Abstract
Arterial spin labeling (ASL) has emerged as a technique for assessing mild traumatic brain injury (mTBI), as it can noninvasively evaluate cerebrovascular physiology. To date, there is substantial variability in methodology and findings of ASL studies of mTBI. While both increased and decreased perfusion are reported after mTBI, more consistency is emerging when perfusion is examined with regard to symptomology. We evaluated 15 teenage athletes two and six weeks after sports-related concussion (SRC group) using pseudo-continuous ASL. We acquired comparison data from 15 matched controls from a single time point. At each time point, we completed whole-brain contrasts to evaluate differences between the SRC group and controls in relative cerebral blood flow (rCBF). Cluster-level findings directed region of interest (ROI) analyses to test for group differences in rCBF across the left dorsal anterior cingulate cortex (ACC) and left insula. Finally, we evaluated ROI rCBF and symptomology in the SRC group. At two weeks post-injury, the SRC group had significantly higher rCBF in the left dorsal ACC and left insula than controls; at six weeks post-injury, elevated rCBF persisted in the SRC group in the left dorsal ACC. Perfusion in the left dorsal ACC was higher in athletes reporting physical symptoms six weeks post-injury compared with asymptomatic athletes and controls. Overall, these findings are inconsistent with reports of reduced rCBF after mTBI but coherent with studies that report increased perfusion in persons with greater or persistent mTBI-related symptomology. Future work should continue to assess how CBF perfusion relates to symptomology and recovery after mTBI.
Keywords: : adolescents, arterial spin labeling, mild traumatic brain injury, sports-related concussion, symptomology
Introduction
Comprehensive understanding of sports-related concussion (SRC) and other forms of mild traumatic brain injury (mTBI) relies on advances in behavioral and neuroimaging methodologies. Mild TBI is thought to represent a physiological, rather than anatomical, injury,1 but the physiological consequences of mTBI have not been well defined. Conventional imaging techniques are unable to consistently detect abnormalities, so more sensitive techniques are required to identify and monitor mTBI-induced pathophysiology. Arterial spin labeling (ASL), a neuroimaging method that evaluates absolute or relative cerebral blood flow (aCBF and rCBF, respectively), can noninvasively evaluate altered cerebrovascular physiology—a common consequence of TBI2—unlike conventional anatomic magnetic resonance imaging (MRI).
After moderate or severe brain injury, ASL has identified global decreases in CBF,3,4 and, more recently, studies have used ASL to examine CBF after mTBI.5 Unlike other techniques (e.g. positron emission tomography [PET]), ASL does not rely on an external contrast agent to measure perfusion, which increases its utility in the clinical and pediatric populations.6 As a relatively new method, however, there is substantial variability in the literature in both study design and findings, which is compounded by variability inherent in the mTBI literature at large. Subsequently, there are a finite number of ASL studies in mTBI populations, and they vary widely.
Variability in ASL studies of mTBI exists within the populations that are studied, including differences in age, mechanism of injury, and time between injury and testing. There are also differences in how CBF perfusion is acquired; researchers use pulsed ASL and pseudo-continuous ASL (also called pulsed-continuous ASL). Pulsed ASL labels the arterial blood magnetically in a spatially selective manner, whereas pseudo-continuous ASL labels the arterial blood in the feeding arteries continuously by a radiofrequency (RF) pulse train.
Studies also differ in whether they collect and/or report rCBF or aCBF values. The rCBF values provide an index of how well perfused a region of interest (ROI) is relative to the rest of the brain, whereas absolute values provide a CBF quantity for a ROI that is calculated independently of other regions. Compared with rCBF, aCBF has increased sensitivity to changes in physiological factors such as breathing rate or uptake of caffeine, and, subsequently, researchers have shown that rCBF has enhanced specificity to focal CBF abnormalities.7 Finally, studies vary in whether they further classify and group participants by secondary characteristics, such as presence or absence of symptoms and recovery status.
Unsurprisingly, variability in methodology results in heterogeneous findings that are difficult to synthesize and summarize—yielding uncertainty about the use of CBF as a possible biomarker for mTBI. Some researchers observe reduced CBF after injury; others observe dynamic changes in CBF across recovery, while others observe increases in CBF. Somewhat surprisingly, there are few studies that demonstrate no change in CBF, which might be expected given the heterogeneity of published findings. This may reflect publication bias toward significant results or reflect publication of studies where expected results were not defined a priori and, rather, reported findings are ones that arose merely by chance. To date, there appear to be 11 published studies that have used ASL in populations with a history of mTBI; this number excludes studies of moderate and severe TBI, animal studies, review papers, and single case studies. Table 1 summarizes those 11 studies to illustrate the variability in study design and findings.
Table 1.
