Abstract
The major nutrients available to human colonic Bacteroides species are glycans exemplified by pectins, a network of covalently linked plant cell wall polysaccharides containing galacturonic acid (GalA). Metabolism of complex carbohydrates by the Bacteroides genus is orchestrated by polysaccharide utilisation loci or PULs. In Bacteroides thetaiotaomicron, a human colonic bacterium, the PULs activated by the different pectin domains have been identified, however, the mechanism by which these loci contribute to the degradation of these GalA-containing polysaccharides is poorly understood. Here we show that each PUL orchestrates the metabolism of specific pectin molecules, recruiting enzymes from two previously unknown glycoside hydrolase (GH) families. The apparatus that depolymerizes the backbone of rhamnogalacturonan-I (RGI) is particularly complex. This system contains several GHs that trim the remnants of other pectin domains attached to RGI, while nine enzymes contribute to the degradation of the backbone comprising a rhamnose-GalA repeating unit. The catalytic properties of the pectin degrading enzymes are optimized to protect the glycan cues that activate the specific PULs ensuring a continuous supply of inducing molecules throughout growth. The contribution of Bacteroides spp. to the metabolism of the pectic network is illustrated by cross-feeding between organisms.
The human gut microbiota (HGM) impacts on host physiology and health2,3. Understanding the mechanisms of nutrient acquisition by the HGM, exemplified by glycan metabolism4–6, underpins the development of probiotic and prebiotic strategies that maximize human health. While glycan acquisition by human colonic Bacteroides species is well established7–10, it should be emphasised that Firmicutes are more abundant in the HGM of Western populations, however, the mechanism by which they metabolise complex carbohydrates is less well understood4. Indeed, it is likely that Firimicutes make a substantial contribution to the degradation of dietary and host glycans in the HGM. The glycan degrading systems of Bacteroidetes are encoded by polysaccharide utilization loci (PULs) that are activated by the target carbohydrate4. These systems comprise surface glycan binding proteins (SGBPs), outer membrane oligosaccharide transporters; SusC and SusD homologues (SusCH and SusDH, respectively), and surface and periplasmic carbohydrate active enzymes (CAZymes) that are grouped into sequence based families in the CAZy database11. Relevant to this work are glycoside hydrolase (GH) and polysaccharide lyase (PL) families12.
Pectins are d-galacturonic acid (d-GalA) rich plant cell wall polysaccharides that are abundant in fruits and vegetables. The two major pectins (see1 for review) are homogalacturonan (HG) and rhamnogalacturonan-I (RGI) (Fig. 1a). HG comprises α-1,4-linked d-GalA and the backbone of RGI is a repeating unit of the disaccharide α-1,2-l-rhamnose (Rha)-α-1,4-d-GalA. Depending on the plant species, the RGI backbone is decorated with galactans (β-1,4-d-galactose (D-Gal) units) and/or arabinans (α-1,5-linked l-arabinofuranose (l-Araf) units with additional l-Araf side-chains)13. The backbones of HG and RGI are covalently linked14. Although individual microbial pectin degrading enzymes have been described15, the mechanism by which these biocatalysts participate in the concerted degradation of intact pectin remains opaque. Bacteroides thetaiotaomicron, a member of the HGM, utilizes all known pectins structures and discreet PULs activated by these glycans have been identified16. Here we have characterized the function of PULs associated with pectin metabolism, and explored how they contribute to interactions within the HGM foodweb. The data show how these loci coordinate the complex degradative interactions between the backbone and oligosaccharide decorations of these acidic polysaccharides.
