Abstract
Background
Surveillance in the era of antiretroviral therapy (ART) requires estimates of HIV prevalence as well as the proportion eligible for ART. We estimated HIV prevalence and assessed field staging of individuals to estimate the burden of HIV disease needing treatment in rural Malawi.
Methods
Adults aged 18–59 years in a demographic surveillance system were interviewed, examined, and HIV counselled and tested. Staging that used a simplified version of the WHO criteria (‘field checklist’) was compared with staging by a medical assistant using a ‘clinic checklist’ and to CD4 cell results.
Results
A total of 2129 of 2303 eligible adults (92.4%) were traced, and 2047 (96.1%) participated. Of the 1443 participants (70.5%) tested, 11.6% were HIV positive. ART eligibility classification by the field and clinic checklists were concordant in 122 of 133 HIV-positive individuals. Compared with the clinic checklist, the field checklist had a sensitivity of 50% and a specificity of 96%. Including those already known to be on ART, staging by the field and clinic checklists estimated ART eligibility at 16.3 and 17.7% of HIV-positive individuals, respectively. Using CD4 cell count under 250 cells/μl or WHO stage III/IV, the Malawi national programme criteria, 38% of HIV-positive individuals were eligible for ART, compared with 31% based on the 2006 WHO criteria of CD4 cell count under 200 cells/μl or WHO stage IV or CD4 cell count of 200–350 cells/μl and WHO stage III.
Conclusion
The field checklist was not a suitable tool for individual staging. Criteria for ART eligibility based on clinical staging alone missed two-thirds of those eligible by clinical staging and CD4 cell count.
Introduction
Whereas antiretroviral therapy (ART) rollout programmes throughout sub-Saharan Africa have sought to meet the challenge of monitoring the number of individuals receiving treatment, none have tried directly to measure the number of HIV-infected individuals who meet treatment criteria in the community. Instead, treatment need has generally been estimated indirectly using sentinel surveillance data [1,2] and theoretical models, with various assumptions [3–5].
The rollout of free ART in Malawi started in 2004 [6]. The achievements and challenges of the programme have been described in detail elsewhere [6–10]. In order to facilitate a rapid scale-up in a healthcare system in which even basic laboratory services are often not available [11], ART eligibility is primarily determined by clinical staging [10]. All patients in World Health Organization (WHO) clinical stages 3 or 4 are generally eligible; if laboratory services are available, patients in stages 1 or 2 with a CD4 cell count less than 250 cells/μl (previously < 200 cells/μl) or with a total lymphocyte count of less than 1200 copies/μl are also eligible for ART. This approach is supported by the WHO [12]. The estimated number of individuals needing ART in Malawi, as of December 2006, was 190 000 and ART coverage was estimated to be 43% [13].
ART rollout in Karonga district, northern Malawi, started in June 2005. As part of data collection procedures for operational research and as a clinical tool to lead the clinician systematically through all the AIDS-defining criteria (thus supplementing their initial clinical assessment), a checklist of symptoms and signs was devised, with an associated algorithm for determining the eligibility for ART.
An on-going demographic surveillance system in the district [14] provided a unique opportunity to estimate HIV prevalence in the general population, the burden of HIV disease needing treatment, and to assess the appropriateness of clinical eligibility criteria for ART therapy in Malawi. Given the shortage of medical personnel available in resource-constrained countries such as Malawi [15], we also tested a simple field-based staging checklist. This provides the first population-based data on the distribution of clinical and immunological stages in Malawi.
Methods
The Karonga demographic surveillance system was established as part of the Karonga Prevention Study, a large epidemiological study of leprosy and tuberculosis, which has been running since 1979. Two total population surveys were conducted in the 1980s. Data from these surveys and all subsequent studies by the project, including data on all diagnosed tuberculosis cases in the district and on Karonga District rollout programme ART recipients, are held in a linked database.
The demographic surveillance covers a population of 32 000 in an area of 135 km2 in the southern part of the district. It is divided into 230 ‘clusters’ with an average of 30 households per cluster. After an initial census, information has been updated using key informants, reporting at monthly sessions, and visits by field staff when vital events and changes of household membership are reported, followed by an update census after 2 years.
Choosing clusters
Stratified sampling of clusters was used to ensure representative coverage of the whole area. Clusters with an expected high HIV prevalence were oversampled to maximize the statistical power in the assessment of the field checklist. In order to define the ‘high’, ‘medium’ and ‘low’ prevalence strata, project interviewers independently scored clusters according to their own perception of the likely HIV prevalence in that cluster. Next, the ordered clusters were divided into quintiles of the total population. The top risk quintile was considered to be a ‘high-risk’ stratum, the second and third quintiles a ‘medium-risk’ stratum, and the fourth and fifth quintiles a ‘low-risk’ stratum. Using probability proportional to size (PPS) sampling, the clusters within each stratum were assigned cumulated population numbers and selected at random, and all households within a cluster were included, to give a total of 750 adults from each stratum. The sample size changed slightly as the membership list for some households was updated at the first visit to reflect recent changes in membership. It was estimated that 150 HIV-positive individuals in this sample would contribute to the study comparison of interest.
