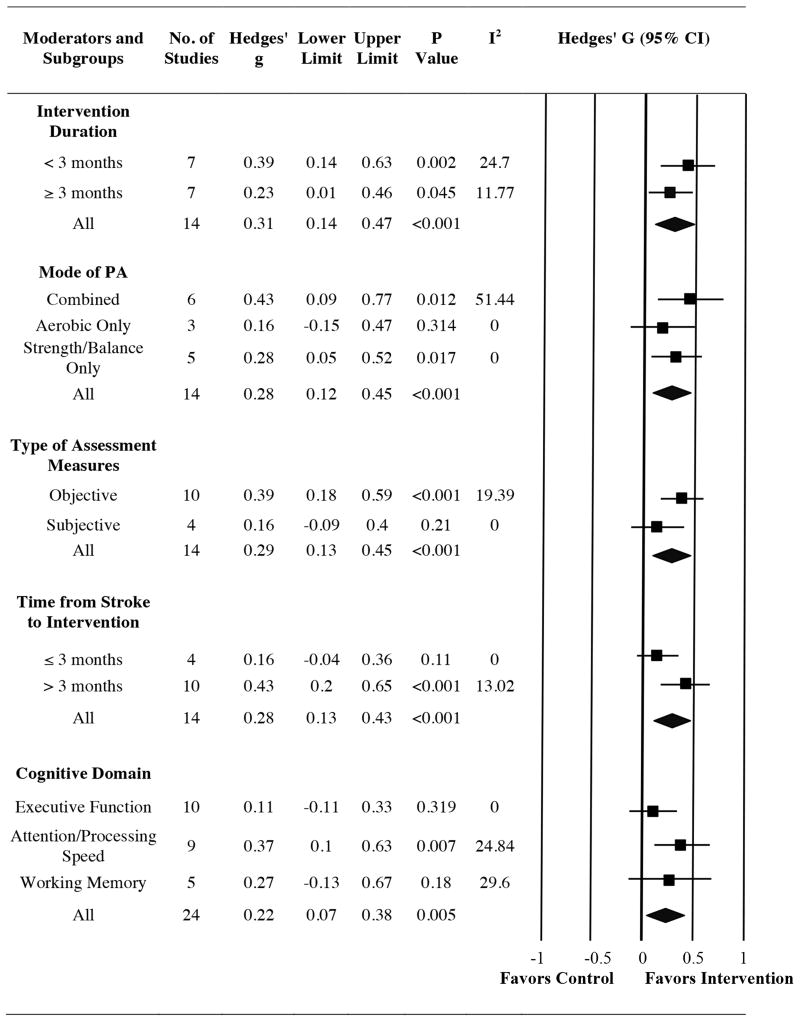
Mixed-effects analysis revealed that longer intervention trials were not associated with a greater magnitude of cognitive gains relative to brief interventions (QB = 0.82; p = 0.367). Specifically, PA training lasting less than 3 months (Hedges' g [CI] = 0.39 [0.14, 0.63]) and interventions involving at least 3 months of PA training (Hedges' g [CI] = 0.23 [0.01, 0.46]) were associated with improvements in cognitive performance compared to controls (Figure 3).
Figure 3.
Forest plot showing results from moderation analyses, including pooled effects for subgroups. Positive values of Hedges' g reflect improvements in cognitive performance among those in the PA group relative to controls.
To assess the influence of the type of training, studies were stratified based on whether the PA training involved 1) a combination of aerobic and strength/balance training (6 studies; 297 participants) 2) aerobic exercise only (3 studies; 158 participants) or 3) stretching/toning/balance training without an aerobic component (5 studies; 281 participants). Participants in combined strength and aerobic training programs experienced the largest cognitive benefits (Hedges' g [CI] = 0.43 [0.09, 0.77]), although cognitive gains were also apparent among subjects that underwent a PA regimen involving only strength/balance training (Hedges' g [CI] = 0.28 [0.05, 0.52]) The effect size for aerobic only trials was not significantly different from zero (Hedges' g [CI] = 0.16 [-0.15, 0.47]), and the between-groups difference did not reach statistical significance (QB = 1.35; p = 0.508).
The type of cognitive assessment was significant such that positive, moderate effects were observed among studies that employed objective cognitive assessments (Hedges' g [CI] = 0.39 [0.18, 0.59]), while the pooled effect size for trials using subjective cognitive measures did not significantly differ from zero (Hedges' g [CI] = 0.16 [-0.09, 0.40]). However, the interaction effect did not reach statistical significance (QB = 1.93; p = 0.165).
