Abstract
Traumatic brain injury (TBI) may affect the pharmacodynamics of centrally acting drugs. Paired-pulse transcranial magnetic stimulation (ppTMS) is a safe and noninvasive measure of cortical gamma-aminobutyric acid (GABA)-mediated cortical inhibition. Huperzine A (HupA) is a naturally occurring acetylcholinesterase inhibitor with newly discovered potent GABA-mediated antiepileptic capacity, which is reliably detected by ppTMS. To test whether TBI alters cerebral HupA pharmacodynamics, we exposed rats to fluid percussion injury (FPI) and tested whether ppTMS metrics of cortical inhibition differ in magnitude and temporal pattern in injured rats. Anesthetized adult rats were exposed to FPI or sham injury. Ninety minutes post-TBI, rats were injected with HupA or saline (0.6 mg/kg, intraperitoneally). TBI resulted in reduced cortical inhibition 90 min after the injury (N = 18) compared to sham (N = 13) controls (p = 0.03). HupA enhanced cortical inhibition after both sham injury (N = 6; p = 0.002) and TBI (N = 6; p = 0.02). The median time to maximum HupA inhibition in sham and TBI groups were 46.4 and 76.5 min, respectively (p = 0.03). This was consistent with a quadratic trend comparison that projects HupA-mediated cortical inhibition to last longer in injured rats (p = 0.007). We show that 1) cortical GABA-mediated inhibition, as measured by ppTMS, decreases acutely post-TBI, 2) HupA restores lost post-TBI GABA-mediated inhibition, and 3) HupA-mediated enhancement of cortical inhibition is delayed post-TBI. The plausible reasons of the latter include 1) low HupA volume of distribution rendering HupA confined in the intravascular compartment, therefore vulnerable to reduced post-TBI cerebral perfusion, and 2) GABAR dysfunction and increased AChE activity post-TBI.
Keywords: : cerebral pharmacodynamics, epilepsy, huperzine A, transcranial magnetic stimulation, traumatic brain injury
Introduction
Huperzine A (HupA) is a naturally occurring compound found in the firmoss Huperzia Serrata. HupA is an acetylcholinesterase (AChE) inhibitor, in active investigation as a treatment for disorders of cognition and memory, such as Alzheimer's disease.1–3 As well, our laboratory recently identified a potent anticonvulsive HupA capacity that is mediated by enhancement of cortical GABAergic inhibition.4,5 HupA also has analgesic and neuroprotective properties.6–10 Taken together, these findings indicate a potential to deploy HupA in the setting of acute brain injury, perhaps as a direct neuroprotective intervention, and also to mitigate pain and suppress acute seizures, which can worsen the neurological outcome.11,12
Yet, although potentially useful in an acute brain injury setting, HupA has a volume of distribution of 0.061 L/kg,13 which suggests that it is vastly confined in the intravascular compartment, and that its pharmacodynamics are likely to be affected by events, such as brain injury, that alter cerebral perfusion. Accordingly, we tested whether the magnitude and timing of HupA-mediated enhancement of cortical inhibion is altered in an established rat traumatic brain injury (TBI) model. Specifically, we measured cortical inhibiton by paired-pulse transcranial magnetic stimulation (ppTMS) combined with needle electromyography (EMG) in the rat fluid percussion TBI model.
In ppTMS, the motor cortex is stimulated, noninvasively, by small intracranial electrical currents generated by a fluctuating extracranial magnetic field,14,15 and the resultant motor output is quantified as the amplitude of the motor evoked potential (MEP) obtained from a limb muscle. Notably, ppTMS is available in both rats and in humans and thus is emerging a valuable translational experimental tool.16–18 In common translational ppTMS protocols, the pair of stimuli, termed conditioning and test stimulus, are separated by 50- to 300-ms interstimulus intervals to produce a pair of MEPs. When analyzed as pairs, the size of the second (test) MEP is predictably smaller than the size of the first (conditioning) MEP, most likely attributed to gamma-aminobutyric acid (GABA)A receptor (GABAAR)-mediated long-interval intracortical inhibition of the test response that is triggered by the initial conditioning stimulus.4,17
In the subacute and chronic (days to weeks post-TBI) post-traumatic time windows, TBI causes an increase in cortical excitation/inhibition (E:I) ratio, as measured by immunohistochemistry and by ppTMS, that is attributed to the loss of GABAergic synaptic inhibition.19–21 Yet, acute (minutes or hours post-injury) TBI effects on cortical inhibitory tone are unknown and may be relevant to pharmacological management of injured patients by GABAergic drugs such as HupA. Thus, we tested whether 1) cortical E:I ratio is affected in an acute TBI setting and 2) HupA-mediated enhancement of intracortical inhibition is altered by TBI.