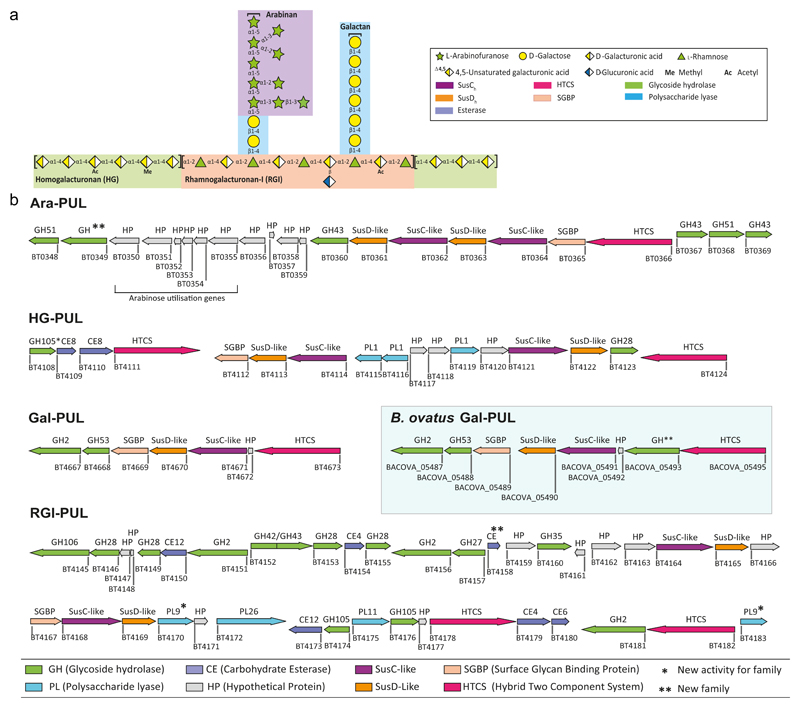
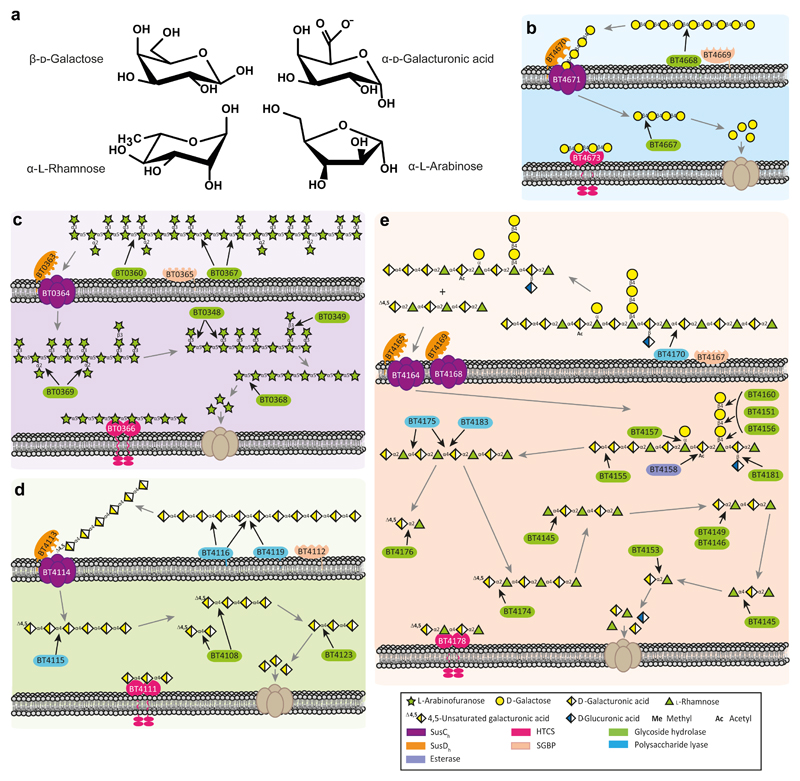
Figure 1. Genomic organization of pectin PULs.
a, Schematic of pectin structure showing the different polysaccharides highlighted with different coloured backgrounds. The respective linkages and monosaccharide composition are represented according to the Symbol Nomenclature for Glycans system50. b, genes encoding proteins of known or predicted functionalities are colour coded. GHs, CEs and PLs located in a known CAZy family are indicated by GHXX, CEXX or PLXX where XX indicates the number of the family.
