Abstract
Background
Survival patterns after HIV infection in African populations in the era before antiretroviral therapy (ART) form an important baseline for measuring future successes of treatment programmes. Few studies have followed seroconverters for 10 or more years to describe such patterns.
Methods
The Kisesa open cohort study conducted four rounds of village-based HIV testing and 20 rounds of household-based demographic surveillance between 1994 and 2006. Approximate infection dates were established for individual seroconverters by allocating a date between the last negative and first positive test. Person-years lived post-infection were computed, allowing for left truncation and right censoring, and Kaplan–Meier survival functions were constructed, truncating the analysis at the start of 2005 when ART first became available in the community. Weibull models were fitted to estimate median survival time, and parametric regression methods were used to investigate the influence of sex and age at infection.
Results
A total of 369 seroconverters were identified, providing 890 person-years of follow-up during which 44 deaths were observed. The Kaplan–Meier function showed 67% surviving 9 years post-infection, and the overall predicted median survival was 11.5 years. Survival was strongly related to age at infection (hazard ratio 1.06 for each additional year of age, and weakly to sex. A strong effect of age was evident even after allowing for mortality from non-HIV-related causes using cause deletion methods to estimate net mortality.
Conclusion
The survival of HIV-infected individuals was comparable to that reported in developed country studies before the introduction of HAART. Survival patterns in Kisesa are marginally more favourable than those reported in cohort studies in Uganda.
Keywords: HIV/AIDS, mortality, survival, Tanzania
Background
HIV/AIDS has had a devastating impact on mortality in Africa, which in 2006 experienced 2.1 million of the 2.9 million deaths attributed to AIDS worldwide [1]. HIV is currently estimated to account for between 35 and 75% of deaths of adults in the prime productive ages in east African populations [2,3]. It is, however, not easy to obtain accurate information on HIV-related mortality patterns in Africa, because most affected countries lack vital registration data that reliably record the cause of death. HIV cohort studies have an important role to play in this respect, as their ability to measure HIV incidence and the survival of infected individuals allows analysts to make better use of the widely available data on national HIV prevalence trends [4].
The most informative comparative measures of HIV-related mortality are based on life table estimates of survival after infection, obtained by follow-up of known seroconverters [2]. Survival patterns after HIV infection before the introduction of antiretrovitral therapy (ART) also form an important baseline for measuring the future success of treatment programmes. Previously published survival estimates in African populations suggested median times from infection to death ranging from 5 years (commercial sex workers in Nairobi [5]) to 9.8 years (community study in Masaka district, Uganda [6]). These estimates are lower than the average of 10.5 years observed in studies of infected individuals in high income countries [7].
The Kisesa open cohort is one of three ongoing African community-based studies that have followed seroconverters for 10 or more years [8,9]. The study is located in northwest Tanzania, approximately 20 km east of the regional capital Mwanza (Tanzania’s second city), along the main road to Kenya. It has collected data on demographic change (20 rounds) and serological status (four rounds) since 1994, as described in several earlier publications [10,11].
By 2005, shortly before ART became available in the area, Kisesa ward had a population of approximately 27 000, living in six villages, categorized geographically as ‘remote rural areas’ and ‘roadside areas’, the latter including an urbanized trading centre. The majority of residents are from the Sukuma tribe, the largest ethnic group in Tanzania. Economic activities revolve around farming, and petty trade of agricultural products. Per capita income in 2004 was estimated to be less than US$120 per year [12,13].
This paper presents estimates of survival in the Kisesa cohort study, using fitted Weibull models to extend direct estimates as necessary.
Methods
Fieldwork and laboratory methods
Demographic surveillance was carried out during house-hold surveys, at approximately half-yearly intervals, in which village-based enumerators collected information on births, deaths and household movements. Proxy information was accepted from the head of household or other competent adult, and respondents’ estimates of the date of death were accepted if they lay between the demographic round at which the death was first reported, and the date of the previous round at which the subject was known to be alive and resident in the household. For reported dates of death lying outside of these intervals, and for unknown dates of death, a date halfway between household interviews was used. To examine the relationship between mortality and migration out of the study area, in the last round of demographic surveillance (round 20 in 2006) information was also obtained on the survival status (alive or dead) and date of death for respondents who had moved out of the household at an earlier round.
Epidemiological serosurveys were carried out in 1994/1995, 1996/1997, 1999/2000 and 2003/2004 in special village survey centres, to which eligible respondents (permanent residents aged 15 years and over) were invited. Study participants gave verbal consent for interview and consent for their blood to be tested for HIV on the understanding that the results would not be disclosed to them, and would not be linked on a named basis by the study staff. On-site voluntary counselling and testing was offered in rounds 3 and 4. Eligible participants and their children were offered free medical care (but not ART) at temporary clinics, which were better supplied with essential drugs than local dispensaries.
HIV testing was carried out at a regional reference laboratory in Mwanza City. In the first survey venous blood was collected; subsequently dried blood spots from fingerpricks were used. In the first three surveys HIV testing was carried out using Vironostika HIV-MIXT B (Organon, Boxtel, the Netherlands) and Enzygnost HIV-1/HIV-2 (Dade Behring, Marburg, Germany). In the fourth round Uniform 2 (Organon) and Enzygnost HIV-1/HIV-2 (Dade Behring) were used. In all survey rounds samples for which both enzyme-linked immunosorbent assay results were reactive were considered to be HIV positive. Western blot tests were used to discriminate between discrepant results in round 1, in subsequent rounds the two enzyme-linked immunosorbent assay tests were repeated, and if still discrepant the sample was excluded from analysis (27 samples).
