Abstract
Atrophy is thought to be a primary mode of muscle loss in neuromuscular injuries. The differential effects of central and peripheral injuries on atrophy and degeneration/regeneration in skeletal muscle tissue have not been well described. This study investigated skeletal muscle atrophy and degeneration/regeneration in an animal model of traumatic brain injury (TBI). Eight 8-month-old wild-type C57BL6 mice underwent either a sham craniotomy or TBI targeting the motor cortex. Atrophy (fiber area; FA) and degeneration/regeneration (centralized nuclei proportions; CN) of the soleus and tibialis anterior (TA) muscles were measured 2 months post-injury. Injured soleus FAs were smaller than sham soleus (p = 0.02) and injured TA (p < 0.001). Mean CNs were higher in the TBI-injured TA than in other muscles. Differential TBI-induced atrophy and degeneration/regeneration in lower limb muscles suggests that muscle responses to cortical injury involve more complex changes than those observed with simple disuse atrophy.
Keywords: : atrophy, controlled cortical impact, degeneration, regeneration, skeletal muscle, traumatic brain injury
Introduction
Muscle atrophy is thought to be a key phenotype underlying the loss of functional contractile tissue in neuromuscular injury and may result from any number of changes in neuromuscular activity and connectivity, including disuse, denervation, or inhibition of muscle activity secondary to altered motor drive or pain.1 Muscle degeneration (e.g., myophagocytosis, membrane, and cytoplasmic disruption) and subsequent regeneration (centralization of myonuclei), are processes that are distinct from atrophy, and have been observed in human musculoskeletal degenerative conditions that manifest with functional deficits.2 Human muscle biopsy data in these conditions demonstrate that an overwhelming majority of muscle regions show signs of fiber degeneration, even in the absence of atrophy.2 However, the stimuli inducing atrophy versus degenerative/regenerative phenotypes remain unclear. In particular, it is unknown whether these degenerative phenotypes are a result of localized or peripheral inflammation, denervation, or impaired motor drive from the central nervous system (CNS).
Current experimental models for comparing the differential effects of CNS, peripheral nervous system, and peripheral tissue injury on skeletal muscle are sparse.3 There is a wealth of data describing independent effects of various peripheral tissue adaptations and injury models on skeletal muscle (i.e., immobilization,4 tenotomy,5 cardiotoxin,6,7 lengthening contraction exercise8,9), and similarly, the effects of peripheral nerve injury (i.e., denervation,10,11 botulinum toxin12). However, few studies assess the effect of CNS injury on degeneration/regeneration in skeletal muscle; most are limited to measuring and reporting atrophy and/or fiber type shifts in conditions such as stroke and spinal cord injury.13,14 These comparisons are important because few models of peripheral muscle and/or nerve injury reproduce the human muscle degenerative phenotype observed in chronic musculoskeletal pathology, suggesting that inducing degeneration may require a combination of both central and peripheral injuries to the muscle and/or nerve over time. Therefore, identifying key atrophy and degeneration/regeneration-related morphological features after CNS injury, and comparing these features with those resulting from peripheral nerve or direct muscle injury is important in understanding if and how CNS injury causes muscle degeneration. This is an important precursor to understanding the mechanisms contributing to degenerative muscle changes, their functional sequelae, and ultimately the appropriate treatments to reverse muscle loss.
Traumatic brain injury (TBI) is a CNS injury in which external mechanical forces on the brain induce focal and/or diffuse neuronal injuries. Similar to other models of CNS injury, TBI ultimately leads to long-term muscle impairments, which often are associated with pain and result in poor functional recovery. The TBI model of CNS injury has been well developed in animals,15,16 and this injury model is known to induce secondary alterations in peripheral tissues due to disuse and altered motor drive.17,18 Simulated TBIs have successfully been used to mimic the muscle functional losses (i.e., weakness) observed in humans following injuries to the motor cortex.15 However, muscle morphological phenotypes following TBI and other CNS injuries are currently not well described. The purpose of this study was to investigate skeletal muscle–related changes (atrophy and degeneration/regeneration) resulting from controlled cortical impact in a mouse model of TBI. We hypothesized that traumatic injury to the motor cortex would induce atrophy, but not degeneration/regeneration in lower limb skeletal muscles (soleus and tibialis anterior).
