Abstract
Acetylcholine is an excitatory neurotransmitter in the central nervous system that plays a key role in cognitive function, including learning and memory. Previous studies have shown that experimental traumatic brain injury (TBI) reduces cholinergic neurotransmission, decreases evoked release of acetylcholine, and alters cholinergic receptor levels. Galantamine (U.S. Food and Drug Administration approved for the treatment of vascular dementia and Alzheimer's disease) has been shown to inhibit acetylcholinesterase activity and allosterically potentiate nicotinic receptor signaling. We investigated whether acute administration of galantamine can reduce TBI pathology and improve cognitive function tested days after the termination of the drug treatment. Post-injury administration of galantamine was found to decrease TBI-triggered blood-brain barrier (BBB) permeability (tested 24 h post-injury), attenuate the loss of both GABAergic and newborn neurons in the ipsilateral hippocampus, and improve hippocampal function (tested 10 days after termination of the drug treatment). Specifically, significant improvements in the Morris water maze, novel object recognition, and context-specific fear memory tasks were observed in injured animals treated with galantamine. Although messenger RNAs for both M1 (Nos2, TLR4, and IL-12ß) and M2 (Arg1, CCL17, and Mcr1) microglial phenotypes were elevated post-TBI, galantamine treatment did not alter microglial polarization tested 24 h and 6 days post-injury. Taken together, these findings support the further investigation of galantamine as a treatment for TBI.
Keywords: : blood–brain barrier permeability, cholinergic neurotransmission, GABAergic neurodegeneration, memory impairments, microglial polarization
Introduction
Acetylcholine (ACh) is widely distributed in the brain and plays a central role in cognitive function. The important role of ACh in learning and memory is evident in Alzheimer's disease (AD)-associated learning and memory impairments, which are accompanied by a decrease in cholinergic signaling in the hippocampus and cortex.1 Current AD treatments include inhibitors of acetylcholinesterase (galantamine and donepezil) that increase synaptic ACh levels and boost acetylcholine receptor (AChR) signaling. ACh mediates its effect by engaging muscarinic (mAChR) and nicotinic (nAChR) receptors that are widely expressed in the brain. mAChRs are G-protein coupled and can mobilize intracellular calcium through Gq/11 (M1, M3, and M5 subtypes) proteins, or decrease cyclic adenosine monophosphate levels through Gi/o (M2 and M4 subtype) proteins. nAChRs are pentameric ion channels consisting of different combinations of alpha and beta subunits that are permeable to Na+ and K+ ions. Some of these receptors are also permeable to Ca2+, with alpha7 nicotinic receptors (nAChR7) being the most permeable. Signaling through nAChR7 has been demonstrated to play a prominent role in neuroplasticity, expression of neurotrophic factors, neurite outgrowth, learning and memory, and neuroprotection.2–5
Cognitive impairments are hallmarks of traumatic brain injury (TBI). Experimental TBI has been shown to reduce cholinergic neurotransmission, decrease evoked release of ACh, and alter cholinergic receptor levels.6–9,10 For example, it has been reported that TBI results in reductions in muscarinic M2 receptor binding within hours of the injury in the hippocampus.11,12 In addition, scopolamine, an mAChR antagonist, administered before, or shortly after, TBI normalizes [3H]QNB binding and improves behavioral performance in injured rats,13,14 suggesting that acute decreases in mAChR signaling is protective. In contrast to the acute effects of mAChR signaling, cholinergic deficits in the subacute/chronic period of TBI are thought to be detrimental for cognitive function. Consistent with this premise, administration of the blood–brain barrier (BBB) permeable ACh precursor, CDP-choline, to TBI animals improves navigational memory.15 However, studies using donepezil have reported mixed results.16,17 For example, a study by Yu and colleagues found that post-injury administration of donepezil improved the performance of injured animals in the Morris water maze task, an effect not observed in a similar study performed by Shaw and colleagues.16,17
Galantamine increases the synaptic availability of acetylcholine and is widely used for the treatment of AD. When used in experimental TBI, de la Tremblaye and colleagues showed that galantamine improves water maze learning and memory when tested in the presence of the drug.18 Galantamine also acts as a positive allosteric modulator for nAChR7.19–21 Beneficial effects of targeting peripheral nAChR7 have been demonstrated in models of sepsis.22 These effects have been shown to result from a decrease in systemic inflammation. We have recently demonstrated that administration of the selective alpha7 receptor agonist, PNU-282987, decreases both systemic and central inflammation and reduces BBB permeability post-TBI.23 Thus, by the combined action of its acetylcholinesterase inhibitory and nAChR positive allosteric modulatory activities, galantamine may have utility as a treatment for TBI. In this report, we evaluated the effect of acute post-injury galantamine treatment on TBI-triggered BBB permeability. In addition, we examined inflammation, neuronal loss, and memory function starting 10 days after termination of galantamine treatment. Our results show that post-TBI administration of galantamine significantly reduced BBB permeability, reduced the loss of hippocampal GABAergic neurons, and improved memory function. However, contrary to our predictions, galantamine had no effect on measures of post-TBI inflammation.
Methods
Materials
Galantamine hydrobromide was purchased from Tocris (Bristol, UK). Antibodies against von Willebrand factor (vWF; catalog no.: F3520; 1:3000) were bought from Sigma-Aldrich (St. Louis, MO), Claudin-5 (catalog no.: 35-2500; 1:500) from Invitrogen (Carlsbad, CA), glutamic acid decarboxylase 67 kDa (GAD67; catalog no.: MAB5406; 1:1000) from Millipore (Billerica, MA), and doublecortin (catalog no.: 4604; 1:1000) from Cell Signaling (Danvers, MA). Sprague–Dawley rats were purchased from Envigo (Houston, TX). Animals were housed under temperature-controlled conditions with a 12-h light/dark cycle and ad libitum access to water and food. Animal protocols were approved by the Institutional Animal Welfare Committee and were in compliance with the National Institute of Health's (NIH) Guide for Care and Use of Laboratory Animals.
Controlled cortical impact injury
An electromagnetic controlled cortical impact (CCI) device was used to cause moderate brain injury as has been previously described.24,25 Male Sprague–Dawley rats (275–300 g) were anesthetized with isoflurane, and a cranietomy (6-mm diameter) was made over the right parietal cortex. Injury consisted of a single impact at 5.0 m/sec, 2.5 mm deformation to the right parietal cortex. Sham animals were anesthetized and received an incision, but not the craniotomy or impact. Acute neurological responses (i.e., suppression of pain reflexes, righting response) were monitored immediately after the injury to ensure comparability across injuries. Body temperature was monitored and maintained at 37°C using a Deltatherm heating blanket attached to a rectal thermometer. After injury, animals were allowed to recover in a warmed chamber before being returned to their home cages. Male C57BL/6 mice were used for BBB permeability assays and immunohistological evaluation of cerebral vasculature. Mice were anesthetized with isoflurane and a craniotomy (5.0 mm) performed on the right cranial vault midway between lambda and bregma. A single impact at 3.0 m/sec, 1.0 mm deformation to the right parietal cortex was delivered to cause brain injury.
Drug preparation and administration
Galantamine hydrobromide was solubilized in sterile saline. Previous reports have indicated that the plasma half-life of galantamine is 7 h (in humans), with the recommended doses for use in AD patients being 8–12 mg, given twice a day. In rats, the half-life has been determined to be 3.5 h (in males) and approximately 2 h in mice.26,27 The doses chosen for these studies (3 mg/kg in mice, 1 mg/kg in rats) were based on the human dose range, after correction for body-surface-area differences (human/rat = 1:6.2; human/mouse = 1:12.328). Because of its relatively shorter half-life in mice compared to rats, we administered 3 mg/kg in order to extend its duration of effectiveness. Drug (or vehicle) was initially administered (intravenously; i.v.) 30 min post-injury, with an additional dose given approximately 7 h later. Additional doses were given twice-daily for 3 additional days, or until time of euthanasia. Although we have not directly measured the blood concentration of galantamine, it is anticipated that administration of two doses a day (separated by 7 h) would result in two short-lived peaks.
Measurement of blood–brain barrier permeability
BBB permeability was assessed by measuring the extravasation of Evans Blue dye as described previously.25,29 Twenty-four hours after CCI injury, animals were anesthetized and Evans Blue (3% in saline) was injected slowly through the jugular vein (4 mL/kg) and allowed to circulate for 1.5 h. After the circulation period, animals were given an overdose of pentobarbital (100 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) followed by PBS containing 4% paraformaldehyde (PFA). Brains were removed, and ipsilateral hemispheres were incubated in 0.5 mL of formamide at 55°C for 24 h. After incubation, the formamide solution was cleared by centrifugation at 20,000g for 20 min. The supernatant was collected, and the optical density at 620 nm was measured to determine the relative amount of dye in each sample. A standard curve was simultaneously generated to confirm the linearity of the recorded values.