Summary of Arterial Spin Labeling Studies in Mild Traumatic Brain Injury Populations*
| Population | Mean age (SD) TBI subjects | #TBI; #Controls | Technique | Δ in CBF | Symptoms and CBF Δ | Summary of findings | |
|---|---|---|---|---|---|---|---|
| Doshi et al., 2015 | 3 h–10 days post-mTBI | 27.1 (5.5) | 7 TBI; 12 controls | Pulsed ASL | Increase | No significant relationship | Significantly higher rCBF in frontal lobe, occipital lobe, and left striatum |
| Wang et al., 2015 | 3–12 months after SRC | 15.1 (.9) | 14 TBI; 15 controls | Pulsed ASL | Decrease | Not statistically evaluated | Significantly reduced aCBF in bilateral frontotemporal lobes |
| Meier et al., 2015 | 1 day, 1 week, and 1 month post-SRC | 20.6 (1.2) | 17 TBI; 27 controls | Pseudo-continuous ASL | Decrease | Inverse relationship in rCBF and depression and anxiety symptoms | Initial reduction in rCBF in right insular and superior temporal cortex; resolved by 1 month post-injury |
| Sours et al., 2015 | 3–10 days, 21–51 days, and 173–228 days post-mTBI | 38.9 (15.9) | 28 TBI; 28 controls | Pulsed ASL | N/A | Increased rCBF in task positive nodes in symptomatic mTBI | Atypical ratio of rCBF perfusion between functional networks 3–10 days and 21–51 days post-mTBI |
| Militana et al, 2016 | 3–6 days post-SRC | 19.7 (1.2) | 7 TBI; 11 controls | Pseudo- continuous ASL | None | Not statistically evaluated | Increased cerebrovascular reactivity, but no significant findings in aCBF |
| Mutch et al., 2016 | 33–993 days post-SRC with PCS | 17.3 (SD not reported) | 15 TBI; 15 controls | Pseudo- continuous ASL | Mixed | Not statistically evaluated | Regional differences in rCBF: some areas with increased rCBF and other areas with decreased rCBF |
| Wang et al., 2016 | 24 h and 8 days post-SRC | 17.8 (1.5) | 18 TBI; 19 controls | Pseudo- continuous ASL | Decrease | Not statistically evaluated | Global decrease in aCBF at 24 h post-SRC, further decreases 8 days post-SRC |
| Peng et al., 2016 | 72 h, 3 days–3 weeks, and ≥3 months post-mTBI | 39.1 (5.6) | 20 TBI; 20 controls | Pulsed ASL | Decrease | Not statistically evaluated | Global reduction in aCBF 72 h and 3 days–3 weeks post-mTBI; resolved at 3 months post-mTBI; no findings in insula |
| Lin et al., 2016 | 8–16 days post-mTBI | 51.6 (6.7) | 23 TBI; 22 controls | Pseudo-continuous ASL | Decrease | Higher aCBF linked to more physiological symptoms | Significant reduction in aCBF in bilateral frontal and left occipital cortex |
| Churchill et al., 2017 | 1–7 days post-SRC | 20 (SD not reported) | 26 TBI; 26 controls | Pulsed ASL | Mixed | Not statistically evaluated | Patients 1–3 days post-injury had higher aCBF; those 5–7 day post-injury had decreased aCBF |
| Barlow et al., 2017 | 36–44 days post-mTBI | 14 (SD not reported) | 27 symptomatic TBI; 24 asymptomatic TBI; 21 controls | Pseudo- continuous ASL | Mixed | Increased aCBF in symptomatic mTBI | Symptomatic children had increased aCBF; asymptomatic children had lower aCBF |
SD, standard deviation; TBI, traumatic brain injury; CBF, cerebral blood flow; Δ, change; mTBI, mild traumatic brain injury; ASL, arterial spin labeling; rCBF, relative cerebral blood flow; SRC, sports-related concussion; aCBF, absolute cerebral blood flow; PCS, post-concussive syndrome.
Excluded articles: moderate and severe TBI, animal studies, review papers, single case studies.
It does not appear that consistency in age, time since injury, or ASL technique rendered similar findings. Some similarity is seen across studies, however, when examining the relationship between CBF perfusion and the presence of post-concussive symptoms. One study found that children with persistent mTBI symptoms had significantly higher aCBF globally in comparison with controls, whereas asymptomatic children had significantly lower aCBF globally.8 Similarly, another study found that adults with persistent mTBI symptoms were more likely to have increased rCBF in nodes of the task positive network relative to the nodes of the default mode network.9 A third study found that increased symptoms were positively associated with increased aCBF in the left frontal lobe.10 Only one study observed an inverse relationship between rCBF perfusion and symptoms; lower rCBF was associated with more symptoms of anxiety and depression.11
It appears that examining CBF and the presence or absence of symptoms may help to explain heterogeneity of ASL findings. It is difficult to make that conclusion, however, because each of the reported studies evaluated symptoms differently, and six of the 11 total studies did not report or statistically evaluate symptoms with CBF perfusion.12–17 Further, one study examined the relationship between CBF perfusion and symptoms and failed to find a significant relationship, although this study included only seven participants, which may have resulted in insufficient statistical power.5 Nevertheless, when compared with all other elements of variability within ASL studies, symptom status appears to be an appropriate candidate for further inquiry and may help explain heterogeneity of ASL findings.
In this study, we examined rCBF perfusion and symptom status in adolescents with sports-related mTBI who were within two weeks post-injury and again when they were six weeks post-injury. We compared rCBF from those two time points with rCBF values collected at a single time point from a comparison cohort of never-concussed age- and sex-matched peers. We hypothesized that we would observe different rCBF perfusion in the mTBI group compared with never-concussed peers. We also anticipated that the total number of self-reported symptoms may influence rCBF values within the mTBI group. Finally, we hypothesized that the type of symptoms (e.g., physical, emotional, cognitive, or sleep-related) may differentially influence rCBF in the mTBI group.