Methods
Animals
Eight 8-month-old male wild-type C57BL6 mice were used in this experiment.
Controlled cortical impact model
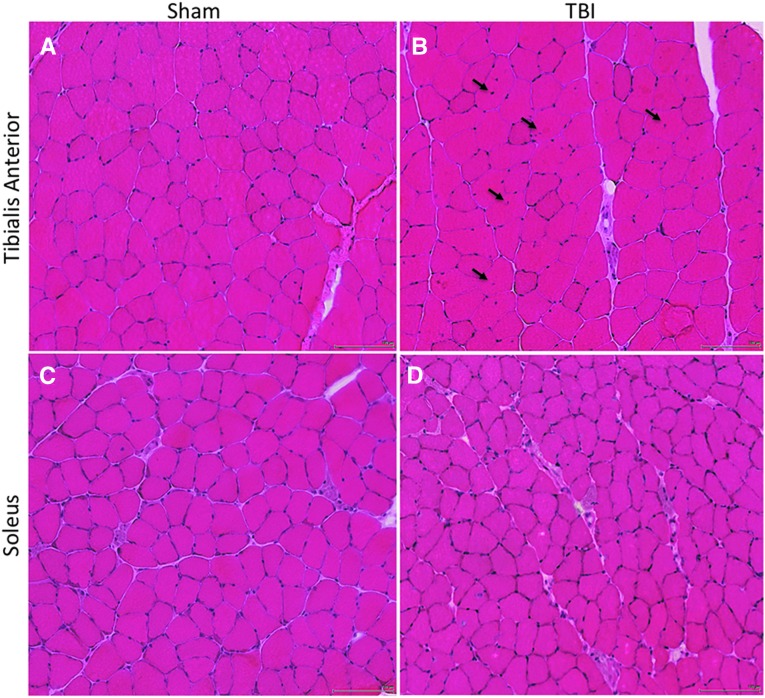
A craniotomy over the right primary and secondary motor cortex and parietal-temporal cortex (+1 to −4 anterior-posterior from the bregma, 4 mm laterally from the sagittal suture) was performed on anesthetized mice fixed to a stereotactic frame.16 A 3-mm diameter piston was centered over the motor cortex at approximately +0.5 to −2.5 mm bregma and 3 mm lateral to the sagittal suture. Using a stereotaxic impactor (Impact One; myNeuroLab.com), the piston was accelerated at a speed of 3 m/sec to an impact depth of 1 mm below the cortical surface. Severity of motor functional deficits and behavioral changes using this injury model are quantified elsewhere.16 Four mice were subjected to either sham (craniotomy only) or controlled cortical impact (CCI). Two months later, mice were euthanized and the soleus (Sol) and tibialis anterior (TA) muscles contralateral to the CCI were dissected, pinned at in vivo length and flash frozen in liquid nitrogen cooled isopentane, and stored at −80° until histological analysis. All procedures were approved by the institutional animal care and use committee. Hematoxylin and eosin stains were used to visualize overall tissue morphology, and wheat germ agglutinin with 4′6-diamidino-2-phenylindole to visualize muscle basal lamina and nuclei for measurements of fiber cross-sectional areas (FA) and centralized nuclei (CN; Fig. 1).19
FIG. 1.
Hematoxylin and eosin–stained sections of the tibialis anterior (TA) muscle (A, B; top row) and soleus (Sol) muscle (C, D; bottom row) in Sham-injured mice (A, C; left column) and traumatic brain injury (TBI) mice (B, D; right column). Several centralized nuclei (black arrows) can be seen in the injured TA muscle (B), and smaller fiber areas can be appreciated in the injured Sol muscle (D), compared with sham-injured animals. Micrographs were obtained at 20 × magnification. Scale bar is 100 μm.