Immunohistochemistry and fluorescence quantification
For assessment of BBB components, animals were euthanized 24 h post-injury, then brains were removed and quickly frozen in −80°C isopentane. Thirty-micron-thick coronal sections were prepared and mounted directly on gelatin-subbed slides. After air drying, the sections were fixed with 100% methanol for 20 min at −20°C and then rinsed in Tris-buffered saline (TBS) with 0.25% Triton X-100 (TBS-TX) for 20 min. The sections were blocked in 5% goat serum in TBS-TX at room temperature for 1 h followed by incubation with primary antibodies in 2.5% goat serum in TBS-TX at 4°C for 24 h and then with species-specific secondary antibodies in 2.5% goat serum in TBS-TX for 3 h at room temperature. Fluorescence intensity was quantified as described previously.25,30 Briefly, images of immunofluorescence were captured using a Zeiss Axiovert S100 microscope through a Zeiss EC Plan-Neofluar 20 × /0.5 lens and a MicroFIRE camera. The parameters used for image acquisition (including laser power, iris size, brightness, offset, etc.) were adjusted to minimize the background and optimize the signal using a tissue section from an injured animal. These parameters were then kept constant across all subsequent groups. ImageJ software (NIH, Bethesda, MD) was used to quantify the fluorescence intensity. Fluorescent images from three nonoverlapping regions in the ipsilateral cortex (0.5 mm from injury core) from each section, and two sections from each animal (n = 5 mice/group), were captured and quantified
Immunohistochemistry and cell counts
For assessing neuronal nuclei (NeuN), GAD67, and microtubule-associated protein 2 (MAP2), rats were overdosed 1 month post-injury with sodium pentobarbital (100 mg/kg) and transcardially perfused with PBS followed by 4% PFA. For assessing doublecortin immunoreactivity, rats were euthanized as described above on day 3 post-injury. Brains were removed, post-fixed overnight in perfusant, then cryoprotected in a 30% sucrose solution. Cryosections (40-μm thickness) spanning the rostral-caudal extent of the hippocampus were prepared. Free-floating sections were incubated overnight in primary antibody (0.5–1.0 μg/mL) in TBS containing 2% bovine serum albumin (BSA) and 2.5% normal goat serum. After extensive washing, immunoreactivity was detected using species-specific secondary antibodies coupled to Alexa Fluors. A blind counting methodology was employed for determination of GAD67-positive inhibitory neurons in the hilus of the ipsilateral hippocampus. Every 10th section through the ipsilateral dorsal hippocampus was processed for GAD67 immunoreactivity as described above. Positive cells within the hilus were counted using the optical dissector technique (Stereo Investigator; MicroBrightField Bioscience, Williston, VT).31 Cells in the outermost planes of focus (5 μm) were omitted to avoid counting cell caps. The number of GAD67-labeled neurons in approximately 20 computer-chosen areas within the hilus was scored for each section. The counting frame was 108 × 108 μm. The size of the counting frame, and the number of grid sections, was determined based on preliminary cell counts. The number of GAD67-labeled cells/mm2 for each section was obtained from the estimated cells divided by the contour area. The number of cells/mm2 for each animal (n = 4 rats/group) was calculated as the average of the number of cells/mm2 from each section examined. For counting doublecortin-positive newborn neurons, coded slides were counted by two independent observers who were blind to the treatment groups. Doublecortin-positive cells implanted in the dentate gyrus were counted and the lengths of the dentate gyrus measured. The number of cells/mm were calculated from three sections for each animal (n = 4/group).
Blood collection and plasma preparation
At 24 h and 6 days, animals were overdosed with sodium pentobarbital. Once the animal failed to respond to tail and foot pinch, the heart was exposed and blood was collected by cardiac puncture using a 16-Ga needle attached to a 3-mL syringe. Ethylenediaminetetraacetic acid was added as the anticoagulant. Platelet-poor plasma was prepared by centrifuging the blood at 1000g for 10 min to remove the erythrocytes, leukocytes, and platelets. The supernatant solution was removed and centrifuged again at 10,000g for 10 min to generate a platelet-poor plasma fraction. Plasma was aliquoted and frozen at −80°C until needed.
Enzyme-linked immunosorbent assays
Rat plasma and tissue interleukin (IL)-1ß levels were assessed using sandwich-style enzyme-linked immunosorbent assays (ELISAs; Quantikine IL-1ß assay; R&D Systems, Minneapolis, MN). The range of the standards was based on the vendors' instructions and on our previous experience with these techniques. The concentrations of each sample (assayed in triplicate) were calculated by comparison to the appropriate reference standard curve. For tissue extracts (prepared in assay buffer provided in the ELISA kit), a microBCA (bicinchoninic acid) assay was performed to determine protein concentration. ELISA results were normalized to the total protein amount in the sample.
Western blots
High-mobility group box protein 1 (HMGB1) levels in serum were assayed using western blots as previously described.32 Briefly, equal volumes of plasma (50 μL) were centrifuged on an Ultracel 100K Amicon centrifugal filter for 15 min at 14,000g. The flow-through was collected, 10 μL of which was boiled in gel load buffer and separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels. After transfer to polyvinylidene difluoride membranes (Millipore), membranes were stained with Ponceau S and imaged. Membranes were then blocked in 5% BSA in TBS overnight at room temperature, then incubated in anti-HMGB1 antibodies (0.5 μg/mL) for 3 h followed by extensive washing and incubation in an horseradish peroxidase–conjugated secondary antibody. Immunoreactivity was detected using a chemiluminescence substrate (SuperSignal West Pico; Life Technologies, Woburn, MA) followed by imaging and quantification using a LiCor C-Digit imager. Immunoreactive signals were normalized by two different methods. The protein concentration of the flow-through from the 100K Amicon centrifugal filter was determined by a microBCA assay, and the HMGB-1 immunoreactive signals were normalized by the relative protein amount in each sample. In addition, to account for potential transfer problems, the optical density of the stained proteins (<50 kDa) within each lane of the Ponceau-stained membrane was quantified and used to normalize HMGB-1 immunoreactivity.
Quantitative polymerase chain reaction for microglial phenotype markers
Cortical tissue in the penumbra region of the brain injured animals (or sham-operated controls) was removed and sonicated in Trizol (Invitrogen) and total RNA was extracted. A 1-μg sample of total RNA was reverse transcribed using the SuperScript® VILO™ complementary DNA (cDNA) synthesis kit (Invitrogen), according to the manufacturer's protocol. The level of expression of each target mRNA was quantified by amplification of the cDNA in triplicate using a CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Polymerase chain reaction (PCR) primers and dual-labeled fluorescent probes were purchased from Bio-Rad (Prime PCR Assay™). The amplification protocol consisted of one cycle at 95°C for 3 min followed by 40 cycles at 95°C for 10 sec and pre-determined annealing temperature (described below) for 30 sec. A single amplified product was confirmed by melt curve analysis. The annealing temperature was pre-determined for each primer set in the range from 58°C to 62°C. For quantification, a standard curve was generated for each amplification using increasing concentration of the pooled cDNA from the injured animals to determine the linear range and amplification efficiency. The threshold cycle of each sample was fitted to the standard curve to calculate the relative DNA abundance in the sample. The amplification efficiency was within 80–100%, and the regression coefficient R2 was >0.90.
Assessment of recognition memory
Recognition memory was assessed using the novel object recognition (NOR) task. The NOR task relies on the behavior that a rat will spend more time exploring a novel object than it does an object with which it is familiar.33 In this task, animals were pre-exposed to a testing chamber (100 × 100 cm box) twice-daily for 2 days. After the habituation period, two identical objects were introduced and the rat was allowed to explore them for a period of 10 min. Twenty-four hours later, the rat was placed back into the training chamber, with one of the familiar objects replaced by a novel object that had a different shape but was the same color and relative size. The time spent exploring the novel and familiar objects was recorded during a 4-min testing session. The difference in time spent exploring the novel object versus the familiar one was used as a measure of recognition memory.