Methods
Participants
Fifteen adolescents, ages 13 to 17, with SRC were recruited from clinical encounters (e.g., concussion clinic) and enrolled in this study. Inclusion criteria for the concussion group included: presence of a recent concussion (a witnessed blow to the head or body that occurred during organized sport participation with subsequent onset of at least two concussion-induced symptoms), loss of consciousness (when present) of 15 min or fewer and post-traumatic amnesia of 24 h or fewer, and Glasgow Coma Scores from emergency department admission (when available) of 13 to 15. Exclusion criteria for the concussion group included: history of chronic medical disorder, current inpatient hospitalization, current use of narcotic pain medicine, history of moderate or severe TBI, or history of mTBI without return to baseline. Fifteen never-concussed adolescent athletes, ages 13 – 17, were also recruited using flyers, word-of-mouth, and radio advertisements and enrolled in this study. Exclusion criteria for the control group included a history of TBI, including concussion. None of the participants within the SRC or control group met criteria for pre-injury behavioral or educational diagnoses based on structured parent interview. The Johns Hopkins Medicine Institutional Review Board approved this study, written informed consent was obtained from a parent or legal guardian, and assent was acquired from adolescent participants.
Imaging acquisition and processing
Imaging was performed on a 3T MR system (Philips Medical System, Best, The Netherlands) using the body coil for transmission and an eight-channel head coil for reception. A 4.5-min T1-based magnetization-prepared, rapid acquisition gradient echo (T1-MPRAGE) image was first acquired for anatomical reference and spatial normalization using the following parameters: 150 axial slices, voxel size 1 × 1 × 1 mm3, matrix = 224 × 224, field of view = 224 × 180, time to repeat/echo time = 8.1 msec/3.7 msec. Pseudo-continuous ASL (PCASL) was then used to obtain estimates of resting CBF.18, 19 Thirty-five control and label pairs of images were acquired with the following parameters: labeling duration = 1650 msec, post-labeling delay = 1525 msec, time to repeat/echo time = 4000 msec/12 msec, flip angle = 90 degrees, matrix size = 80 × 80, voxel size = 3 × 3 × 7 mm3, 17 slices, no gap, and duration = 288 sec.
The PCASL data were processed to generate CBF maps using the fully automated ASL-MRICloud tool.18,19 The ASL-MRICloud is a cloud-based tool for ASL data analysis under the infrastructure of MRICloud.org20,21 and follows the quantification model described in the ASL white paper.22 In the automated pipeline, T1-MPRAGE was used to normalize the CBF maps into Montreal Neurological Institute (MNI) space. Anatomic ROIs, generated from the T1-based brain segmentation tool under mricloud.org infrastructure20 were applied to yield structure-based CBF values. Considering the baseline differences across imaging sessions and across subjects, rCBF images and ROI values, normalized by the whole-brain average, were used for further analysis.
Study procedure
Adolescents with SRC were evaluated at two time points. Neuroimaging data were acquired from 12 of the 15 eligible adolescents at Visit 1 (mean: 8.8 days post-injury; standard deviation [SD]: 3.4); two participants did not complete neuroimaging because of scanner malfunction, and one participant did not complete neuroimaging because of scheduling constraints. Neuroimaging data were acquired from 14 of the 15 eligible adolescents at Visit 2 (mean: 43.2 days post-injury; SD: 12.5), because one participant did not return for testing. Controls participants were evaluated at a single time point.
Cluster-based analysis
The Statistical Parametric Mapping (SPM12) second-level analyses were used to evaluate between-group differences in rCBF. Each participant's rCBF map was used in independent sample t tests to evaluate differences at Visit 1 between the SRC group and controls and at Visit 2 between the SRC group and controls. The voxel-level threshold was established at p < 0.001, and family-wise error (FWE) correction was used for multiple comparisons at a cluster-level threshold of p < 0.05.
ROI-based analysis
The MNI coordinates of the peak activated voxel, where cluster-level between-group differences were observed, were entered into the Wake Forest University Pickatlas toolbox23,24 to identify the corresponding anatomic structures, using the Automated Anatomical Labeling Atlas.25 Relative CBF values from these anatomic ROIs were entered into t tests in Statistical Package for the Social Sciences (SPSS) to evaluate differences between the SRC group and controls and to evaluate differences between time points within the SRC group. Control analyses, Pearson correlations, and analyses of covariance (ANCOVAs), were also completed in SPSS to ensure that neither age nor sex was influencing group differences.
Symptom and rCBF analyses
The self-reported symptoms from the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT®:www.impacttest.com.) fall into four categories: physical, emotional, cognitive, and sleep-related symptoms. Both the SRC group and control group were asked to report symptoms on the ImPACT.® We divided the SRC group into a high symptom or low symptom subgroup by using the median number of symptoms in each symptom category at Visit 1 and a symptoms present or symptoms absent subgroup at Visit 2.
For Visit 1, participants with a symptom count greater than or equal to the median were included in the high symptom subgroup while those with a symptom count less than the median were included in the low symptom subgroup. For Visit 2, because many participants reported no symptoms in symptom categories, those who were still reporting symptoms were included into the symptoms present subgroup (individuals with one or more symptoms) and those with no reported symptoms were included in the symptoms absent subgroup. Between-group differences in rCBF were then evaluated with univariate ANCOVAs (to account for age) using a model threshold of p ≤ 0.003 (calculated by p = 0.05 divided by 16, the total number of ANCOVA analyses) and Bonferroni corrected pairwise comparisons.
