Abstract
Background
High blood pressure is the second most important risk factor of cardiovascular diseases (CVDs) in Iran. It is imperative to estimate the burden of CVDs that can be averted if high blood pressure is controlled at population level. The aim of the current study was to estimate the avertable CVD mortality in the setting of Golestan Cohort Study (GCS).
Methods
Over 50,000 participants were recruited and followed for a median of 7 years. The exposures of interest in this study were non-optimal systolic blood pressure (SBP) and hypertension measured at baseline. Deaths by cause have been precisely recorded. The Population Attributable Fraction (PAF) of deaths and Years of Life Lost (YLLs) due to CVDs attributable to exposures of interest were calculated.
Results
Overall, 223 deaths due to ischemic heart disease (IHD), 207 deaths due to cerebrovascular accidents (CVA), and 460 deaths due to all CVDs could be averted if the SBP of all subjects in the study were optimal. Similarly, 5,560 YLLs due to IHD, 4,771 YLLs due to CVA, and 11,135 YLLs due to CVD could be prevented if SBP were optimal. In all age groups, the avertable deaths and YLLs were higher due to IHD compared with CVA. Deaths and YLLs attributable to non-optimal SBP in women were less than men.
Conclusions
A very large proportion of CVD deaths can be averted if blood pressure is controlled in Iran. Effective interventions in primary and secondary health care setting are mandatory to be implemented as early as possible.
Keywords: blood pressure, hypertension, mortality, years of life lost
Introduction
Cardiovascular diseases are now the most common cause of mortality in many low- and middle-income countries, including Iran. The Global Burden of Disease study has reported that the share of deaths due to cardiovascular diseases in Iran increased from 31.9% in 1990 to 46.8% in 2010 1–4. It is also reported that high systolic blood pressure is the second most important risk factor for all deaths as well as cardiovascular deaths in Iran and has retained its rank since 1990. Additionally almost one-half of deaths caused by cardiovascular diseases are attributable to high systolic blood pressure in Iran 1–3. A study by Farzadfar and colleagues, using data from the 1st National Surveillance of Risk Factors of Non-Communicable Diseases in 2005, reported that the fraction of all deaths attributed to high systolic blood pressure ranged from 45% to 63% in different sub-regions of Iran 5, 6. Another study by Karami and colleagues, using data from the 5th National Surveillance of Risk Factors of Non-Communicable Diseases in 2009, reported that the fraction of incident strokes attributable to hypertension was approximately 41% 6, 7. However, these reports were based on relative risks estimated through comparative risk assessments methods at the global scale 4, 8–12, not relative risks obtained from studies conducted in Iran, as results from large-scale, long-term cohort studies in Iran were not available.
The aim of this study was to assess the fraction of deaths and Years of Life Lost (YLLs) due to ischemic heart disease (IHD), cerebrovascular accidents (CVA), and all cardiovascular diseases combined (CVDs) that are attributable to non-optimal systolic blood pressure (SBP) and hypertension in the Golestan Cohort Study (GCS).
Methods
Golestan Cohort Study
The details of the GCS are described elsewhere 13, 14. In short, 50,045 participants aged 40 to 75 years living in Gonbad City and 326 villages, all in Golestan Province in northeastern Iran, were recruited from January 2004 to June 2008. Rural participants were recruited from the whole population of 326 villages of the Golestan province. Urban dwellers were recruited from Gonbad city by cluster sampling. The only exclusion criteria were unwillingness to participate in the study or being a temporary resident. At baseline, data on demographic and lifestyle characteristics, past medical history, family history, and medication history were collected by trained interviewers using structured questionnaires. Anthropometric and blood pressure measurements were done by trained health personnel in the primary health care of Iran, who are called “Behvarz” and are responsible for vaccination, family planning, vital registration, and primary care for communicable and non-communicable diseases. Blood pressure was measured twice from each arm in the sitting position, with a 10-minute interval between measurements. Measurements were done using Richter auscultatory sphygmomanometers.
All GCS participants were followed annually. Follow-up was primarily done using annual phone calls to the participant, but other measures were also used, such as contacting friends and family members and contacting the Behvarz in charge of participant’s health. Using these methods, the success rate of follow up was over 99%. If a death was reported by telephone calls or Behvarz or the death registry, a physician performed a verbal autopsy, and at the same time all medical documents available in Golestan Province or the neighboring provinces for that participant were collected.
For 65% of patients, extensive medical documents, as well as verbal autopsy, were used to determine the cause of death. For the remaining 35%, medical documents were not available and only verbal autopsy was used 13. Previous studies have demonstrated that verbal autopsy is accurate in this population for determining the cause of death, especially for major categories of causes of death. In this study, two separate internists reviewed all existing documents and determined the cause of death based on ICD-10 codes. If the internists didn’t reach agreement, a third more experienced internist made the final diagnosis13.
Statistical analysis
For this analysis, we used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association of exposures and outcomes of interest. All subjects suffering from heart disease, CVA, or other vascular diseases at baseline were excluded from this analysis.
The exposures of interest were non-optimal SBP and hypertension. Non-optimal SBP was defined as SBP equal to or more than 120 mmHg. Adjusted HRs were calculated for SBP, which was divided into 4 categories defined by the Joint National Committee 7th report (unchanged in 8th report): optimal (<120 mmHg), prehypertension (120–139 mmHg), hypertension stage I (140–159 mmHg), and hypertension stage II (160 mmHg and above)15. Prehypertension is equivalent to normal and high normal SBP based on the guideline of European Society of Hypertension and the European Society of Cardiology (ESH/ESC). Hypertension stage II is equivalent to Stages II and III defined by ESH/ESC 16. Hypertension, as a binary variable, was defined as high systolic or diastolic blood pressure >=140/90 mmHg or being under treatment for hypertension.