Methods
Animals and pharmaceuticals
Thirty-one adult male Sprague–Dawley rats (240–400 g) were used for this set of experiments. Rats were assigned to four groups: sham-vehicle (n = 5), sham-HupA (n = 6), TBI-vehicle (n = 5), and TBI-HupA (n = 6). Nine additional rats were used only for baseline recording. Animals were kept in standard cages with ad libitum water and food supply in a climate-controlled facility on a 12-h light/dark cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital and in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The following stock concentrations were used in the current experiments: urethane (0.2 g/mL); HupA (1 mg/mL); and pentobarbital (50 mg/mL). HupA (99% pure; Biscayne Neurotherapeutics, Inc., Miami, FL) was dissolved in normal saline. An equal volume of normal saline was injected intraperitoneally (i.p.) to the vehicle controls.
Fluid percussion injury
Rats were anesthetized with isoflurane (4% for induction, 2% for maintenance) before their heads were secured on the stereotactic frame. After making a 1-cm sterile midline incision, a circular 4-mm burr hole was drilled on the left posterior parietal bone, adjacent to the temporal bone and the lambdoid suture. Fluid percussion injury (FPI), modified per our previously published protocol, was induced with a fluid percussion device (AmScien Instruments, Henrico, VA).22 A percussion wave of 4.3 ± 0.3 atm was delivered to the exposed dura. Rats were closely observed after FPI for the duration of apnea. Animals with less than 10 sec of apnea were excluded from the study. For sham injury, a sterile incision was made and the posterior parietal bone was lightly scratched with the drill bit in a circular fashion without turning the drill on, but no craniectomy was performed.
After surgical wound closure, animals were injected with urethane (1.2 g/kg, i.p.) for long-term anesthesia and buprenorphine (0.1 mg/kg, subcutaneously) for analgesia. Urethane was injected in 2 equal doses separated by 25 min.
Paired-pulse transcranial magnetic stimulation
Anesthetized animals were mounted on the stereotactic frame and focal single TMS pulses were applied over the left motor cortex by a customized 70-mm figure-8 coil (40 mm external diameter of each lobe) using Magstim 200 magnetic stimulators (Magstim, Wales, UK). The coil positioning and motor threshold (MT) determination were adapted from the methods described in our lab.23 After obtaining the MT of the right brachioradialis muscle, 15 pairs of TMS pulses with 100-ms interstimulus intervals were applied at 120% MT every 20 sec over 5 min. Immediately after the last pair, 0.6 mg/kg i.p. of HupA or saline was injected 90 min post-FPI. Then, six blocks of resting (10 min) and EMG recording (5 min) for a total of 90 min followed the injection. We previously demonstrated that 0.6 mg/kg of HupA increases GABAergic transmission in uninjured rats.4 In those experiments, higher HupA doses caused cholinergic side effects (i.e., hypersalivation and muscle fasciculations), which interfered with data acquisition. We therfore used 0.6 mg/kg of HupA in our experiments.
Statistical analysis
Data were analyzed using STATISTICA (version 10; StatSoft, Inc., Palo Alto, CA) and SAS software (version 9.2; SAS Institute Inc., Cary, NC) with the significance level of p < 0.05. The contralateral brachioradialis MEPs were recorded for every ppTMS. Log transform of the test/conditioning MEP values were used to avoid floor effect. Hence, lower values correspond to greater cortical inhibition. We hereby present the log values as the percent cortical inhibition, that is, −0.25 is 25% cortical inhibition. Seven sets of MEPs were averaged for every time point (baseline, 15, 30, 45,60, 75, and 90 min).
ppTMS measures among groups were compared by repeated-measures analysis of variance, with factors GROUP and TIME. To compare the baseline values for sham (n = 13) and verum (n = 18) injury groups, the Mann-Whitney U test was used. The best fit polynomial curves were generated by Microsoft Excel (2013; Microsoft Corporation, Redmond, WA), and the abscissas of their vertices were calculated from the quadratic polynomial equations. The time to maximum effect for each animal was compared with the Mann-Whitney U test. The chi-squared test was used to compare the cortical inhibition ratios.