Results
The PULs that orchestrate pectin degradation were identified from transcriptomic data16. PULs upregulated in response to arabinan (bt0348-bt0369, Ara-PUL), galactan (bt4667-bt4673, Gal-PUL), RGI backbone (bt4145-bt4183, RGI-PUL) and HG (bt4108-bt4124, HG-PUL) were identified (Fig. 1b). To determine the mechanisms by which these loci mediate pectin degradation, the biochemical functions of recombinant proteins encoded by the PULs were determined (Supplementary Tables 1-5).
Initial degradation of the pectins by B. thetaiotaomicron is mediated by endo-acting CAZymes on the surface of the bacterium (Fig. 2c and Supplementary Fig. 1). These enzymes are essential for pectin utilization as they generate glycans with an appropriate degree of polymerization (DP) for transport into the periplasm17. Consistent with this premise, deletion of the genes encoding the single outer membrane endo-acting enzymes encoded by RGI-PUL (BT4170) and Gal-PUL (BT4668), Supplementary Fig. 2 and Fig. 2c, prevented growth on the respective pectin (Fig. 2a). The surface location of these enzymes was consistent with whole cell assays of B. thetaiotaomicron under aerobic conditions (Fig. 2b), which report only the activity of surface proteins. To explore the function of the rhamnogalacturonan lyase BT4170, a key component of the RGI degrading apparatus, the crystal structure of the enzyme was determined in complex with ligands. The data (Supplementary Fig. 3) showed that the catalytic apparatus of BT4170 and a HG lyase (Pel9A, 1RU4) both located in family PL9, comprising a Brønstead base (Lys285 in BT4170) and a calcium, was conserved. Specificity determinants were identified in subsites distal to the active site, explaining why Pel9A and BT4170 target distinct substrates (see Supplementary Discussion and Supplementary Fig. 3).
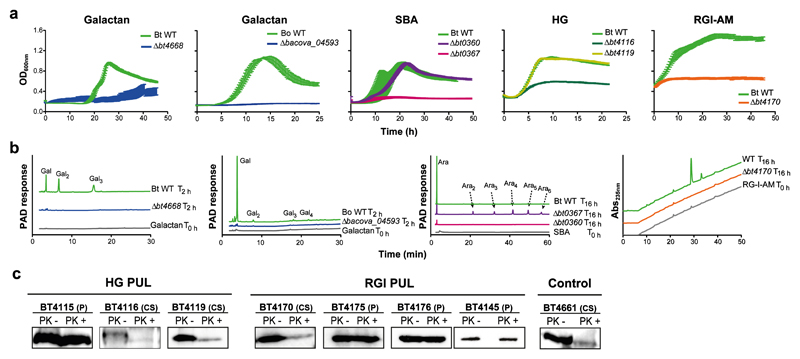
Figure 2. Depolymerization of pectins at the cell surface of B. thetaiotaomicron cell surface.
a, Growth of wild-type and mutants of B. thetaiotaomicron (BtWT and Δbtxxxx) or B. ovatus (BoWT and Δbacovaxxxx) in minimal media containing the indicated pectic polysaccharide; HG, homogalacturonan; SBA, sugar beet arabinan; RGI-AM, rhamnogalacturonan I backbone from Arabidopsis mucilage (biological replicates, n=3, error bars denote s.e.m). b, BtWT, BoWT and mutants lacking functional outer membrane enzymes were incubated with appropriate polysaccharides in aerobic conditions for the times indicated. Under these conditions substrate is only available to the surface enzymes. Products released from the glycans were monitored by High performance anion exchange chromatography (HPAEC) with pulsed amperometric detection (PAD) or UV detection at 235 nm (Abs235nm). The degree of polymerisation of the peaks corresponding to the galactose (Gal) and arabinose (Ara) oligosaccharides are shown in subscript numbers. c, Western blot detection of selected B. thetaiotaomicron enzymes encoded by the HG-PUL and RGI-PUL after treatment with proteinase K (PK+) or untreated (-). BT4661 is a known surface glycan binding protein (control)7. The cellular localization is indicated as periplasmic (P) or cell surface (CS). The example is from biological replicates n=3. The full western blots are shown in Supplementary Fig. 1.