Assessment of spatial learning and memory
Rats were tested for their spatial learning and memory using the standard hidden platform version of the Morris water maze.34–37 Beginning on day 14 post-injury, animals were given four training trials per day (with an intertrial interval of 4 min) for 6 days. If the animal failed to locate the platform within 60 sec on any given trial, it was led there by the experimenter. Twenty-four hours after the last day of training, spatial memory was tested by removing the platform from the water maze and allowing the animals to search for a period of 60 sec. Time to first platform crossing and number of platform crossings were recorded and used as indices of memory. After the completion of the memory test, reversal learning was assessed by placing the platform back into the tank at a position 180 degrees from its previous location. Rats were given four training trials with the platform in the new location to assess their cognitive flexibility. Movement within the maze was monitored using a video camera linked to tracking software (EthoVision; Noldus Information Technology, Wageningen, the Netherlands).
Context fear conditioning
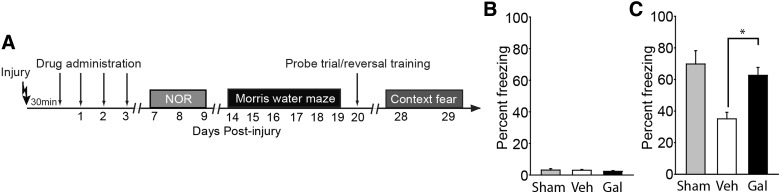
Beginning on day 28 post-injury, rats were trained in a one-trial fear-conditioning task. Rats were placed in the training chamber and allowed to freely explore their surroundings for a period of 2.5 min. Freezing behavior was monitored (in 2-sec increments) throughout the 2.5-min period by an observer blind to the injury status of the animals. A 2-sec, 0.7-mA footshock was then delivered. Thirty seconds after the footshock, the rats were removed from the training chamber and returned to their home cage. Twenty-four hours later, context-specific fear memory was tested by placing the animal back in the training chamber for a period of 3 min and scoring freezing behavior.
Statistical analysis
The number of animals used for each experiment was determined using a power analysis and based upon our experience with the techniques/tests utilized. Statistical comparisons were carried out using the statistics package within SigmaPlot (version 11.0; Systat Software, Inc., San Jose, CA). Data were subjected to a Shapiro-Wilk normality test to ensure a normal distribution and an equal variance test. Two group comparisons at a single time point (Evans Blue dye extravasation, immunohistochemistry, and probe trial data) was statistically tested using a Student's two-tailed t-test for unpaired variables. Two group comparisons across time (e.g., water maze learning, inflammation markers) were statistically evaluated using repeated-measures two-way analyses of variance (ANOVAs). Novel object recognition was evaluated within groups (e.g., novel vs. familiar) using a two-tailed Student's t-test for paired variables. All data were analyzed using raw recorded data, before transformation into percent control for presentation. Data were considered significant at p < 0.05 and presented as mean ± standard error of the mean.
Results
Galantamine decreases blood–brain barrier permeability
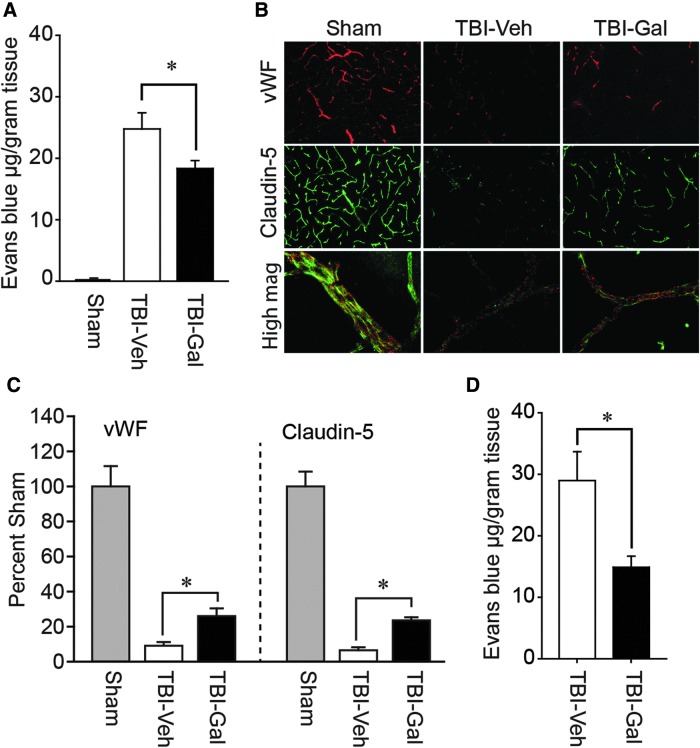
Disruption of the BBB post-TBI is a prominent secondary pathology that contributes to nonselective entry of blood-borne materials, vasogenic edema, and exacerbated tissue damage.38 We therefore examined whether post-injury administration of galantamine reduces BBB permeability in both mice and rats. Mice were injured, then 30 min, 8 h, and 20 h later i.v. injected with either 3.0 mg/kg of galantamine (n = 8 mice) or an equal volume of vehicle (n = 6 mice). Twenty-four hours post-injury, mice were injected with Evans Blue dye and the amount of extravasated dye quantified. A group of sham-operated mice (n = 8 mice) was used as baseline controls, but not statistically compared. Figure 1A shows that, by comparison to vehicle-treated injured animals, galantamine significantly reduced Evans Blue extravasation (p = 0.035), suggesting partial protection of BBB components.
FIG. 1.
Post-injury administration of galantamine reduces BBB permeability after TBI in both mice and rats. (A) Injured mice treated with 3.0 mg/kg of galantamine (n = 8) had significantly less Evans Blue extravasation in the ipsilateral cortex compared to vehicle-treated injured animals (n = 6). Data from sham-operated controls are presented to demonstrate baseline extravasation. (B) Representative images of von Willebrand factor (vWF; a marker of vascular endothelial cells) and the tight junction protein, Claudin-5, immunoreactivity in tissue sections taken from a sham, a TBI animal treated with vehicle (TBI-Veh), and a TBI animal treated with galantamine (3.0 mg/kg; TBI-Gal). Images were taken from the pericontusion region of injured animals and the corresponding area from shams. (C) Summary data (n = 5/group) show that systemic galantamine administration reduced the loss of both vWF and Claudin-5. *p < 0.05 by t-test between vehicle- and galantamine-treated animals. (D) Injured rats treated with 1.0 mg/kg of galantamine (n = 8) had significantly less Evans Blue extravasation in the ipsilateral cortex compared to vehicle-treated injured animals (n = 8). Data are presented as mean ± standard error of the mean. BBB, blood–brain barrier; TBI, traumatic brain injury.
Immunohistochemical examination of BBB components, namely endothelial cells (indicated by vWF immunoreactivity) and tight junction proteins (e.g., Claudin-5), revealed that TBI dramatically reduced the immunoreactivity of these markers in the pericontusion region by 24 h post-injury (Fig. 1B). Post-injury administration of 3.0 mg/kg of galantamine appears to partially preserve vWF and Claudin-5 immunoreactivity. The summary results shown in Figure 1C indicate that vWF (p = 0.029) and Claudin-5 (p < 0.001) immunoreactivities are significantly increased in galantamine-treated injured animals (n = 5 mice) compared to vehicle-treated injured controls (n = 5 mice).
To test whether galantamine can offer protection of the BBB in rats, male Sprague–Dawley rats were injured then injected with either 1.0 mg/kg of galantamine (i.v.) or an equal volume of vehicle 30 min post-injury (n = 8 rats/group). Additional doses were given 8 and 20 h post-injury. When Evans Blue extravasation was examined 24 h post-injury, a significant (p = 0.011) decrease in BBB permeability was observed in the galantamine-treated injured rats compared to vehicle-treated injured controls (Fig. 1D). Because both mice and rats showed reductions in BBB permeability as a result of galantamine administration, all subsequent experiments were performed using only rats.
