Abstract
Intestinal inflammation is a key element in inflammatory bowel disease (IBD) and is related to a combination of factors, including genetics, mucosal barrier dysfunction, bacteria translocation, deleterious host-microbe interactions, and dysregulated immune responses. Over the past decade, it has been appreciated that these inflammatory lesions are associated with profound tissue hypoxia. Interestingly, an endogenous adaptive response under the control of hypoxia signaling is enhancement in adenosine signaling, which impacts these different endpoints, including promoting barrier function and encouraging anti-inflammatory activity. In this review, we discuss the hypoxia-adenosine link in IBD, intestinal ischemia/reperfusion injury, and colon cancer. Additionally, we provide a summary of clinical implications of hypoxia and adenosine signaling in intestinal inflammation and disease.
Keywords: Adenosine, Adenosine Receptors, Hypoxia, HIF, Intestinal Inflammation, Inflammatory Bowel Disease, Ischemia/Reperfusion Injury, Colon Cancer
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is a chronic disorder of the intestinal tract characterized by intestinal inflammation and epithelial injury. The exact set of causes of IBD remains unclear. However, significant evidence indicates dysfunction of the mucosal immune system plays an important role in the pathogenesis of IBD. Strong evidence also implicates genetic susceptibility, deleterious host-microbe interactions, mucosal barrier dysfunction, and environmental factors (1, 2). There is also now significant evidence these inflamed lesions become profoundly hypoxic. Inflamed tissues experience significant changes in tissue metabolism. Oxygen supply and other metabolic factors are limited to tissues due to vascular occlusion, damaged blood supply, and/or compression of the tissue (3, 4). The metabolic demand of inflammatory cells also is a burden. For example, activated neutrophils consume significant amounts of oxygen, so much so that they imprint on the tissue environment, making it hypoxic (5). Over the past decade, it has been appreciated that inflammatory-hypoxia (tissue inflammation leading to tissue hypoxia (3)) increases extracellular adenosine/adenosine signaling and serves as an essential endogenous anti-inflammatory pathway that protects tissues on multiple levels (6, 7). Though hypoxia is an inflammatory stimulus, there are examples of low oxygen promoting tissue protection (8–15). In this review, we discuss the hypoxia-adenosine link, particularly hypoxia-inducible factors (HIFs) in regulating adenosine pathway genes. We also discuss the hypoxia-adenosine link in IBD, intestinal ischemia/reperfusion injury, and colon cancer and conclude with a summary of clinical implications of these pathways in intestinal inflammation and disease.
Adenosine Signaling during Intestinal Inflammation
Extracellular adenosine and adenosine signaling has shown to be an essential endogenous anti-inflammatory pathway in a number of conditions and diseases, including acute lung injury (16–21), myocardial injury (14, 22–25), intestinal ischemia/reperfusion injury (26–28), and IBD (11, 29–33). During inflammation, adenosine triphosphate (ATP) is released from stressed, apoptotic and/or necrotic cells, and bacteria (34, 35). ATP is converted to adenosine by cell surface ectonucleotidases, ectonucleoside triphosphate diphosphohydrolase-1 (CD39) and ecto-5′nucleotidase (CD73). CD39 generates adenosine diphosphate (ADP) and monophosphate (AMP) from ATP, and CD73 generates adenosine from AMP. Adenosine signals through membrane-spanning adenosine receptors, A1R, A2AR, A2BR, and A3R and is terminated by adenosine re-entering the cell through equilibrative or concentrative nucleoside transporters (ENTs, CNTs) or through activity of adenosine deaminase (ADA) (34–36). A2BR is the predominant adenosine receptor expressed on intestinal epithelial cells (32, 33, 37), whereas A2AR is expressed by most immune cells (34).
Mucosal Epithelial Cells
Epithelial cells play an important role in IBD pathogenesis. The breakdown of their barrier encourages bacterial translocation as well as promotes the release of pro-inflammatory cytokines, exciting tissue-damaging immune cells. The role of adenosine signaling in protecting tissue barriers was appreciated three decades ago, beginning with outstanding studies on polymorphonuclear leukocyte (PMN; neutrophil)-derived secretagogue (38–40). PMNs were found to release purine nucleotides (AMP (40) and ATP (41)) during transmigration. Subsequently, ATP/AMP is converted to adenosine by endothelial/epithelial cell CD39 or CD39 family members and CD73 and tissue barriers resealed by A2BR (39, 40). Adenosine signaling induces barrier protection by inducing actin polymerization (42) and through changes in the actin cytoskeleton, involving vasodilator-stimulated phosphoprotein (VASP) (43, 44). Indeed, a significant phenotype of mice deficient for adenosine pathway genes is barrier dysfunction. For example, wild-type mice gavaged with CD73 inhibitor, APCP experience severe intestinal epithelial barrier permeability in hypoxia (12). Similar, CD73 (13) and A2BR (8) deficient mice exposed to low oxygen suffer massive barrier breakdown and inflammatory cell accumulation in several tissues. As well, CD39 (29) and CD73 (30) deficient mice and mice with global or tissue-specific deletion of A2BR (32, 33) suffer more severe disease during experimental colitis and is associated with the breakdown of the intestinal epithelial barrier. A2BR on intestinal epithelial cells also regulates inflammatory cytokines, limiting immune cell infiltration. For example, cytokine interleukin-10 (IL-10) levels are reduced in inflamed mucosa from A2BR deficient mice (33). The anti-inflammatory role of IL-10 is significant in intestinal inflammation; IL-10 deficient mice develop spontaneous colitis (45). Extracellular adenosine/A2BR also reduces intestinal epithelial cell expression of pro-inflammatory cytokines interleukin-8 (IL-8) (46), interferon-αA, interleukin-1β, and tumor necrosis factor-α (TNF-α) (47). Studies using bone-marrow chimeras show A2BR activity on non-immune cells is essential in suppressing inflammatory cytokine and chemokine production during sepsis (48). A2BR activity on intestinal epithelial cells also promotes electrogenic chloride secretion, which flushes the lumen of toxins and pathogens (secretory diarrhea) (37, 38) and is another level of protection during intestinal inflammation. Loose stools are a clinical concern with IBD. Accordingly, there is some concern of severe diarrhea being a side effect with A2BR agonists.