We also built a longitudinal model using a linear mixed-effects regression model with random intercept, slope of time, and slope of time2 terms to account for within-subject correlations. The model included linear and quadratic effects of time and their interactions with group to differentiate the trajectories of two groups. This model allowed us to compare the patterns in which the curves reached their peak and returned to their baseline. The final model selection was made using Akaike information criterion.
Results
Cortical inhibition decreases acutely after traumatic brain injury
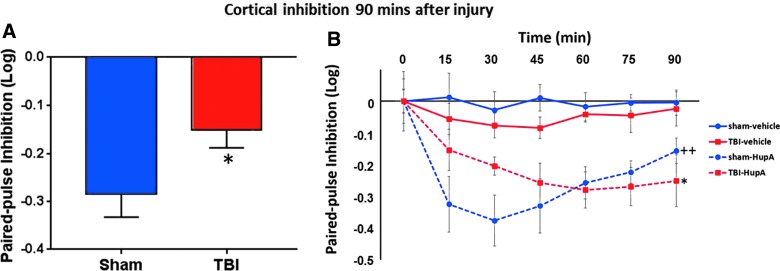
Cortical excitability ranged from 1% to 60% inhibition (median, 24% inhibition) in the sham group, whereas it ranged from 50% inhibition to 8% excitation (median, 14% inhibition) in the TBI group. Baseline median cortical inhibition 90 min post-TBI was significantly less than that after sham injury (p = 0.038; Fig. 1A). In addition, 8 animals (44%) in the TBI group, as opposed to only 1 animal (8%) in the sham group, had less than 10% cortical inhibition 90 min post-TBI (χ2(1) = 4.95; p = 0.026).
FIG. 1.
Cortical inhibition measurements by ppTMS. Given that log transform of conditioning/test MEP values were used to avoid floor effect, lower values correspond to greater inhibition (A). Decrease in cortical inhibition 90 min post-TBI. Ninety minutes after sham or verum injury at baseline, the median cortical inhibition was 24% and 14% in sham (n = 13) and TBI (n = 18) groups, respectively (p = 0.038). (B) Enhanced cortical inhibition after HupA. Cortical inhibition changes were observed for 90 min after HupA (0.6 mg/kg) or vehicle injection. Injections were 90 min after the surgery and immediately after the baseline ppTMS session. HupA enhanced cortical inhibition after both sham injury (++p = 0.002) and TBI (*p = 0.017). HupA, huperzine A; ppTMS, paired-pulse transcranial magnetic stimulation; TBI, traumatic brain injury.
Huperzine A enhances cortical inhibition in sham and traumatic brain injury animals
In sham-injured animals, cortical inhibition was increased after HupA injection compared to saline-injected animals (F(1,9) = 17.84; p = 0.002; Fig. 1B). This confirms the previous findings, which showed a HupA-mediated increase in GABAergic transmission.4 In addition, our findings show the time course of HupA-mediated inhibition over 90 min. Post-TBI, HupA also enhanced cortical inhibition compared to saline-injected animals (F(1,9) = 8.5; p = 0.017; Fig. 1B).