Galantamine reduces hippocampal cell loss
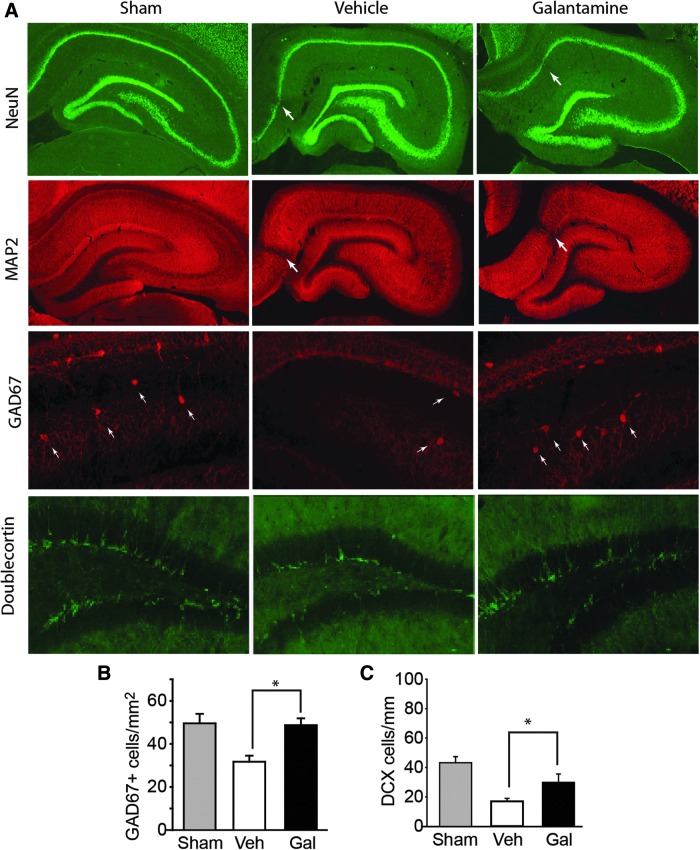
Hippocampal function is critical for learning and memory, and cell loss in this structure has been associated with learning and memory dysfunction post-TBI. Both adult pyramidal and granule neurons, as well as GABAergic interneurons in the hilus, have been shown to die post-TBI.38–41 To assess whether galantamine can offer neuronal protection post-TBI, rats were injured, then treated with either vehicle or 1.0 mg/kg galantamine 30 min post-injury, then twice-daily for 3 additional days (n = 4 rats/group). A group of sham-operated rats (n = 4) was prepared as baseline controls, but not statistically compared. Animals were euthanized 1 month post-injury by exsanguination with 4% PFA and their brains removed for histological evaluation. Microscopic evaluation of tissue sections stained with the neuronal marker, NeuN, did not reveal any demonstrable differences in TBI-triggered cell death within the ipsilateral hippocampus (arrows) between the vehicle- and galantamine-treated groups (Fig. 2A). Further, no differences in dendritic disruptions, evidenced by loss of MAP-2 immunoreactivity, were observed between the two groups. However, when GABAergic neurons (immunostained for GAD67) were evaluated, the TBI-triggered loss of these cells in the hilus of the dentate gyrus appeared to be lessened as a result of galantamine treatment (Fig. 2A). Stereological cell counts revealed that TBI animals treated with galantamine had significantly more GAD67-positive cells than did vehicle-treated injured controls (p = 0.003; Fig. 2B).
FIG. 2.
Galantamine treatment improves the survival of GAD67- and doublecortin-positive cells in the ipsilateral hippocampus of injured rats. (A) Representative photomicrographs showing NeuN and MAP2 immunoreactivity within the ipsilateral hippocampus. Arrow indicates sites of neuronal loss/dendritic damage. No visible difference between vehicle- and galantamine-treated injured rats was observed. In contrast, galantamine treatment appears to reduce the loss of GAD67 immunoreactivity within the hilus and increase the number of doublecortin-positive cells in the dentate gyrus of the ipsilateral hippocampus. (B) Summary data showing that the injury-induced loss of GAD67-positive cells is significantly reduced in animals treated post-injury with galantamine (n = 4/group). (C) Summary data showing that the injury-induced loss of doublecortin-positive cells is reduced in animals treated post-injury with galantamine (n = 4/group). *p < 0.05 between vehicle- and galantamine-treated injured animals. Data are presented as mean ± standard error of the mean. DCX, doublecortin; GAD67, glutamic acid decarboxylase 67 kDa; Gal, galantamine; MAP2, microtubule-associated protein 2; NeuN, neuronal nuclei; Veh, vehicle.
In addition to adult neurons, TBI has been demonstrated to cause the loss of newborn hippocampal neurons (originating from precursor cells in the subgranular zone), with maximal loss observed 24–72 h post-injury.42,43 To assess whether post-injury galantamine can protect against the loss of newborn neurons, rats were injured then injected with either vehicle (n = 4 rats) or 1.0 mg/kg of galantamine (n = 4 rats) 30 min post-injury. Additional doses were given daily for 3 additional days. A group of sham-operated animals (n = 4 rats) was used as a baseline control, but not statistically compared. Three days post-injury, rats were transcardially perfused with 4% PFA, their brains were removed, and tissue sections prepared for doublecortin immunostaining. Figure 2A shows representative photomicrographs of the ipsilateral hippocampus showing doublecortin immunoreactivity from a sham, a CCI rat treated with vehicle, and a CCI rat treated with galantamine. As reported previously,42,43 CCI causes a visible loss of doublecortin-immunopositive cells compared to that observed in the sham-operated control. Galantamine treatment appears to increase newborn neuron survival. Quantification of doublecortin-positive cells revealed that TBI animals treated with galantamine had significantly more doublecortin-positive immature neurons than vehicle-treated injured controls (p = 0.048; Fig. 2C).
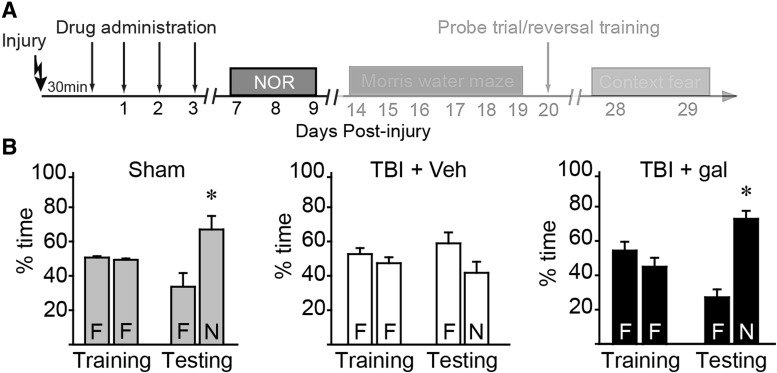
Post-injury galantamine improves recognition memory
To test the consequences of post-injury galantamine administration on cognitive performance, rats were injured and acute neurological responses monitored. When the acute neurological responses were compared between animals subsequently receiving vehicle and those receiving galantamine, no significant differences were detected in either suppression of pain reflexes (tail pinch: vehicle = 358 ± 27 sec, galantamine = 351 ± 26 sec; p = 0.845; toe pinch: vehicle = 350 ± 25 sec, galantamine = 334 ± 27 sec; p = 0.655) or righting response (vehicle = 405 ± 17 sec, galantamine = 399 ± 24 sec; p = 0.829). Galantamine (1.0 mg/kg or vehicle) was injected 30 min post-injury (n = 10 rats/group). Additional doses were given (twice-daily) on days 1–3 post-injury (Fig. 3A). A group of sham-operated rats (n = 6) was used as baseline controls. On days 7–9 post-injury (3 days after discontinuation of the drug), animals were trained and tested in the NOR task as outlined in the Methods section. Figure 3B shows that sham-operated controls explored the two objects equally during the familiarization period, indicating no pre-existing biases or preferences. When one of these objects was replaced with a novel object, sham-operated rats spent significantly (p = 0.023) more time exploring the novel object (N) than the familiar one (F). In contrast, vehicle-treated injured rats (TBI + veh) spent similar percentages of time exploring the novel and familiar objects (p = 0.218), indicating impaired recognition memory. Post-injury galantamine treatment (TBI + gal) improved recognition memory, as indicated by treated rats spending significantly more time exploring the novel object than the familiar one (Fig. 3B; p < 0.001).
FIG. 3.
Recognition memory is improved in injured rats treated post-injury with galantamine. (A) Timeline of behavioral testing and drug administration. Novel object recognition (NOR) testing was carried out on days 7–9 post-injury. (B) Summary data showing % time spent exploring objects in the NOR task. F = familiar, N = novel. Whereas both sham- (n = 6) and galantamine-treated injured animals (n = 10) animals spent significantly more time exploring the novel object than the familiar one during testing, vehicle-treated injured animals did not. *p < 0.05 by paired t-test between familiar and novel objects. Data are presented as mean ± standard error of the mean. gal, galantamine; TBI, traumatic brain injury; Veh, vehicle.