Innate Immune Cells
Besides epithelial barrier breakdown, dysfunction of the mucosal immune system is significant in the pathogenesis of IBD. Several lines of evidence support A2AR on immune cells promotes profound endogenous anti-inflammatory responses (49, 50) Accordingly, interests in harnessing the immunosuppressive actions of extracellular adenosine/adenosine signaling (e.g., graft versus host disease and IBD) as well as to inhibit this response (e.g., tumors) for therapeutic benefit have been a recent topic of interest. A2AR activity on neutrophils inhibits neutrophil adhesion to and killing of endothelial/epithelial cells as well as modulates the production and release of pro-inflammatory cytokines (e.g., TNF-α) (51), chemokines (52), and prostaglandins (e.g., PGE2) (53, 54). A2AR activity on neutrophils, en route to sites of inflammation, is tissue protective by inhibiting the release of toxic oxygen species (55, 56). A2AR activity on macrophages limits their production of pro-inflammatory cytokines (e.g., interleukin-12 (IL-12) and TNF-α) and promotes their release of anti-inflammatory cytokine, IL-10 (57–61). Similar, A2AR on mature dendritic cells shifts their cytokine profile, reducing IL-12, IL-6, and interferon-α (IFN-α) (pro-inflammatory) and increasing IL-10 (62–64). A2BR activity on macrophages increases IL-10 (65) and also dampens TNF-α (59); dampening TNF-α appears operational only when not masked by A2AR (59). A2AR and A2BR activity on macrophages can also promote alternative macrophage activation, which may be important in IBD (66–68). In IBD, anti-inflammatory factor, netrin-1 interacts with A2BR, which dampens the infiltration of neutrophils into the intestinal mucosa (11, 31, 69). Adenosine receptors, A1R and A3R are less described, but show to support pro-inflammatory events (e.g., chemotaxis) leading up to A2AR and A2BR activity (56). Impaired innate immunity is implicated particularly in Crohn’s disease (70).
Adaptive Immune Cells
T cells are particularly sensitive to the immunosuppression/immunomodulatory activity of A2AR. During inflammation, A2AR is up-regulated on effector T cells. Subsequent activation of A2AR on these cells inhibits their proliferation, expansion, cytotoxic activity, and cytokine production (71–73). A2AR activity decreases both T helper 1 (Th1) and 2 (Th2) development and effector function (74). A2AR activity on these effector T cells also enhances the formation of Tregs and their expression of programmed cell death protein 1 (PD-1) and CTLA-4, negative regulators of inflammation (75). Naïve T cells express CD73 and is downregulated upon activation and differentiation, whereas Tregs express both CD39 and CD73 (76). Studies by Simon Robson have shown an essential regulatory loop, whereby extracellular adenosine generated by Tregs activates A2AR on effector T cells, suppressing effector T cell activity (77). Extracellular adenosine also activates A2AR on Tregs, promoting their expansion and immunoregulatory activity (self-reinforcing loop) (78). Accordingly, A2AR deficiency on Tregs reduces their immunosuppressive efficacy (79). A2AR agonists prevent colitis induced by pathogenic T cells in the absence of Tregs (80), whereas adoptive transfer studies show Tregs from wild-type mice fail to prevent colitis induced by pathogenic T cells from A2AR deficient mice (80). A2AR on myeloid cells is also required for controlling intestinal inflammation, as cotransfer of Tregs and pathogenic T cells from wild-type mice into rag/A2AR double deficient mice still leads to colitis (80). Interestingly, Th17 cells function similar to Tregs to suppress pro-inflammatory responses as well as show effector Th17 features, such as producing interleukin-17 and low levels of A2AR. CD39 is involved in the transition of Th17 cells into suppressor-like Th17 cells (81, 82). Many studies are directed at understanding the immunopathogenesis of IBD and relatedness of adenosine signaling. These studies will provide greatly to new ideas for treating intestinal inflammation.
Hypoxia Signaling
Inflamed lesions are often severely hypoxic. Tissues have evolved essential mechanisms to detect and adapt to low oxygen. Prolyl hydroxylases are essential to sensing oxygen and hypoxia inducible factors (HIFs) to initiating adaptive responses. HIFs are αβ-heterodimeric transcription factors that interact with hundreds of genes that encode glycolytic enzymes, inhibit mitochondrial respiration, regulate apoptosis, modulate inflammation, and promote angiogenesis (83). Oxygen-dependent prolyl hydroxylases (PHDs) and von Hippel-Lindau (VHL), an E3 ubiquitin ligase, control the stability of HIFs (84–88). In normoxia, HIF-1α and HIF-2α are hydroxylated at proline residues by PHDs, promoting their ubiquitination by VHL and proteosomal degradation (89). Mice deficient in PHDs or VHL show features of increased HIF activity, including increased vascularization and high levels of erythropoiesis (90–94). Factor inhibiting HIF (FIH), an oxygen-dependent asparaginyl hydroxylase, also controls HIFs through hydroxylation of asparagyl residues of α-subunits, which blocks the association of HIFs with transcriptional coactivators (95, 96). In hypoxia, PHDs and FIH activity is inhibited by the low availability of oxygen. HIF-α subunits combine with HIF-1β and subsequently bind to HIF-responsive elements (HREs) in gene promoters. HIF-1α and HIF-2α share a few, but largely have distinct target genes (97, 98). Recently, HIF-2α has been indicated as having a non-transcriptional role, indicating that much more remains to be discovered regarding HIF activity. HIFs also can be stabilized by inflammatory factors, such as bacterial component lipopolysaccharide (LPS), involving Toll-like receptor-4 (99), as well as bacterial release of iron-binding siderophopres, which stabilize HIFs by forming chelated complexes with iron ions, inhibiting PHDs (100). While PHDs require oxygen as a cofactor, iron is also necessary. PHDs are not limited to targeting HIFs. For example, studies by Cormac Taylor have shown that IKKβ (subunit of IκB kinase complex, member of the NF-κβ pathway) contains a conserved prolyl hydroxylation site similar to HIF-1α, and that hypoxia increases IKKβ stability through inhibition of IKKβ hydroxylation by PHD1, directing NF-κB disassociation from its inhibitor, thus promoting NF-κB to induce pro-inflammatory gene expression (101). NF-κβ can directly regulate HIF-1α and, in turn, NF-κβ can directly regulate HIF-1α (102). Moreover, studies involving macrophages demonstrate TNF-α stabilizes HIF-1α in normoxia (103). Recent studies involving Type 1 regulatory cells (Tr1), aryl hydrocarbon receptor, and CD39 provide additional insight into hypoxia signaling in integrating immunological, metabolic, and environmental signals to regulate immune responses (104). Taken together, HIF signaling in epithelial and immune cells is essential to tissue adaptation to low oxygen.