Traumatic brain injury delays maximal huperzine A effect
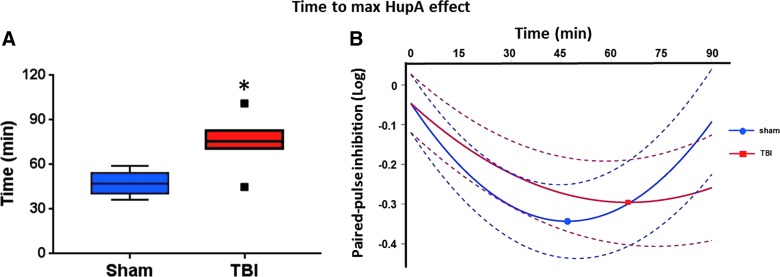
Based on the magnitude of HupA cortical inhibition over time, the polynomial best fit curves were generated. The abscissas of the vertices represent the time-to-maximal HupA effect, which was 36–59 min (median, 46.4) in the sham group and 45–101 min (median, 76.5) in the TBI group (p = 0.03; Fig. 2). The linear mixed-effect model demonstrated that the HupA effect-time curves had significantly steeper slopes both linearly (FPI vs. sham; β = 0.00496; p = 0.04) and quadratically (FPI vs. sham; β = −0.00008; p = 0.007) in the TBI group than in the sham group. That is, relative to sham, both reaching the maximal HupA effect and returning to baseline were delayed in the TBI group.
FIG. 2.
Time to maximal HupA effect. (A) Comparison of sham and TBI groups in terms of time to maximal HupA effect demonstrated as Tukey box plot. Time to maximal HupA effect was delayed in the TBI group (p = 0.03). (B) Polynomial best fit curves with 95% confidence intervals and the vertices for sham and TBI groups shown. Round and squared dots represent the vertices of sham (n = 6) and TBI (N = 6) groups, respectively. HupA, huperzine A; TBI, traumatic brain injury.
Discussion
Our data show that cortical inhibition is depressed acutely post-TBI. Notably, published pre-clinical data indicate that the first measurable decrease in paired-pulse cortical inhibition is 2 weeks post-TBI, though in those experiments, cortical inhibition was not measured until 1 week post-injury.19 Thus, we provide insight into the mechanism of acute post-TBI seizures and also add an acute time point to the study of the mechanisms of prolonged suppression of cortical inhibition that may lead to lasting post-traumatic consequences, such as post-traumatic epilepsy.
With respect to rescue of lost cortical inhibition, HupA treatment enhanced not only inhibition in sham-injured animals, but also post-TBI. Hence, we show that, despite injury, the brain retains a capacity for HupA-mediated enhancement of cortical inhibitory tone. Yet, the peak cortical inhibitory HupA effect is delayed post-TBI. This underscores that the acute TBI setting may require specialized considerations of drug dose and timing.
The plausible reasons for altered HupA pharmacodynamics post-TBI include 1) GABAAR dysfunction, 2) lowered HupA bioavailability attributed to reduced cerebral perfusion, and 3) increased AChE activity.24–26 Relevant to the ppTMS data interpretation indicating that the HupA effect is mediated by increased GABA release, published in vitro results show that pathologically altered chloride homeostasis with a shift toward intracellular chloride accumulation renders GABAARs depolarizing, and thus excitatory, given that their activation would result in chloride efflux rather than influx.27,28 Animal studies also suggest that GABAAR subunits (α1, α2, α5, β2, β3, γ2, and δ) and α7nAChR expression are reduced, thus favoring net excitation, in the acute TBI setting.24,29 Yet, these pathophysiologic changes, in our view, are more likely to result in dampened, rather than delayed, response to HupA. On the other hand, either increased post-TBI AChE activity or decreased cerebral perfusion may retard intrathecal HupA accumulation and explain the delayed time to peak ppTMS response post-TBI.25,26 Further experiments investigating these pathophysiological mechanisms may provide insight into acute TBI patient care in terms of the drug of choice and dosing schedules. Hence, we propose that HupA dosing should be adjusted acutely post-TBI to elicit antiseizure or other relevant beneficial effects.
We note that the present experiments relied on only a single HupA dose, which was adequate to answer the binary question of whether or not cerebral pharmacodynamics are distinct in groups separated by presence or absence of TBI. The use of a single HupA dose and absence of a dose-response curve is a limitation of our study. We thus anticipate further characterization of the TBI contribution to cerebral pharmacodynamics in follow-up studies, beyond the scope of this report, that will include a range of agents and doses.
Beyond HupA, our data also support the notion that centrally acting drugs are affected to various degrees by TBI, and to improve the probabability of a desired pharmacological effect, the dosing of such agents should accomodate pharmacodynamic and pharmacokinetic changes that follow brain injury.