All the surveys were given ethical approval by the Medical Research Co-ordinating Committee of the Tanzanian Ministry of Health.
Analytical methods
For analytical purposes, two kinds of individuals are defined as ‘seroconverters’. The first group (360 individuals) are those who recorded a negative HIV test followed by a positive test at a later serosurvey. This group includes 56 individuals who missed a serosurvey round between their last negative and first positive test. The second group (46 individuals) are young persons aged 18 years and under when they attended their first serosurvey, who were already HIV positive at that time, and who had been resident in Kisesa ward at the time of the previous survey, but were too young to be eligible to participate in the earlier survey. These young prevalent cases were assumed to have been uninfected at the time of the earlier survey, and their assumed last negative test date was set at the median test date for the subvillage in which they were resident at the time of the earlier survey.
Intervals between serosurveys are much longer (approximately 3 years) than interviews between demographic rounds (approximately 6 months), which would result in pronounced heaping of seroconversion dates by calendar year if seroconversion times were assigned to the midpoint between serosurvey rounds. A multiple imputation technique was therefore used to estimate seroconversion dates and overcome the problem of interval censoring [14,15]. Seroconverters (and young prevalent cases) were allocated a random date between their last negative test date (or time of previous serosurvey) and their first positive test date, with random numbers drawn in such a way as to produce a uniform distribution of seroconversion dates in each interval for the population as a whole.
Survival analyses were performed using Stata 9 (Stata survival analyses and epidemiological tables; Stata Corp., College Station, Texas, USA). Person-years lived post-infection were computed, and Kaplan–Meier survival functions constructed, allowing for left truncation (time between seroconversion and first positive test) and right censoring (time after exit from study or household interview at demographic round 19) [16]. Seroconverters who left the study area very soon after their first positive test and were no longer resident in a subsequent demographic round were dropped from the analysis (37 individuals) as no follow-up time could be recorded for them. No deaths were reported between the serosurvey and the following demographic round among these excluded individuals.
Demographic round 19 (September 2004 to February 2005) was chosen as the cutoff point for survival analysis as this round was completed shortly before the introduction of a referral scheme for study participants, whereby those who had undergone voluntary counselling and testing and discovered they were infected were offered assessment for ART eligibility at Bugando Medical Centre, the referral hospital in Mwanza city. ART was on offer at this hospital from early 2005, but a search of hospital records showed no Kisesa residents had attended the ART clinic before the initiation of the referral scheme. To investigate trends in HIV-related mortality by calendar year of infection, seroconversions were divided into those occurring before and after testing in the third serosurvey, which lasted from August 1999 to August 2000.
Maximum likelihood regression methods were used to fit Weibull parametric models to all the observed person-year episodes. These regressions were used to investigate the influence of sex and infection age on survival, and to predict median survival times in subpopulations in which follow-up time was too short to estimate median survival directly.
Symbolically, s, the proportion surviving to time t is predicted for each individual using the Weibull function:
s = exp{ −λtϕ}
where the level parameter, λ, can be made to depend on the age and sex covariates, and the shape parameter, ϕ, is the same for all subjects.
To illustrate the pattern of change of mortality risks with time since infection, hazard rates were estimated using a bandwidth of 2 years to smooth the point hazard estimates, and confidence bands were calculated using log transformations.
Comparable survival times for never-infected individuals were calculated based on survival from the first negative test. Uninfected individuals distributed by sex and age at the first negative test were weighted by the distribution of seroconverters by sex and age at infection to produce life tables with a common demographic starting point. Using age-weighted survival probabilities for uninfected individuals, life tables ‘net’ of mortality unrelated to HIV were constructed for the seroconverters, using standard cause deletion methods [17].
Results
Numbers included in analyses
Participation rates in the serosurveys ranged from 66 to 75%, with higher attendance rates by women than men and better attendance in the remote rural villages than in the roadside villages. The most common reason for non-attendance was temporary absence from home. At each serosurvey, over 99% of those who attended provided a blood sample for HIV testing. Among age-eligible Kisesa residents who had at least one HIV-negative blood test in one of the first three serosurveys, 39% did not provide a second sample that would have enabled them to be identified as seroconverters, the main reason for not testing again was permanent outmigration from the study site (76%). Among those who remained resident in the ward throughout the entire period of the study, 97% tested on two or more occasions.
In the demographic rounds, in which proxy information was accepted on behalf of those who were temporarily absent, overall loss to follow-up over the 20 rounds among residents aged 15 years and over was 0.9%. Dates of death were unknown or misreported in 6% of cases, and had to be imputed using household interview dates.