The outcomes of interest were death and YLLs caused by ischemic heart disease (IHD), cerebrovascular accidents (CVA), and all cardiovascular diseases (CVDs), defined as the combination of IHD, CVA and all other cardiovascular diseases classified based on ICD-10 codes. Other cardiovascular diseases included: hypertensive heart diseases, cardiac arrest, heart failure, pulmonary heart diseases, and peripheral vascular diseases. YLL was calculated as the highest predicted life expectancy (predicted for Japanese women in 2010) at the age of death for each subject 17(webtable).
In the adjusted analyses, HRs were adjusted for age, sex, ethnicity (Turkmen/non-Turkmen), residence (urban/rural), marital status (married/non-married), wealth score measured by multiple correspondence analysis (MCA)18, body mass index (BMI), physical activity (metabolic equivalent task)19, tobacco use, opium use, alcohol use, diabetes, and education. We used education and wealth score as indicators of socio-economic status. Education (highest level attained) was categorized into illiterate, under 5 years of schooling, 6–8 years of schooling, high school diploma, and university education. Among confounders, sex, ethnicity, residence, marital status, tobacco use, opium use, alcohol use, and diabetes were binary. Age, MCA, BMI, and physical activity were continuous. Education was defined as a categorical variable.
To handle missing or implausible data, values of SBP outside the plausible range (40–300 mmHg) were categorized as missing based on the definition of WHO for plausible range 6. Deaths caused by unknown diseases were also categorized as missing. However, as missing values were minimal (75 cases), complete case analysis was done.
We computed the proportional reduction of disease specific deaths that would occur if exposure to non-optimal SBP in the real world (the baseline scenario) were reduced to optimal SBP (alternative or fantasy scenario) while all other risk factors were assumed to remain the same. As a second fantasy scenario, we computed the proportional reduction of deaths that would occur if no one in the population was hypertensive while all other risk factors were assumed to remain the same. This metric, called the Population Attributable Fraction (PAF), was calculated based on the following formula:
where RRi is the RR for exposure category i, Pi is the fraction of the population in exposure category i, and n is the number of exposure categories
These 2 scenarios are parallel and separate definitions of optimal blood pressure at population level that have been compared with each other in this study. PAFs and their 95% uncertainty intervals (95% UI) were computed for all participants, and were also stratified by gender and by age groups categorized into: 40–49, 50–59, 60–69, and 70 and above years old. PAFs were then used to calculate deaths and YLLs due to IHD, CVA, and all CVDs that could be averted if SBP were optimal or if no participant in GCS study had hypertension. All analyses were done in Stata statistical software version 12 (StataCorp, College Station, TX) 20.
The study was approved by the ethics committee of Tehran University of Medical Sciences, and a written informed consent was signed by all participants in the study. Illiterate invitees were asked to visit the study center and to observe the procedures before signing the written informed consent.
Results
The analysis in the current study included 46,674 subjects free from heart disease, CVA, or any other cardiovascular disease at baseline, followed up until October 30th, 2013. Participants were followed for a total of 328,079 person-years, with a median follow up of 7.1 years. Approximately 58% of the study participants were women, 76% were of Turkmen ethnicity, 81% were rural dwellers, 88% were married, and 70% were illiterate.
The mean age of participants at baseline was 51.6 years (SD: 8.8). The mean SBP ± SD was 127.0 ± 23.9 mmHg and the mean DBP ± SD was 77.0 ± 13.7 mmHg. The mean ± SD for SBP by sex and age are shown in Table 1. SBP and DBP were significantly higher in women than in men (p<0.001), and were significantly higher in older than in younger age groups in both men and women (p<0.001). High DBP (≥90 mmHg) was also significantly more common among women than men (20.7% vs 17.1%, p-value<0.001). Likewise, a higher percentage of women were under anti-hypertensive treatment than men (19.6% vs 7.7%, p-value<0.001).
Table 1.