A post hoc subgroup analysis assessed whether cognitive gains were observed specifically in studies that screened for and included subjects with mobility limitations, including chronic hemiparesis and an inability to ambulate without an assistive device. Across the 8 included studies, results revealed a moderate, positive treatment effect in favor of the PA group (Hedges' g [CI] = 0.33 [0.10, 0.56]; I2 = 0%).
Time from Stroke to Intervention
We also investigated whether the magnitude of cognitive gains differed depending on when a PA regimen was introduced in the course of stroke recovery. Among the 4 studies initiated within 3 months post-stroke (382 participants), one started PA training within one week post-stroke,18 one began, on average, 3 weeks following a stroke,15 and two began approximately 6-10 weeks post-stroke,14,16 with a pooled average of approximately 35 days post-stroke. The time from stroke to intervention among the 10 trials including chronic stroke survivors (> 3 months post-stroke) (354 participants) ranged from 3 months to nearly 5 years, with an approximate overall average of 2.62 years since stroke onset.
Results indicated that PA training introduced in the chronic stroke phase resulted in significant, moderate, and positive effects on cognition (Hedges' g [CI] = 0.43 [0.20, 0.65]), while the effect size for trials that initiated PA training within 3 months post-stroke was not significantly different from zero (Hedges' g [CI] = 0.16 [-0.04, 0.36]). However, mixed-effects analysis did not demonstrate a significant between-group difference. Effect size estimates were statistically homogenous within subgroups for all moderation analyses, aside from the combined training subgroup (I2 = 51.44; p = 0.069). Primary and moderation analyses are represented in Figure 3 and Table II (supplementary materials, please see http://stroke.ahajournals.org).
Subgroup analysis: Domain specificity
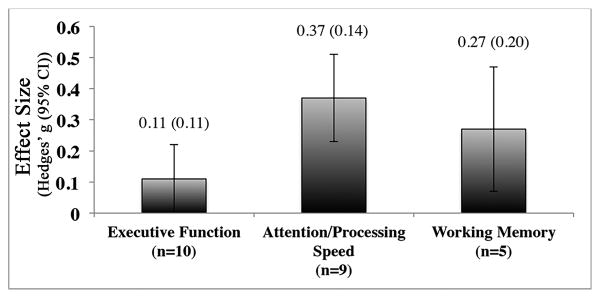
We also examined the domain specificity of PA training. This mixed-effects analysis consisted of 24 effect sizes derived from 5 studies that utilized comprehensive neuropsychological batteries, representing data from 153 participants. Analyses were conducted on 10 measures of executive function, 9 assessments of attention/processing speed, and 5 measures of working memory, yielding a pooled effect size estimate for each domain. The overall effect size across all 3 domains was 0.22 (CI = 0.07, 0.38 p = 0.005). Of the 3 domains, effects were only significantly different from zero for attention and processing speed (Hedges' g [CI] = 0.37 [0.10, 0.63]) and not for executive function (Hedges' g [CI] = 0.11 [-0.11, 0.33]) or working memory (Hedges' g [CI] = 0.27 [-0.13, 0.67]) (Figure 4). When subjected to a between-group analysis, results revealed no significant difference in effect size across the 3 domains (QB = 2.18; p = 0.336).
Figure 4.
Mean effect (Hedges' g) of PA as a function of cognitive domain, based on a subgroup of included studies. No significant between-group difference (p>0.05) was observed. Error bars show standard errors. N=number of data points within each domain.
Discussion
Post-stroke cognitive impairment has gained increased attention in recent years due to its high prevalence, persistence, and implications on health, quality of life, and recovery among stroke survivors. Our results indicate that PA training enhances cognitive performance following a stroke. In particular, small to moderate cognitive gains were achieved among stroke survivors who participated in structured PA training relative to controls. These findings suggest that PA may reduce the burden of cognitive deficits in stroke survivors.
One prior meta-analysis assessed the effects of PA training on cognitive outcomes in stroke and found a small but significant benefit of structured PA training across the 9 included studies. Seven additional randomized controlled trials were published since this review, which allowed us to expand upon the previous meta-analysis in several important ways. Specifically, we were able to impose stricter inclusion criteria, excluding studies with mixed populations and interventions consisting of a single bout of PA. Furthermore, we were able to 1) investigate factors influencing the efficacy of PA interventions and 2) evaluate and characterize the effects of PA training on different cognitive domains. We found that PA training resulted in moderate, positive improvements on measures of attention and processing speed, while the executive function and working memory domains did not reach statistical significance.