A total of 369 seroconverters followed up while resident in the ward were included in the main analysis (184 men and 185 women), providing 890 person-years of follow-up, during which 44 deaths were observed (Table 1). Infections occurred between 1994 and 2004; the median calendar year for infections was 1999. The mean follow-up time was 4.2 years, and the longest individual follow-up time was 9.9 years; the median follow-up time was 3.8 years, with an interquartile range of 2.6–5.9 years. The mean ages at infection of male and female seroconverters were very close: 30.0 years for men and 29.9 years for women, the peak 5-year age group for seroconversion was 20–24 years for both sexes. The earliest recorded death was 0.6 years after seroconversion, the last death occurred 9.1 years after seroconversion.
Table 1. Survival of seroconverters from estimated infection time, Kisesa 1994–2005.
| Population | Seroconverters followed-up while resident in Kisesa | Including those followed-up after moving out | Excluding those with seroconversion interval > 3.5 years | Excluding those already positive at first age-eligible survey | ||||
|---|---|---|---|---|---|---|---|---|
| No. at start | 369 | 390 | 301 | 325 | ||||
| Total deaths | 44 | 52 | 37 | 44 | ||||
| Person-years | 890 | 1050 | 790 | 822 | ||||
| Max observation time | 9.9 | 10.1 | 9.9 | 9.9 | ||||
| Years post-infection | Proportion surviving | 95% CL | Proportion surviving | 95% CL | Proportion surviving | 95% CL | Proportion surviving | 95% CL |
| 1 | 0.974 | 0.898–0.993 | 0.975 | 0.904–0.994 | 0.957 | 0.837–0.989 | 0.969 | 0.881–0.992 |
| 2 | 0.954 | 0.887–0.981 | 0.956 | 0.894–0.983 | 0.931 | 0.829–0.973 | 0.947 | 0.970–0.979 |
| 3 | 0.923 | 0.860–0.959 | 0.929 | 0.869–0.962 | 0.899 | 0.806–0.949 | 0.913 | 0.841–0.954 |
| 4 | 0.865 | 0.798–0.911 | 0.874 | 0.812–0.916 | 0.838 | 0.747–0.898 | 0.851 | 0.777–0.902 |
| 5 | 0.814 | 0.742–0.868 | 0.818 | 0.751–0.868 | 0.799 | 0.708–0.865 | 0.798 | 0.720–0.857 |
| 6 | 0.759 | 0.680–0.821 | 0.751 | 0.678–0.809 | 0.754 | 0.659–0.826 | 0.742 | 0.658–0.808 |
| 7 | 0.707 | 0.619–0.778 | 0.711 | 0.633–0.775 | 0.711 | 0.612–0.789 | 0.689 | 0.598–0.764 |
| 8 | 0.707 | 0.619–0.778 | 0.711 | 0.633–0.775 | 0.711 | 0.612–0.789 | 0.689 | 0.598–0.764 |
| 9 | 0.666 | 0.544–0.762 | 0.682 | 0.586–0.761 | 0.667 | 0.534–0.770 | 0.649 | 0.527–0.746 |
| 10 | 0.625 | 0.477–0.742 | ||||||
CL, Confidence limit
Routine follow-up of subjects ceases when they leave the study area, but in demographic round 20, respondents were asked to report the survival status of former household members who were no longer resident in the ward, and the approximate date of death for those outmigrants who died. This extra information increased the number of seroconverters for whom follow-up data were available from 369 to 390, and the number of reported deaths increased from 44 to 52. Follow-up times for outmigrants reported at round 20 were censored at the median interview time for round 19, to ensure that the experience covered by these reports did not overlap with the time during which ART started to become available. The maximum follow-up time was thereby increased from 9.9 to 10.1 years, and person-years of follow-up from 890 to 1050.
Non-parametric survival analysis for total population
Kaplan–Meier survivor functions based on the experience of seroconverters who were followed up while resident in Kisesa (Table 1, first panel) are compared with the experience of those for whom survival information was available at round 20 (second panel). The confidence limits overlap throughout and there is no systematic difference between the two survivor functions or discernible separation over time. Even with the inclusion of migrants followed up outside the area, there is not enough information to estimate the median survival time directly, as 62% were still alive after 10 years [95% confidence interval (CI) 48–74%].
Of the 369 seroconverters who are the focus of the main analyses, 68 were identified after an interval in which they missed a serosurvey, as a result they have seroconversion intervals ranging from 4 to 8 years. Including these long intervals introduces a large uncertainty with respect to the estimated time of infection, and a possibility of bias if early deaths are missed. The third panel of Table 1 shows the survival function obtained after excluding those with longer seroconversion intervals. Although the confidence intervals overlap, in this case the difference between survival functions is somewhat larger, and the direction of the difference is systematic: the life table that excludes those with long seroconversion intervals has lower survival proportions for up to 6 years after infection. We have kept in the analyses those who had a long seroconversion interval, but we investigate the effect of this factor using parametric and non-parametric approaches.
Among the 46 young prevalent cases, 44 were followed up after their positive test, and are included in the 369 seroconverters in the main analyses. Their estimated ages at seroconversion ranged from 12 to 18 years. No deaths were observed among this group, but their follow-up times were relatively short, with a mean of 3.2 years (range 1.1–7.8). The last panel of Table 1 shows that excluding these young people from the analysis has a small but systematic impact on overall survival, lowering the estimated proportion surviving by one or two percentage points throughout, although the resulting survival function is still well within the confidence intervals of the population used for the main analyses.