The Hypoxia-Adenosine Link during Intestinal Inflammation
The normal intestinal mucosa exists in a state of hypoxia (physiological hypoxia) (6, 105, 106) and has shown to be particularly resistant to low oxygen (105), a feature important to priming the tissue for oxygen changes and adaptation. Accordingly, HIFs are found stabilized in normal gut mucosa and are elevated in IBD patients (107). While searching for barrier protective pathways, Sean Colgan recognized CD73 and CD39 were increased several fold in hypoxic intestinal mucosa and that CD73 is a target of HIF-1α (12). Extensions of these studies identified that Sp1 increases CD39 expression in hypoxia (14). Sp1 is strongly implicated in targeting hypoxia adaptive genes, such as vascular endothelial growth factor (VEGF) (108). In contrast, HIFs repress ENT1 (9) and ENT2 (109) expression and reduce the conversion of adenosine to AMP by adenosine kinase (AK) (110, 111). AK is repressed by HIF-1α (111). ENT1 and ENT2 are bidirectional adenosine transporters, controlling adenosine efflux or intracellular uptake according to the concentration gradient (112). Studies involving radio-labeled adenosine showed extracellular adenosine uptake slows in hypoxia and that both ENT1 and ENT2 expression significantly decreases in intestinal epithelial models. ENT1 and ENT2 promoters also contain HREs and are targets of HIF-1α (9, 109). Consistent is that intestinal epithelial-targeted HIF-1α deficient mice show increased expression of ENTs (9, 109). Moreover, ENT inhibitor, dipyridamole promotes intestinal epithelial barrier function and attenuates mucosal inflammation in murine models of hypoxia (109). Collectively, ENT1 and ENT2 down-regulation by HIFs are significant in raising the levels of extracellular adenosine/adenosine signaling during hypoxia. Indeed, dipyridamole tissue protection is reduced in A2BR deficient mice (18). A2BR appears to have a select role in barrier protection in hypoxia (8). Intestinal epithelial models show A2BR is a HIF-1α target (113). HIF-2α appears to target A2AR (114). Netrin-1 (which interacts with A2BR) is also targeted by HIF-1α (11). The tissue environment also promotes HIF-mediated adenosine signaling. For example, low oxygen or tissue inflammation greatly favors the release of extracellular ATP/ADP (3, 35). As well, colitis studies show that transmigrating neutrophils consume local oxygen, making the tissue even more hypoxic (5). Inflammation in general also induces tissue hypoxia. To summarize, hypoxia/HIFs increase adenosine pathway gene expression that support an increase in extracellular adenosine/adenosine signaling and at the same time decreases genes that reduce extracellular adenosine levels/adenosine signaling (Figure 1).
Figure 1.
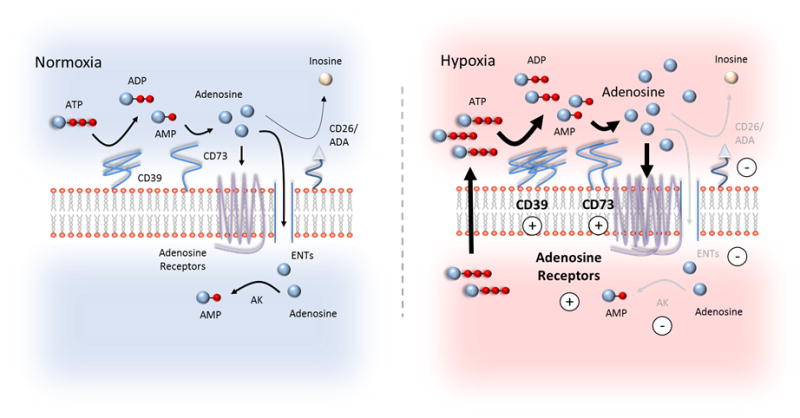
Increased extracellular adenosine and adenosine signaling by hypoxia. In normoxia, the concentration of adenine nucleosides (ATP, ADP, and AMP) at the cell surface is low. Extracellular ATP is converted to adenosine by two phosphohydrolysis reactions by cell surface nucleotidases, CD39 and CD73. CD39 converts ATP/ADP to adenosine and CD73 converts AMP to adenosine. Adenosine can activate adenosine receptors (A1R, A2AR, A2BR, A3R); be transported into the cell via equilibrative nucleoside transports (ENTs); or be converted to inosine at the cell surface by CD26-bound adenosine deaminase (ADA). In normoxia, adenosine is primarily distributed between high affinity adenosine receptors (e.g., A1R, A2AR, and A3R) and ENTs. In hypoxia, adenosine signaling is enhanced. Hypoxia increases the release of extracellular ATP/ADP via lytic and non-lytic pathways. Additionally, HIFs up-regulate (indicated by positive circles) adenosine metabolizing and signaling genes, including CD73 and adenosine receptors (e.g., A2AR and A2BR) while down-regulating (indicated by negative circles) genes that dampen adenosine signaling, including ADA, ENTs, and adenosine kinase (AK). CD39 is up-regulated in hypoxia by Sp1. In the gut, CD39 is largely restricted to immune cells and vascular endothelium. ATP/ADP metabolism on epithelial cells may involve ectonucleoside triphosphate diphosphohydrolase 7 (ENTPD7), a CD39 family member, and alkaline phosphatases. Together, these events increase extracellular adenosine and adenosine signaling, which dampens tissue inflammation and protects tissue barriers.
In recent years ample evidence has indicated that hypoxia/HIFs and extracellular adenosine/adenosine signaling play a prominent role in modulating immune cells during inflammation. Indeed, pharmacological and genetic studies strongly indicate that no other mechanism/pathway can compensate for the immune modulating/immunosuppression actions of extracellular adenosine (49, 50, 115). As well, HIF-1α importantly regulates the metabolic switch of immune cells from aerobic energy to glycolysis and multiple facets of myeloid and T cell biology, including development, proliferation, survival, and cytokine production (116). As discussed, hypoxia/HIFs increase extracellular adenosine levels/adenosine signaling, whereby activation of A2AR and/or A2BR on many immune cells is a potent “off” signal to the cells. In addition to promoting inflammation, hypoxia can promote immunosuppression (116). Many studies support a potential link between hypoxia and adenosine in immune cells during inflammation. Hypoxia/HIFs impair T cell receptor (TCR)-mediated activation and reduce proliferation, IFN-γ production, and cytotoxicity. Similar, A2AR and A2BR activity inhibits T cell TCR-mediated activation, proliferation, and cytokine (e.g., IFN-γ) production. Both are considered to act in concert to mediate these effects (117, 118). Notably, studies also indicated adenosine receptor-independent mechanisms (118). The exact mechanisms are yet to be clearly defined. HIF-1α additionally plays a role in influencing Treg differentiation and proliferation and also may coordinate with extracellular adenosine/adenosine signaling to mediate this response (77, 119–121). Metabolic control of Tr1cells is balanced through coordination of hypoxia/HIF-1α target genes and ATP/adenosine metabolism (104). Moreover, HIF-1α-induced netrin-1 interacts with A2BR on PMNs, attenuating their transepithelial migration and limiting tissue damage. Recently, HIF-1α was shown to up-regulate A2BR on alternatively activated macrophages in chronic inflammatory models (122). Suffice to say, there is great excitement for the possible benefit of therapeutic agents targeting hypoxia/adenosine signaling in regulating immune responses in number of health conditions and disease.