Statistical analysis
Separate two-way analyses of variance were performed (injury × muscle) to compare FA and CN means between experimental groups. Post hoc Fisher's least significant difference comparisons (uncorrected t-tests) were performed when main/interaction effects were found to be significant. The p values were considered significant at <0.05. All statistics were performed in PRISM 7.0 (GraphPad Software, Inc., 2017).
Results
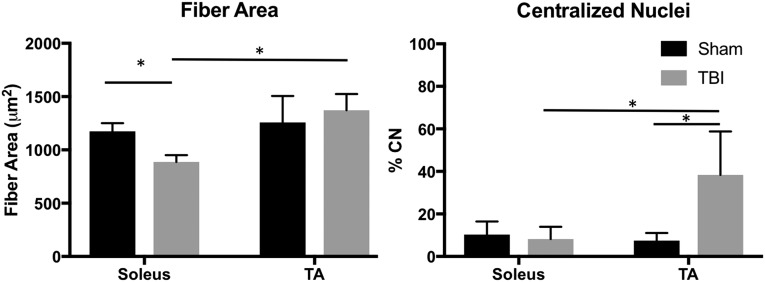
There was no main effect of injury on FA (p = 0.29), but there was a significant interaction (p = 0.02) with smaller injured Sol fiber FAs in the TBI group (887.90 ± 62.89 μm2), compared with both sham Sol (1173.77 ± 77.11; p = 0.02) and TBI injured TA (1371.69 ± 152.08 μm2; p < 0.001) muscles (Fig. 2). There were higher CN percentages in the TBI injured (23.21 ± 21.28%), compared with the sham (8.84 ± 4.92%; p = 0.02) groups. There was also a significant interaction (p = 0.01) driven by the high percentage of CN in injured TA muscles (38.29 ± 20.44%), compared with sham TA (7.43 ± 3.59%; p = 0.002), sham Sol (10.25 ± 6.18%; p = 0.004), and injured Sol (8.12 ± 5.76%; p = 0.003) muscles (Fig. 2).
FIG. 2.
Mean fiber area (left) and centralized nuclei (right) measurements for the soleus.
Discussion
These data provide the first morphological evidence for skeletal muscle degeneration/regeneration following CNS injury, created using an in vivo mouse model of TBI. CCI to the motor cortex induced muscle degeneration/regeneration but not atrophy in the TA, and atrophy but not degeneration/regeneration in the Sol. The evidence of degeneration/regeneration in muscle without peripheral nerve or direct muscle injury is novel. The concurrent presence of high levels of CN in the injured TA without evidence of atrophy may indicate a highly successful regenerative process for this muscle.
The differential presence of CN and atrophy between muscles may be a result of the different fiber type proportions in the mouse TA and Sol. The soleus of a rodent is almost completely composed of type I, or oxidative, fiber types, whereas the TA is primarily composed of type II, or fast-glycolytic, fiber types.20 The effects of injury on muscles with different fiber type compositions may suggest that muscles with higher proportions of type II fibers are more responsive (degeneration and regeneration) to CNS injury, compared with muscles with primarily type I fiber composition. In humans, clinical studies of muscle in patients with stroke and chronic hemiplegia demonstrate shifts towards type II fibers.21–23 However, the overall effect of these fiber type changes on muscle protection from atrophy or recovery from degeneration are unknown.
Comparisons to peripheral injury models
Peripheral denervation injuries result in rapid decreases in muscle fiber areas, with conflicting evidence of muscle degeneration/regeneration.24 These injuries induce selective atrophy of type II fibers, as well as fiber-type shifting.25,26 In muscle disuse models, loss of contractile protein content via the ubiquitin-proteosome pathway is often observed, such as in the case of immobilization.4,27 In contrast, direct muscle injury results in immediate activation of an inflammatory response, with large-scale muscle fiber necrosis, and high levels of nuclear migration coupled with high satellite cell activation in the cases of cardiotoxin, tenotomy, and lengthening contraction exercise.9,28,29 These changes are consistent with an active degeneration/regeneration response, and are distinct from peripheral denervation in that robust recovery occurs within 1–3 weeks of injury and no long-term changes in muscle size or fiber type are observed.30 Our CCI injury model induced different phenotypes between lower limb muscles, in that the phenotype observed in the TA was similar to that typically observed with direct muscle injury (degeneration/regeneration without atrophy), but the Sol was similar to the phenotype seen with disuse (atrophy without degeneration/regeneration).