Acknowledgments
The authors thank Dr. Mustafa Hameed and Mr. Sameer Dhamne for their valuable suggestions on animal surgeries and EMG recordings.
Author Disclosure Statement
This study was supported by NIH/NINDS 5R01NS088583 (to A.R.) and the Boston Children's Hospital Translational Research Program (to A.R.). Dr. Steven Schachter is the inventor on a patent for the use of Huperzine A for treatment of epilepsy, which is licensed by Harvard Medical School to Biscayne Neurotherapeutics, Inc., in which he holds less than 5% equity and for which he serves as chair of the scientific advisory board. Drs. Joshua T. Johnstone and Stephen Collins are the employees of Biscayne Neurotherapeutics, Inc.
References
- 1.Santos M.A., Chand K., and Chaves S. (2016). Recent progress in repositioning Alzheimer's disease drugs based on a multitarget strategy. Future Med. Chem. 2016. October 24. doi: 10.4155/fmc-2016-0103 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Shao Z.Q. (2015). Comparison of the efficacy of four cholinesterase inhibitors in combination with memantine for the treatment of Alzheimer's disease. Int. J. Clin. Exp. Med. 8, 2944–2948 [PMC free article] [PubMed] [Google Scholar]
- 3.Rafii M.S., Walsh S., Little J.T., Behan K., Reynolds B., Ward C., Jin S., Thomas R., and Aisen P.S. (2011). A phase II trial of huperzine a in mild to moderate Alzheimer disease. Neurology 76, 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gersner R., Ekstein D., Dhamne S.C., Schachter S.C., and Rotenberg A. (2015). Huperzine a prophylaxis against pentylenetetrazole-induced seizures in rats is associated with increased cortical inhibition. Epilepsy Res. 117, 97–103 [DOI] [PubMed] [Google Scholar]
- 5.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., and White H.S. (2015). Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EİLAT Xİİ). Epilepsy Res. 111, 85–141 [DOI] [PubMed] [Google Scholar]
- 6.Damar U., Gersner R., Johnstone J.T., Schachter S., and Rotenberg A. (2017). Huperzine A: a promising anticonvulsant, disease modifying, and memory enhancing treatment option in Alzheimer's disease. Med. Hypotheses 99, 57–62 [DOI] [PubMed] [Google Scholar]
- 7.Zhu S.Z., Huang W.P., Huang L.Q., Han Y.L., Han Q.P., Zhu G.F., Wen M.Y., Deng Y.Y., and Zeng H.K. (2016). Huperzine a protects sepsis associated encephalopathy by promoting the deficient cholinergic nervous function. Neurosci. Lett. 631, 70–78 [DOI] [PubMed] [Google Scholar]
- 8.Lu H., Jiang M., Lu L., Zheng G., and Dong Q. (2015). Ultrastructural mitochondria changes in perihematomal brain and neuroprotective effects of huperzine A after acute intracerebral hemorrhage. Neuropsychiatr. Dis. Treat. 11, 2649–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu D., Thakor D.K., Han I., Ropper A.E., Haragopal H., Sidman R.L., Zafonte R., Schachter S.C., and Teng Y.D. (2013). Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proc. Natl. Acad. Sci. U. S. A. 110, E746–E755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park P., Schachter S., and Yaksh T. (2010). Intrathecal huperzine a increases thermal escape latency and decreases flinching behavior in the formalin test in rats. Neurosci. Lett. 470, 6–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascarella A., Trojano L., Loreto V., Bilo L., Moretta P., and Estraneo A. (2016). Long-term outcome of patients with disorders of consciousness with and without epileptiform activity and seizures: a prospective single centre cohort study. J. Neurol. 263, 2048–2056 [DOI] [PubMed] [Google Scholar]
- 12.Majidi S., Makke Y., Ewida A., Sianati B., Qureshi A. I., and Koubeissi M.Z. (2017). Prevalence and risk factors for early seizure in patients with traumatic brain injury: analysis from national trauma data bank. Neurocrit. Care 27, 90–95 [DOI] [PubMed] [Google Scholar]