Log rank tests indicate that the length of the seroconversion interval is not significantly associated with survival, (χ2(1) = 0.65, P = 0.42) even after stratifying by age at infection (stratification is necessary as 69% of those with short seroconversion intervals were infected at young ages compared with only 40% of those with long intervals). With respect to the time of seroconversion, it is only possible to compare survivor functions for 5 years, as those infected after the third serosurvey (approximately from 2000 onwards) do not have the possibility of a longer follow-up. At 5 years post-infection, the proportion surviving among those infected before the third serosurvey was 79% (CI 70–86%), whereas among those infected later, the equivalent proportion was 82% (CI 58–93%), suggesting that those infected later may have slightly lower mortality in the first few years after infection. Again, the log rank test of the survival functions needs to be stratified by age at infection, because the proportions infected at ages below 30 years before and after the third serosurvey are 61 and 51%, respectively. The stratified log rank test suggests that the effect of infection date is not significant, (χ2(1) = 0.83, P = 0.36).
Stratified analyses and parametric regression
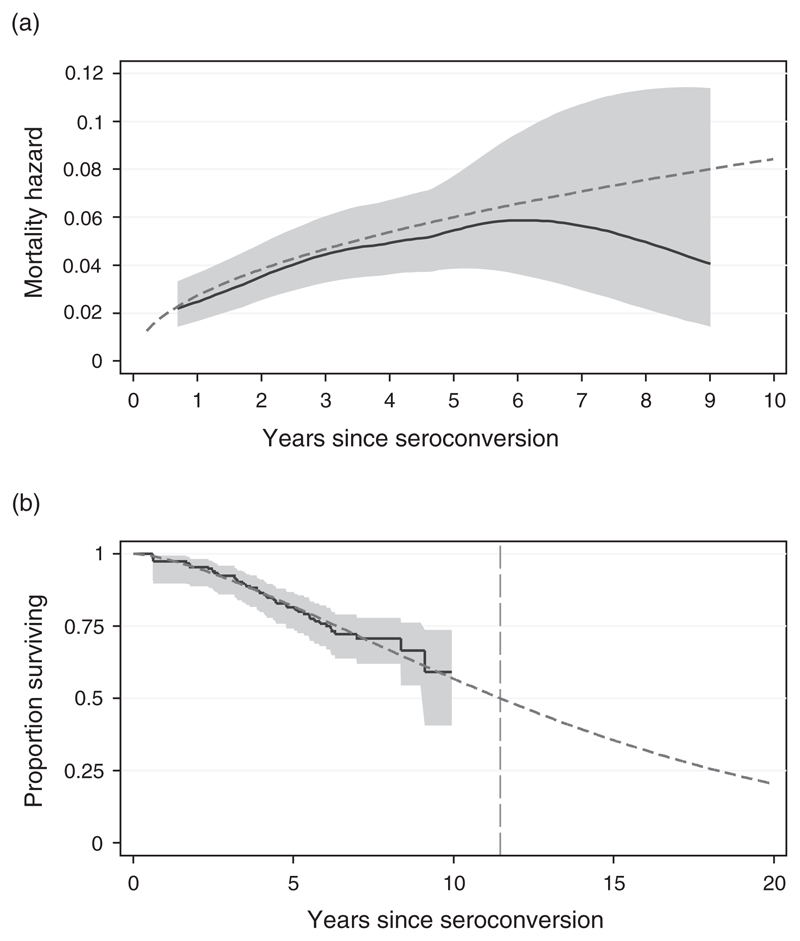
Analytical smoothing of the hazard rates (Fig. 1a) indicates that the risk of dying increases steadily with the time since infection, at least up to 6 years post-infection. After that, the smoothed hazard curve appears to level off, and eventually there is a downturn; however, the results for 6 plus years are based on only seven deaths observed during 120 person-years of follow-up, which means that confidence limits become too wide at this stage to define the shape with reasonable certainty. The fitted Weibull hazard curve was very close to the smoothed hazard curve for durations less than 6 years, and remained within the 95% confidence limits throughout the range for which data were available.
Fig. 1. Comparison of observed and model representation of mortality of seroconverters in Kisesa, 1994–2005.
(a) Mortality hazards.  95% confidence intervals;
95% confidence intervals;  smoothed hazard function;
smoothed hazard function;  Weibull fitted hazard function; (b) Proportion surviving, with out of sample prediction.
Weibull fitted hazard function; (b) Proportion surviving, with out of sample prediction.  95% confidence intervals;
95% confidence intervals;  survivor function;
survivor function;  Weibull fitted survivor function.
Weibull fitted survivor function.
The same maximum-likelihood fitted Weibull function was used to extend the observed survival curve to predict the median survival time for infected individuals in this population, producing an overall estimate of 11.5 years (95% CI 10.4–12.5; Fig. 1b). The extended curve implies that 20% of infected individuals would still be alive 20 years after infection; this does not reflect the downturn in mortality hazards apparent in Fig. 1a, as the theoretical Weibull curve implies mortality rates that increase steadily with time from infection.