In conclusion, our data support the presence of muscle-specific degeneration/regeneration or atrophy in response to CNS injury via TBI, demonstrating that muscle degeneration/regeneration can occur independent of a direct injury to the peripheral tissues. Although preliminary in nature, these findings have important implications for our understanding of initiating factors and mechanisms of muscle degeneration/regeneration, as well as motor rehabilitation following TBI, and highlight the need for future research with larger sample sizes and in a variety of neurological conditions.
Acknowledgments
This work was funded by BX003671 and NS073653 awarded to BPH, and TL1TR001443 awarded to BS.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Bonaldo P. and Sandri M. (2013). Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 6, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbons M. C., Singh A., Anakwenze O., Cheng T., Pomerantz M., Schenk S., Engler A. J., and Ward S. R. (2017). Histological evidence of muscle degeneration in advanced human rotator cuff disease. J. Bone Joint Surg. Am. 99, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thépenier C., Pascal Q., Guguin A., Gayraud-Morel B., Cavaillon J. M., Tajbakhsh S., Rocheteau P., and Chrétien F. (2016). Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11, e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark B. C. (2009). In vivo alterations in skeletal muscle form and function after disuse atrophy. Med. Sci. Sports Exerc. 41, 1869–1875 [DOI] [PubMed] [Google Scholar]
- 5.Jamali A. A., Afshar P., Abrams R. A., and Lieber R. L. (2000) Skeletal muscle response to tenotomy. Muscle Nerve 23, 851–862 [DOI] [PubMed] [Google Scholar]
- 6.Garry G. A., Antony M. L., and Garry D. J. (2016). Cardiotoxin induced injury and skeletal muscle regeneration. Methods Mol. Biol. 1460, 61–71 [DOI] [PubMed] [Google Scholar]
- 7.d'Albis A., Couteaux R., Janmot C., Roulet A., and Mira J. C. (1988). Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur. J. Biochem. 174, 103–110 [DOI] [PubMed] [Google Scholar]
- 8.Fridén J. and Lieber R. L. (1992) Structural and mechanical basis of exercise-induced muscle injury. Med. Sci. Sports Exerc. 24, 521–530 [PubMed] [Google Scholar]
- 9.Lieber R. L. and Fridén J. (1999). Mechanisms of muscle injury after eccentric contraction. J. Sci. Med. Sport 2, 253–265 [DOI] [PubMed] [Google Scholar]
- 10.Siu P. M. and Alway S. E. (2009). Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front. Biosci. (Landmark Ed.) 14, 432–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biral D., Kern H., Adami N., Boncompagni S., Protasi F., and Carraro U. (2008). Atrophy-resistant fibers in permanent peripheral denervation of human skeletal muscle. Neurol. Res. 30, 137–144 [DOI] [PubMed] [Google Scholar]
- 12.Mathevon L., Michel F., Decavel P., Fernandez B., Parratte B., and Calmels P. (2015). Muscle structure and stiffness assessment after botulinum toxin type A injection. A systematic review. Ann. Phys. Rehabil. Med. 58, 343–350 [DOI] [PubMed] [Google Scholar]
- 13.English C., McLennan H., Thoirs K., Coates A., and Bernhardt J. (2010). Loss of skeletal muscle mass after stroke: a systematic review. Int. J. Stroke 5, 395–402 [DOI] [PubMed] [Google Scholar]
- 14.Shields R. K. (2002). Muscular, skeletal, and neural adaptations following spinal cord injury. J. Orthop. Sports Phys. Ther. 32, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps H. W. (2016). Systematic review of traumatic brain injury animal models. Methods Mol. Biol. 1462, 61–88 [DOI] [PubMed] [Google Scholar]