Results
Participant characteristics
At Visit 1, the SRC group included 12 participants (mean age: 15.6 [SD = 1.2]; four females [33%]). At Visit 2, the SRC group included 14 participants (mean age: 15.7 [SD = 1.4]; four females [29%]). The control group included 15 participants (mean age: 15.2, [SD = 1.7]; five females [33%]). No significant differences existed in age or sex at Visit 1 or Visit 2 between the SRC and control groups, p values >0.05.
Cluster-based group differences
At Visit 1, results from whole-brain contrasts revealed that the SRC group (mean = 1.33, SD = 0.15) had significantly higher rCBF than controls (mean = 1.01, SD = 0.16) in a cluster (contiguous voxels [kE] = 1017) with the peak activated voxel (MNI coordinates −44, −30, 20) residing in the left insula, p < 0.001. In addition, at Visit 1, the SRC group (mean = 1.17, SD = 0.09) had significantly higher rCBF than controls (mean = 0.95, SD = 0.14) in a cluster (contiguous voxels [kE] = 1126) with the peak activated voxel (MNI coordinates −14, 4, 44) residing in the left cingulate cortex, p < 0.001.
At Visit 2, results from whole-brain contrasts revealed that the SRC group (mean = 1.15, SD = 0.12) had significantly higher rCBF than controls (mean = 0.90, SD = 0.14) in a cluster (contiguous voxels [kE] = 1672) with the peak activated voxel (MNI coordinates −14, 4, 44) residing in the left cingulate cortex, p < 0.001.
ROI-based group differences
Given the cluster-based group differences, two ROIs from the anatomically parcellated map—left insula and left dorsal anterior cingulate cortex (left dorsal ACC)—were selected and evaluated for potential group differences. First, we conducted control analyses to evaluate potential influence of sex and age on ROI rCBF values. There were no significant differences in ROI rCBF values between males and females within the control group, p values >0.45, nor differences between males and females within the SRC group at either time point, p values >0.32. In the control group, age was significantly correlated with left insula rCBF, r = 0.55, p = 0.03, but was not related to left dorsal ACC rCBF, p = 0.53. In the SRC group, age was significantly correlated with left dorsal ACC rCBF at Visit 1, r = 0.91, p < 0.001 but not at Visit 2, p = 0.15, but there was no significant relationship between age and left insula rCBF at Visit 1, p = 0.79, or Visit 2, p = 0.39.
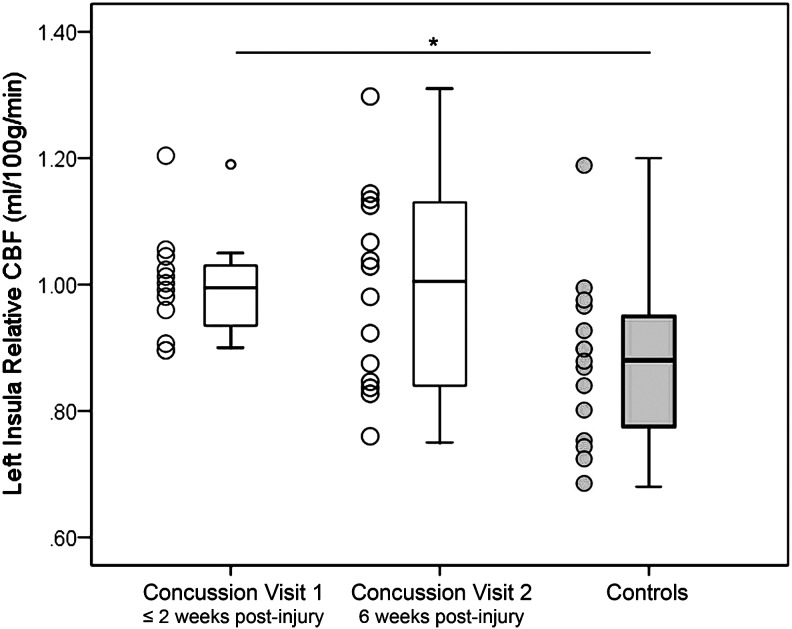
In the left insula, at Visit 1, when controlling for age, the SRC group (mean = 1.0, SD = 0.08) had significantly higher rCBF than controls (mean = 0.88, SD = 0.13), t (1, 25) = −2.71, p = 0.02. At Visit 2, when controlling for age, the SRC group (mean = 0.99, SD = 0.16) no longer had significantly different rCBF than controls (mean = 0.88, SD = 0.13), p = 0.07. No significant differences existed between Visit 1 and Visit 2 within the SRC group, p = 0.93; see Figure 1. In the SRC group, the number of days since injury did not correlate to rCBF in the left insula, p = 0.91.
FIG. 1.
Between-group differences in left insula relative cerebral blood flow (rCBF). Adolescents with sports-related concussion (SRC, white boxes) had significantly higher rCBF than never-concussed controls (gray boxes) in the left insula at Visit 1 (≤ two weeks post-injury). At Visit 2 (six weeks post-injury), adolescents with SRC had numerically higher rCBF than controls, but this was not statistically significant when analyses controlled for age. No differences existed in rCBF between Visits 1 and 2 in the SRC group.