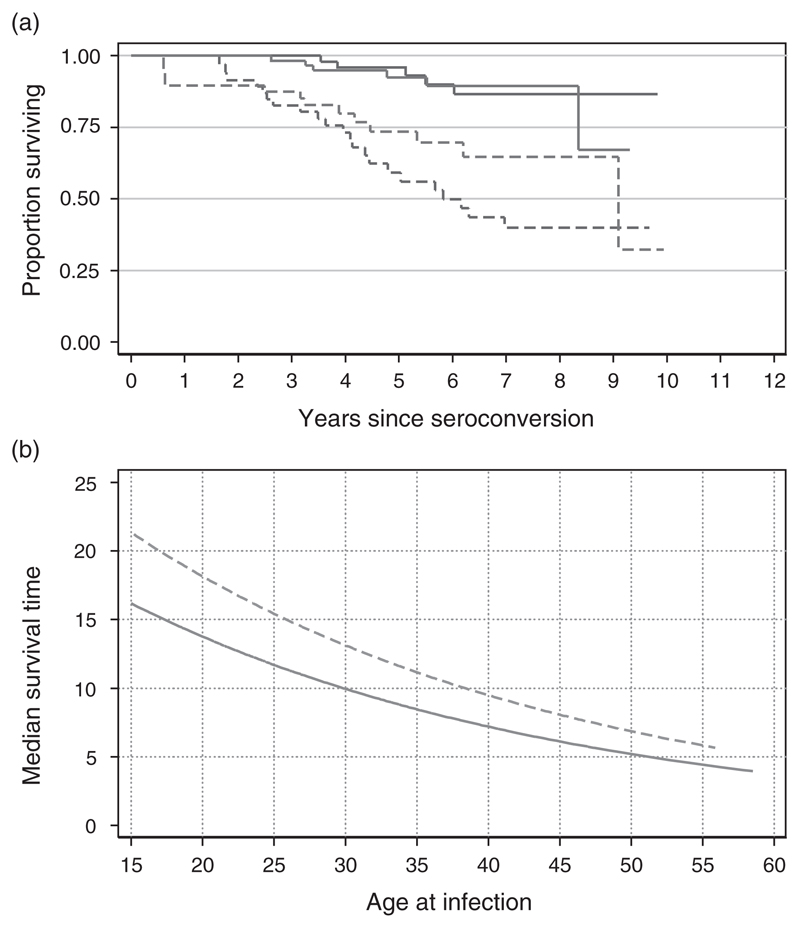
Figure 2a shows the Kaplan–Meier survival curves for seroconverters stratified by sex and age (<30, 30+); clearly those infected before age 30 years have much more favourable survival patterns. Log rank tests comparing these survival distributions indicate that the relationship between age at infection and survival is statistically significant (χ2(1) = 23.7, P < 0.0001 for both sexes), whereas the relationship of survival to sex is much weaker (χ2(1) = 2.5, P = 0.11) for all ages, although older women may have a slight advantage over older men (χ2(1) = 3.3, P = 0.07) for those infected at ages 30 years plus.
Fig. 2. Effect of sex and age on survival post-infection, Kisesa, 1994–2005.
(a) Observed proportion surviving by time since infection, stratified by broad age group and sex.  Male < 30 years;
Male < 30 years;  male > 30 years;
male > 30 years;  female < 30 years;
female < 30 years;  female > 30 years. (b) Predicted median survival time by sex and exact age at infection, based on Weibull regression.
female > 30 years. (b) Predicted median survival time by sex and exact age at infection, based on Weibull regression.  Male;
Male;  female.
female.
Parametric regression based on the Weibull function was used to model the impacts of age, sex, time of infection and length of seroconversion interval (Table 2). The crude regression models confirm that age at infection has a dramatic impact on survival, causing the mortality hazard to increase by 6% for each additional year of age. Sex has a marginal effect, with slightly lower mortality rates for women than men, although this does not quite attain statistical significance. The crude effects of the calendar year of infection and interval length are smaller than the effect of sex, and are statistically less significant. After allowing for the effects of sex and age at infection, the time of infection and length of seroconversion interval are unstable and lose statistical significance.
Table 2. Determinants of survival of seroconverters in Kisesa, 1994–2005: hazard ratios for covariates estimated using Weibull parametric regression.
| Crude |
Adjusted for sex and age |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable categories | N | HR | Pr>|z| | 95% CI | HR | Pr>|z| | 95% CI | ||
| Age at infection (continuous) | 369 | 1.06 | 0.000 | 1.03 | 1.09 | 1.06 | 0.000 | 1.03 | 1.10 |
| Sex | |||||||||
| male | 184 | 1 | 1 | ||||||
| female | 185 | 0.64 | 0.145 | 0.35 | 1.17 | 0.62 | 0.128 | 0.34 | 1.15 |
| Time of infection | |||||||||
| before 2000 | 190 | 1 | 1 | ||||||
| after 2000 | 179 | 0.96 | 0.922 | 0.39 | 2.34 | 0.61 | 0.484 | 0.16 | 2.40 |
| Seroconversion interval | |||||||||
| < 3.5 yrs | 301 | 1 | 1 | ||||||
| ≥ 3.5 yrs | 68 | 1.03 | 0.947 | 0.49 | 2.15 | 0.91 | 0.868 | 0.29 | 2.84 |
CI Confidence Interval; HR Hazard Ratio; Pr>|z| two tailed probability that HR is different from 1.0
The numerical values of the Weibull parameter estimates are given by:
λ = exp(−5.383 + 0.0544 * age − 0.464 * sex)
ϕ = exp{0.516}
where age (a continuous variable) is the estimated age at infection for the individual, and sex is a categorical variable coded zero for men, one for women.