The demographic, life style, and disease history of study participants
| Number (%) of participants § | Mean (SD) of SBP | P-value | Optimal SBP N (%)* |
Prehypertension SBP N (%)* |
Hypertensive SBP Stage I N (%)* |
Hypertensive SBP Stage II N (%)* |
Mean (SD) of DBP | P-value | Hypertension N (%)* |
|
|---|---|---|---|---|---|---|---|---|---|---|
| All | 46,674 (100) | 127.0 (23.9) | 17,914 (38.4) | 15,754 (33.8) | 8,036 (17.2) | 4,961 (10.6) | 77.0 (13.7) | 18,694 (40.1) | ||
| Sex | ||||||||||
| Female | 26,848 (57.5) | 128.0 (24.6) | <0.001 | 9,996 (37.2) | 8,852 (33.0) | 4,804 (17.9) | 3,196 (11.9) | 77.7 (13.8) | <0.001 | 11,999 (44.7) |
| Male | 19,817 (42.5) | 125.5 (22.8) | 7,918 (40.0) | 6,902 (34.8) | 3,232 (16.3) | 1,765 (8.9) | 76.2 (13.5) | 6,695 (33.8) | ||
| Age | ||||||||||
| 40–49 years | 22,971 (49.2) | 121.0 (20.9) | <0.001 | 11,005 (47.9) | 7,872 (34.3) | 2,801 (12.2) | 1,291 (5.6) | 75.6 (13.4) | <0.001 | 6,855 (29.9) |
| 50–59 years | 14,672 (31.4) | 129.8 (24.4) | 4,871 (33.2) | 4,960 (33.8) | 2,984 (20.3) | 1,852 (12.6) | 78.1 (13.9) | 6,653 (45.4) | ||
| 60–69 years | 6,607 (14.2) | 136.3 (25.6) | 1,585 (24.0) | 2,173 (32.9) | 1,594 (24.1) | 1,254 (19.0) | 79.0 (13.7) | 3,664 (55.5) | ||
| 70 years and above | 2,424 (5.2) | 140.5 (25.2) | 453 (18.7) | 749 (30.9) | 657 (27.1) | 564 (23.3) | 78.6 (13.3) | 1,522 (62.8) | ||
| Ethnicity | ||||||||||
| Non-Turkmen | 11,690 (25.1) | 126.1 (22.9) | <0.001 | 4,670 (40.0) | 4,045 (34.6) | 1,877 (16.1) | 1,098 (9.4) | 77.1 (12.3) | 0.547 | 4,361 (37.3) |
| Turkmen | 34,984 (74.9) | 127.2 (24.2 | 13,244 (37.9) | 11,709 (33.5) | 6,159 (17.6) | 3,863 (11.1) | 77.0 (14.1) | 14,333 (40.1) | ||
| Area of residence | ||||||||||
| Rural | 37,715 (80.8) | 127.0 (24.0) | 0.539 | 14,395 (38.2) | 12,719 (33.7) | 6,541 (17.4) | 4,053 (10.8) | 76.9 (14.0) | <0.001 | 15,081 (40.0) |
| Urban | 8,958 (19.2) | 126.8 (23.4) | 3,518 (39.3) | 3,035 (33.9) | 1,495 (16.7) | 908 (10.1) | 77.7 (12.2) | 3,613 (40.3) | ||
| Education | ||||||||||
| Illiterate | 32,578 (69.8) | 128.6 (24.9) | <0.001 | 11,734 (36.0) | 10,725 (32.9) | 6,063 (18.6) | 4,048 (12.4) | 77.1 (14.1) | <0.001 | 14,280 (43.9) |
| Under 5 years of education | 7,994 (17.1) | 124.2 (21.6) | 3,393 (42.5) | 2,817 (35.2) | 1,200 (15.0) | 583 (7.3) | 77.0 (12.9) | 2,667 (33.4) | ||
| 6–8 years of education | 2,085 (4.5) | 122.3 (20.1) | 941 (45.1) | 752 (36.1) | 262 (12.6) | 130 (6.2) | 76.1 (12.2) | 598 (28.7) | ||
| High school diploma | 3,008 (6.4) | 121.6 (19.5) | 1,387 (46.1) | 1,092 (36.3) | 381 (12.7) | 148 (4.9) | 76.1 (12.2) | 839 (27.9) | ||
| University education | 1,009 (2.5) | 122.1 (19.5) | 459 (45.5) | 368 (36.5) | 130 (12.9) | 52 (5.2) | 77.3 (11.7) | 310 (30.7) | ||
| Marital Status | ||||||||||
| Married | 41,178 (88.2) | 126.2 (23.5) | <0.001 | 16,223 (39.4) | 13,987 (34.0) | 6,873 (16.7) | 4,088 (9.9) | 76.9 (13.6) | <0.001 | 15,847 (38.5) |
| Non-married | 5,496 (11.8) | 132.3 (26.1) | 1,691 (30.8) | 1,767 (32.2) | 1,163 (21.2) | 873 (15.9) | 78.1 (14.0) | 2,847 (51.8) | ||
| Ever cigarette smoking | ||||||||||
| No | 38,726 (83.0) | 128.0 (24.0) | <0.001 | 14,169 (36.6) | 13,221 (34.2) | 6,916 (17.9) | 4,413 (11.4) | 77.7 (13.6) | <0.001 | 16,358 (42.3) |
| Yes | 7,948 (17.0) | 121.9 (22.5) | 3,745 (47.1) | 2,533 (31.9) | 1,120 (14.1) | 548 (6.9) | 74.0 (13.5) | 2,336 (29.4) | ||
| Ever opium abuse | ||||||||||
| No | 38,956 (83.5) | 127.7 (23.7) | <0.001 | 14,349 (36.8) | 13,431 (34.5) | 6,913 (17.8) | 4,256 (10.9) | 77.7 (13.5) | <0.001 | 16,107 (41.4) |
| Yes | 7,718 (16.5) | 123.1 (24.5) | 3,565 (46.2) | 2,323 (30.1) | 1,123 (14.6) | 705 (9.1) | 73.8 (14.2) | 2,587 (33.5) | ||
| Ever alcohol use | ||||||||||
| No | 45,135 (96.7) | 127.0 (23.9) | 0.002 | 17,283 (38.3) | 15,225 (33.7) | 7,780 (17.2) | 4,838 (10.7) | 77.0 (13.7) | <0.001 | 18,156 (40.2) |
| Yes | 1,539 (3.3) | 125.1 (22.1) | 631 (41.0) | 529 (34.4) | 256 (16.6) | 123 (8.0) | 77.2 (12.7) | 538 (35.0) | ||
| History of diabetes mellitus | ||||||||||