- 13.Li Y.X., Zhang R.Q., Li C.R., and Jiang X.H. (2007). Pharmacokinetics of huperzine a following oral administration to human volunteers. Eur. J. Drug Metab. Pharmacokinet. 32, 183–187 [DOI] [PubMed] [Google Scholar]
- 14.Hallett M. (2007). Transcranial magnetic stimulation: a primer. Neuron 55, 187–199 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M., and Pascual-Leone A. (2003). Transcranial magnetic stimulation in neurology. Lancet. Neurol. 2, 145–156 [DOI] [PubMed] [Google Scholar]
- 16.Vahabzadeh-Hagh A.M., Muller P.A., Gersner R., Zangen A., and Rotenberg A. (2012). Translational neuromodulation: Approximating human transcranial magnetic stimulation protocols in rats. Neuromodulation 15, 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh T.H., Dhamne S.C., Chen J.J., Pascual-Leone A., Jensen F.E., and Rotenberg A. (2012). A new measure of cortical inhibition by mechanomyography and paired-pulse transcranial magnetic stimulation in unanesthetized rats. J. Neurophysiol. 107, 966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vahabzadeh-Hagh A.M., Muller P.A., Pascual-Leone A., Jensen F.E., and Rotenberg A. (2011). Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J. Neurophysiol. 105, 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh T.H., Lee H.H., Hameed M.Q., Pascual-Leone A., Hensch T.K., and Rotenberg A. (2016). Trajectory of parvalbumin cell impairment and loss of cortical inhibition in traumatic brain injury. Cereb. Cortex 2016. November 30. doi: 10.1093/cercor/bhw318 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantu D., Walker K., Andresen L., Taylor-Weiner A., Hampton D., Tesco G., and Dulla C.G. (2015). Traumatic brain injury increases cortical glutamate network activity by compromising gabaergic control. Cereb. Cortex 25, 2306–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huusko N., and Pitkänen A. (2014). Parvalbumin immunoreactivity and expression of GABAA receptor subunits in the thalamus after experimental TBI. Neuroscience 267, 30–45 [DOI] [PubMed] [Google Scholar]
- 22.Hameed M.Q., Goodrich G.S., Dhamne S.C., Amandusson A., Hsieh T.H., Mou D., Wang Y., and Rotenberg A. (2014). A rapid lateral fluid percussion injury rodent model of traumatic brain injury and post-traumatic epilepsy. Neuroreport 25, 532–536 [DOI] [PubMed] [Google Scholar]
- 23.Rotenberg A. (2010). Prospects for clinical applications of transcranial magnetic stimulation and real-time EEG in epilepsy. Brain Topogr. 22, 257–266 [DOI] [PubMed] [Google Scholar]
- 24.Drexel M., Puhakka N., Kirchmair E., Hörtnagl H., Pitkänen A., and Sperk G. (2015). Expression of GABA receptor subunits in the hippocampus and thalamus after experimental traumatic brain injury. Neuropharmacology 88, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y., Pan Y., Wang M., Huang X., Yin Y., Wang Y., Jia F., Xiong W., Zhang N., and Jiang J.Y. (2012). Blood-brain barrier permeability is positively correlated with cerebral microvascular perfusion in the early fluid percussion-injured brain of the rat. Lab. Invest. 92, 1623–1634 [DOI] [PubMed] [Google Scholar]
- 26.Donat C.K., Schuhmann M.U., Voigt C., Nieber K., Schliebs R., and Brust P. (2007). Alterations of acetylcholinesterase activity after traumatic brain injury in rats. Brain Inj. 21, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 27.Van Den Pol A.N., Obrietan K., and Chen G. (1996). Excitatory actions of GABA after neuronal trauma. J. Neurosci. 16, 4283–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staley K.J., Soldo B.L., and Proctor W.R. (1995). Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269, 977–981 [DOI] [PubMed] [Google Scholar]
- 29.Hoffmeister P.G., Donat C.K., Schuhmann M.U., Voigt C., Walter B., Nieber K., Meixensberger J., Bauer R., and Brust P. (2011). Traumatic brain injury elicits similar alterations in alpha7 nicotinic receptor density in two different experimental models. Neuromolecular Med. 13, 44–53 [DOI] [PubMed] [Google Scholar]