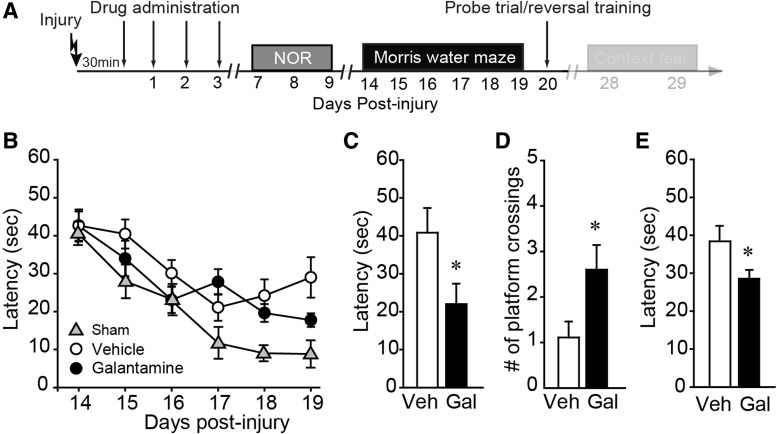
Post-injury galantamine administration improves spatial memory
Spatial learning and memory was tested using the standard hidden platform version of the Morris water maze task. Training was initiated 10 days after the termination of treatment (on day 14 post-injury) and continued for an additional 5 days (Fig. 4A). Sham animals were trained simultaneously and used to demonstrate normal learning. Figure 4B shows that there was no significant difference in spatial learning between the vehicle- and galantamine-treated injured animals (two-way repeated-measures ANOVA: F = 1.706; p = 0.209). Memory was assessed 24 h after the completion of training by monitoring the latency to first platform crossing and the number of platform crossings in a single probe trial in which the platform was removed from the tank and animals were allowed to search for a period of 60 sec. Galantamine-treated animals (Gal) required significantly less time to cross the previous location of the hidden platform (p = 0.039; Fig. 4C), and crossed the platform location more times (p = 0.029; Fig. 4D) than did vehicle-treated injured rats (Veh), indicating improved spatial memory. After the probe trial, reversal learning was assessed by reintroducing the platform into the maze in a location 180 degrees from its previous location. Rats were given four trials to learn the new location. Figure 4E shows that, on average, galantamine-treated rats were able to locate the new platform location more quickly than vehicle-treated controls (p = 0.045), indicative of improved cognitive flexibility.
FIG. 4.
Post-injury galantamine treatment of injured rats improves spatial memory and cognitive flexibility. (A) Timeline of behavioral testing and drug administration. Water maze training and testing was carried out on days 14–20 post-injury. (B) Acquisition curves were not significantly different between injured animals treated with 1.0 mg/kg of galantamine and vehicle-treated controls (n = 10 rats/group). Data from a group of sham animals presented for reference. Post-injury treatment with galantamine improved spatial memory as indicated by (C) reduced latencies to first platform crossing and (D) increased number of platform crossings when tested 24 h after training. (E) When reversal learning was tested, galantamine-treated animals learned to find the new platform location significantly faster than vehicle-treated injured animals, indicating improved cognitive flexibility. *p < 0.05 by t-test between vehicle- and galantamine-treated injured animals. Data are presented as mean ± standard error of the mean. Gal, galantamine; Veh, vehicle.
Post-injury galantamine treatment improves contextual fear memory
One-trial contextual fear has been demonstrated to be dependent on hippocampal neurogenesis.44 Because we observed that post-injury galantamine treatment significantly reduces the TBI-associated death of newborn neurons, we tested whether this cellular protection was associated with improved memory in this task. Training was carried out 28 days post-injury because newly generated neurons require 4–6 weeks to mature and integrate into the hippocampal circuit where they can influence behavior (Fig. 5A). Fear was assessed by monitoring freezing behavior (refraining from all movement except that needed for respiration). Figure 5B shows that before the footshock, all groups displayed minimal freezing behaviors, suggesting no pre-existing fear of the training chamber or generalized anxiety. When tested for fear memory 24 h after the training, sham animals displayed enhanced freezing behavior when placed back in the training chamber, indicating intact contextual fear memory (Fig. 5C). In contrast, injured animals treated with vehicle had impaired contextual fear memory, displaying reduced freezing behavior compared to sham controls. Galantamine-treated animals, by comparison, displaying freezing percentages similar to that observed in sham controls. When the freezing behaviors were compared, the galantamine-treated injured animals were found to freeze significantly more (p < 0.001) than their vehicle-treated, injured counterparts, indicating improved contextual memory.
FIG. 5.
Contextual fear memory is improved in injured rats treated with galantamine. (A) Timeline of behavioral testing and drug administration. Contextual fear conditioning and testing was carried out on days 28–29 post-injury. (B) Summary data showing the percent freezing of sham- (n = 6), vehicle-treated injured (n = 10), and galantamine-treated injured (n = 10) animals preceding the footshock. (C) Vehicle-treated injured animals had poor contextual memory as indicated by significantly less freezing than sham controls when placed back into the conditioning chamber 24 h after training. This effect was blunted in injured animals treated with galantamine. *p < 0.05 by Student's t-test between vehicle- and galantamine-treated injured rats. Data are presented as mean ± standard error of the mean. Gal, galantamine; Veh, vehicle.
Post-injury administration of galantamine does not reduce markers of inflammation
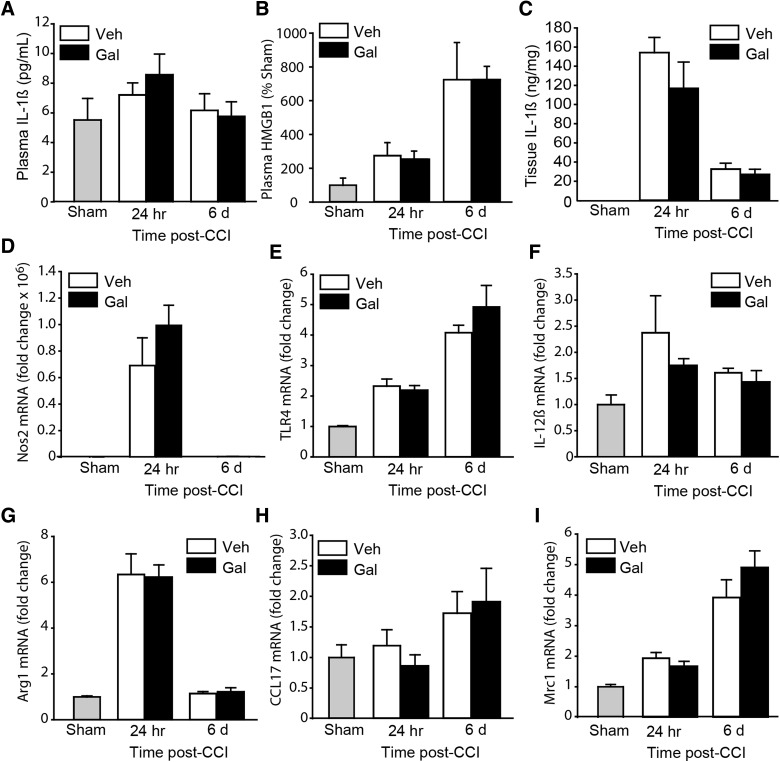
It has been demonstrated, in a model of sepsis, that pre-treatment with galantamine reduces systemic inflammation.45 Further, we have shown that post-TBI treatment with an nAChR7 agonist reduces the levels of circulating IL-1ß and HMGB1 (a pro-inflammatory damage-associated molecular pattern protein).46 To examine whether galantamine offers similar protection after brain injury, we examined the serum levels of IL-1ß (by ELISA) and HMGB1 (by western blotting). Animals were euthanized 24 h and 6 days post-injury (n = 4 rats/time point/group) and blood and tissues collected. The time points chosen were based on previous studies that have examined these pro-inflammatory molecules post-TBI.47,48 Figure 6A shows that plasma IL-1ß levels only modestly increased at the time points examined, and that these levels were not significantly altered as a result of galantamine treatment (F = 0.577; p = 0.458). Likewise, although injury dramatically increased HMGB1 levels, the levels observed in injured rats treated with galantamine were not significantly different from those in vehicle-treated injured animals (F = 0.417; p = 0.528; Fig. 6B). We next examined whether local IL-1ß levels in the injured brain tissue were altered as a result of treatment. Figure 6C shows that the tissue levels of IL-1ß were dramatically increased by 24 h post-injury, with increased levels still observed by 6 days post-injury compared to the levels detected in sham-operated controls. Galantamine treatment did not significantly affect the levels of IL-1ß in the injured cortical tissue (F = 1.264; p = 0.278).
FIG. 6.