The findings should be interpreted with caution, however, as one study was overrepresented in the analysis due to the inclusion of a larger cognitive battery, and the working memory effect size estimate was based on only 5 evaluations. Furthermore, it is possible that exercise may improve performance in cognitive domains that are not well represented in the current literature, including language, visuospatial, and memory abilities, and may also preferentially influence subtypes of executive function. Therefore, this study is an important first step in elucidating domain-specific effects of PA training. However, future randomized controlled PA trials utilizing detailed cognitive assessments of both global and focal (e.g., aphasia, hemispatial neglect) cognitive deficits are needed to confirm and expand upon these findings.
We evaluated several moderators to characterize the features of PA interventions that may maximize cognitive gains. Mixed-effects analysis demonstrated that PA had favorable effects regardless of program length. Importantly, this suggests that participation in brief structured PA programs is sufficient to induce cognitive gains. However, trials that were longer in duration (≥ 3 months) tended to involve fewer PA sessions per week, suggesting that a frequency-duration trade-off may have also contributed to the lack of effect size differences between the two groups.
We also investigated differences in effect size as a function of type of PA training. Our analysis indicated that strength/balance training and interventions that combined both aerobic and strength training protocols achieved significant effects, although the effect size for combined trials was nearly double that of strength/balance training only studies. Interventions involving only aerobic exercise did not result in significant cognitive gains, although we may have been insufficiently powered to detect an effect in this subgroup (n=3 studies). The relative superiority of combined interventions is consistent with findings in healthy aging,4 and has also been shown to yield the largest improvements in quality of life among stroke survivors.26 Our results suggest that combined aerobic and strength training programs may generate the largest cognitive gains, and support the recent guidelines highlighting the need for cardiovascular exercise protocols to be integrated into standard subacute and chronic stroke rehabilitation programs.27
There are likely many cellular and molecular mechanisms by which PA influences cognitive function. Animal models have shown that aerobic exercise following a stroke increases brain-derived neurotropic factor, insulin-like growth factor-1 synaptogenesis, and dendritic branching,28 reduces lesion volume in affected regions, and protects perilesional tissue from inflammation and oxidative damage.29 Exercise training soon after stroke also exerts greater effects on many of the aforementioned processes than delayed initiation of PA training.28,29 Yet, here, only trials that introduced PA more than 3 months after stroke onset demonstrated a significant overall treatment effect. The modest effect size among trials introducing PA within 3 months post-stroke may, in part, be a consequence of the small number of studies conducted within this time frame (4 studies; 382 participants), as well as the exclusive use of subjective neuropsychological assessments in half of the trials. According to our moderation analyses, subjective measures may not be as sensitive as objective assessments at detecting PA-induced changes in cognitive performance. Furthermore, two of the studies did not include an aerobic component, and only 1 trial involved a combined strength and aerobic training program. Determining whether cognitive recovery can be maximized by introducing PA during this critical window of heightened neuroplasticity constitutes an important focus for future work.
We report that PA training is capable of improving cognitive performance in chronic stroke. Participants within this subgroup began PA training, on average, approximately 2.62 years after stroke, and our results showed positive and moderate treatment effects. These findings emphasize the importance of including both strength and aerobic training in standard postacute rehabilitation programs, including community and home-based services, in the interest of promoting cognitive recovery in chronic stroke.
Safety is an important consideration in this population, particularly given the prevalence of mobility impairments and heightened risk of injury and falls post-stroke.27 Unfortunately, many of the studies included here had inadequate reporting regarding adverse events and whether the events were serious, expected (e.g., muscle soreness), or related/unrelated to the intervention protocol (see http://stroke.ahajournals.org for details). Eight of the included trials, to some degree, reported on adverse events that occurred during intervention sessions and found no incidence of injury or serious adverse events during PA sessions. Improved reporting of adverse events in future studies is critical to determine interventions that minimize the likelihood of adverse events in this population. Notably, when we exclusively analyzed studies that included participants with motor impairments, we found that PA training led to improvements in cognitive performance that were moderate in magnitude. These results indicate that participants with motor deficits were successfully able to engage in PA training to such an extent that cognitive gains were achieved. Thus, determining safe, effective and feasible methods of PA training and customizing these to the needs and abilities of the patient is an important area for future work.