| No | 43,754 (93.7) | 126.4 (23.7) | <0.001 | 17,157 (39.2) | 14,779 (33.8) | 7,352 (16.8) | 4,458 (10.2) | 76.9 (13.7) | <0.001 | 16,882 (38.6) |
| Yes | 2,920 (6.3) | 134.7 (24.7) | 757 (25.9) | 975 (33.4) | 684 (23.4) | 503 (17.2) | 79.6 (13.4) | 1,812 (62.1) | ||
| BMI | ||||||||||
| Underweight | 3,018 (6.5) | 117.2 (23.2) | <0.001 | 1,713 (56.8) | 811 (26.9) | 311 (10.3) | 182 (6.0) | 70.8 (13.5) | <0.001 | 704 (23.3) |
| Normal weight | 16,227 (34.8) | 122.9 (23.1) | 7,438 (45.9) | 5,217 (32.2) | 2,257 (13.9) | 1,312 (8.1) | 74.1 (13.3) | 4,989 (30.8) | ||
| Overweight | 15,750 (33.7) | 128.6 (23.6) | 5,530 (35.1) | 5,595 (35.5) | 2,860 (22.3) | 1,703 (14.6) | 78.1 (13.2) | 6,702 (42.6) | ||
| Obese | 11,679 (25.0) | 133.0 (23.7) | 3,233 (27.7) | 4,131 (35.4) | 2,608 (22.3) | 1,703 (14.6) | 81.3 (13.4) | 6,299 (54.0) |
SBP: Systolic Blood Pressure
DBP: Diastolic Blood Pressure
BMI: Body Mass Index
Optimal SBP: SBP<120 mmHg
Prehypertensive SBP: 120–139 mmHg
Hypertensive SBP stage I: 140–159 mmHg
Hypertensive SBP stage II: 160 mmHg and above
Hypertension: SBP/DBP >= 140/90 mmHg OR being treated for hypertension
: percentages are reported for columns
: percentages are reported for rows
Overall, 38.4% of the participants had optimal SBP, 33.8% had prehypertension, 17.2% had hypertension stage I, and 10.6% had hypertension stage II. A higher percentage of women than men fell into the categories of hypertension stages I and II. As expected, higher percentages of older than younger participants were also found in the categories of hypertension. Both non-optimal SBP and hypertension were significantly more prevalent in those who were Turkmen, illiterate, or non-married. Unexpectedly, these blood pressure abnormalities were also more prevalent in participants with no history of smoking, opiate, or alcohol use. Non-optimal SBP and hypertension were also more frequent in diabetic participants, and their prevalence increased with rising BMI (Table 1).
In total, 829, 483, and 1,438 deaths occurred due to IHD, CVA, and overall CVDs, respectively. The corresponding YLLs were 20,593, 11,097, and 34,800 years (Table 2). The number of deaths was highest in the age group of 60–69 years, while YLLs were highest in the age group of 50–59 years (Table 2). Deaths due to IHD, CVA, and CVD in women were less than men. The same observed for YLLs except for those due to CVA, which were higher in women.
Table 2.
Deaths and Years of Life Lost due to Cardiovascular Diseases, by gender and age groups
| IHD deaths numbers |
IHD death rates |
IHD YLLs numbers |
IHD YLLs rates |
CVA deaths numbers |
CVA death rates |
CVA YLLs numbers |
CVA YLLs rates |
CVD deaths numbers |
CVD death rates |
CVD YLLs numbers |
CVD YLLs rates |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||||
| Women | 359 | 188.3 | 9 160 | 4 803.3 | 241 | 126.4 | 5 636 | 2 955.4 | 653 | 342.4 | 16 177 | 8 482.9 |
| Men | 470 | 341.2 | 11 433 | 8 300.4 | 242 | 175.7 | 5 461 | 3 964.7 | 785 | 569.9 | 18 623 | 13 520.4 |
| Age groups | ||||||||||||
| 40 – 49 years | 159 | 97.0 | 5 939 | 3 623.4 | 65 | 39.7 | 2 391 | 1 458.8 | 247 | 150.7 | 9 197 | 5 611.1 |
| 50 – 59 years | 259 | 249.7 | 7 345 | 7 079.9 | 126 | 121.5 | 3 621 | 3 490.3 | 421 | 405.8 | 12 023 | 11 589.0 |
| 60 – 69 years | 249 | 552.2 | 5 054 | 11 207.8 | 177 | 392.5 | 3 492 | 7 743.9 | 462 | 1 024.5 | 9 265 | 20 546.2 |
| 70 years and above | 162 | 1 032.0 | 2 255 | 14 365.6 | 115 | 732.6 | 1 593 | 10 148.3 | 308 | 1 962.1 | 4,315 | 27 489.0 |
| Total | 829 | 252.4 | 20 593 | 6 269.9 | 483 | 147.1 | 11 097 | 3 378.7 | 1 438 | 437.8 | 34 800 | 10 595.5 |
PAFs and their 95% uncertainty intervals attributable to non-optimal SBP and hypertension are shown in Table 3. The PAFs (95% UI) for IHD, CVA, and all CVDs due to hypertension were 30% (25%–35%), 48% (43%–54%), and 35% (32%–39%), respectively in all participants. The PAFs for non-optimal SBP were only slightly (3–5%) lower in each category of death. Generally, the PAFs were higher for CVA mortality than those for IHD mortality.
Table 3.