Ara-PUL and HG-PUL each encode two surface enzymes. The enzymes derived from Ara-PUL, BT0360 and BT0367, are α1,5-arabinanases that display endo- and endo-processive activity, respectively (Supplementary Fig. 4 and Supplementary Discussion). Only Δbt0367 led to the loss of the arabinan utilization phenotype (Fig. 2a). Gene deletion studies showed that of the two surface PLs (BT4116 and BT4119, Supplementary Fig. 4 and Supplementary Table 2) encoded by HG-PUL, only BT4116 was essential for growth on HG (Fig. 2a). The functional significance of BT0360 and BT4119 are unclear, but may reflect the targeting of substrates not evaluated here.
Gene deletion studies explored the functional significance of the SusCH/SusDH pairs encoded by each pectin PUL (Supplementary Fig. 5). In HG-PUL, which contains two SusCH/SusDH pairs, only the Δbt4114 mutant displayed no growth on HG, indicating that BT4114 plays a key role in the import of this pectic glycan. Similarly, the Ara-PUL encodes two pairs of SusCH/SusDH transporters (BT0361/BT0362 and BT0363/BT0364)16. Deletion of bt0364, but not bt0362, prevented growth on arabinan. This indicates that only the BT0363/BT0364 complex is capable of transporting arabinooligosaccharide products. The rationale for the presence of two SusCH/SusDH pairs in the HG-PUL and Ara-PUL remains unknown, but likely increases access to additional pectins.
SGBPs contribute to glycan degradation by bringing substrates into proximity of membrane bound enzymes8. Here a single SGBP encoded by each PUL was identified and shown to be specific for the target polysaccharide (Supplementary Table 5). Only SusDHs encoded by Gal-PUL and HG-PUL displayed affinity for their cognate glycans (Supplementary Table 5). The lack of glycan recognition by SusDHs associated with the arabinan and RGI degrading systems suggests that the corresponding SusCH partner is required for ligand recognition. Recent structural data demonstrate that the tight association of SusCH-DH pairs18, supporting the concept that initial ligand recognition can require participation of both protein partners.
The oligosaccharides imported into the periplasm were degraded by GHs and PLs. The oligosaccharides generated from galactan and arabinan were depolymerized exclusively by exo-GHs, with HG and the RGI backbone by endo-PLs and exo-GHs (Supplementary Fig. 2 and 6).
With respect to galactan degradation only a single GH2 β1,4-galactosidase (BT4667) depolymerized galactooligosaccharides generated by the surface endo-galactanase (Fig. 2b and Supplementary Table 3). Surprisingly the Δbt4667 mutant displayed no growth defect on galactan (Supplementary Fig. 5). This may reflect an element of redundancy within the large number of predicted B. thetaiotaomicron β-galactosidases19.
Periplasmic degradation of arabinan-derived oligosaccharides was mediated by three exo-α-l-arabinofuranosidases (Supplementary Fig. 6). BT0369 removed α-1,2-l-arabinofuranose side chains20. Here we demonstrate the GH51 enzymes BT0348 and BT0368 target arabinan side chains, likely α-1,3-arabinofuranosyl linkages, and the backbone α-1,5-arabinofuranosyl linkages, respectively (Supplementary Table 4). BT0349 released β-l-arabinose from an arabinan derived oligosaccharide (Supplementary Fig. 7 and Supplementary Table 7). The enzyme reveals a previously unknown GH family now designated GH146 (Supplementary Fig. 8 and Supplementary Discussion).