*Indicates p < 0.05. Error bars represent standard deviation.
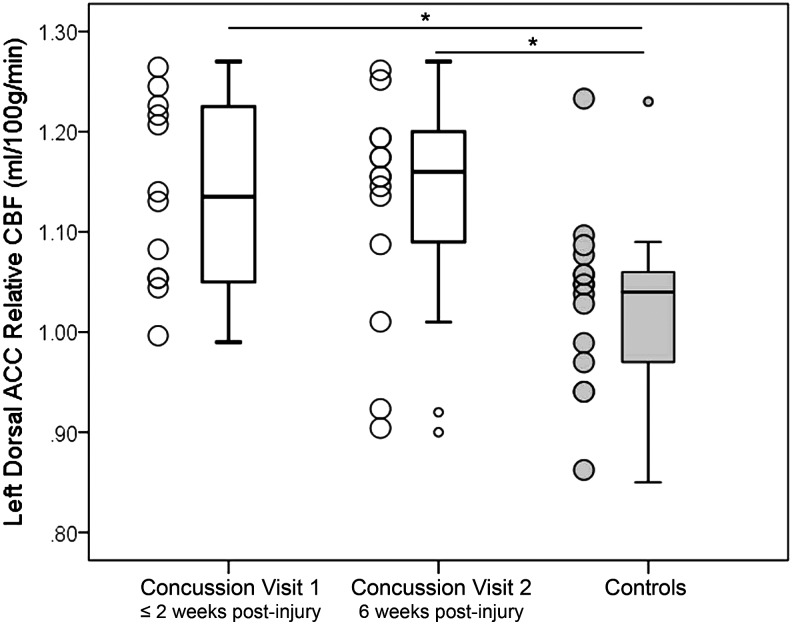
In the left dorsal ACC, at Visit 1, when controlling for age, the SRC group (mean = 1.14, SD = 0.10) had significantly higher rCBF than controls (mean = 1.02, SD = 0.09), t ( 25) = - 3.25, p = 0.01. At Visit 2, when controlling for age, the SRC group (mean = 1.13, SD = 0.11) continued to have significantly higher rCBF than controls (mean = 1.02, SD = 0.09), t (26) = - 2.85, p = 0.01. No significant differences existed between Visit 1 and Visit 2 in the left dorsal ACC rCBF within the SRC group, p = 0.84; see Figure 2. In the SRC group, the number of days since injury did not correlate to rCBF in the left dorsal ACC, p = 0.86.
FIG. 2.
Between group differences in left dorsal anterior cingulate cortex (ACC) relative cerebral blood flow (rCBF). Adolescents with sports-related concussion (SRC, white boxes) had significantly higher rCBF than never-concussed controls (gray boxes) in the left dorsal ACC at Visit 1 (≤ two weeks post-injury) and Visit 2 (six weeks post-injury). These differences persisted when analyses controlled for age. No differences existed in rCBF between Visits 1 and 2 in the SRC group.
*Indicates p < 0.05. Error bars represent standard deviation.
Symptom and rCBF results
At Visit 1, ANCOVA analyses did not reach the established model threshold of p ≤ 0.003 when comparing rCBF of the left insula or left dorsal ACC of controls and SRC subgroups divided by any symptom category (i.e., physical, emotional, cognitive, sleep-related). At Visit 2, ANCOVA analyses did not reach the established model threshold of p ≤ 0.003 when comparing rCBF of the left insula of controls and SRC subgroups divided by any symptom category; ANCOVA analyses also did not reach p ≤ 0.003 when comparing rCBF of the left dorsal ACC of controls and SRC subgroups divided by emotional, cognitive, or sleep-related symptoms. Significant between-group differences were observed, however, when comparing rCBF of the left dorsal ACC of controls and SRC subgroups divided by physical symptoms.
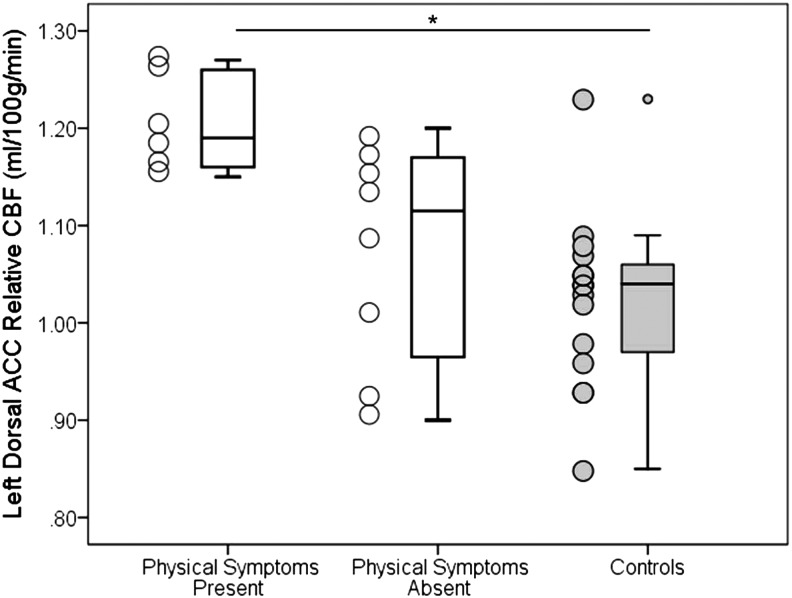
At Visit 2, the median number of physical symptoms was 0, placing six participants in the physical symptoms present subgroup (individuals with one or more symptoms) and eight participants in the physical symptoms absent subgroup (individuals reporting 0 symptoms). The ANCOVA revealed a significant effect of group on rCBF in the left dorsal ACC, F (2, 25) = 7.47, p = 0.003. Post-hoc analyses revealed that the physical symptoms present subgroup (mean = 1.20, SD = 0.05) had significantly higher rCBF in the left dorsal ACC compared with controls (mean = 1.02, SD = 0.09), adjusted p = 0.002 and numerically higher rCBF than the physical symptoms absent subgroup (Mean = 1.08, SD = 0.09), adjusted p = 0.058. No significant differences existed between the low physical symptom subgroup and controls, adjusted p = 0.679; see Figure 3.