All adult members aged between 18 and 59 years within each selected household were eligible to participate in the study. Repeated visits were made if necessary. At each household, the study was introduced and explained to all members, and then each individual was asked privately for consent to study participation, physical examination and HIV testing.
Study procedures
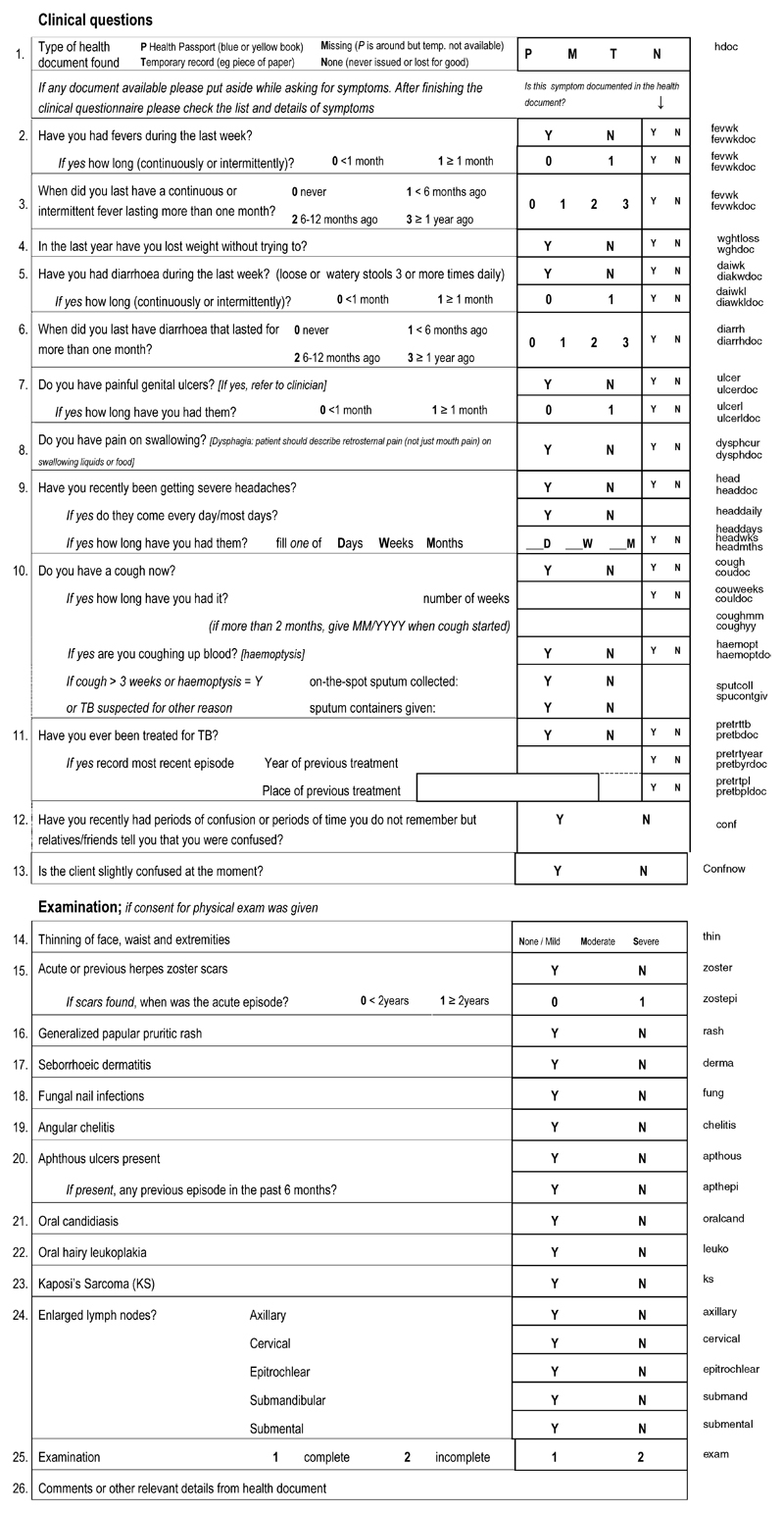
A ‘field checklist’ (Fig. 1) based on a simplified version of the WHO HIV/AIDS staging criteria was administered by a health assistant who already had experience examining for leprosy and skin diseases and drawing blood for HIV testing. The physical examination was limited to the face, mouth, lymph nodes, arms and lower legs. Other areas were only examined if the individual reported a rash, lesion or skin condition elsewhere. Some of these visits were conducted by nurses and medical assistants when available. Staging was later performed by the use of a computer algorithm.
Fig. 1. Field checklist form.