- 16.Egawa J., Schilling J. M., Cui W., Posadas E., Sawada A., Alas B., Zemljic-Harpf A. E., Fannon-Pavlich M. J., Mandyam C. D., Roth D. M., Patel H. H., Patel P. M., and Head B. P. (2017). Neuron-specific caveolin-1 overexpression improves motor function and preserves memory in mice subjected to brain trauma. F.A.S.E.B. J. 31, 3403–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osier N. D., Carlson S. W., DeSana A., and Dixon C. E. (2015). Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J. Neurotrauma 32, 1861–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niesman I. R., Schilling J. M., Shapiro L. A., Kellerhals S. E., Bonds J. A., Kleschevnikov A. M., Cui W., Voong A., Krajewski S., Ali S. S., Roth D. M., Patel H. H., Patel P. M., and Head B. P. (2014). Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflammation 11, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamoto V. B., Hulst J. B., Lim M., Peace W. J., Bremner S. N., Ward S. R., and Lieber R. L. (2007). Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev. Med. Child Neurol. 49, 907–914 [DOI] [PubMed] [Google Scholar]
- 20.Eng C. M., Smallwood L. H., Rainiero M. P., Lahey M., Ward S. R., and Lieber R. L. (2008). Scaling of muscle architecture and fiber types in the rat hindlimb. J. Exp Biol. 211, 2336–2345 [DOI] [PubMed] [Google Scholar]
- 21.von Walden F., Jakobsson F., Edström L., and Nader G. A. (2012). Altered autophagy gene expression and persistent atrophy suggest impaired remodeling in chronic hemiplegic human skeletal muscle. Muscle Nerve 46, 785–792 [DOI] [PubMed] [Google Scholar]
- 22.Scelsi R., Lotta S., Lommi G., Poggi P., and Marchetti C. (1984). Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol. 62, 324–331 [DOI] [PubMed] [Google Scholar]
- 23.Castro M. J., Apple D. F., Staron R. S., Campos G. E., and Dudley G. A. (1999). Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J. Appl. Physiol. (1985) 86, 350–358 [DOI] [PubMed] [Google Scholar]
- 24.Carlson B. M. (2014). The biology of long-term denervated skeletal muscle. Eur. J. Transl. Myol. 24, 3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daemen M. A., Kurvers H. A., Bullens P. H., Slaaf D. W., Freling G., Kitslaar P. J., and van den Wildenberg F. A. (1998). Motor denervation induces altered muscle fibre type densities and atrophy in a rat model of neuropathic pain. Neurosci. Lett. 247, 204–208 [DOI] [PubMed] [Google Scholar]
- 26.Patterson M. F., Stephenson G. M., and Stephenson D. G. (2006). Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am. J. Physiol. Cell Physiol. 291, C518–C528 [DOI] [PubMed] [Google Scholar]
- 27.Urso M. L. (2009). Disuse atrophy of human skeletal muscle: cell signaling and potential interventions. Med. Sci. Sports Exerc. 41, 1860–1868 [DOI] [PubMed] [Google Scholar]
- 28.Lin Shiau S. Y., Huang M. C., and Lee C. Y. (1976). Mechanism of action of cobra cardiotoxin in the skeletal muscle. J. Pharmacol. Exp. Ther. 196, 758–770 [PubMed] [Google Scholar]
- 29.Vignaud A., Hourdé C., Butler-Browne G., and Ferry A. (2007). Differential recovery of neuromuscular function after nerve/muscle injury induced by crude venom from Notechis scutatus, cardiotoxin from Naja atra and bupivacaine treatments in mice. Neurosci. Res. 58, 317–323 [DOI] [PubMed] [Google Scholar]
- 30.Ciciliot S. and Schiaffino S. (2010). Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 16, 906–914 [DOI] [PubMed] [Google Scholar]