FIG. 3.
Left dorsal anterior cingulate cortex (ACC) relative cerebral blood flow (rCBF) in physical symptom subgroups at Visit 2. Adolescents with sports-related concussion (SRC, white boxes) in the physical symptoms present subgroup had significantly higher rCBF in the left dorsal ACC compared with never-concussed controls (gray boxes). The physical symptoms present subgroup also had numerically higher rCBF compared with the physical symptoms absent subgroup. No significant differences existed between the physical symptoms absent subgroup and control participants.
*Indicates p < 0.05. Error bars represent standard deviation.
As a posteriori analysis, we also evaluated rCBF in the left dorsal ACC in controls who were divided into physical symptoms present (n = 8) and physical symptoms absent (n = 7) subgroups (using the same criteria to create groups). Relative CBF in the left dorsal ACC was not significantly different between controls in the physical symptoms present subgroup (mean = 0.99, SD = 0.07) and controls in the physical symptoms absent subgroup (mean = 1.06, SD = 0.09), p = 0.112. Although the reported p value is nearing a statistical trend, it is important to note that the pattern is in the opposite direction of what was observed in the SRC group (i.e., controls in the physical symptoms absent subgroup have higher rCBF), and this p value was not adjusted for multiple comparisons.
Discussion
We evaluated cerebral perfusion in adolescents with SRC and a comparison cohort of never-concussed age- and sex-matched peers. At Visit 1, which occurred within two weeks post-injury, we observed that, in comparison with controls, the SRC group had significantly higher rCBF in both the left insula and left dorsal ACC. At Visit 2, which occurred approximately six weeks post-injury, the SRC group continued to have significantly higher rCBF in the left dorsal ACC than controls. While some researchers have also found increased CBF in participants with mTBI,5,8,12,14 others report the exact opposite—decreased CBF after mTBI.10,11,15–17
While previous work has observed decreased CBF after severe TBI,4 the mixed findings after mTBI suggest that there may be more variability in CBF after mTBI. There is heterogeneity in findings even when we compare our data with a subset of previous studies that included participants of similar ages. Wang and colleagues16 observed globally reduced aCBF in acute SRC (24 h and eight days post-injury) and regionally reduced aCBF in chronic SRC (3–12 months post-injury),17 which contradicts our findings of increased regional rCBF. Mutch and colleagues14 evaluated rCBF in participants with post-concussion syndrome and found both regional increases and decreases in rCBF, which is partially consistent with our findings.
It is possible that evaluating aCBF rather than rCBF could render contrasting results. As noted in the Introduction, aCBF is more sensitive to changes in physiological factors such as breathing rate and caffeine uptake.7 Therefore, it is possible that reported between-group differences in aCBF are influenced by confounding variables unrelated to mTBI. Barlow and associates8 observed globally increased aCBF in symptomatic participants with mTBI and globally reduced aCBF in asymptomatic participants, however, suggesting that symptom status may be a more important factor than aCBF versus rCBF.
We next examined our findings with respect to those of Meier and colleagues11 who also used PCASL to evaluate rCBF after SRC in a marginally older cohort at similar time points. Despite considerable overlap between study designs, Meier and colleagues11 observed regional reductions in rCBF one day and one week post-injury that resolved one month after injury, while we observed regional increases in rCBF approximately two weeks and six weeks post-injury. We considered that the slight age difference between the studies may have contributed to the contrasting results, but we observed that age was positively, not inversely, associated with rCBF, making age an unlikely contributor. Collectively, these examples further illustrate the vast variability in ASL studies and highlight the need for additional research—especially studies that consider the role of symptoms status and/or recovery in post-injury CBF.
Here, we observed group differences in the left insula and the dorsal ACC. These regions are known to be functionally linked as nodes of the salience network (SN),26 suggesting an explanation for what we identified—altered CBF in these two specific regions. The SN is known to be activated in response to novel or behaviorally relevant stimuli, including internal stimuli such as pain,27 and altered SN connectivity has been observed after mTBI and linked to persistent injury-related complaints,28 which may represent increased perception of internal stimuli. In addition, acute stress has been found to disrupt intrinsic functional connectivity of the SN.29
While connectivity analyses are beyond the scope of the data currently presented, conceivably increased rCBF perfusion—as reported here—could be associated with altered intrinsic connectivity of the SN because of mTBI and the acute stress elicited by mTBI. If this were the case, then changes within the SN may subsequently influence awareness or perception of mTBI-related symptoms. In this hypothetical scenario, it remains unclear whether CBF and connectivity within the SN mediate symptomology after mTBI or if individual differences in symptom perception mediate SN activity. Future studies should directly examine the relationship of rCBF perfusion and intrinsic functional connectivity of the SN and other neural networks, along with behavioral performance and symptom reporting after SRC and other forms of mTBI.