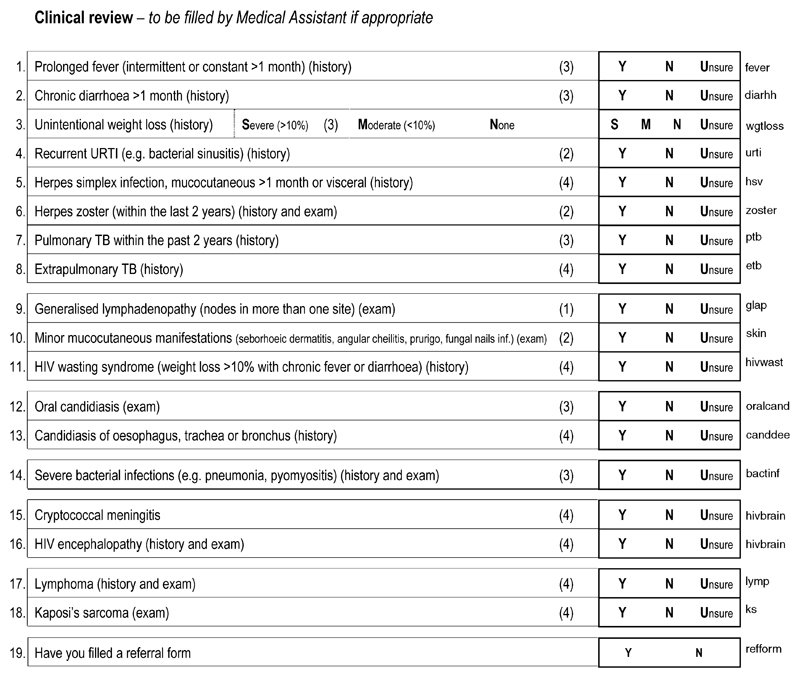
Participants who, at the time of pretest counselling, had indicated that they would like to receive their HIV result were visited for a second time. At this second visit, all HIV-positive participants were asked for a second blood sample for CD4 cell count testing and were physically examined and restaged by a medical assistant using the ‘clinic checklist’ of history, signs and symptoms used in the ART clinic when ascertaining ART eligibility (Fig. 2). Participants identified by the medical assistant to be in stage III/IV, or stage II with a CD4 cell count less than 250 cells/μl, were given a referral document to facilitate access to screening by the local ART clinic. Follow-ups were conducted for those who failed to present at the ART clinic. A random sample of HIV-negative participants from each cluster were asked to give a blood sample for CD4 cell count testing, and to undergo a second medical review in order to maintain confidentiality of HIV status within the study and to provide an estimate of the distribution of CD4 cell counts among HIV-negative individuals in this setting.
Fig. 2. Clinical review form.
Ethics approval for this study was given by the Malawi National Health Sciences Research Committee (2005, protocol 354), the London School of Hygiene and Tropical Medicine, UK (2005, protocol 3054), and WHO (2005, protocol RPC 130).
HIV testing was conducted with parallel enzyme-linked immunosorbent assay (Organon, Vironostika, Durham, North California, USA) and particle agglutination (Edgware modification of Serodia) tests. Discordant samples were repeated in duplicate. A second sample was sought if the original sample remained discordant. The same algorithm was applied to the second sample, if given, and any remaining unresolved samples were tested using Unigold and Determine rapid tests.
Analyses
All analyses were conducted using Stata version 9.2 (Stata Corp., College Station, Texas, USA). HIV prevalence within each cluster was calculated using test results available from the cluster and assuming that the prevalence was not significantly different among those who refused to test. To calculate an overall HIV prevalence for the demographic surveillance area, the prevalence estimates for the three strata (low, medium and high-risk) were weighted to reflect the sampling method. The WHO stage assigned was based on a modified version of the WHO’s revised clinical staging guidelines [12]. Persistent generalized lymphadenopathy was defined as lymphadenopathy in two or more sites (excluding inguinal). For weight loss, the stage II definition was modified to reported unintentional weight loss but no obvious thinning, and stage III was defined as reported unintentional weight loss and noticeable thinning. Chronic diarrhea was defined as the report of three or more loose or watery stools per day for more than one month, continuously or intermittently. Esophageal candidiasis was assessed by reported pain or difficulty in swallowing. It was decided that cryptosporidiosis and isosporiasis would be indistinguishable from chronic diarrhea in a field setting, and many of the other stage IV diagnoses, such as pneumocystis pneumonia or toxoplasmosis of the central nervous system, could not be reliably made in the field, so these were not included in the checklist. See Figure 1 for details.
Assessment of the utility of the field checklist was only conducted among participants who had not already started ART at the time of the study visit. Classification of HIV-positive participants as eligible for ART (stage III/IV) versus not eligible (stage I/II) by the field checklist was compared with classification of the same participant using the ART clinic checklist. Whether nurses or medical assistants, i.e. staff with more medical training than health assistants, administering the field checklist resulted in higher concordancy with the assigned stage at the second visit (defined as whether or not each review concluded the individual was eligible for ART) was also explored. Discordant classifications were reviewed to identify limitations in the field checklist. Classification based on clinical staging alone was compared with classification based on clinical information and CD4 cell count.