RGI released from the pectin network contains remnants of arabinan, galactan and HG. Prior to RGI backbone depolymerisation these accessory structures must be removed, explaining why RGI-PUL is so complex. To characterise these accessory enzymes we used RGI from potato galactan (RGI-P), which contains many of these remnants. Galactan substitutions were cleaved from the RGI-P backbone by the synergistic action of three exo-β-1,4-galactosidases, BT4151, BT4156 and BT4160 (Supplementary Table 1). BT4160 targeted galactooligosaccharides, while the other two enzymes released galactose only from RGI-P. The lack of functional arabinofuranosidase genes in RGI-PUL likely reflects the role of single β1,4-D-Gal units in linking arabinan chains to the RGI backbone13. Enzyme cocktail data indicate that BT4151 and BT4156 play a pivotal role in exposing the backbone of RGI to enzymatic attack (Supplementary Fig. 9). RGI-PUL also encodes the esterase BT4158 (Supplementary Table 1), which releases acetyl groups from D-GalA in the RGI backbone, was also shown to be important for the depolymerisation of the glycan (Supplementary Fig. 9). A GH28 α-d-galacturonidase (BT4155), which targets HG (Supplementary Table 1), removed D-GalA from RGI-P but not from the glycan in Arabidopsis mucilage (RGI-AM), which contains no HG decorations. The crystal structure of BT4155 (Supplementary Fig. 10) revealed the expected β-helix for a GH28 enzyme21. In the center of the helix is a pocket that houses three carboxylate residues that comprise the predicted catalytic apparatus based on conservation with other GH28 enzymes22 and mutagenesis data (Supplementary Table 6). The pocket extends into a channel-like structure that likely accommodates the conformation adopted by HG but not the RGI backbone.
In addition to enzymes classically associated with pectin degradation, RGI-PUL encodes BT4157, which is located in the apparent “non-pectinase family” GH27. The enzyme (Supplementary Table 1) was shown to be a α-galactosidase, which likely targets single α-galactose units that decorate the RGI backbone from Okra plants23. Another example of enzyme diversity is the β-D-glucuronidase activity displayed by BT4181 against sugar beet arabinan in which the RGI backbone is known to contain GlcA24 (Supplementary Fig. 6). It is evident, therefore, that the pectin degrading systems are able to accommodate diversity in the fine-chemistry of RGI structures from a variety of plants.
In contrast to exo-cleavage of arabinan and galactan, the backbone of RGI and HG were initially cleaved by endo-PLs and the products depolymerized by exo-GHs (Fig. 1 and Supplementary Fig. 2 and 6). The different degradative strategy likely reflects the high DP of the imported RGI- and HG-derived oligosaccharides compared to the neutral glycans. Thus, the initial concentration of available substrate for exo-GHs is low, but is increased by the endo-PLs. RGI-PUL encodes three periplasmic PLs, respectively. Of particular note is BT4175, which was shown to accommodate glycans appended to backbone rhamnose units (Supplementary Fig. 2), ensuring that cleavage of the (Rha-GalA)n polymer occurred in concert with, and not subsequent to, side-chain removal.
The β-elimination of the RGI and HG backbone by the PLs generated Δ4,5-GalA. The unsaturated residues were removed from RGI oligosaccharides with a DP of 2 or ≥4 by BT4176 or BT4174, respectively (Supplementary Table 1); and HG oligosaccharides by BT4108 (Supplementary Table 2), which expands the activity for the GH105 family. BT4108 products were then depolymerized to GalA by the exo-α-galacturonidase BT4123 (Supplementary Table 2 and Supplementary Fig. 6). The RGI-AM oligosaccharides were degraded through the successive action of a RGI-specific GH106 α-l-rhamnosidase (BT4145) and one of three GH28 rhamnogalacturonidases (BT4146, BT4153 and BT4149) that target [d-GalAp-α-1,2-l-Rhap]n with a DP of 2, ≥2 or ≥4, respectively (Supplementary Table 1). BT4145 cleaved rhamnosidic linkages through an inverting mechanism (Supplementary Fig. 11). The biological rationale for galacturonidases that target substrates with different DPs is unclear. Surprisingly deletion of BT4145 only extended lag phase (Supplementary Fig. 5), likely reflecting the slow but complete degradation of RGI-AM by the PLs and Δ4,5-unsaturated-α-rhamnogalacturonidases.