Galantamine does not reduce markers of inflammation in brain-injured rats. (A) ELISA measurements of IL-1ß in serum samples obtained from sham, injured animals receiving vehicle, and injured animals receiving 1.0 mg/kg of galantamine at 24 h and 6 days post-injury (n = 4/group/time point). The modest increase in plasma IL-1ß (observed 24 h post-injury) was not affected by galantamine treatment. (B) The increases in plasma HMGB1 (measured using western blots) in TBI animals were unaffected by post-injury galantamine. (C) Tissue IL-1ß was markedly increased in the injured cortical tissue compared to sham (undetectable by ELISA). This increase was not influenced by galantamine. (D–F) Quantification of mRNA for markers of the M1 microglial/macrophage phenotype: nitric oxide synthase 2 (Nos2; D), Toll-like receptor 4 (TLR4; E), and interleukin-12ß (IL-12ß; F) indicate increases in M1 activation after TBI. However, galantamine did not change the levels of these markers. (G–I) Quantification of mRNA markers of the M2 microglial/macrophage phenotype: arginase1 (Arg1; G), chemokine (C-C motif) ligand 17 (CCL17; H), and mannose receptor C-type 1 (Mrc1; I) after TBI. The mRNA levels of these markers were unaffected by galantamine. CCI, controlled cortical impact; ELISA, enzyme-linked immunosorbent assay; Gal, galantamine; HMGB1, high-mobility group box 1; mRNA, messenger RNA; TBI, traumatic brain injury; Veh, vehicle.
Microglial activation and polarization post-TBI has also been suggested to be a marker of inflammation.49 When we examined markers of microglial/macrophage polarization, we observed that the messenger RNA (mRNA) levels for both M1 (nitric oxide synthase 2 [Nos2], Fig. 6D; Toll-like receptor 4 [Tlr4], Fig. 6E; interleukin-12ß [IL-12ß], Fig. 6F) and M2 (arginase1 [Arg1], Fig. 6G; chemokine [C-C motif] ligand 17 [Ccl17], Fig. 6H; mannose receptor C-type 1 [Mrc1], Fig. 6I) phenotypes were increased as a result of injury. No change in markers of microglial polarization was observed as a result of post-injury galantamine treatment.
Discussion
The present investigation assessing the therapeutic potential of galantamine revealed three key findings: 1) Post-injury administration of galantamine reduces TBI-triggered BBB compromise both in rats and mice; 2) post-injury administration of galantamine to injured rats improves the survival of both hippocampal GABAergic and newborn neurons; and 3) post-TBI acute treatment with galantamine to injured rats improves multiple modalities of memory, tested days after discontinuation of the drug. However, galantamine treatment did not significantly decrease indices of inflammation nor microglia polarization in the injured brain.
Central cholinergic signaling plays an important role in cognitive function. Cholinergic stimulation has been shown to facilitate long-term potentiation (a cellular mechanism for learning and memory) both in vitro50 and by stimulation of the medial septal area in vivo.51 In contrast, cholinergic antagonists (e.g., scopolamine) were found to cause memory impairments in rodents and in healthy volunteers.52 Thus, augmentation of cholinergic signaling has been suggested to be beneficial for diseases characterized by memory impairments. Consistent with this, administration of the ACh precursor, CDP-choline, improves cognitive function in brain-injured animals when tested during the period of drug administration.15 A recent study by de la Tremblaye and colleagues has shown that galantamine, when given for 19 days post-injury, improves water maze learning and memory when tested on days 14–19 (in the presence of the drug). Although cholinesterase inhibitors have also been suggested to improve attention and memory in chronic TBI patients,53–56 a recent small-scale clinical study has reported that galantamine does not improve cognitive symptoms in chronic mild TBI patients.57 However, the effect of acute administration of galantamine on TBI pathology and outcome have not been examined.
Several studies have suggested that blockade of muscarinic acetylcholine receptors before or soon after TBI may offer neuroprotection and improve motor/cognitive function.58–60 For example, a single bolus injection of scopolamine (a pan-muscarinic receptor antagonist) given acutely post-injury significantly improved motor function and reduced TBI-associated body weight loss.61 Post-injury administration of the selective muscarinic M2 receptor antagonist, BIBN99, has been demonstrated to significantly improve cognitive performance when given soon after TBI, but not when the administration was delayed.62 In addition to muscarinic receptors, studies are beginning to also demonstrate a role for nicotinic ACh receptors in TBI pathology. For example, Gatson and colleagues have shown that post-injury administration of a positive allosteric modulator of α7 nicotinic acetylcholine receptors reduces hippocampal apoptosis in rats subjected to TBI.63 Additional studies have examined beneficial effects of acetylcholinesterase inhibitors. For example, rivastigmine, a centrally acting anticholinesterase inhibitor, reduced motor and cognitive dysfunction when administered into the injured brain within minutes of the injury.64 In addition, Yu and colleagues found that daily post-injury administration of the acetylcholinesterase inhibitor, donepezil (U.S. Food and Drug Administration approved for AD), improves performance in the Morris water maze task when tested 2 weeks after the termination of treatment.16 However, Shaw and colleagues did not observe any benefit in either motor or cognitive performance in response to donepezil treatment.17 In the present study, we used the acetylcholinesterase inhibitor galantamine that also acts as an agonist for the alpha7 nicotinic receptor19–21 and examined its effect on neuroprotection and cognitive outcome. Our findings show that transient, acute treatment can have a lasting benefit on cognitive function post-TBI.
Previously, it has been reported that GABAergic neurons in the hilus of the hippocampus are vulnerable to TBI.40 It was proposed that the loss of these neurons can alter the balance of excitatory and inhibitory neuronal transmission, thereby compromising hippocampal function.65 When we quantified the number of GABAergic neurons 30 days post-injury (27 days after the termination of galantamine treatment), we observed that the number of these neurons in galantamine-treated injured animals was comparable to that observed in sham-operated controls. This preservation of GABAergic neurons may be an underlying mechanism for the improved hippocampal function we observed. In addition to preservation of GABAergic neurons, we also observed that galantamine treatment significantly improved the survival of newborn neurons. It has been estimated that newborn neurons require 4–6 weeks to mature and functionally integrate themselves into the hippocampal circuit. A number of studies have shown that these neurons play an important role in pattern separation,66,67 and a recent study by Hen and colleagues has shown that genetic ablation of these neurons inhibits context fear when tested using a one-trial paradigm.68 Based on these studies, we tested whether the preservation of newborn neurons observed after galantamine treatment improved one-trial contextual fear when tested 4 weeks post-injury and observed an improvement. These results suggest that galantamine improves cognitive performance in brain-injured animals, at least in part, by increasing the survival of newborn neurons. At present, it is not clear whether these benefits are attributed to galantamine's ability to inhibit acetylcholinesterase activity and/or modulate nAChR7 receptors. Although Yu and colleagues have suggested that the therapeutic effects of donepezil (tested using the Morris water maze) occur independent of its beneficial effect on newborn neurons, studies have reported conflicting results concerning the role of neurogenesis in spatial learning and memory.44,69,70 Whether donepezil can improve one-trial fear or contextual discrimination in TBI animals was not tested by Yu and colleagues.
At present, it is not known whether the protections afforded by galantamine on the BBB and on GABAergic and newborn neuron survival are related or occur independently. Mortazavian and colleagues have reported that galantamine can protect endothelial cells from free-radical–induced cell death.71 Given that free radical production is increased post-TBI,72–75 and endothelial cell loss is thought to be a contributing factor to the disruption of the BBB, it is possible that galantamine may improve BBB integrity (at least in part) through a similar mechanism. Disruption of the BBB has been shown to cause the unregulated entry of blood cells and molecules into the brain, where they can contribute to inflammation, edema, and exacerbate cell loss.76 Although in the present study we did not observe any influence of galantamine on inflammation or microglial polarization, we cannot rule out other effects that preservation of the BBB may have had on GABAergic and newborn neuron survival.
Stimulation of peripheral nAChR, either using agonists or by electrical stimulation of the vagus nerve, has been demonstrated to exert anti-inflammatory effects.77,78 In contrast to agents that stimulate peripheral nAChRs, galantamine has been reported to decrease systemic inflammation through activation of central mAChRs.45 It has been proposed that galantamine activates the vagus efferent circuit, causing release of ACh that stimulates peripheral nAChR7 on macrophages to suppress production and release of pro-inflammatory molecules.45 Contrary to the anti-inflammatory effects of galantamine observed in models of sepsis and colitis,45,79 we did not observe any reduction in the plasma levels of IL-1ß or HMGB1 (Fig. 6). Further, although TBI significantly increased the levels of IL-1ß in the injured brain, this increase was also unaffected by galantamine treatment. Studies have shown that TBI activates resident microglia, as well as causes infiltration of monocytes/neutrophils, resulting in an increase in the expression of MI and M2 markers,80,81 and can contribute to neuronal injury.82 Consistent with this, our quantitative PCR measurements of mRNA for markers of M1 (Nos1, Tlr4, and IL12ß) and M2 (Arg1, Ccl17, and Mrc1) microglial phenotypes revealed overall increases in their levels, indicative of activation of microglia. However, galantamine treatment did not influence the levels of these messenger RNAs (mRNAs), nor did it cause any detectable shift in polarization at the time points examined. The reason for the lack of anti-inflammatory effect by galantamine post-TBI is not clear.