Estimates of the proportion eligible for ART using the different clinical and immunological criteria were calculated with and without including participants who had already started ART.
The project database provided another method of assessing the usefulness of staging based on reported illness by establishing the reliability of reports of a history of tuberculosis and its timing (WHO stage III only if the pulmonary tuberculosis episode is within the past 2 years).
Results
A total of 2129 of 2303 eligible adults (92.4%) were contacted, of whom 2047 (96.1%) agreed to participate in the study. Table 1 presents the characteristics of the population enrolled, overall and by HIV status. Among the 1443 participants who agreed to testing (70.5%), 11.6% were HIV positive (13.7% of women, 9.1% of men). Only 24 individuals stated at the first visit that they did not want to be told their HIV result, two of whom (8.3%) were HIV positive; 37 others said they were unsure whether they wanted to know their status and needed more time to decide.
Table 1. Characteristics of study participants, overall and by HIV status.
| Characteristic | Overall | HIV tested (% of overall) |
HIV positive (% of those tested) |
|---|---|---|---|
| Total | 2047 | 1443 (70.5) | 168 (11.6) |
| Sex* | |||
| Female | 1110 | 786 (70.8) | 108 (13.7) |
| Male | 937 | 657 (70.1) | 60 (9.1) |
| Women (years) | |||
| < 20 | 101 | 73 (72.3) | 8 (11.0) |
| 20–25 | 259 | 179 (69.1) | 19 (10.6) |
| 25–30 | 200 | 131 (65.5) | 20 (15.3) |
| 30–35 | 148 | 111 (75.0) | 20 (18.0) |
| 35–40 | 111 | 79 (71.2) | 17 (21.5) |
| 40–45 | 99 | 71 (71.7) | 8 (11.3) |
| 45–50 | 87 | 60 (69.0) | 9 (15.0) |
| 50+ | 105 | 82 (78.1) | 7 (8.5) |
| Men** (years) | |||
| < 20 | 102 | 81 (79.4) | 0 (–) |
| 20–25 | 196 | 136 (69.4) | 5 (3.7) |
| 25–30 | 176 | 121 (68.8) | 9 (7.4) |
| 30–35 | 151 | 102 (67.6) | 12 (11.8) |
| 35–40 | 92 | 60 (65.2) | 15 (25.0) |
| 40–45 | 73 | 46 (63.0) | 8 (17.4) |
| 45–50 | 59 | 42 (71.2) | 5 (11.9) |
| 50+ | 88 | 69 (78.4) | 6 (8.7) |
| Education | |||
| None | 41 | 28 (68.3) | 2 (4.9) |
| Primary | 1274 | 930 (73.0) | 111 (8.7) |
| Secondary | 724 | 478 (66.0) | 54 (7.5) |
| Tertiary | 8 | 7 (87.5) | 1 (12.5) |
| Current marital status** | |||
| Married | 1451 | 999 (68.9) | 109 (10.9) |
| Single | 342 | 263 (76.9) | 15 (5.7) |
| Divorced | 177 | 133 (75.1) | 24 (18.1) |
| Widowed | 77 | 48 (62.3) | 20 (41.7) |
| Stage by field checklist** (N = 2033a) | |||
| I Asymptomatic | 1938 | 1355 (69.9) | 121 (8.9) |
| I Symptomatic | 8 | 7 (87.5) | 5b (71.4) |
| II | 52 | 41 (78.9) | 18 (43.9) |
| III | 22 | 18 (81.8) | 9 (50.0) |
| IV | 13 | 12 (92.3) | 5 (41.7) |
P < 0.01.
P < 0.001 for comparison between HIV status groups.
Fourteen other individuals were already on antiretroviral therapy.
All five individuals had enlarged lymph nodes in more than one area; one individual also had zoster scars reported but the timing of the acute episode was not recorded.
HIV prevalence in the area, allowing for stratified sampling, was estimated to be 11.4%, [95% confidence interval (CI) 11.4, 11.5] overall; 12.8% (95% CI 12.7, 12.9) in the presumed high prevalence strata, 13.3% (95% CI 13.2, 13.4) in the medium, and 8.9% (95% CI 8.7, 9.0) in the presumed low prevalence strata. Ten participants who refused to have their blood tested for HIV in this study had been tested by the Karonga Prevention Study within the past year. Including these test results the prevalence estimate was virtually unchanged, 11.5%.
Fourteen participants, including nine women (64%), were identified as already on ART, by self-report at the study visit or through cross-checking with the project-linked database. Only one participant refused to be examined. Table 1 includes the distribution of staging based on the field checklist at the first visit, among those who were not already on ART (N = 2033). Of the HIV-positive individuals not on ART, 121 of 158 (76.6%) had no symptoms and only 14 (8.9%) were classified as eligible for ART. Sixteen HIV-negative participants (1.3%) were also classified as stage III/IV. Of these, 12 (75%) had a report of prolonged fever that contributed to the assigned stage; for six of these prolonged fever was the only symptom recorded.