Figure 2b shows how predicted median survival varies with sex and age, based on this regression model. The expected median survival time for those infected at age 15 years is over 15 years, three times as long as the median survival for those infected at ages 60 years and over.
The model predictions can also be used to calculate expected survival patterns in broad age groups, for comparison with directly observed patterns. For all individuals infected before the age of 30 years, predicted median survival is 14.5 years, and the first quartile survival time is 8.6 years. For those infected after the age of 30 years, predicted median survival is 8.5 years, the first quartile is 5.0 years. These predictions are somewhat more favourable than the equivalent measures in the few cases in which these have been directly observed, but whenever 95% confidence limits can be estimated from the data, the model results lie within this range.
Survival patterns among HIV-negative participants can be compared with those of seroconverters, after allowing for their initial age distribution: 91% of uninfected persons survive for 9 years (95% CI 80%-96%) compared to 67% of seroconverters (95% CI 54% to 76%). The crude death rate for seroconvertors is 49 per thousand (95% CI 37 to 67), for negative participants adjusted to the same age distribution as seroconverters it is 11 per thousand (95% CI 9 to 12), giving an age adjusted rate ratio of 4.7 (95% CI 2.7 to 8.1).
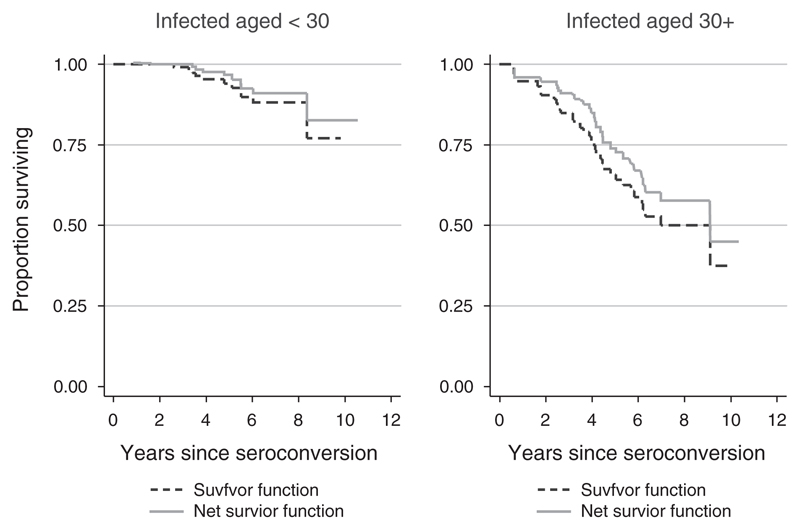
Among HIV-negative participants, mortality also increases with age, as older individuals are more likely to suffer from degenerative diseases of old age. We therefore investigated how much of the difference in survival between young and old might be accounted for by non-HIV-related mortality. The ‘net’ survival functions (survival after removing non-HIV-related mortality) were constructed for those aged under or over 30 years at infection. The results are shown in Fig. 3. The chi-squared statistic summarizing the difference between the ‘net’ life tables for seroconverters aged under and over 30 years at the time of infection is 20.0, only marginally lower than the equivalent statistic comparing the ‘gross’ life tables for young and old seroconverters, 23.7, and still highly significant (P < 0.0001). Clearly, the difference between ‘net’ survival for those infected at ages under 30 years, and ‘net’ survival for those infected at older ages remains very large, implying that it is not background, non-HIV-related mortality that accounts for the impact of age at infection.
Fig. 3. Proportion surviving by time since infection net of background mortality, by age at infection, Kisesa, 1994–2005.
Discussion
From direct observation of deaths occurring to seroconverters in the Kisesa cohort, we found that 67% (95% CI 54–76%) were still alive 9 years after their estimated infection date. This is appreciably higher than the corresponding estimates for the two Ugandan cohorts: Masaka (51%) and Rakai (50%) [8,9]. At 9 years post-infection, however, only 10 seroconverters remain under observation in Kisesa, so the margin of uncertainty in this estimate is wide, comparing proportions surviving at 5 years post-infection, the results are much closer: Kisesa 81% (95% CI 74–87%); Masaka 80%, Rakai 80%. The observed proportions surviving in Kisesa are fairly close to those observed in a pooled analysis of HIV survival in high income countries [7] before HAART, in which the proportion surviving 5 years was 85% (95% CI 84–86%), and the proportion surviving 9 years was 66% (95% CI 64–68%).
The Kisesa results show the same large impact of age at infection previously noted in high income country studies [7] and confirmed in the pooled analysis of low and middle income country data published in this volume [18]. In crude terms, individuals infected at age 30 years and over experience 4.6 times the mortality hazard of those infected under 30 years; a detailed analysis suggests that the overall hazard increases by approximately 6% for every year of age at infection.
Participation rates in the demographic rounds that are used to ascertain survival are very high: less than 1% of adults ever-resident in the study site were censored because they were lost to follow-up, so it is unlikely that the analysis is biased by the large-scale omission of deaths. As demographic rounds were at most 8 months apart, imputation of dates for the 6% of deaths with unknown or misreported dates would result in a maximum displacement of 4 months from the actual date of death for any individual, the direction of this displacement should be random and is therefore not expected to bias results for the population as a whole.