Examples for Hypoxia-Adenosine Link in Intestinal Inflammation and Disease
Above, we have summarized adenosine signaling in protecting the intestinal epithelial barrier and its potent anti-inflammatory responses. Additionally, we discussed hypoxia signaling and the link between adenosine signaling. Below, we discuss the hypoxia-adenosine link in IBD, intestinal ischemia/reperfusion injury, and colon cancer (Figure 2).
Figure 2.
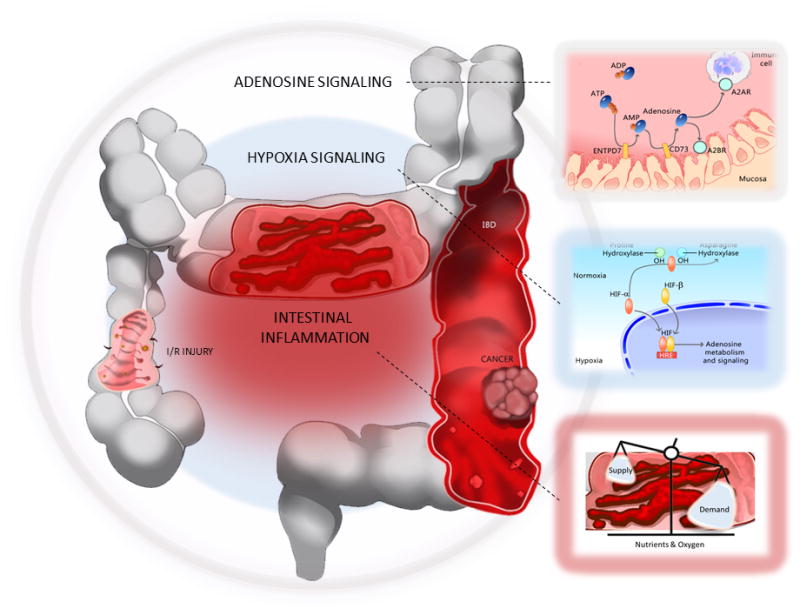
Summary: the hypoxia-adenosine link during intestinal inflammation and disease.
Inflamed tissues often become severely hypoxic, whereas tissue hypoxia can lead to tissue inflammation. HIFs are stabilized in these conditions, binding hypoxia-response elements (HREs) in target genes. Adenosine signaling pathway genes are direct gene targets of hypoxia/HIFs. In general, HIF-1α-mediated increase of adenosine signaling is tissue protective (e.g., IBD and intestinal I/R injury), whereas in cancer, HIFs may target extracellular adenosine/adenosine signaling genes to promote tumorigenesis. Boxes: Lower right: Inflammation alters tissue metabolism, reducing the supply of nutrients and oxygen to tissues. In turn, inflamed tissues become profoundly hypoxic. Middle right: Hypoxia signaling. Both proline hydroxylases (PHDs) and asparaginyl hydroxylase factor inhibiting HIF (FIH; indicated as asparagine hydroxylase) serve an important role as oxygen sensors, controlling the activity of HIFs. In normoxia, HIFs are rapidly targeted for degradation by proline hydroxylase (PHDs) and von Hippel-Lindau (VHL; not shown), an E3 ubiquitin ligase. In hypoxia, HIF-α is stabilized. Oxygen is an essential co-factor of PHDs and asparagine hydroxylase. HIFs (α and β subunits) translocate to the nucleus and dimerize, binding to HREs of target genes. Top right: Adenosine signaling. Multiple adenosine pathway genes are targets of hypoxia/HIFs. The increased release of ATP/ADP to the cell surface and hypoxia/HIF-mediated regulation of extracellular adenosine pathway genes (see Figure 1. for details) together enhance adenosine signaling.
Inflammatory Bowel Disease
IBD is characterized by excessive inflammation and profound hypoxia. Several genetic and pharmacological studies support that hypoxia signaling is protective during IBD (6). For example, mice with deletion of HIF-1α in intestinal epithelial cells are more susceptible to intestinal inflammation and have more severe disease during experimental colitis. Mice experience significant weight loss, colonic shortening, and suffer extensive increases in barrier permeability (105). In as much, mice with intestinal epithelial cell-specific deletion of VHL (105) or deletion of PHD1 (123) are protected during experimental colitis. These mice benefit from much improved intestinal barrier function and reduced inflammation (105, 123). Notably, increased HIF stabilization associates with increased expression of HIF-1α barrier protective genes, including CD73, in VHL deficient mice (105). Several studies show mucosal HIF-1α stabilization as having therapeutic promise for IBD. Stabilization of HIFs in immune cells is also protective. Loss- and gain-of-function studies show HIF-1α induces Treg differentiation, whereby Tregs deficient for HIF-1α fail to limit inflammation in models of T cell-mediated colitis (121). HIF-1α deficiency in dendritic cells also results in the failure to dampen intestinal inflammation, resulting in the increase of pro-inflammatory cytokines and diminished numbers of Tregs (124). HIF-2α appears to have a different role from HIF-1α in IBD; HIF-2α promotes the severity of colitis in mice (125). HIF-2α promotes pro-inflammatory responses (125) and barrier dysfunction (126).
Significant tissue hypoxia during IBD has been shown by 2-nitoimidazole compounds (6, 105, 106). As mentioned, CD73, A2AR (114), and A2BR (113) are targets of HIFs; CD39 is a gene target of hypoxia-induced Sp1. Both cd39−/− (29) and cd73−/− (30) mice experience severe disease during experimental colitis, including significant weight loss, colonic shortening, enhanced immune cell infiltration, and increased barrier permeability. Wild-type mice treated with APCP experience the same devastation (30), whereas wild-type mice receiving apyrase, a soluble factor with enzyme activity identical to CD39, experience significant protection (29). Notably, CD39 polymorphisms are associated with IBD in humans (29), which provides additional support of the importance of this pathway in IBD. Several studies support that adenosine signaling by A2BR and A2AR provides protection during IBD. Indeed, mice with global or tissue-specific deletion of A2BR experience increased severity of colitis (32). Similar, wild-type mice treated with A2BR antagonist, PSB1115 experience increased weight loss, colonic shortening, and leukocyte infiltration. A2A receptor agonist, ATL146e decreases both leukocyte infiltration and the production of inflammatory cytokines by T effector cells in IBD models (127). As mentioned, netrin-1 is directly induced by HIF-1α and interacts with A2BR, dampening neutrophil trafficking (11, 31). Accordingly, netrin-1 deficient mice also suffer significant disease severity during experimental colitis. Taken together, these studies support that HIF-mediated extracellular adenosine/adenosine signaling is protective in IBD. Of note, other studies suggest that A2BR deletion increases the severity of colitis (128–130). Reasons for these differences are unclear, possibly related to differences in experimental models and design.