At least one item was missing from the clinical section of the field checklist for five out of 158 of the HIV-positive individuals (3.2%). For all five individuals the isolated missing data could not, however, affect their assigned stage because they did not have sufficient other symptoms to reach a WHO stage III or IV definition.
A second visit was conducted with 1388 of the individuals who did not refuse to receive their HIV test result and were not already on ART (98.5%). Of these, 150 were HIV positive, 133 of whom (88.7%) were restaged. Twelve HIV-positive participants were referred to the ART clinic by the medical assistants. No participant started ART between the two visits. The median time between the first and second visit was 20 days (interquartile range 14–29 days). Overall, 93.3% of participants tested in the study received their HIV test result.
At the first visit, 30 participants (1.5%) reported having previously been treated for tuberculosis, five of them within the past 2 years. The project database confirmed 17 (57%, 12 of them pulmonary) and identified three other participants who had not reported their tuberculosis treatment history. At the second visit, one additional participant reported a history of tuberculosis in the last 2 years but this was not confirmed by the project database. Table 2 provides a comparison of the classification of treatment eligibility by checklist type. ART eligibility classification by the field checklist and the clinic checklist were concordant in 122 of 133 HIV-positive individuals. Using staging with the clinic checklist as the gold standard, the field checklist missed six of 12 individuals who were eligible for ART (i.e. sensitivity 50%), and incorrectly identified five of 121 individuals as needing referral for ART (specificity 96%). There was no statistically significant difference in concordance of staging by the type of health worker administering the field checklist.
Table 2. Eligibility for antiretroviral therapy by checklist type among HIV-positive individuals.
| Field checklist | Antiretroviral clinic checklist |
Total | |
|---|---|---|---|
| Stage I/II N (%) |
Stage III/IV N (%) |
||
| Stage I/II | 116 (87.2) | 6 (4.5)b | 122 (91.7) |
| Stage III/IV | 5 (3.8)a | 6 (4.5) | 11 (8.3) |
| Total | 121 (91.0) | 12 (9.0) | 133 (100) |
At the first visit, three had reports of prolonged fever for more than one month within the past 6 months, and two had oral candidiasis recorded.
Misclassified antiretroviral therapy eligibility status for four participants because the field questionnaire did not ask about extrapulmonary tuberculosis (one) and serious bacterial infections (three). One participant reported a history of prolonged fever at the second visit only, and another reported severe weight loss and pulmonary tuberculosis within the past 2 years.
Three of the five misclassifications of individuals as eligible for ART by field staging were the result of reports of prolonged fever more than one month within the past 6 months (one of these had also reported a recent history of chronic diarrhea during field staging). The field checklist identified oral candidiasis for the other two individuals, but this was not observed at the second visit.
Four of the six misclassifications of individuals as not eligible for ART by field staging were caused by the field checklist not asking about particular conditions: extrapulmonary tuberculosis (one individual) and serious bacterial infections (three individuals).
Of the 133 HIV-positive restaged individuals, 124 (93.2%) gave blood for CD4 cell count testing although four specimens failed to give usable results. Table 3 shows a comparison of the proportions eligible for ART using clinical and immunological eligibility criteria. All of the criteria suggest that a higher proportion of HIV-positive men are eligible for treatment than HIV-positive women. With the exception of the clinic checklist, however, this difference was not statistically significant and was generally explained by the lower average age of HIV-positive women (Table 1, Table 3). Including those already known to be on ART, staging by the field checklist and the clinic checklist estimated ART eligibility as 16.3 and 17.7% of HIV-positive individuals, respectively, whereas the Malawi national programme criteria and the 2006 WHO criteria estimated 38.1 and 33.6%, respectively.
Table 3. Eligibility for antiretroviral therapy by different criteria among HIV-positive individuals not already on treatment.
| Field checklist | Antiretroviral clinic checklist | CD4 cell count <200 cells/μl | CD4 cell count <250 cells/μl | Malawi national programme criteriaa | 2006 WHO criteriab | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | n/N | % | n/N | % | n/N | % | |
| Sex | ||||||||||||
| Male | 6/56 | 10.7 | 9/47 | 19.1 | 13/46 | 28.3 | 17/46 | 37.0 | 18/46 | 39.1 | 16/46 | 34.8 |
| Female | 8/102 | 7.8 | 3/86 | 3.5 | 12/74 | 16.2 | 18/74 | 24.3 | 19/74 | 25.7 | 15/74 | 20.3 |
| P value | 0.6 | 0.004c | 0.1 | 0.1 | 0.1 | 0.08 | ||||||
| Age (years) | ||||||||||||
| < 30 | 2/61 | 3.3 | 3/53 | 5.7 | 7/48 | 14.6 | 8/48 | 16.7 | 10/48 | 20.8 | 10/48 | |
| 30+ | 12/97 | 12.4 | 9/80 | 11.3 | 18/72 | 25.0 | 27/72 | 37.5 | 27/72 | 37.5 | 21/72 | |
| P value | 0.05 | 0.3 | 0.2 | 0.01d | 0.05 | 0.31 | ||||||
| Total excluding those on ART | 14/158 | 8.9 | 12/133 | 9.0 | 25/120 | 20.8 | 35/120 | 29.2 | 37/120 | 30.8 | 31/120 | 25.8 |
| Total including those on ART | 28/172 | 16.3 | 26/147 | 17.7 | 39/134 | 29.1 | 49/134 | 36.6 | 51/134 | 38.1 | 45/134 | 33.6 |
ART, Antiretroviral therapy.