Population Attributable Fractions of Cardiovascular Diseases due to Non-optimal Systolic Blood Pressure and Hypertension, overall and by gender
| Non-optimal SBP | Hypertension | |||
|---|---|---|---|---|
| PAF | 95% UI* | PAF | 95% UI* | |
| Overall | ||||
| IHD mortality | 0.27 | 0.18–0.36 | 0.30 | 0.25–0.35 |
| CVA mortality | 0.43 | 0.31–0.53 | 0.48 | 0.43–0.54 |
| CVD mortality | 0.32 | 0.25–0.38 | 0.35 | 0.32–0.39 |
| Women | ||||
| IHD mortality | 0.21 | 0.07–0.35 | 0.38 | 0.29–0.45 |
| CVA mortality | 0.44 | 0.25–0.57 | 0.44 | 0.33–0.53 |
| CVD mortality | 0.28 | 0.16–0.38 | 0.39 | 0.32–0.45 |
| Men | ||||
| IHD mortality | 0.30 | 0.18–0.41 | 0.24 | 0.17–0.30 |
| CVA mortality | 0.42 | 0.25–0.55 | 0.51 | 0.44–0.57 |
| CVD mortality | 0.33 | 0.24–0.41 | 0.32 | 0.27–0.37 |
95% uncertainty interval
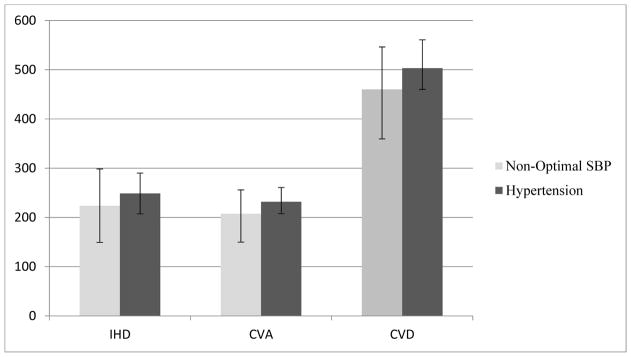
Overall, 223 (149–298) deaths due to IHD, 207 (149–255) deaths due to CVA, and 460 (359–546) deaths due to all CVDs could have been prevented if the isolated SBP of all participants in the study had been in the optimal range. Similarly, 5,560 (3,706–7,413) YLLs due to IHD, 4,771 (3,439, 5,881) YLLs due to CVA, and 11,135 (8,699–13,223) YLLs due to all CVDs could have been prevented if SBP were optimal. On the other hand, 248 (207–290) deaths due to IHD, 231 (207–260) deaths due to CVA, and 503 (460–560) deaths due to all CVDs would have been prevented if no one in the GCS had been hypertensive. Similar figures for YLLs were 6,177 (5,148–7,207) due to IHD, 5,326 (4,771–5,992) due to CVA and 12,179 (11,135–13,571) due to all CVDs.
Figure 1 shows the CVD deaths attributable to non-optimal SBP and hypertension. In all categories of causes of death, more deaths and YLLs were attributable to hypertension than to non-optimal SBP.
Figure 1.
Deaths due to IHD, CVA, and CVD attributable to non-optimal SBP and Hypertension
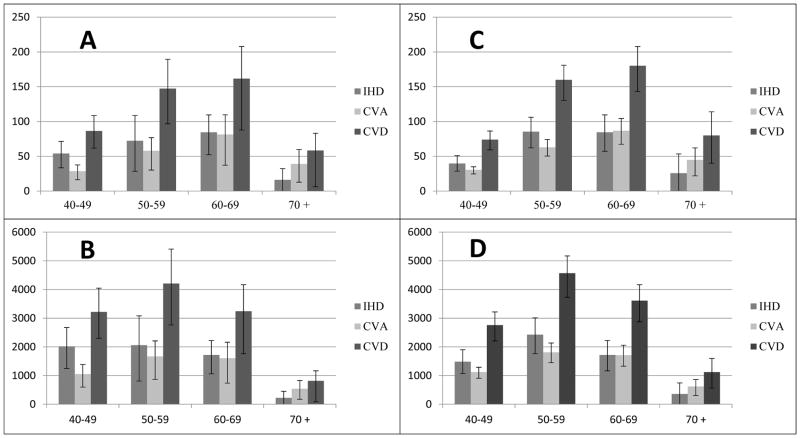
Figure 2 shows these same results stratified by age groups. In all age groups except for the oldest age group, the avertable deaths and YLLs were higher due to IHD than to CVA. The avertable deaths were highest in the age group of 60–69 years. However, the YLLs were higher in the younger 50–59 year age group, which is to be expected.
Figure 2.
Deaths and YLLs attributable to non-optimal SBP and hypertension, by age group. A. Deaths attributable to non-optimal SBP; B: YLLs attributable to non-optimal SBP; C: Deaths attributable to hypertension; D: YLLs attributable to hypertension.