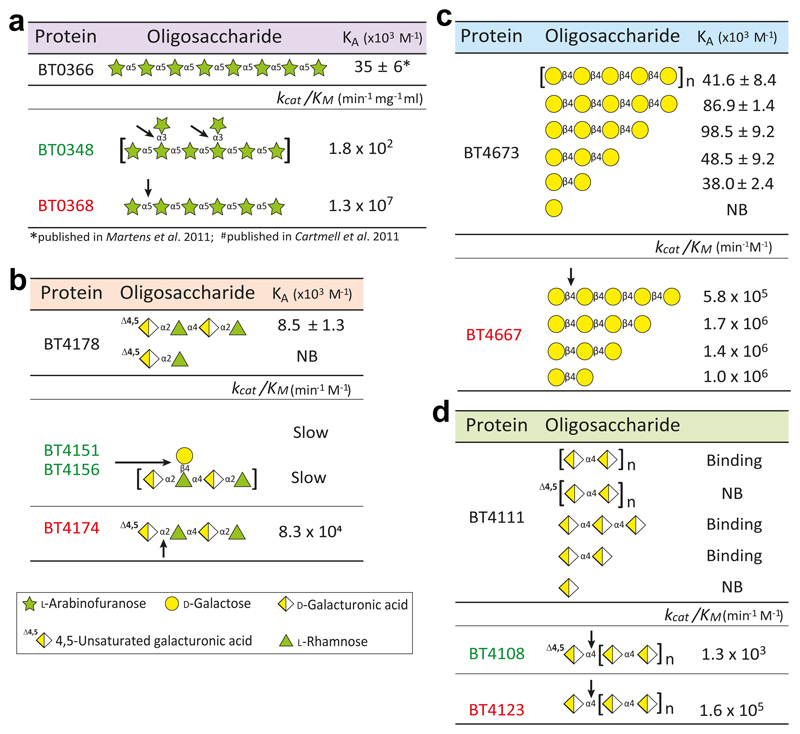
The ligands that activate the pectin degradative system were explored. Previously arabinooligosaccharides with DP ≥6 were shown to activate Ara-PUL16. Here we determined ligands that bound and activated the hybrid two component system (HTCS) of the pectin PULs. The data (Supplementary Table 5 and Supplementary Fig. 12) demonstrate that the HTCS of Ara-PUL bound linear but not decorated arabinan, and the sensor of the Gal-PUL HTCS (BT4673) recognised small galactooligosaccharides. Only the oligosaccharide Δ4,5GalA-α-1,2-Rha-α-1,4-GalA-α-1,2-Rha, a major limit product of the rhamnogalacturonan lyases, bound the HTCS (BT4178) that regulates RGI-PUL. Saturated RGI oligosaccharides failed to bind the sensor, indicating that unsaturation of the non-reducing terminal sugar is a recognition determinant. The HTCS that unregulates HG-PUL recognised only saturated HG-derived oligosaccharides. The mRNA levels of the susCH genes of the pectin PULs showed that activation of RGI-PUL resulted in a small up-regulation of HG-PUL and RGII-PUL1 (Supplementary Fig. 13). This may reflect the need to extract RGI from pectins networks through cleavage of adjacent HG segments, as a prelude to its degradation.
Exo-acting enzymes that target the remnants of RGI side chains and α-1,3-l-Araf units that decorate arabinan were substantially less active than GHs that depolymerised the backbone of the respective glycans (Supplementary Tables 1 and 4). Additionally, BT4108, which removed 4,5ΔGalA from HG-oligosaccharides generating the HTCS activating ligand was slow compared to the other enzymes that act on these pectins (Supplementary Tables 1 and 2). The biological rationale for this difference in catalytic competence, may reflect the need to protect the inducing ligand (Fig. 3), as proposed for the chondroitin sulfate utilization system25. Slow release of the side chain stubs or unsaturated uronic acids will block the rapid degradation of the backbone ensuring that there is continuous production of the activating molecules throughout growth on the respective glycan.
Figure 3. Signal molecule protection.