Stage III/IV or CD4 cell count less than 250 cells/μl.
CD4 cell count less than 200 cells/μl or WHO stage IV or (CD4 cell count 200–350 cells/μl and WHO stage III).
P = 0.01 having adjusted for age using a binary indicator for age less than 30 versus age greater than 30 years.
P = 0.03 having adjusted for sex.
Table 4 compares CD4 cell levels and clinical staging among HIV-positive individuals not already on ART. For example, of 35 individuals with CD4 cell counts less than 250 cells/μl, only nine (25.7%) were staged as WHO stage III/IV. Relying on the CD4 cell count alone (< 250 cells/μl) missed two out of 11 (18.2%) of those at WHO stage III/IV. Using the Malawi national programme criteria, 30.8% of HIV-positive individuals are eligible for ART (Table 3, excluding those already on ART), of whom 11 of 37 (30%) were identified by clinical staging alone.
Table 4. CD4 cell count distribution by WHO stage among HIV-positive individuals not already on antiretroviral therapy.
| WHO stagea |
||||
|---|---|---|---|---|
| CD4 cell count criteria | Stage Ib N (%)c |
Stage II N (%) |
Stage III N (%) |
Stage IV N (%) |
| < 200 | 3 (5) | 17 (40) | 3 (43) | 2 (50) |
| 200–350 | 15 (23) | 5 (12) | 4 (57) | 2 (50) |
| ≥350 | 48 (72) | 21 (48) | 0 (−) | 0(−) |
| <250 | 6 (9) | 20 (47) | 5 (71) | 4 (100) |
| Total (N = 120) | 66 | 43 | 7 | 4 |
Using WHO stage classified by the antiretroviral clinic question-naire.
Including those who were asymptomatic.
Proportion in the WHO stage category meeting the CD4 cell count criteria.
Almost half the individuals in stage II had a CD4 cell count less than 250 cells/μl (20/43; Table 4). Three of these 20 participants reported moderate unintentional weight loss only, two reported recurrent upper respiratory tract infections only, four reported herpes zoster within the past 2 years only, and one had minor mucutaneous manifestations on examination. Eleven (55%) reported multiple stage II conditions, which always included moderate unintentional weight loss.
Eighty-five of the HIV-negative individuals (80.2%) agreed to a second medical review, but 26 refused to give a blood sample for CD4 cell count testing. The median CD4 cell count was 894 cells/μl (interquartile range 704, 1161).
Discussion
Acceptance of HIV testing and uptake of HIV test results were high in this community, and HIV prevalence was estimated to be 11.4%; 13.7% among women and 9.1% among men. These estimates and the age-specific patterns observed are consistent with previous estimates from the same area [16]. A sampling method that made use of local perceptions of the likely HIV prevalence in an area was able to differentiate low prevalence areas, but was less successful in differentiating between medium and high prevalence areas.
The field checklist for WHO clinical staging had high specificity (96%) but low sensitivity (50%) in identifying individuals eligible for ART, compared with the clinic checklist. This result is disappointing. This may be an issue of the small number of individuals who were stage III/IV. Our investigation of the discordant results between staging at the first and second visit suggested that some changes could be made to the field checklist to improve sensitivity. Adding questions for a history of extrapulmonary tuberculosis and serious bacterial infections, and training the field staff to diagnose serious bacterial infections during examination might have increased the sensitivity of the field checklist, but this is not easy without the clinical knowledge to probe. Our study team had previous experience in research studies and filling in forms. Some had special training in dermatology and were excellent in diagnosing skin conditions and lymphadenopathy, whereas others were nurses with other medical training, but there was no significant difference in concordance according to the training of the individual administering the field checklist. The research experience of our field staff may have reduced any differences that might have existed from their formal training, but there is little doubt that they were more experienced than could be hoped for in other field settings. This suggests that the field checklist is not an appropriate screening tool for individual ART eligibility even though, in this study, it gave a similar estimate of the proportion needing ART in the community as did the clinic checklist.