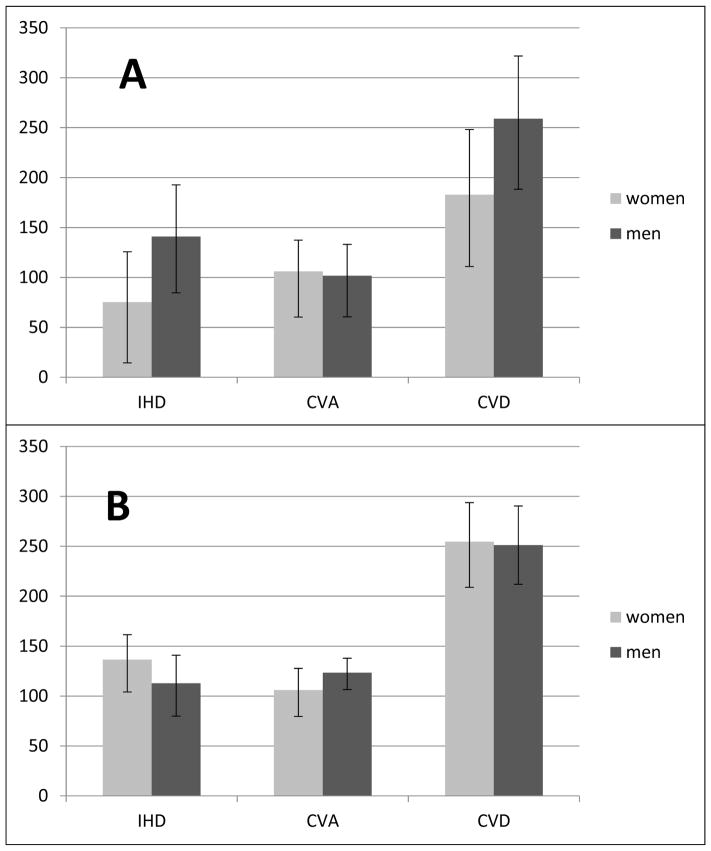
The CVD deaths attributable to non-optimal SBP and hypertension, stratified by sex, are shown in Figure 3. PAFs (Table 3) as well as the number of deaths due to IHD and overall CVDs attributable to non-optimal SBP were less in women than in men, while for CVA, the opposite was true. On the other hand, PAFs and deaths due to IHD and overall CVD that were attributable to hypertension were slightly higher in women than in men, and both PAFs and CVA deaths attributable to hypertension were lower in women than in men.
Figure 3.
Deaths attributable to non-optimal SBP and hypertension in women and men. A: Deaths attributable to non-optimal SBP; B: Deaths attributable to hypertension
Discussion
Our results indicate that during a median follow-up of 7 years in the GCS, over 450 CVD deaths could be prevented if SBP were optimal and over 500 CVD deaths could be averted if no one in the GCS sample were hypertensive in its classical definition. Among all participants, both sexes and all age groups, PAFs of non-optimal SBP ranged from 0.27 for IHD to 0.43 for CVA. Corresponding figures attributable to hypertension were 0.30 for IHD to 0.48 for CVA. PAFs were higher for CVA than for IHD, due to stronger relative risks, but deaths due to CVA caused by non-optimal SBP or hypertension were fewer than those due to IHD, due to the lower incidence of CVA. PAFs of IHD and CVD deaths attributable to hypertension were higher in women than in men because a higher percentage of women were under anti-hypertensive treatment or had high DBP.
There is only one comparable longitudinal study in Iran that estimated the CVD deaths attributable to high blood pressure in the general population free from cardiovascular disease. The researchers of the Tehran Lipid and Glucose Study (TLGS) conducted this study among residents free from CVD in Tehran, the capital of Iran, following them for a median of 9.3 years until 2009. Their initial report of the first 2,378 participants showed that over 45% of incident cases of stroke were attributable to hypertension 21. In a later analysis, the TLGS investigators reported that the PAF of incident coronary heart disease (CHD), incident CVD, and all-cause mortality attributable to type 2 diabetes were 21%, 22%, and 24% respectively among all 6331 participants 22. And in another more recent analysis, they reported PAFs of hypertension in diabetic and non-diabetic participants of the TLGS. PAFs in non-diabetic subjects for incident CHD, incident CVD, fatal CVD, and all-cause mortality were 24.1%, 26.6%, 44.9%, and 29.6%, respectively, and all of these figures were higher in diabetics 23. All of these estimates are consistent with the results of the present study.
The current study, however, has certain strengths compared to this previously conducted study. The GCS is a large-scale population-based study with a nearly complete (over 99%) follow-up rate. The participants include both rural and urban dwellers, and thus this study is more representative of the general population of Iran than the all-urban TLGS study. In addition, the relative risks used for the PAF estimates were derived from the entire GCS population.
Our study has certain limitations as well. Estimated risk functions were based on single measurements rather than ‘usual’ levels, which imposed the regression dilution bias on our results. A second limitation was a probable inconsistency in recording hypertension treatment, as in the setting of rural areas many subjects may report that they take anti-hypertensive medication but in fact they don’t adhere to treatment; and conversely, they may take medications prescribed for them but may not know the names or uses of the drugs they take. This again may lead to dilution of the results. Yet these results reflect the clinical setting realistically, where patients may have similar reporting problems.
The results of the current study have certain significant implications for medically approved clinical guidelines and national and subnational policy making and priority setting in Iran. Extrapolating the results to the national level based on the total number of deaths that are recorded annually in the national death registry can reveal the seriousness of the burden that non-optimal systolic blood pressure imposes on Iranians. Farzadfar et al have estimated that 80,000 deaths could have been averted in Iran in 2005 if SBP were optimal among Iranians5. As Iran transitions in its epidemiologic pattern of morbidity and mortality from predominantly infectious to predominantly chronic diseases, the healthcare system should be more prepared to provide care for risk factors of chronic diseases, such as high blood pressure.
Considering that high blood pressure is often asymptomatic, implementing effective interventions can be quite challenging. Detection of asymptomatic high blood pressure in the general population is relatively difficult, and requires periodic screening and surveillance of individual levels and trends of blood pressure on a national scale. This is the mainstay of primary prevention of morbidity and mortality due to high blood pressure, and it is the first crucial step for evidence-based policy making. The recent approach of WHO towards national surveillance of CVD risk factors is the major strategy that has been adopted so far in several countries, including Iran. This surveillance was performed six times in Iran between 2005 and 20116.