The included studies varied in a number of population characteristics that we were unable to systematically study here, but may influence the effect of PA training on cognition. These variables include age, baseline fitness levels, stroke characteristics, such as type, severity and first versus recurrent stroke, as well as the presence of comorbid cardiovascular and mental health conditions. In addition, we included trials involving cognitively healthy participants as well as cognitively impaired samples. Favorable effects of PA on cognition have been found in neurologically healthy older adults4 as well as those with dementia5, so PA training may exert similar cognitive benefits across a range of deficits post-stroke. Nonetheless, the extent and specificity of PA-induced cognitive changes may differ depending on the level of cognitive dysfunction. Finally, many of the studies that included participants with motor impairments did not provide details regarding the specificity of these motor deficits, so whether these findings are generalizable to individuals with more severe mobility limitations is not well understood. Overall, further assessment of these population characteristics is needed to determine how these factors may influence treatment effects.
This literature is marked by methodological limitations, with only 2 studies meeting low risk of bias in all 6 quality domains. However, the small number of published interventions and inconsistent reporting across trials precluded our ability to examine whether these factors may have accounted for effect size variance across studies. The nature of the control group (e.g., waitlist control, non-PA, or stretching/toning) may also impact the magnitude of effect sizes, however, we were underpowered to assess this in the present study. Similarly, adherence was only sporadically reported and therefore could not be taken into consideration. Finally, some moderation analyses included subgroups containing just 4 or 5 effect sizes. While there is no fixed minimum number of data points required for a meta-analysis, fewer data points may limit the precision of pooled estimates as well as the power to detect effects, particularly among studies with small sample sizes.25
Summary
Results from our quantitative synthesis and analysis indicate that PA training enhances cognitive performance following a stroke. We found that cognitive benefits were achieved in as little as 12 weeks, and combined intervention programs yielded the largest cognitive gains. Further, PA training had favorable effects on cognition even when introduced during the chronic stroke phase. These findings also highlight several critical avenues for future work, including identifying the ideal window of time following a stroke and the optimal training parameters needed to maximize cognitive recovery. Included in these trials should be participants with more severe levels of cognitive and physical impairment to enhance generalizability and help inform the development of methods to customize PA to the needs and abilities of these patients.
Supplementary Material
Acknowledgments
None.
Sources of Funding: LO was supported by an NIH training grant (Multimodal Neuroimaging Training Program, DA022761). KE was supported by the National Institute of Diabetes and Digestive Kidney Diseases (DK095172), National Institute on Aging (AG053952, AG024827), National Institute of Mental Health (MH90333) and National Cancer Institute (CA196762). JB was supported by a National Health and Medical Research Council Established Fellowship (1058365).
Footnotes
Disclosures: None.
References
- 1.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive Impairment after Stroke: Clinical Determinants and Its Associations with Long-Term Stroke Outcomes. Journal of the American Geriatrics Society. 2002;50:700–706. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- 2.Jokinen H, Melkas S, Ylikoski R, Pohjasvaara T, Kaste M, Erkinjuntti T, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. European Journal of Neurology. 2015;22:1288–1294. doi: 10.1111/ene.12743. [DOI] [PubMed] [Google Scholar]
- 3.Barker-Collo S, Feigin V, Parag V, Lawes C, Senior H. Auckland stroke outcomes study part 2: Cognition and functional outcomes 5 years poststroke. Neurology. 2010;75:1608–1616. doi: 10.1212/WNL.0b013e3181fb44c8. [DOI] [PubMed] [Google Scholar]
- 4.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults a meta-analytic study. Psychological science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 5.Groot C, Hooghiemstra A, Raijmakers P, van Berckel B, Scheltens P, Scherder E, et al. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing research reviews. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Cumming TB, Tyedin K, Churilov L, Morris ME, Bernhardt J. The effect of physical activity on cognitive function after stroke: a systematic review. International Psychogeriatrics. 2012;24:557–567. doi: 10.1017/S1041610211001980. [DOI] [PubMed] [Google Scholar]