This study suggests that prolonged fever is not useful as an independent indicator of WHO stage III in an area with endemic malaria. A similar finding was reported in Uganda [17]. Our study also suggests that reports of tuberculosis may not be reliable when collected in a survey-style approach, although tuberculosis occurring outside the district would not have been recorded in the project database. If an ‘improved version’ of our field checklist were to be used in other settings, linking tuberculosis self-reports to those collected by the district tuberculosis programme may be advantageous.
In the absence of direct estimates of ART need, UNAIDS guidelines (2003) suggested that 15% of all individuals estimated to have HIV infection should be assumed to have advanced stage HIV disease [18] when trying to estimate treatment need. This is approximately equal to our estimate of overall ART eligibility based on clinical staging alone if those already on ART are included. We only included individuals aged 18 years or older, so slightly higher estimates are expected. A deterministic model for the whole of Karonga district estimated that approximately 17.5% of HIV-positive adults (aged 15 years and above) were likely to need ART in 2005 [4].
These estimates are all lower than the estimate obtained using CD4 cell counts alone or in addition to clinical criteria. This could partly be caused by bias, if individuals refusing a second examination and blood test were less likely to have advanced HIV disease. There is, however, no evidence that this is the case. Second examinations were performed for 122 of 144 individuals staged I/II in the field examination (85%), compared with 11 of 14 of those staged III/IV (79%), and CD4 cell counts were available for 109 of 121 of those clinically staged I/II (90%), compared with 11 of 12 of those staged III/IV (92%). As an extreme, if all those initially screened but without further information are assumed not to be eligible for ART, the proportion eligible would be 23% (37/158).
The Malawi national programme criteria provide a higher estimate of the proportion needing treatment than the 2006 WHO criteria because they suggest that individuals who are WHO stage III (or stage II and with a CD4 cell count less than 250 cells/μl) should also receive treatment. The Malawi criteria are rarely applied in full within Malawi because of the scarcity of CD4 cell count testing resources. Malawi’s national programme acknowledges that CD4 cell counts are helpful in the care of HIV-infected individuals and has set priorities for CD4 cell testing [11]. Work is ongoing to find cheaper ways of monitoring CD4 cell counts [19–21] as well as alternatives to eligibility criteria that include CD4 cell counts, such as body mass index, hemoglobin, total lymphocyte count [22] and the appearance of grey or distal-banded nails [23].
This study demonstrates that focusing upon the clinical stage alone probably misses two-thirds of those who would probably benefit from starting treatment. The importance of CD4 cell counts in staging has been shown elsewhere, in clinical settings [24–26], but our community surveillance removes any biases associated with barriers in accessing care. Limiting ART to those who are symptomatic may contribute to high early mortality rates. In settings in which there is not the capacity to start everyone who becomes eligible, the constraint will remain the national programme’s capacity to initiate patients on ART each year.
Acknowledgements
The authors wish to thank the National Health Sciences Research Committee of Malawi and the Malawi Ministry of Health for their continued support. They would also like to thank Jesus Maria Garcia Calleja and Victoria Hosegood for their contribution to the study design and the manuscript.
Sponsorship: The Karonga Prevention Study is supported by the Wellcome Trust, UK, and the British Leprosy Relief Association (LEPRA). This study was supported by the World Health Organization (contract no. SANTE/2004/089-735, Second Generation Surveillance on HIV/AIDS). J.R.G. is partly supported by the UK Department of Health.
Footnotes
Conflicts of interest: None.
References
- 1.Diaz T, Loth G, Whitworth J, Sutherland D. Surveillance methods to monitor the impact of HIV therapy programmes in resource-constrained countries. AIDS. 2005;19(Suppl 2):S31–S37. doi: 10.1097/01.aids.0000172875.67262.21. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Guidelines on construction of core indicators. Geneva: UNAIDS; 2005. [Google Scholar]
- 3.Spectrum policy modelling system (computer program), version 2.37Beta1. Futures Inst; Ct. USA: 2005. [Google Scholar]
- 4.White RG, Vynnycky E, Glynn JR, Crampin AC, Jahn A, Mwaungulu F, et al. HIV epidemic trend and antiretroviral treatment need in Karonga District, Malawi. Epidemiol Infect. 2007;135(6):922–932. doi: 10.1017/S0950268806007680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS/WHO. Estimation and projection package (computer program), version 2.0b. Geneva: UNAIDS/WHO; 2005. [Google Scholar]