As well, A2AR is essential to regulating adaptive immunity in IBD (34). A2AR agonists in the absence of Tregs prevent colitis by pathogenic T cells, whereby A2AR deficient mice suffer severe disease even with transfer of wild-type Tregs (80). These studies show that A2AR expression on both CD45RBhigh and CD45RBlow cells are important to controlling T cell-mediated colitis by suppressing pro-inflammatory cytokine expression while sparing anti-inflammatory activity mediated by IL-10 and transforming growth factor-β (TGF-β) (80). Adoptive transfer studies show that A2AR activity on lymphoid and nonlymphoid cells are additionally important for suppressing immune responses in colitis (131). Taken together, the ability of HIF-1α to increase extracellular adenosine/adenosine signaling in both immune and mucosal cells is essential in protecting tissues during IBD.
Intestinal Ischemia/Reperfusion Injury
Intestinal ischemia is a life-threatening condition associated with thrombosis, hypotension, necrotizing enterocolitis, bowel transplantation, trauma, and chronic inflammation. Extracellular adenosine/adenosine signaling has long been linked to ischemia protection in tissues (22, 132–134). Mounting evidence suggests a protective role of adenosine signaling in intestinal ischemia/reperfusion (I/R) injury. For example, A2BR expression is increased in intestinal mucosal scrapings following I/R in mice (26), whereby A2BR deficient mice suffer more profound intestinal I/R injury (26). Similar, A2BR antagonism enhances intestinal inflammation and injury during I/R in wild-type mice (26), whereas A2BR agonist treatment protects from intestinal injury, inflammation, and barrier breakdown (26). Adenosine treatment also attenuates intestinal I/R injury (27, 28). In general, CD73 and A2BR deficient mice experience more severe tissue injury with I/R (22, 26), and ischemia results in a robust increase in HIFs (15). Notably, studies of heart ischemia show HIF-1α-mediated cardioprotection is dependent on CD73 and A2BR signaling. Here, wild-type mice receive significant cardioprotective benefit from HIF activator, dimethyloxalylglycine (DMOG) treatment, whereas DMOG treatment in CD73 and A2BR deficient mice provides no protection against ischemia injury (15). As well, Sp1-mediated increase of CD39 provides significant barrier protection during tissue ischemia (14, 135). Though additional studies are needed, these studies provide consideration of a link between hypoxia and adenosine in intestinal I/R injury. Therapeutic agents targeting hypoxia or adenosine signaling are widely available. Future laboratory studies will hopefully examine the potential of these agents in intestinal I/R injury.
Colon Cancer
Colitis-associated cancer (CSC) is a primary example of the induction of cancer by chronic inflammation. Ulcerative colitis patients carry an 18% life-time risk of developing colon cancer (136). CSC and sporadic colon cancer have different development pathways, however share similar inflammatory pathways. Inflammation in sporadic colon cancer can develop by oncogenes inducing inflammatory transcriptomes (137, 138) as well as through microbiota-mediated mechanisms (139). In general, all solid tumors eventually outpace the supply of oxygen which in turn promotes inflammation. Similar to IBD (125), HIF-1α and HIF-2α may have different roles. For example, HIF-1α stabilization is associated with poor prognosis, whereas HIF-2α shows no prognostic value and is inversely associated with high tumor grade and HIF-1α (colon tumors, n=731) (140). Additionally, xenograft studies show HIF-1α deficiency inhibits colon tumor growth, whereas HIF-2α deficiency stimulates tumor growth (141). Moreover, inhibiting HIF-1α dimerization with acriflavine halts the progression of CSC in immunocompetent mice (142). Interestingly, HIF-1α overexpression does not increase tumorigenesis in sporadic colon and CSC cancer models and does not result in spontaneous tumor formation in mice (143). In contrast, intestinal epithelial disruption of Vhl shows to increase tumor progression, which is HIF-2α dependent (144). Suffice to say, there is much excitement surrounding the possible clinical benefit of targeting HIFs. However, at the same time, much remains to be understood.
Extracellular adenosine/adenosine signaling is also associated with many hallmarks of cancer, particularly immunosuppression. Both tumor promoting inflammation and antitumor immunity co-exist in tumors. The immunosuppressive actions of adenosine in cancer have been demonstrated in CD39, CD73, and A2AR deficient mice and by pharmacological and genetic studies in immune competent and immunodeficient mice (145–149). Recently, studies show co-inhibition of CD73 and A2AR is superior in improving antitumor immune responses (150). A2BR also is immunosuppressive. Tumors grow considerably slower in A2BR deficient mice and wild-type mice treated with A2BR antagonists (ATL-801, PSB1115) (151–153). Here, immune cell immunity is necessary for the antitumor effect, as A2BR antagonist treatment is not effective in nude mice (153) and T cell-deficient animals (152). Reduced tumor growth by ATL-801 or PSB1115 increases T cell and reduces Treg and myeloid-derived suppressor cell (MDSC) infiltration. A2BR is found up-regulated in colon tumors and cell lines and appears essential for colon cancer growth (154). A3R is up-regulated in colon cancer, however is largely shown to inhibit cancer growth (155, 156). Interestingly, treatment of colon cancer cells with caffeine (pan-adenosine receptor antagonist) inhibits A3R-stimulated HIF-1α stabilization (157). Studies in melanoma also show adenosine increases HIF-1α stabilization in a dose-dependent and time-dependent manner exclusively by A3R (158). IB-MECA (A3R agonist) also inhibits the growth of melanoma tumors in syngeneic models (159) and lung metastases models (160, 161). It is not clear if A3R-mediated increase in HIF-1α promotes tumor progression or supports the tumor suppressive actions of A3R.
Recent studies show whole body exposure to hyperoxic atmosphere (60% oxygen) reduces tumor growth (162, 163). Notably, adenosine, CD39, CD73, A2AR, and A2BR are reduced in these tumors and enhanced antitumor immune activity is seen. Accordingly, hyperoxia may have therapeutic benefit in cancer. These studies also strongly support the presence of the hypoxia-adenosine link in tumors. Indeed, another recent study has demonstrated that HIFs directly induce ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2), a family member of CD39, to prevent MDSC differentiation in hepatocellular cancer (164). Taken together, there is a real opportunity to target hypoxia and adenosine signaling in CSC/colon cancer. However, more studies are necessary for understanding these pathways in colon tumors.