In addition, enhancing the knowledge and awareness of both health care providers and the general public by mass media and other public venues can be substantially effective24. A recent study by Malekzadeh et al has demonstrated that only 46% of hypertensive participants in the GCS were aware of their high blood pressure25. Providers should be oriented toward the necessity of screening in primary or secondary care settings, and people should also be encouraged to perform self-care, which is a very important strategy in controlling risk factors as well as overt CVDs 26.
After detection of high blood pressure in asymptomatic individuals, it is still difficult to change the attitude of these people and their health care providers towards the necessity of adopting a healthier life style. Such healthier behaviors include reducing salt intake, weight loss, and increasing fish oil consumption, all leading to reduced hypertension and a reduced risk of CVDs. However, it is worth noting that evidence regarding the long-term effectiveness of non-pharmacological interventions is controversial 27. Still, trials on the effectiveness of decreasing salt intake in controlling and preventing high blood pressure are numerous.28–32 Salt reduction can be achieved through individual-level consultations teaching people to use less salt in cooking and to add less salt to foods at the table. And at a population level, salt intake can be reduced by devising and implementing policies to supervise food industries and set standards on the amount of salt allowed in processed food 33.
Pharmacological interventions are the last strategy that can be adopted for controlling high blood pressure. The efficacy of interventions for such secondary prevention is well established15. However, recent evidence suggests that medical treatment can also be useful for primary prevention, especially for those older than 50 years. In their pivotal paper in 2003, Wald and Law suggested that primary prevention with anti-hypertensive treatment in individuals older than 55 years could potentially prevent over 80% of incident CVDs 34. To overcome the problem of adherence to multidrug treatment, they suggested a combination of statins, three blood pressure lowering drugs, folic acid, and aspirin – collectively named Polypill – to be prescribed for the primary prevention of cardiovascular diseases 34. Ongoing clinical trials are testing the effectiveness of various combinations of such Polypills 35, 36, and some of these studies have affirmed the potential effectiveness of Polypill prescription in primary prevention 37–39. A few meta-analyses have been done in this regard as well40. However, the cost-effectiveness, feasibility, and acceptability of this prevention strategy remain to be investigated.
In effect, considering the prevailing and rising epidemic of CVDs in Iran, it is mandatory that clinical guidelines and population-based primary care interventions be developed for monitoring and controlling the major risk factors of CVDs as soon as possible41, 42.
Conclusion
Non-optimal systolic blood pressure and hypertension impose a considerable burden on the health of Iranians, as well as citizens of many other low- and middle-income countries. A high percentage of CVD deaths in Iran are attributable to hypertension. Considering the rapid rise of CVD risk factors, effective interventions are crucial and should be implemented as quickly as possible. Government can play an important role in controlling risk factors by devising and implementing policies and encouraging the inter-sectoral collaboration. To be successful, such interventions will require logistics, funds, skilled human resources, and sustained commitment.
Acknowledgments
Supports and funding
This study was supported in part by the intramural research program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute, NIH, by the Digestive Disease Research Center of Tehran University of Medical Sciences (grant No 82-603), by Cancer Research UK (CRUK), and by the International Agency for Research on Cancer.
This study was supported in part by the intramural research program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute, NIH, by the Digestive Disease Research Institute of Tehran University of Medical Sciences (grant No 82–603), by Cancer Research UK (CRUK), and by the International Agency for Research on Cancer. We sincerely thank the Golestan Cohort study staff for their valuable helps. We also thank the study participants for their cooperation and health workers (Behvarz) in the study area for their help.
Footnotes
Conflicts of interest: None
References
- 1.Forouzanfar MH, Sepanlou SG, Shahraz S, Dicker D, Naghavi P, Pourmalek F, et al. Evaluating causes of death and morbidity in Iran, global burden of diseases, injuries, and risk factors study 2010. Arch Iran Med. 2014;17(5):304–320. [PubMed] [Google Scholar]
- 2.Naghavi M, Shahraz S, Sepanlou SG, Dicker D, Naghavi P, Pourmalek F, et al. Health transition in Iran toward chronic diseases based on results of Global Burden of Disease 2010. Arch Iran Med. 2014;17(5):321–335. [PubMed] [Google Scholar]
- 3.Shahraz S, Forouzanfar MH, Sepanlou SG, Dicker D, Naghavi P, Pourmalek F, et al. Population health and burden of disease profile of Iran among 20 countries in the region: from Afghanistan to Qatar and Lebanon. Arch Iran Med. 2014;17(5):336–342. [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzadfar F, Danaei G, Namdaritabar H, Rajaratnam JK, Marcus JR, Khosravi A, et al. National and subnational mortality effects of metabolic risk factors and smoking in Iran: a comparative risk assessment. Popul Health Metr. 2011;9(1):55. doi: 10.1186/1478-7954-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. STEPwise approach to surveillance (STEPS) [ http://www.who.int/chp/steps/en/]