- 6.Libamba E, Makombe S, Mhango E, de Ascurra TO, Limbambala E, Schouten EJ, et al. Supervision, monitoring and evaluation of nationwide scale-up of antiretroviral therapy in Malawi. Bull WHO. 2006;84:320–326. doi: 10.2471/blt.05.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harries AD, Gomani P, Teck R, de Teck OA, Bakali E, Zachariah R, et al. Monitoring the response to antiretroviral therapy in resource-poor settings: the Malawi model. Trans R Soc Trop Med Hyg. 2004;98:695–701. doi: 10.1016/j.trstmh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Harries AD, Schouten EJ, Makombe SD, Libamba E, Neufville HN, Some E, et al. Ensuring uninterrupted supplies of anti-retroviral drugs in resource-poor settings: an example from Malawi. Bull WHO. 2007;85:152–155. doi: 10.2471/BLT.06.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libamba E, Makombe SD, Harries AD, Schouten EJ, Yu JK, Pasulani O, et al. Malawi’s contribution to “3 by 5”: achievements and challenges. Bull WHO. 2007;85:156–160. doi: 10.2471/BLT.05.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libamba E, Makombe S, Harries AD, Chimzizi R, Salaniponi FM, Schouten EJ, et al. Scaling up antiretroviral therapy in Africa: learning from tuberculosis control programmes – the case of Malawi. Int J Tuberc Lung Dis. 2005;9:1062–1071. [PubMed] [Google Scholar]
- 11.Malawi Ministry of Health. Guidelines for the use of antiretro-viral therapy in Malawi. 2nd ed. Lilongwe: Malawi Ministry of Health; 2006. [Google Scholar]
- 12.WHO. Case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: WHO; 2006. [Google Scholar]
- 13.WHO. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. Progress report. Geneva: WHO; 2007. [Google Scholar]
- 14.Jahn A, Crampin AC, Glynn JR, Mwinuka V, Mwafilaso J, Mwaiyeghele E, et al. Evaluation of a village-informant driven demographic surveillance system in Karonga, Northern Malawi. Demograph Res. 2007;16:219–248. [Google Scholar]
- 15.Harries AD, Schouten EJ, Libamba E. Scaling up antiretroviral treatment in resource-poor settings. Lancet. 2006;367:1870–1872. doi: 10.1016/S0140-6736(06)68809-0. [DOI] [PubMed] [Google Scholar]
- 16.Crampin AC, Glynn JR, Ngwira BM, Mwaungulu FD, Ponnighaus JM, Warndorff DK, et al. Trends and measurement of HIV prevalence in northern Malawi. AIDS. 2003;17:1817–1825. doi: 10.1097/00002030-200308150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Morgan D, Maude GH, Malamba SS, Okongo MJ, Wagner HU, Mulder DW, et al. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet. 1997;350:245–250. doi: 10.1016/S0140-6736(97)01474-8. [DOI] [PubMed] [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS. Monitoring the declaration of commitment on HIV/AIDS: guidelines on construction of core indicators. Geneva: UNAIDS; 2003. ISBN 92-9173-238-9:25-26. 2003. [Google Scholar]
- 19.Fryland M, Chaillet P, Zachariah R, Barnaba A, Bonte L, Andereassen R, et al. The Partec CyFlow Counter could provide an option for CD4+ T-cell monitoring in the context of scaling-up antiretroviral treatment at the district level in Malawi. Trans R Soc Trop Med Hyg. 2006;100:980–985. doi: 10.1016/j.trstmh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Zijenah LS, Kadzirange G, Madzime S, Borok M, Mudiwa C, Tobaiwa O, et al. Affordable flow cytometry for enumeration of absolute CD4+ T-lymphocytes to identify subtype C HIV-1 infected adults requiring antiretroviral therapy (ART) and monitoring response to ART in a resource-limited setting. J Transl Med. 2006;4:33. doi: 10.1186/1479-5876-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakrishnan P, Dunne M, Kumarasamy N, Crowe S, Subbulakshmi G, Ganesh AK, et al. An inexpensive, simple, and manual method of CD4 T-cell quantitation in HIV-infected individuals for use in developing countries. J Acquir Immune Defic Syndr. 2004;36:1006–1010. doi: 10.1097/00126334-200408150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tassie JM, Marquardt T, Damisoni H, Odhiambo OD, Mulemba M, Szumilin E, et al. Indirect markers to initiate highly active antiretroviral therapy in a rural African setting. AIDS. 2004;18:1226–1228. doi: 10.1097/00002030-200405210-00025. [DOI] [PubMed] [Google Scholar]
- 23.Scarborough M, Gordon SB, French N, Phiri C, Musaya J, Zijlstra EE. Grey nails predict low CD4 cell count among untreated patients with HIV infection in Malawi. AIDS. 2006;20:1415–1417. doi: 10.1097/01.aids.0000233575.26349.cc. [DOI] [PubMed] [Google Scholar]
- 24.Diomande FV, Bissagnene E, Nkengasong JN, Maurice C, Monga B, Laga M, et al. The most efficient use of resources to identify those in need of antiretroviral treatment in Africa: empirical data from Cote d’Ivoire’s Drug Access Initiative. AIDS. 2003;17(Suppl. 3):S87–S93. doi: 10.1097/00002030-200317003-00012. [DOI] [PubMed] [Google Scholar]
- 25.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings – the case of Cote d’Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 26.Martinson N. Does WHO clinical stage reliably predict who should receive ART treatment?; Third International AIDS Society Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. 24–27 July 2005; Abstract no We F00304. [Google Scholar]