In conclusion, our results indicate that acute, post-injury administration of galantamine can reduce two prominent TBI pathologies: disruption of the BBB and neuronal death. Further, we show that galantamine improves hippocampal function tested using three different hippocampal-dependent tasks: NOR, water maze, and one-trial contextual fear. At present, however, we cannot determine the relative contribution of improved BBB integrity and neuroprotection on the improved cognitive outcome we observed. It is possible that the protective effects of this drug are attributed to a combination of its actions.
Acknowledgments
The authors are thankful of the Senator Lloyd and B.A. Bentsen Center for Stroke Research and NIH (NS087149 and NS088298) who provided funding support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bartus R.T., Dean R.L., III, Beer B., and Lippa A.S. (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–414 [DOI] [PubMed] [Google Scholar]
- 2.Egea J., Rosa A.O., Sobrado M., Gandia L., Lopez M.G., and Garcia A.G. (2007). Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience 145, 866–872 [DOI] [PubMed] [Google Scholar]
- 3.Kalappa B.I., Sun F., Johnson S.R., Jin K., and Uteshev V.V. (2013). A positive allosteric modulator of alpha7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br. J. Pharmacol. 169, 1862–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmermann D.B., Gronlien J.H., Kohlhaas K.L., Nielsen E.O., Dam E., Jorgensen T.D., Ahring P.K., Peters D., Holst D., Christensen J.K., Malysz J., Briggs C.A., Gopalakrishnan M., and Olsen G.M. (2007). An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J. Pharmacol. Exp. Ther. 323, 294–307 [DOI] [PubMed] [Google Scholar]
- 5.Belluardo N., Mudo G., Blum M., Amato G., and Fuxe K. (2000). Neurotrophic effects of central nicotinic receptor activation. J. Neural Transm. Suppl. 227–245 [DOI] [PubMed] [Google Scholar]
- 6.Gorman L.K., Fu K., Hovda D.A., Murray M., and Traystman R.J. (1996). Effects of traumatic brain injury on the cholinergic system in the rat. J. Neurotrauma 13, 457–463 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt R.H., and Grady M.S. (1995). Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J. Neurosurg. 83, 496–502 [DOI] [PubMed] [Google Scholar]
- 8.Dixon C.E., Bao J., Johnson K.M., Yang K., Whitson J., Clifton G.L., and Hayes R.L. (1995). Basal and scopolamine-evoked release of hippocampal acetylcholine following traumatic brain injury in rats. Neurosci. Lett. 198, 111–114 [DOI] [PubMed] [Google Scholar]
- 9.Hayes R.L., Jenkins L.W., and Lyeth B.G. (1992). Neurotransmitter-mediated mechanisms of traumatic brain injury: acetylcholine and excitatory amino acids. J Neurotrauma 9, Suppl. 1, S173–S187 [PubMed] [Google Scholar]
- 10.Shin S.S., and Dixon C.E. (2015). Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J. Neurotrauma 32, 1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeAngelis M.M., Hayes R.L., and Lyeth B.G. (1994). Traumatic brain injury causes a decrease in M2 muscarinic cholinergic receptor binding in the rat brain. Brain Res. 653, 39–44 [DOI] [PubMed] [Google Scholar]
- 12.Lyeth B.G., Jiang J.Y., Delahunty T.M., Phillips L.L., and Hamm R.J. (1994). Muscarinic cholinergic receptor binding in rat brain following traumatic brain injury. Brain Res. 640, 240–245 [DOI] [PubMed] [Google Scholar]
- 13.Lyeth B.G., Dixon C.E., Jenkins L.W., Hamm R.J., Alberico A., Young H.F., Stonnington H.H., and Hayes R.L. (1988). Effects of scopolamine treatment on long-term behavioral deficits following concussive brain injury to the rat. Brain Res. 452, 39–48 [DOI] [PubMed] [Google Scholar]
- 14.Jiang J.Y., Lyeth B.G., Delahunty T.M., Phillips L.L., and Hamm R.J. (1994). Muscarinic cholinergic receptor binding in rat brain at 15 days following traumatic brain injury. Brain Res. 651, 123–128 [DOI] [PubMed] [Google Scholar]
- 15.Dixon C.E., Ma X., and Marion D.W. (1997). Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J. Neurotrauma 14, 161–169 [DOI] [PubMed] [Google Scholar]
- 16.Yu T.S., Kim A., and Kernie S.G. (2015). Donepezil rescues spatial learning and memory deficits following traumatic brain injury independent of its effects on neurogenesis. PLoS One 10, e0118793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw K.E., Bondi C.O., Light S.H., Massimino L.A., McAloon R.L., Monaco C.M., and Kline A.E. (2013). Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J. Neurotrauma 30, 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Tremblaye P.B., Bondi C.O., Lajud N., Cheng J.P., Radabaugh H.L., and Kline A.E. (2017). Galantamine and environmental enrichment enhance cognitive recovery after experimental traumatic brain injury but do not confer additional benefits when combined. J. Neurotrauma 34, 1610–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias E., Gallego-Sandin S., Villarroya M., Garcia A.G., and Lopez M.G. (2005). Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic receptors. J. Pharmacol. Exp. Ther. 315, 1346–1353 [DOI] [PubMed] [Google Scholar]
- 20.Schrattenholz A., Pereira E.F., Roth U., Weber K.H., Albuquerque E.X., and Maelicke A. (1996). Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol. Pharmacol. 49, 1–6 [PubMed] [Google Scholar]
- 21.Samochocki M., Zerlin M., Jostock R., Groot Kormelink P.J., Luyten W.H., Albuquerque E.X., and Maelicke A. (2000). Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol. Scand. Suppl. 176, 68–73 [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., and Tracey K.J. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 23.Dash P.K., Zhao J., Kobori N., Redell J.B., Hylin M.J., Hood K.N., and Moore A.N. (2016). Activation of alpha 7 cholinergic nicotinic receptors reduce blood-brain barrier permeability following experimental traumatic brain injury. J. Neurosci. 36, 2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 25.Zhao J., Moore A.N., Redell J.B., and Dash P.K. (2007). Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 27, 10240–10248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van B.L., Geerts R., Verhaeghe T., Willems B., Bode W., Lavrijsen K., and Meuldermans W. (2004). Pharmacokinetics and tissue distribution of galantamine and galantamine-related radioactivity after single intravenous and oral administration in the rat. Arzneimittelforschung 54, 85–94 [DOI] [PubMed] [Google Scholar]
- 27.Monbaliu J., Verhaeghe T., Willems B., Bode W., Lavrijsen K., and Meuldermans W. (2003). Pharmacokinetics of galantamine, a cholinesterase inhibitor, in several animal species. Arzneimittelforschung 53, 486–495 [DOI] [PubMed] [Google Scholar]
- 28.Nair A.B. and Jacob S. (2016). A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wester P., Watson B.D., Prado R., and Dietrich W.D. (1995). A photothrombotic ‘ring’ model of rat stroke-in-evolution displaying putative penumbral inversion. Stroke 26, 444–450 [DOI] [PubMed] [Google Scholar]
- 30.Dash P.K., Orsi S.A., Zhang M., Grill R.J., Pati S., Zhao J., and Moore A.N. (2010). Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 5, e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coggeshall R.E. and Lekan H.A. (1996). Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 364, 6–15 [DOI] [PubMed] [Google Scholar]
- 32.Bruchfeld A., Qureshi A.R., Lindholm B., Barany P., Yang L., Stenvinkel P., and Tracey K.J. (2008). High Mobility Group Box Protein-1 correlates with renal function in chronic kidney disease (CKD). Mol. Med. 14, 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ennaceur A. and Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 31, 47–59 [DOI] [PubMed] [Google Scholar]
- 34.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 35.Dash P.K., Moore A.N., and Dixon C.E. (1995). Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 15, 2030–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dash P.K., Mach S.A., and Moore A.N. (2002). The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience 114, 755–767 [DOI] [PubMed] [Google Scholar]
- 37.Royo N.C., LeBold D., Magge S.N., Chen I., Hauspurg A., Cohen A.S., and Watson D.J. (2007). Neurotrophin-mediated neuroprotection of hippocampal neurons following traumatic brain injury is not associated with acute recovery of hippocampal function. Neuroscience 148, 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chodobski A., Zink B.J., and Szmydynger-Chodobska J. (2011). Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2, 492–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colicos M.A. and Dash P.K. (1996). Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: possible role in spatial memory deficits. Brain Res. 739, 120–131 [DOI] [PubMed] [Google Scholar]