- 7.Karami M, Soori H, Monfared AB. Estimating the contribution of selected risk factors in attributable burden to stroke in iran. Iran J Public Health. 2012;41(5):91–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 9.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Measuring the Global Burden of Disease and Risk Factors, 1990–2001. In: Lopez AD, et al., editors. Global Burden of Disease and Risk Factors. Washington (DC): 2006. [PubMed] [Google Scholar]
- 10.Lopez AD, Murray CJ. The global burden of disease, 1990–2020. Nat Med. 1998;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 11.Morrow RH, Hyder AA, Murray CJ, Lopez AD. Measuring the burden of disease. Lancet. 1998;352(9143):1859–1861. doi: 10.1016/S0140-6736(05)79929-3. [DOI] [PubMed] [Google Scholar]
- 12.Murray CJ, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks conceptual framework and methodological issues. Popul Health Metr. 2003;1(1):1. doi: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khademi H, Etemadi A, Kamangar F, Nouraie M, Shakeri R, Abaie B, et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One. 2010;5(6):e11183. doi: 10.1371/journal.pone.0011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourshams A, Khademi H, Malekshah AF, Islami F, Nouraei M, Sadjadi AR, et al. Cohort Profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39(1):52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Institutes of Health; 2004. [Google Scholar]
- 16.2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 17.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 18.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 20.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata Journal. 2013;13(4):672–698. [Google Scholar]
- 21.Fahimfar N, Khalili D, Mohebi R, Azizi F, Hadaegh F. Risk factors for ischemic stroke; results from 9 years of follow-up in a population based cohort of Iran. BMC Neurol. 2012;12:117. doi: 10.1186/1471-2377-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozorgmanesh M, Hadaegh F, Sheikholeslami F, Azizi F. Cardiovascular risk and all-cause mortality attributable to diabetes: Tehran lipid and glucose study. J Endocrinol Invest. 2012;35(1):14–20. doi: 10.3275/7728. [DOI] [PubMed] [Google Scholar]
- 23.Bozorgmanesh M, Hadaegh F, Mohebi R, Ghanbarian A, Eskandari F, Azizi F. Diabetic population mortality and cardiovascular risk attributable to hypertension: a decade follow-up from the Tehran Lipid and Glucose Study. Blood Press. 2013;22(5):317–324. doi: 10.3109/08037051.2013.769294. [DOI] [PubMed] [Google Scholar]
- 24.Whelton PK, Beevers DG, Sonkodi S. Strategies for improvement of awareness, treatment and control of hypertension: results of a panel discussion. J Hum Hypertens. 2004;18(8):563–565. doi: 10.1038/sj.jhh.1001738. [DOI] [PubMed] [Google Scholar]
- 25.Malekzadeh MM, Etemadi A, Kamangar F, Khademi H, Golozar A, Islami F, et al. Prevalence, awareness and risk factors of hypertension in a large cohort of Iranian adult population. J Hypertens. 2013;31(7):1364–1371. doi: 10.1097/HJH.0b013e3283613053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadeghi M, Shiri M, Roohafza H, Rakhshani F, Sepanlou SG, Sarrafzadegan N. Developing an appropriate model for self-care of hypertensive patients: first experience from EMRO. ARYA Atheroscler. 2013;9(4):232–240. [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen T, Jacobsen RK, Toft U, Aadahl M, Glumer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ. 2014;348:g3617. doi: 10.1136/bmj.g3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6):363–384. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 30.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor R, Hooper L, Ebrahim S. Dietary salt and cardiovascular disease. Lancet. 2011;378(9808):1993. doi: 10.1016/S0140-6736(11)61865-5. [DOI] [PubMed] [Google Scholar]
- 32.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;(7):CD009217. doi: 10.1002/14651858.CD009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Guideline: Sodium intake for adults and children. World Health Organization (WHO); Geneva: 2012. [PubMed] [Google Scholar]
- 34.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326(7404):1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Pais P, Afzal R, Xavier D, Teo K, Eikelboom J, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373(9672):1341–1351. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf S, Pais P, Sigamani A, Xavier D, Afzal R, Gao P, et al. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the Second Indian Polycap Study (TIPS-2) investigators. Circ Cardiovasc Qual Outcomes. 2012;5(4):463–471. doi: 10.1161/CIRCOUTCOMES.111.963637. [DOI] [PubMed] [Google Scholar]
- 37.Malekzadeh F, Pourshams A, Marshall T. The preventive polypill--much promise, insufficient evidence. Arch Iran Med. 2007;10(3):430–431. [PubMed] [Google Scholar]
- 38.Rastegarpanah M, Malekzadeh F, Thomas GN, Mohagheghi A, Cheng KK, Marshall T. A new horizon in primary prevention of cardiovascular disease, can we prevent heart attack by “heart polypill”? Arch Iran Med. 2008;11(3):306–313. [PubMed] [Google Scholar]
- 39.Malekzadeh F, Marshall T, Pourshams A, Gharravi M, Aslani A, Nateghi A, et al. A pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factors. Int J Clin Pract. 2010;64(9):1220–1227. doi: 10.1111/j.1742-1241.2010.02412.x. [DOI] [PubMed] [Google Scholar]
- 40.Sepanlou SG, Farzadfar F, Jafari E, Danaei G. Cardiovascular disease prevention using fixed dose pharmacotherapy in Iran: updated meta-analyses and mortality estimation. Arch Iran Med. 2012;15(9):531–537. [PubMed] [Google Scholar]
- 41.Sepanlou SG, Kamangar F, Poustchi H, Malekzadeh R. Reducing the burden of chronic diseases: a neglected agenda in Iranian health care system, requiring a plan for action. Arch Iran Med. 2010;13(4):340–350. [PubMed] [Google Scholar]
- 42.Sepanlou SG, Poustchi H, Kamangar F, Malekzadeh R. Effectiveness and feasibility of lifestyle and low-cost pharmacologic interventions in the prevention of chronic diseases: a review. Arch Iran Med. 2011;14(1):46–53. [PubMed] [Google Scholar]