Clinical Implications
Targeting HIFs have proven to have therapeutic benefit for patients with kidney disease (165). Many more studies are ongoing for a number of conditions and diseases (7). DMOG, FG-4497, and TRC160334, all pan-PHD inhibitors, are profoundly protective in models of colitis (166–168). A concern is that in contrast to HIF-1α (143), chronic activation of HIF-2α results in robust spontaneous intestinal inflammation (125), barrier dysfunction (126, 169), and tumorigenesis (144). Therefore, optimal therapies activating HIFs in IBD may be agents that specifically increase HIF-1α. PHD inhibitor, AKB-4924 may be promising. AKB-4924 robustly activates HIF-1α with only modest HIF-2α activation (170). AKB-4924 is currently being developed for use in IBD (NCT02914262). Similar, lower dosages of DMOG are capable of inducing HIF-1α with minimal effects on HIF-2α. Interestingly, HIF-2α–induced inflammation can be reduced with nonsteroidal anti-inflammatory drug (NSAID), nimesulide (125). Thus, combining PHD inhibitors with NSAIDs may have therapeutic potential for IBD patients. Additional concerns of PHD inhibitors are that they are non-specific for PHD isoforms (PHD1, PHD2, and PHD3 (84, 171)) and that many tissues express PHDs. Here, oral administration of PHD inhibitors, including AKB-4924 may minimize side effects. In a head-to-head comparison, though oral delivery of AKB-4924 reduced colon epithelial HIF-1α stabilization compared to intraperitoneal injection, oral AKB-4924 was sufficient enough to induce HIF gene targets and reduce disease severity in colitis models (172). Oral delivery reduced HIF stabilization and HIF target gene expression in extraintestinal tissues (172). Accordingly, with AKB-4924 there may be a trade-off between potency and toxicity. Roxadustat (FG-4592), another oral-available PHD inhibitor, is currently in clinical trials for kidney disease (7). Studies also support the therapeutic benefit of targeting adenosine signaling in IBD; both A2AR (127) and A2BR (32) agonists provide significant benefit in colitis models. Given that many tissues express adenosine receptors, strategies for local delivery of A2AR and A2BR agonists may be worthwhile to assess. Studies also suggest that autologous Treg–based therapies, involving the enrichment of patient’s endogenous CD39+ Tregs and reconditioning the cells with cytokines, may lead to better control of inflammation in IBD patients (173). The role of A3R in IBD is controversial (174, 175), however A3R agonists may have clinical benefit (176). Patients with intestinal I/R injury may also benefit from therapeutic strategies that increase HIF stabilization and adenosine signaling (26) (Figure 3).
Figure 3.
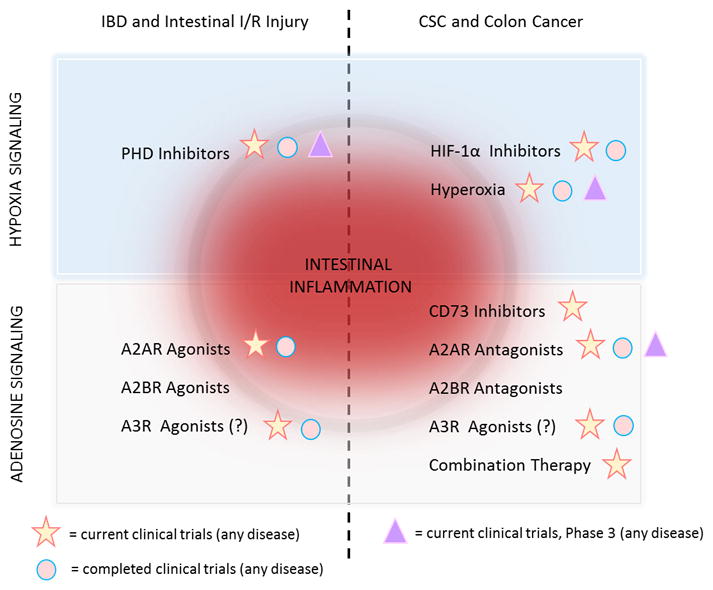
Targeting hypoxia and adenosine signaling in intestinal inflammation and disease. Increasing HIF-1α by PHD inhibitors or increasing adenosine signaling by adenosine receptor agonists (e.g., A2AR, A2BR, and possibly A3R) may provide therapeutic benefit for IBD and intestinal I/R injury patients. In contrast, inhibiting HIF-1α (e.g., HIF inhibitors, hyperoxia) or inhibiting extracellular adenosine (e.g., CD73 inhibitors)/adenosine signaling (e.g., A2AR, A2BR antagonists) may prove beneficial for patients with CSC or sporadic colon cancer. A2BR agonists and antagonists are currently used only in basic science laboratories. PHD inhibitors, Vadadustat (AKB-6548), Daprodustat (GSK1278863), and Roxadustat (FG-4592) are in clinical trials for anemia associated with chronic kidney disease (7). HIF-1α inhibitors and hyperoxia are in clinical trials of various cancers and respiratory conditions/diseases, respectively. A2AR agonist, Regadenoson is in clinical trials for sickle cell anemia (NCT01788631). A2AR antagonists, PBF-509 (NCT02403193) and CPI-444 (NCT02655822) or CD73 inhibitor, MEDI9447 (NCT02503774) are in clinical trials in solid tumors as anticancer therapies to boost immune responses against tumor cells. Completed clinical trials for A2AR antagonists include Parkinson’s disease (e.g., NCT01691924, NCT02111330). A3R agonist, CF-102 may reduce tumor burden and improve survival in hepatocellular cancer (NCT02128958).
In contrast, inactivation of hypoxia/adenosine pathways may decrease immunosuppression activity in tumors in addition to reducing metastasis and resistance to therapy (75). Hypoxia/adenosine signaling can potentially be targeted at different steps in CSC/colon cancer (75), such as improving tissue oxygenation either by HIF-1α inhibitors (177) or hyperoxic atmosphere (162, 163); reducing extracellular adenosine by inhibiting CD73 (146–149); or modulating adenosine signaling using agonists and antagonists (145) (Figure 3). Given the complexity of anti-tumor immunity, the combination of immune therapies may have the most promise for patients. Preclinical studies show anti-hypoxia/adenosine therapy in combination with immune checkpoint inhibitors, such as anti-CTLA-4 and anti-PD-1 provides great benefit (147, 178, 179). Combination therapies are currently in early phase clinical trials (7). Much hope awaits the completion of these studies.