High mobility rates make it likely that some of those who initially tested negative may have become infected while resident in Kisesa, but moved out of the study area before their infection could be identified by testing in a subsequent serosurvey. This would only affect the estimates of survival post-infection if unidentified seroconverters had different survival patterns from those who remained resident in Kisesa long enough to attend a subsequent serosurvey.
We constructed ‘net’ survival curves from which non-HIV mortality had been eliminated, to see whether background (non-HIV-related) mortality, which also increases rapidly with age, could be responsible for the difference by age at infection. The adjustments made to derive the ‘net’ survival curve for those infected before the age of 30 years were much smaller than the corresponding adjustment to the survival curve of those infected over the age of 30 years, but this was not enough to account for the overall mortality difference associated with age at infection, and the ‘net’ survival function for those infected after the age of 30 years remained significantly steeper than the corresponding curve for those infected before 30 years.
We investigated several factors that could potentially bias our results. Previous research had shown that HIV-infected individuals were highly mobile [19], so active follow-up of those seroconverters who left Kisesa could potentially lower survival estimates, if those who left were likely to die shortly after leaving the area; this might arise if treatment-seeking was an important reason for leaving the ward. Out-of area follow-up marginally increased estimated survival times, although the effect was not statistically significant. Retrospective reporting (in round 20) of the date of death for those who left was very incomplete, another 44 migrant seroconverters left the study area for whom no survival information was obtained. We concluded that it was better to continue censoring the experience of permanent migrants at the time they finally left the ward.
Our analysis includes 68 seroconverters who have had a long interval (over 3.5 years) between the last negative and the first positive test dates, as a result of not attending all the serosurveys for which they were eligible. This opens up the possibility of bias, as any individual becoming infected and dying within such a long interval would not be included in our analysis, although left truncation should minimize this bias in theory. Excluding seroconverters who missed a survey has no significant impact on our overall survival analysis. As the serosurveys in Kisesa are less frequent than in other studies [6], we also investigated the effects of long intervals on survival, using a continuous regression on seroconversion interval length, but again, no significant impact was detected.
Exclusion from the analysis of young prevalent cases would have the effect of slightly decreasing survival times. We have chosen to include these individuals, because without them the age distribution of seroconverters would not reflect the real situation in Kisesa. Including this group implies that 19% of infections occur below the age of 20 years; excluding them would leave only 8% of infections in this age group. We investigated whether these young prevalent cases might have been infected through mother-to-child transmission of the virus rather than sexual transmission, but none were found to have had an HIV-infected mother. The relatively young distribution of age at infection may explain why survival times are longer in Kisesa than in the two Ugandan sites [8,9], where the proportion of infections under 20 years is approximately 12%.
If we had used conventional estimates of seroconversion times (halfway between the last negative and the first positive test) the main effect would be to narrow the time range in which deaths were observed to between 1.0 and 8.7 years after seroconversion compared with 0.6–9.1 years when imputed times are used based on the average age, sex and area-specific incidence rates to allow for interval censoring. The narrower age range for deaths increases proportions surviving at shorter durations and decreases proportions surviving at longer durations. The smoothed hazard curve becomes more pronouncedly n-shaped, and therefore less like the Weibull model, the theoretical shape that has been proposed for representing AIDS mortality [4].
The confidence interval width in Fig. 1a suggests that the downturn in hazard rates at long durations could be an artefact of the small number of individuals with long follow-up duration, but it might also indicate that there are significant frailty effects caused by factors we have not allowed for. Kisesa cohort investigations have not yet included viral typing or human genetic factors that may introduce heterogeneity in mortality responses among infected individuals that might explain the downturn in hazards [20,21]. A similar levelling off in the annual risks of dying 8 years after seroconversion was reported in a study from the pre-HAART era in high income countries [22].
The dataset used for this analysis, compiled after the 19th round of demographic follow-up in 2005, represents the last opportunity for studying the natural history of HIV in the Kisesa cohort, because a referral system for ART started shortly afterwards, and by March 2007 more than 100 residents of Kisesa ward had commenced treatment [23]. The current analysis will therefore form an important baseline against which we will be able to judge the success of the ART programme in prolonging the lives of those infected by HIV in this rural part of Tanzania.
Footnotes
Conflicts of interest: None.
References
- 1.UNAIDS. AIDS epidemic update, December 2006. [Accessed: April 2007]; Available at: http://data.unaids.org/pub/EpiReport/2006/2006_EpiUpdate_en.pdf.