Given that some researchers have observed relationships between symptom status and CBF perfusion8–11 and the hypothesized link between the SN and symptoms, we evaluated how SRC-related symptomology was associated with rCBF perfusion. At Visit 1, we did not observe a relationship between symptoms and rCBF values. At Visit 2, we observed that, in the left dorsal ACC, SRC participants with physical symptoms (those with one or more symptoms) had significantly higher rCBF than never-concussed controls and numerically higher rCBF than SRC participants without physical symptoms. Importantly, this pattern was not observed in controls when they were divided into subgroups based on the presence of physical symptoms. This suggests that the presence of physical symptoms is linked to rCBF perfusion after SRC, because asymptomatic individuals had rCBF perfusion much more similar to controls than symptomatic SRC participants.
Our finding that physical symptoms were reported within both the SRC and control groups reinforces that many SRC-induced symptoms (e.g., headaches) lack specificity to SRC, because they are also experienced by healthy persons with no history of SRC, and yet are used clinically to diagnosis the presence of and recovery from concussion.30 While the differing brain-behavior relationship that we preliminarily identified between groups suggests that context is important for interpretation of symptoms, the lack of specificity of symptoms alone highlights the importance of research designed to identify consistent biomarkers of SRC, such as rCBF.
These findings are consistent with previous work where persons with more symptoms had higher CBF values.9,10 Like us, Barlow and colleagues8 observed that children with mTBI who were symptomatic had significantly higher global CBF than controls. In contrast to their findings, however, we did not observe that asymptomatic individuals had lower CBF than controls.8 Nonetheless, CBF perfusion may be a marker of physiological status after concussion. It is possible that increased CBF contributes to reported symptoms, or the presence of symptoms may modulate CBF.
Our findings do not replicate results where rCBF was inversely associated with emotional symptoms.11 With our relatively small sample size and stringent statistical threshold, however, we may have had insufficient statistical power to see the influence of emotional symptoms. Few adolescents reported emotional symptoms at either visit. With many participants reporting zero symptoms, it was difficult to complete correlation analyses or between-group comparisons to adequately evaluate the influence of emotional symptoms. With a greater sample size, or earlier evaluation, we could have improved our ability to determine how emotional symptoms, or other symptom types, influenced rCBF values. Nevertheless, these and others' findings suggest that the presence or absence of symptoms might be associated with rCBF values, and symptom status is an important consideration for future ASL studies.
Future studies could also address some of our study's limitations. For example, we evaluated the SRC group at two time points but only evaluated the control group at a single time point. Therefore, we are unable to determine whether rCBF is constant or fluctuates among nonconcussed adolescents. In addition, we chose to use a cohort of noninjured and never-concussed controls. Control participants with a different type of injury (e.g., orthopedic) may have been a better comparison group and should be considered for future study designs. We also acknowledge that we did not acquire rCBF data in the acute (<48 h) stage of injury. Future studies should consider examining rCBF and symptom status in acute and subacute injury and at the point of recovery from injury. In addition, pre-injury evaluation could provide even more precise information about SRC-related changes in rCBF.
Future work should continue to examine how factors like quantity and type of SRC-related symptoms influence rCBF. We posit that these and other unknown factors likely affect rCBF, and more research is needed to best understand how much injury influences rCBF relative to other factors. In addition, future work should examine how variations in rCBF influence behavioral performance and recovery over time. This information could serve to improve the utility of rCBF as a biomarker after SRC and other forms of mTBI.
Conclusion
In comparison with never-concussed controls, we found that adolescents with SRC had increased rCBF localized to the left insula and left dorsal ACC at approximately two and six weeks post-injury. These findings are consistent with some previous findings while contrasting others. To date, there is little consistency regarding the direction of change in rCBF after mTBI, but our findings and those of others suggest that presence or absence of symptoms may play a role. Future work is needed to understand how symptoms and other factors relate to rCBF changes after mTBI.