- 7.El-Tamawy MS, Abd-Allah F, Ahmed SM, Darwish MH, Khalifa HA. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. NeuroRehabilitation. 2014;34:209–213. doi: 10.3233/NRE-131020. [DOI] [PubMed] [Google Scholar]
- 8.Immink MA, Hillier S, Petkov J. Randomized controlled trial of yoga for chronic poststroke hemiparesis: motor function, mental health, and quality of life outcomes. Topics in stroke rehabilitation. 2014;21:256–271. doi: 10.1310/tsr2103-256. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Gonzalo R, Fernandez-Gonzalo S, Turon M, Prieto C, Tesch PA, del Carmen García-Carreira M. Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: a pilot randomized controlled trial. Journal of neuroengineering and rehabilitation. 2016;13:1. doi: 10.1186/s12984-016-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu-Ambrose T, Eng JJ. Exercise training and recreational activities to promote executive functions in chronic stroke: A proof-of-concept study. Journal of Stroke and Cerebrovascular Diseases. 2015;24:130–137. doi: 10.1016/j.jstrokecerebrovasdis.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachten T, Jansen P. The effects of golf training in patients with stroke: a pilot study. International Psychogeriatrics. 2015;27:865–873. doi: 10.1017/S1041610214002452. [DOI] [PubMed] [Google Scholar]
- 12.Tang A, Eng JJ, Krassioukov AV, Tsang TS, Liu-Ambrose T. High-and low-intensity exercise do not improve cognitive function after stroke: A randomized controlled trial. Journal of rehabilitation medicine. 2016;48:841. doi: 10.2340/16501977-2163. [DOI] [PubMed] [Google Scholar]
- 13.Moore SA, Hallsworth K, Jakovljevic DG, Blamire AM, He J, Ford GA, et al. Effects of Community Exercise Therapy on Metabolic, Brain, Physical, and Cognitive Function Following Stroke A Randomized Controlled Pilot Trial. Neurorehabilitation and neural repair. 2014 doi: 10.1177/1545968314562116. 1545968314562116. [DOI] [PubMed] [Google Scholar]
- 14.Studenski S, Duncan PW, Perera S, Reker D, Lai SM, Richards L. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. 2005;36:1764–1770. doi: 10.1161/01.STR.0000174192.87887.70. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson L, Carlsson J, Danielsson A, Fugl-Meyer A, Hellström K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clinical rehabilitation. 2001;15:515–527. doi: 10.1191/026921501680425234. [DOI] [PubMed] [Google Scholar]
- 16.Özdemir F, Birtane M, Tabatabaei R, Kokino S, Ekuklu G. Comparing stroke rehabilitation outcomes between acute inpatient and nonintense home settings. Archives of physical medicine and rehabilitation. 2001;82:1375–1379. doi: 10.1053/apmr.2001.25973. [DOI] [PubMed] [Google Scholar]
- 17.Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomized trial of exercise or relaxation. Journal of the American Geriatrics Society. 2007;55:892–899. doi: 10.1111/j.1532-5415.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 18.Fang Y, Chen X, Li H, Lin J, Huang R. A study on additional early physiotherapy after stroke and factors affecting functional recovery. Clinical rehabilitation. 2003;17:608–617. doi: 10.1191/0269215503cr655oa. [DOI] [PubMed] [Google Scholar]
- 19.Chen X. Effect of community-based-rehabilitation on activities of daily life and cognitive function in stroke patients. Chinese Journal of Clinical Rehabilitation. 2006;10:4–6. [Google Scholar]
- 20.Quaney BM, Boyd LA, McDowd JM, Zahner LH, He J, Mayo MS, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabilitation and neural repair. 2009;23:879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 4. West Sussex, England: John Wiley & Sons Ltd; 2011. [Google Scholar]
- 23.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis: Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Lipsey MW, Wilson DB. Practical meta-analysis. 1st Ed. Thousand Oaks, California: Sage Publications, Inc; 2001. [Google Scholar]
- 26.Chen MD, Rimmer JH. Effects of Exercise on Quality of Life in Stroke Survivors. Stroke. 2011;42:832–837. doi: 10.1161/STROKEAHA.110.607747. [DOI] [PubMed] [Google Scholar]
- 27.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical Activity and Exercise Recommendations for Stroke Survivors A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 28.Ploughman M, Austin MW, Glynn L, Corbett D. The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Translational stroke research. 2015;6:13–28. doi: 10.1007/s12975-014-0357-7. [DOI] [PubMed] [Google Scholar]
- 29.Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neuroscience research. 2014;87:8–15. doi: 10.1016/j.neures.2014.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.