- 40.Lowenstein D.H., Thomas M.J., Smith D.H., and McIntosh T.K. (1992). Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 12, 4846–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxwell W.L., Dhillon K., Harper L., Espin J., MacIntosh T.K., Smith D.H., and Graham D.I. (2003). There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J. Neuropathol. Exp. Neurol. 62, 272–279 [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y., Gibb S.L., Zhao J., Moore A.N., Hylin M.J., Menge T., Xue H., Baimukanova G., Potter D., Johnson E.M., Holcomb J.B., Cox C.S., Jr., Dash P.K., and Pati S. (2016). Wnt3a, a protein secreted by mesenchymal stem cells is neuroprotective and promotes neurocognitive recovery following traumatic brain injury. Stem Cells 34, 1263–1272 [DOI] [PubMed] [Google Scholar]
- 43.Yu T.S., Zhang G., Liebl D.J., and Kernie S.G. (2008). Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 28, 12901–12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxe M.D., Battaglia F., Wang J.W., Malleret G., David D.J., Monckton J.E., Garcia A.D., Sofroniew M.V., Kandel E.R., Santarelli L., Hen R., and Drew M.R. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 103, 17501–17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov V.A., Parrish W.R., Rosas-Ballina M., Ochani M., Puerta M., Ochani K., Chavan S., Al-Abed Y., and Tracey K.J. (2009). Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23, 41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dash P.K., Zhao J., Kobori N., Redell J.B., Hylin M.J., Hood K.N., and Moore A.N. (2016). Activation of alpha 7 cholinergic nicotinic receptors reduce blood-brain barrier permeability following experimental traumatic brain injury. J. Neurosci. 36, 2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinoshita K., Chatzipanteli K., Vitarbo E., Truettner J.S., Alonso O.F., and Dietrich W.D. (2002). Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery 51, 195–203 [DOI] [PubMed] [Google Scholar]
- 48.Bachstetter A.D., Rowe R.K., Kaneko M., Goulding D., Lifshitz J., and Van Eldik L.J. (2013). The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J. Neurosci. 33, 6143–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morganti J.M., Riparip L.K., and Rosi S. (2016). Call off the dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS One 11, e0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auerbach J.M., and Segal M. (1996). Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J. Physiol. 492, Pt. 2, 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galey D., Destrade C., and Jaffard R. (1994). Relationships between septo-hippocampal cholinergic activation and the improvement of long-term retention produced by medial septal electrical stimulation in two inbred strains of mice. Behav. Brain Res. 60, 183–189 [DOI] [PubMed] [Google Scholar]
- 52.Drachman D.A., and Leavitt J. (1974). Human memory and the cholinergic system. A relationship to aging? Arch. Neurol. 30, 113–121 [DOI] [PubMed] [Google Scholar]
- 53.Warden D.L., Gordon B., McAllister T.W., Silver J.M., Barth J.T., Bruns J., Drake A., Gentry T., Jagoda A., Katz D.I., Kraus J., Labbate L.A., Ryan L.M., Sparling M.B., Walters B., Whyte J., Zapata A., and Zitnay G. (2006). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma 23, 1468–1501 [DOI] [PubMed] [Google Scholar]
- 54.Whitlock J.A., Jr. (1999). Brain injury, cognitive impairment, and donepezil. J. Head Trauma Rehabil. 14, 424–427 [DOI] [PubMed] [Google Scholar]
- 55.Masanic C.A., Bayley M.T., VanReekum R., and Simard M. (2001). Open-label study of donepezil in traumatic brain injury. Arch. Phys. Med. Rehabil. 82, 896–901 [DOI] [PubMed] [Google Scholar]
- 56.Tenovuo O. (2005). Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 61–67 [DOI] [PubMed] [Google Scholar]
- 57.McAllister T.W., Zafonte R., Jain S., Flashman L.A., George M.S., Grant G.A., He F., Lohr J.B., Andaluz N., Summerall L., Paulus M.P., Raman R., and Stein M.B. (2016). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 41, 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin S.S., and Dixon C.E. (2015). Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J. Neurotrauma 32, 1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyeth B.G., Ray M., Hamm R.J., Schnabel J., Saady J.J., Poklis A., Jenkins L.W., Gudeman S.K., and Hayes R.L. (1992). Postinjury scopolamine administration in experimental traumatic brain injury. Brain Res. 569, 281–286 [DOI] [PubMed] [Google Scholar]
- 60.Lyeth B.G., Liu S., and Hamm R.J. (1993). Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 617, 69–75 [DOI] [PubMed] [Google Scholar]
- 61.Lyeth B.G., Ray M., Hamm R.J., Schnabel J., Saady J.J., Poklis A., Jenkins L.W., Gudeman S.K., and Hayes R.L. (1992). Postinjury scopolamine administration in experimental traumatic brain injury. Brain Res. 569, 281–286 [DOI] [PubMed] [Google Scholar]
- 62.Pike B.R., and Hamm R.J. (1995). Post-injury administration of BIBN 99, a selective muscarinic M2 receptor antagonist, improves cognitive performance following traumatic brain injury in rats. Brain Res. 686, 37–43 [DOI] [PubMed] [Google Scholar]
- 63.Gatson J.W., Simpkins J.W., and Uteshev V.V. (2015). High therapeutic potential of positive allosteric modulation of alpha7 nAChRs in a rat model of traumatic brain injury: proof-of-concept. Brain Res. Bull. 112, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 65.Zhang B., Chen X., Lin Y., Tan T., Yang Z., Dayao C., Liu L., Jiang R., and Zhang J. (2011). Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res. 1382, 165–172 [DOI] [PubMed] [Google Scholar]
- 66.Leutgeb J.K., Leutgeb S., Moser M.B., and Moser E.I. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 315, 961–966 [DOI] [PubMed] [Google Scholar]
- 67.Sahay A., Wilson D.A., and Hen R. (2011). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drew M.R., Denny C.A., and Hen R. (2010). Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav. Neurosci. 124, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jessberger S., Clark R.E., Broadbent N.J., Clemenson G.D., Jr., Consiglio A., Lie D.C., Squire L.R., and Gage F.H. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garthe A., Huang Z., Kaczmarek L., Filipkowski R.K., and Kempermann G. (2014). Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav. 13, 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mortazavian S.M., Parsaee H., Mousavi S.H., Tayarani-Najaran Z., Ghorbani A., and Sadeghnia H.R. (2013). Acetylcholinesterase inhibitors promote angiogenesis in chick chorioallantoic membrane and inhibit apoptosis of endothelial cells. Int. J. Alzheimers Dis. 2013, 121068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall E.D., and Braughler J.M. (1993). Free radicals in CNS injury. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 71, 81–105 [PubMed] [Google Scholar]
- 73.Hall E.D., Wang J.A., Bosken J.M., and Singh I.N. (2016). Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr. 48, 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kontos H.A., and Povlishock J.T. (1986). Oxygen radicals in brain injury. Cent. Nerv. Syst. Trauma 3, 257–263 [DOI] [PubMed] [Google Scholar]
- 75.Povlishock J.T., and Kontos H.A. (1992). The role of oxygen radicals in the pathobiology of traumatic brain injury. Hum. Cell 5, 345–353 [PubMed] [Google Scholar]
- 76.Alves J.L. (2014). Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 92, 141–147 [DOI] [PubMed] [Google Scholar]
- 77.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., and Tracey K.J. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 [DOI] [PubMed] [Google Scholar]
- 78.Levine Y.A., Koopman F.A., Faltys M., Caravaca A., Bendele A., Zitnik R., Vervoordeldonk M.J., and Tak P.P. (2014). Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One 9, e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji H., Rabbi M.F., Labis B., Pavlov V.A., Tracey K.J., and Ghia J.E. (2014). Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7, 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bedi S.S., Hetz R., Thomas C., Smith P., Olsen A.B., Williams S., Xue H., Aroom K., Uray K., Hamilton J., Mays R.W., and Cox C.S., Jr. (2013). Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem Cells Transl. Med. 2, 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morganti J.M., Jopson T.D., Liu S., Riparip L.K., Guandique C.K., Gupta N., Ferguson A.R., and Rosi S. (2015). CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J. Neurosci. 35, 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evilsizor M.N., Ray-Jones H.F., Ellis T.W., Jr., Lifshitz J., and Ziebell J.M. (2015). Microglia in experimental brain injury: implications on neuronal injury and circuit remodeling, in: Kobeissy F.H. (ed). SourceBrain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC/Taylor & Francis: Boca Raton, FL: [PubMed] [Google Scholar]