- 2.Porter K, Zaba B. The empirical evidence for the impact of HIV on adult mortality in the developing world: data from serological studies. AIDS. 2004;18(Suppl. 2):S9–S17. doi: 10.1097/00002030-200406002-00002. [DOI] [PubMed] [Google Scholar]
- 3.Lopman BA, Barnabas R, Hallett TB, Nyamukapa C, Mundandi C, Mushati P, et al. Assessing adult mortality in HIV-1-afflicted Zimbabwe (1998–2003) Bull WHO. 2006;84:189–197. doi: 10.2471/blt.05.025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS Reference Group on Estimates, Modelling and Projections. Improved methods and assumptions for estimating the HIV/AIDS epidemic and its impact. AIDS. 2002;16:W1–W14. doi: 10.1097/00002030-200206140-00024. [DOI] [PubMed] [Google Scholar]
- 5.Anzala OA, Nagelkerke NJ, Bwayo JJ, Holton D, Moses S, Ngugi EN, et al. Rapid progression to disease in African sex workers with human immunodeficiency virus type 1 infection. J Infect Dis. 1995;171:686–689. doi: 10.1093/infdis/171.3.686. [DOI] [PubMed] [Google Scholar]
- 6.Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JAG. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU concerted action. Time from HIV-1 sero-conversion to AIDS and death before the widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 8.Van der Paal L, Shafer LA, Todd J, Mayanja BN, Whitworth JAG, Grosskurth H. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS. 2007;21(Suppl. 6):S21–S29. doi: 10.1097/01.aids.0000299407.52399.05. [DOI] [PubMed] [Google Scholar]
- 9.Lutalo T, Gray RH, Wawer M, Sewankambo N, Serwadda D, Laeyendecker O, et al. Survival of HIV infected treatment naive persons with known dates of seroconversion in Rakai, Uganda. AIDS. 2007;21(Suppl. 6):S15–S19. doi: 10.1097/01.aids.0000299406.44775.de. [DOI] [PubMed] [Google Scholar]
- 10.Mwaluko G, Urassa M, Isingo R, Zaba B, Boerma JT. Trends in HIV and sexual behaviour in a longitudinal study in a rural population in Tanzania, 1994–2000. AIDS. 2003;17:2645–2651. doi: 10.1097/00002030-200312050-00012. [DOI] [PubMed] [Google Scholar]
- 11.Urassa M, Boerma JT, Isingo R, Ngalula J, Ng’weshemi J, Mwaluko G, Zaba B. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;15:2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- 12.Boerma JT, Urassa M, Nnko S, Ng’weshemi JZL, Isingo R, Zaba B, Mwaluko G. Socio-demographic context of the AIDS epidemic in a rural area in Tanzania with a focus on people’s mobility and marriage. Sex Transm Infect. 2002;78(Suppl. 1):97–105. doi: 10.1136/sti.78.suppl_1.i97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom S, Urassa M, Isingo R, Ng’weshemi J, Boerma J. Community effects on the risk of HIV infection in rural Tanzania. Sex Transm Infect. 2002;78:261–266. doi: 10.1136/sti.78.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Law CG, Brookmeyer R. Effect of mid-point imputation on the analysis of double censored data. Stat Med. 1992;11:1569–1578. doi: 10.1002/sim.4780111204. [DOI] [PubMed] [Google Scholar]
- 15.Mwita W, Urassa M, Isingo R, Ndege M, Marston M, Slaymaker E, et al. HIV prevalence and incidence in rural Tanzania: results from 10 years of follow-up in an open cohort study. J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e31815a571a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2nd. New York: Springer; 2003. pp. 167–168. [Google Scholar]
- 17.Marston M, Todd J, Glynn JR, Nelson K, Rangsin R, Lutalo T, et al. Estimating ‘net’ HIV-related mortality and the importance of background mortality rates. AIDS. 2007;21(Suppl. 6):S65–S71. doi: 10.1097/01.aids.0000299412.82893.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd J, Glynn JR, Marston M, Lutalo T, Biraro S, Mwita W, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS. 2007;21(Suppl. 6):S55–S63. doi: 10.1097/01.aids.0000299411.75269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishamawe C, Vissers DC, Urassa M, Isingo R, Mwaluko G, Borsboom GJ, et al. Mobility and HIV in Tanzanian couples: both mobile persons and their partners show increased risk. AIDS. 2006;20:601–608. doi: 10.1097/01.aids.0000210615.83330.b2. [DOI] [PubMed] [Google Scholar]
- 20.Kanari Y, Clerici M, Abe H, Kawabata H, Trabattoni D, Capito SL, et al. Genotypes at chromosome 22q12–13 are associated with HIV-1-exposed but uninfected status in Italians. AIDS. 2005;19:1015–1024. doi: 10.1097/01.aids.0000174447.48003.dd. [DOI] [PubMed] [Google Scholar]
- 21.Peeters M, Toure-Kane C, Nkengasong JN. Genetic diversity of HIV in Africa: impact on diagnosis, treatment, vaccine development and trials. AIDS. 2003;17:2547–2560. doi: 10.1097/01.aids.0000096895.73209.89. [DOI] [PubMed] [Google Scholar]
- 22.Koblin BA, van Benthem BH, Buchbinder SP, Ren L, Vittinghoff E, Stevens CE, et al. Long-term survival after infection with human immunodeficiency virus type 1 (HIV-1) among homosexual men in hepatitis B vaccine trial cohorts in Amsterdam, New York City, and San Francisco, 1978–1995. Am J Epidemiol. 1999;150:1026–1030. doi: 10.1093/oxfordjournals.aje.a009926. [DOI] [PubMed] [Google Scholar]
- 23.TAZAMA Project. Phase 2 implementation plan and budget for the monitoring and evaluation component. Report to GFATM. National Institute for Medical Research; Mwanza, Tanzania: 2007. [Google Scholar]