Conclusions
The understanding of the link between hypoxia and adenosine and their potential as therapeutic targets has advanced considerably over the years. It is now appreciated that inflammatory lesions are profoundly hypoxic and that the enhancement of adenosine signaling by HIFs is an essential endogenous adaptive response. A significant challenge that remains is the movement of agents targeting these pathways into clinical practice. A focus on advancing cell or tissue-specific delivery of agents as well as defining challenges that have hampered the success of prior clinical studies of adenosine signaling agents will be important (180). Additionally, we must continue to gain new insights into these pathways. These efforts will certainly provide additional support in moving these pathways closer to being treatments and may make all the difference.
Acknowledgments
The authors thank the Department of Academic Technology at UTHealth, Houston, TX for the artwork.
Funding: International Anesthesia Research Society Mentored Research Award to JLB. National Institute of Health Grants P50-CA098258 and DK056338 to JLB and R01-DK097075, R01-HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowser JL, Lee JW, Yuan X, Eltzschig HK. The Hypoxia-Adenosine Link during Inflammation. J Appl Physiol (1985) 2017 doi: 10.1152/japplphysiol.00101.2017. jap 0010102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 12.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 16.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 17.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckle T, Hughes K, Ehrentraut H, Brodsky KS, Rosenberger P, Choi DS, Ravid K, Weng T, Xia Y, Blackburn MR, Eltzschig HK. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 2013;27:3078–3089. doi: 10.1096/fj.13-228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP, Eltzschig HK. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol. 2014;192:1249–1256. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 21.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 23.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 25.Rose JB, Naydenova Z, Bang A, Eguchi M, Sweeney G, Choi DS, Hammond JR, Coe IR. Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. Am J Physiol Heart Circ Physiol. 2010;298:H771–777. doi: 10.1152/ajpheart.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 27.Taha MO, Miranda-Ferreira R, Simoes RS, Abrao MS, Oliveira-Junior IS, Monteiro HP, Santos JM, Rodrigues PH, Rodrigues JV, Alves AE, Nascimento EC, Silva TL, Zeviani WM, Caricati-Neto A. Role of adenosine on intestinal ischemia-reperfusion injury in rabbits. Transplant Proc. 2010;42:454–456. doi: 10.1016/j.transproceed.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Haddad MA, Miranda-Ferreira R, Taha NS, Maldonado VC, Daroz RR, Daud MO, Neto JL, Muniz DA, Silva PC, Monteiro HP, Fagundes DJ, Caricati-Neto A, Taha MO. Effect of adenosine on injury caused by ischemia and reperfusion in rats: functional and morphologic study. Transplant Proc. 2012;44:2317–2320. doi: 10.1016/j.transproceed.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DJ, Kunzli BM, YIAR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 31.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A, Glover LE, Evans CM, Jedlicka P, Furuta GT, de Zoeten EF, Colgan SP, Eltzschig HK. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol. 2015;8:1324–1338. doi: 10.1038/mi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 35.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 38.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 42.Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K, Jr, Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest. 2016;126:220–238. doi: 10.1172/JCI79380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol. 2002;282:C1235–1245. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 44.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002;16:583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- 45.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jijon HB, Walker J, Hoentjen F, Diaz H, Ewaschuk J, Jobin C, Madsen KL. Adenosine is a negative regulator of NF-kappaB and MAPK signaling in human intestinal epithelial cells. Cell Immunol. 2005;237:86–95. doi: 10.1016/j.cellimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Bowser JL, Broaddus RR. CD73s protection of epithelial integrity: Thinking beyond the barrier. Tissue Barriers. 2016;4:e1224963. doi: 10.1080/21688370.2016.1224963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, Blackburn MR, Deitch EA, Hasko G. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 51.Thiel M, Chouker A. Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-alpha of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med. 1995;126:275–282. [PubMed] [Google Scholar]
- 52.McColl SR, St-Onge M, Dussault AA, Laflamme C, Bouchard L, Boulanger J, Pouliot M. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 2006;20:187–189. doi: 10.1096/fj.05-4804fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cadieux JS, Leclerc P, St-Onge M, Dussault AA, Laflamme C, Picard S, Ledent C, Borgeat P, Pouliot M. Potentiation of neutrophil cyclooxygenase-2 by adenosine: an early anti-inflammatory signal. J Cell Sci. 2005;118:1437–1447. doi: 10.1242/jcs.01737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pouliot M, Fiset ME, Masse M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–5286. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 55.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 58.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 59.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 60.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 63.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–1397. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 64.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 65.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, Virag L, Leibovich SJ, Hasko G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, Pacher P, Bai P, Hasko G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181:815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 70.Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 72.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 73.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 74.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohta A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bono MR, Fernandez D, Flores-Santibanez F, Rosemblatt M, Sauma D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 77.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 81.Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A, Quintana FJ, Robson SC. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One. 2014;9:e87956. doi: 10.1371/journal.pone.0087956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med (Berl) 2017 doi: 10.1007/s00109-017-1545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 84.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 85.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 86.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 88.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 90.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 91.Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeda K, V, Ho C, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 100.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, Neumann D, Colgan SP, Kempf VA. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Ignazio L, Rocha S. Hypoxia Induced NF-kappaB. Cells. 2016:5. doi: 10.3390/cells5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr, Reichner JS. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2001;281:C1971–1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- 104.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl) 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 107.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 109.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 110.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 111.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 112.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 113.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106:10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 116.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 117.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, Ohta A, Lentsch AB, Lukashev D, Sitkovsky MV. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS One. 2007;2:e853. doi: 10.1371/journal.pone.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohta A, Madasu M, Subramanian M, Kini R, Jones G, Chouker A, Ohta A, Sitkovsky M. Hypoxia-induced and A2A adenosine receptor-independent T-cell suppression is short lived and easily reversible. Int Immunol. 2014;26:83–91. doi: 10.1093/intimm/dxt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 121.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Philip K, Mills TW, Davies J, Chen NY, Karmouty-Quintana H, Luo F, Molina JG, Amione-Guerra J, Sinha N, Guha A, Eltzschig HK, Blackburn MR. HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis. FASEB J. 2017;31:4745–4758. doi: 10.1096/fj.201700219R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 124.Fluck K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9:379–390. doi: 10.1038/mi.2015.67. [DOI] [PubMed] [Google Scholar]