Acknowledgments
This research was supported by the National Institutes of Health (J.S. 5T32HD007414); (H.L., R01MH084021, P41 EB015909); (S.S., R21HD080378).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorak J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 2.Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., and Diaz-Arrastia R. (2016). Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 275, 353–366 [DOI] [PubMed] [Google Scholar]
- 3.Kim J., Whyte J., Patel S., Avants B., Europa E., Wang J., Slattery J., Gee J.C., Coslett H.B., and Detre J.A. (2010). Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J. Neurotrauma 27, 1399–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu A.A., Voss H.U., Dyke J.P., Heier L.A., and Schiff N.D. (2011). Arterial spin labeling and altered cerebral blood flow patterns in the minimally conscious state. Neurology 77, 15181523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doshi H., Wiseman N., Liu J., Wang W., Welch R.D., O'Neil B.J., Zuk C., Wang X., Mika V., Szaflarski J.P., Haacke E.M., and Kou Z. (2015). Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS One 10, e0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andre J.B. (2015). Arterial spin labeling magnetic resonance perfusion for traumatic brain injury: technical challenges and potentials. Top. Magn. Reson. Imaging 24, 275–287 [DOI] [PubMed] [Google Scholar]
- 7.Aslan S., and Lu H. (2010). On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn. Reson. Imaging 28, 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow K.M., Marcil L.D., Dewey D., Carlson H.L., MacMaster F.P., Brooks B.L., and Lebel R.M. (2017). Cerebral perfusion changes in post-concussion syndrome: a prospective controlled cohort study. J. Neurotrauma 34, 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sours C., Zhuo J., Roys S., Shanmuganathan K., and Gullapalli R.P. (2015). Disruptions in resting state functional connectivity and cerebral blood flow in mild traumatic brain injury patients. PLoS One 10, e0134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C.M., Tseng Y.C., Hsu H.L., Chen C.J., Chen D.Y., Yan F.X., and Chiu W.T. (2016). Arterial spin labeling perfusion study in the patients with subacute mild traumatic brain injury. PLoS One 11, e0149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier T.B., Bellgowan P.S., Singh R., Kuplicki R., Polanski D.W., and Mayer A.R. (2015). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 72, 530–538 [DOI] [PubMed] [Google Scholar]
- 12.Churchill N.W., Hutchison M.G., Richards D., Leung G., Graham S.J., and Schweizer T.A. (2017). The first week after concussion: blood flow, brain function and white matter microstructure. Neuroimage Clin. 14, 480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Militana A.R., Donahue M.J., Sills A.K., Solomon G.S., Gregory A.J., Strother M.K., and Morgan V.L. (2016). Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging Behav. 10, 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutch W.A., Ellis M.J., Ryner L.N., Ruth Graham M., Dufault B., Gregson B., Hall T., Bunge M., Essig M., Fisher J.A., Duffin J., Mikulis D.J., for The Canada North Concussion Network, and for The University Health Network Cerebrovascular Reactivity Research Group. (2016). Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J. Neurosurg. 125, 648–660 [DOI] [PubMed] [Google Scholar]
- 15.Peng S.P., Li Y.N., Liu J., Wang Z.Y., Zhang Z.S., Zhou S.K., Tao F.X., and Zhang Z.X. (2016). Pulsed arterial spin labeling effectively and dynamically observes changes in cerebral blood flow after mild traumatic brain injury. Neural. Regen. Res. 11, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Nelson L.D., LaRoche A.A., Pfaller A.Y., Nencka A.S., Koch K.M., and McCrea M.A. (2016). Cerebral blood flow alterations in acute sport-related concussion. J. Neurotrauma 33, 1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., West J.D., Bailey J.N., Westfall D.R., Xiao H., Arnold T.W., Kersey P.A., Saykin A.J., and McDonald B.C. (2015). Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Dev. Neuropsychol. 40, 40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Liu P., Li Y., Fan H., Peng S.L., Park D.C., Rodrigue K.M., Jiang H., Faria A., Ceritoglu C., Miller M., Mori S. and Lu H. (2017). Towards a comprehensive online tool for ASL data analysis. Proc. Intl. Soc. Mag. Reson., 25, 3808 [Google Scholar]
- 19.Liu P., Li Y., Herrera A., Faria A., Ceritoglu C., Miller M., Mori S., and Lu H. (2016). ASL in the MRICloud: A platform-independent installation-free tool for arterial spin labeling analysis. Proc. Intl. Soc. Mag. Reson. 24, 2877 [Google Scholar]
- 20.MRICloud. Available at: mricloud.org Accessed: October26, 2017
- 21.Mori S., Wu D., Ceritoglu C., Li Y., Kolasny A., Vaillant M., Faria A., Oishi K., and Miller M. (2016). MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. IEEE Computing in Science & Engineering 18, 21–35 [Google Scholar]
- 22.Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J., Wang D.J., Wong E.C., and Zaharchuk G. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Res. Med. 73, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldjian J.A., Laurienti P.J., and Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21, 450–455 [DOI] [PubMed] [Google Scholar]
- 24.Maldjian J.A., Laurienti P.J., Kraft R.A., and Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 [DOI] [PubMed] [Google Scholar]
- 25.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., and Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 [DOI] [PubMed] [Google Scholar]
- 26.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., and Greicius M.D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borsook D., Edwards R., Elman I., Becerra L., and Levine J. (2013). Pain and analgesia: the value of salience circuits. Prog. Neurobiol. 104, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Horn H.J., Liemburg E.J., Aleman A., Spikman J.M., and van der Naalt J. (2016). Brain networks subserving emotion regulation and adaptation after mild traumatic brain injury. J. Neurotrauma 33, 1–9 [DOI] [PubMed] [Google Scholar]
- 29.Hermans E.J., van Marle H.J., Ossewaarde L., Henckens M.J., Qin S., van Kesteren M.T., Schoots V.C., Cousijn H., Rijpkema M., Oostenveld R., and Fernandez G. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153 [DOI] [PubMed] [Google Scholar]
- 30.Nelson L.D., LaRoche A.A., Pfaller A.Y., Lerner E.B., Hammeke T.A., Randolph C., Barr W.B., Guskiewicz K., and McCrea M.A. (2016). Prospective, head-to-head study of three computerized neurocognitive assessment tools (CNTs): reliability and validity for the assessment of sport-related concussion. J. Int. Neuropsychol. Soc. 22, 24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]