- 125.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie L, Xue X, Taylor M, Ramakrishnan SK, Nagaoka K, Hao C, Gonzalez FJ, Shah YM. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol Cell Biol. 2014;34:3013–3023. doi: 10.1128/MCB.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 128.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingersoll SA, Laroui H, Kolachala VL, Wang L, Garg P, Denning TL, Gewirtz AT, Merlin D, Sitaraman SV. A((2)B)AR expression in non-immune cells plays an important role in the development of murine colitis. Dig Liver Dis. 2012;44:819–826. doi: 10.1016/j.dld.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kurtz CC, Drygiannakis I, Naganuma M, Feldman S, Bekiaris V, Linden J, Ware CF, Ernst PB. Extracellular adenosine regulates colitis through effects on lymphoid and nonlymphoid cells. Am J Physiol Gastrointest Liver Physiol. 2014;307:G338–346. doi: 10.1152/ajpgi.00404.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr Pharm Des. 2008;14:1490–1499. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- 133.Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, Shinozaki Y, Chujo M, Mori H, Inoue M, et al. Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5′-nucleotidase activity and attenuation of adenosine release. Circulation. 1994;89:1237–1246. doi: 10.1161/01.cir.89.3.1237. [DOI] [PubMed] [Google Scholar]
- 134.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hart ML, I, Gorzolla C, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 138.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier RJ, Chung DC. HIF-1alpha and HIF-2alpha have divergent roles in colon cancer. Int J Cancer. 2009;124:763–771. doi: 10.1002/ijc.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shay JE, Imtiyaz HZ, Sivanand S, Durham AC, Skuli N, Hsu S, Mucaj V, Eisinger-Mathason TS, Krock BL, Giannoukos DN, Simon MC. Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis. 2014;35:1067–1077. doi: 10.1093/carcin/bgu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xue X, Ramakrishnan SK, Shah YM. Activation of HIF-1alpha does not increase intestinal tumorigenesis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G187–195. doi: 10.1152/ajpgi.00112.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72:2285–2293. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 148.Allard D, Allard B, Gaudreau PO, Chrobak P, Stagg J. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy. 2016;8:145–163. doi: 10.2217/imt.15.106. [DOI] [PubMed] [Google Scholar]
- 149.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Hasko G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer. 2016;2:95–109. doi: 10.1016/j.trecan.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, Huang Q, Liu J, Takeda K, Teng MWL, Sachsenmeier K, Smyth MJ. Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell. 2016;30:391–403. doi: 10.1016/j.ccell.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 151.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–1409. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ma DF, Kondo T, Nakazawa T, Niu DF, Mochizuki K, Kawasaki T, Yamane T, Katoh R. Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum Pathol. 2010;41:1550–1557. doi: 10.1016/j.humpath.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 155.Gessi S, Cattabriga E, Avitabile A, Gafa R, Lanza G, Cavazzini L, Bianchi N, Gambari R, Feo C, Liboni A, Gullini S, Leung E, Mac-Lennan S, Borea PA. Elevated expression of A3 adenosine receptors in human colorectal cancer is reflected in peripheral blood cells. Clin Cancer Res. 2004;10:5895–5901. doi: 10.1158/1078-0432.CCR-1134-03. [DOI] [PubMed] [Google Scholar]
- 156.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 157.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007;72:395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 158.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, MacLennan S, Baraldi PG, Borea PA. A3 adenosine receptors modulate hypoxia-inducible factor-1alpha expression in human A375 melanoma cells. Neoplasia. 2005;7:894–903. doi: 10.1593/neo.05334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Madi L, Bar-Yehuda S, Barer F, Ardon E, Ochaion A, Fishman P. A3 adenosine receptor activation in melanoma cells: association between receptor fate and tumor growth inhibition. J Biol Chem. 2003;278:42121–42130. doi: 10.1074/jbc.M301243200. [DOI] [PubMed] [Google Scholar]
- 160.Bar-Yehuda S, Barer F, Volfsson L, Fishman P. Resistance of muscle to tumor metastases: a role for a3 adenosine receptor agonists. Neoplasia. 2001;3:125–131. doi: 10.1038/sj.neo.7900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fishman P, Bar-Yehuda S, Barer F, Madi L, Multani AS, Pathak S. The A3 adenosine receptor as a new target for cancer therapy and chemoprotection. Exp Cell Res. 2001;269:230–236. doi: 10.1006/excr.2001.5327. [DOI] [PubMed] [Google Scholar]
- 162.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, Thayer M, Rodig S, Kutok JL, Jackson EK, Karger B, Podack ER, Ohta A, Sitkovsky MV. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hatfield SM, Kjaergaard J, Lukashev D, Belikoff B, Schreiber TH, Sethumadhavan S, Abbott R, Philbrook P, Thayer M, Shujia D, Rodig S, Kutok JL, Ren J, Ohta A, Podack ER, Karger B, Jackson EK, Sitkovsky M. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl) 2014;92:1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, Ng IO, Wong CC. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8:517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting Hypoxia-Inducible Factors for the Treatment of Anemia in Chronic Kidney Disease Patients. Am J Nephrol. 2017;45:187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]
- 166.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 167.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta R, Chaudhary AR, Shah BN, Jadhav AV, Zambad SP, Gupta RC, Deshpande S, Chauthaiwale V, Dutt C. Therapeutic treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates murine colitis. Clin Exp Gastroenterol. 2014;7:13–23. doi: 10.2147/CEG.S51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, Miller L, Kominsky DJ, Jedlicka P, Colgan SP. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci U S A. 2013;110:19820–19825. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Kockritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, Shalwitz RA, Johnson RS, Nizet V. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med (Berl) 2012;90:1079–1089. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 172.Marks E, Goggins BJ, Cardona J, Cole S, Minahan K, Mateer S, Walker MM, Shalwitz R, Keely S. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm Bowel Dis. 2015;21:267–275. doi: 10.1097/MIB.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 173.Gibson DJ, Elliott L, McDermott E, Tosetto M, Keegan D, Byrne K, Martin ST, Rispens T, Cullen G, Mulcahy HE, Cheifetz AS, Moss AC, Robson SC, Doherty GA, Ryan EJ. Heightened Expression of CD39 by Regulatory T Lymphocytes Is Associated with Therapeutic Remission in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2806–2814. doi: 10.1097/MIB.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 174.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 175.Ren T, Grants I, Alhaj M, McKiernan M, Jacobson M, Hassanain HH, Frankel W, Wunderlich J, Christofi FL. Impact of disrupting adenosine A(3) receptors (A(3)(−)/(−) AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren T, Tian T, Feng X, Ye S, Wang H, Wu W, Qiu Y, Yu C, He Y, Zeng J, Cen J, Zhou Y. An adenosine A3 receptor agonist inhibits DSS-induced colitis in mice through modulation of the NF-kappaB signaling pathway. Sci Rep. 2015;5:9047. doi: 10.1038/srep09047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 178.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 179.Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol Res. 2015;3:506–517. doi: 10.1158/2326-6066.CIR-14-0211. [DOI] [PubMed] [Google Scholar]
- 180.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]