Each panel shows the affinities of the signal molecules to respective sensors (top) and the catalytic efficiency of key enzymes implicated in signal molecule degradation (bottom) for a, galactan, b, arabinan and c, RGI. The data were from technical replicates, n = 3.
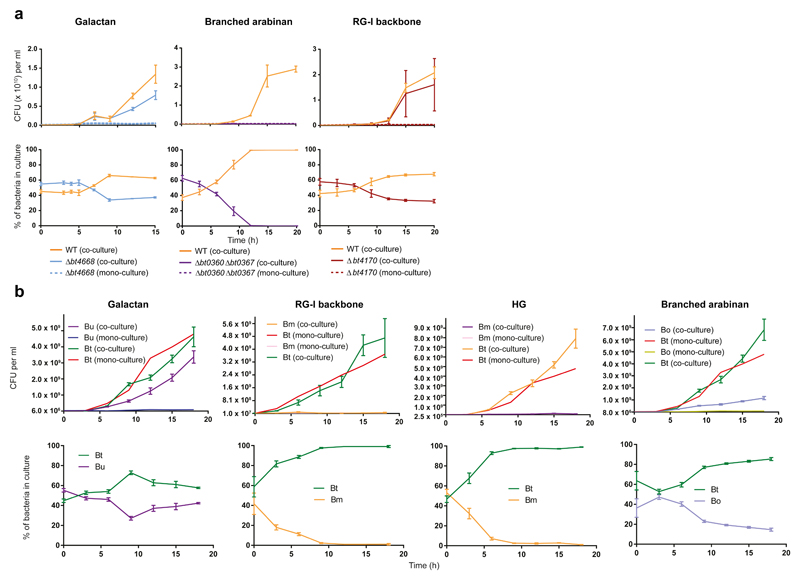
To explore pectin utilization by HGM Bacteroidetes growth of the different species on these GalA-rich polysaccharides was determined. The data showed that only B. ovatus, B. thetaiotaomicron and B. finegoldii utilised all the pectins, although the majority of other organisms could grow on at least some of these glycans (Supplementary Fig. 14 and Supplementary Table 8). Around 70% of the organisms grew on HG and galactan, while only four Bacteroides strains utilise the RGI backbone (RGI-AM). However, 56% and 100% of the strains unable to utilise RGI-AM and potato galactan, respectively, grew on the respective oligosaccharides, demonstrating that these organisms utilise pectin degradation products. A key question is the source of oligosaccharides available to these organisms. Evidence of cross-feeding was provided by mutants of B. thetaiotaomicron engineered to utilize only pectic oligosaccharides [lacking the surface endo-galactanase (Δbt4668) or RGI lyase (Δbt4170)], which grew on the cognate polysaccharide when co-cultured with wild type B. thetaiotaomicron (Fig. 4a). These data show that wild type B. thetaiotaomicron released polysaccharide breakdown products (PBP; Fig. 2f) into culture media, which were available to other organisms. This is consistent with B. uniformis (grows on galactooligosaccharides but not galactan in mono-culture) utilisation of the polysaccharide when co-cultured with wild type B. thetaiotaomicron (Fig. 4b). This pectin cross-feeding between B. thetaiotaomicron and other Bacteroides species, however, is variable. Although B. massiliensis utilize HG or RGI oligosaccharides, the bacterium failed to grow on the cognate polysaccharides when co-cultured with B. thetaiotaomicron (Fig. 4b). This likely reflects the large PBPs generated by B. thetaiotaomicron from these pectins, while B. massilensis appears to import only RGI and HG oligosaccharides with a low DP. The lack of cross-feeding of some pectin-derived PBPs is evident in arabinan utilization. B. ovatus and engineered B. thetaiotaomicron (Δbt0360/Δbt0367, lacking the two surface endo-arabinanases) both grew on arabinooligosaccharides but not arabinan. The two organisms, however, failed to utilise arabinan when co-cultured with wild type B. thetaiotaomicron (Fig 4b). Although B. thetaiotaomicron released arabinooligosaccharides (Fig. 2f), the high DP of these molecules (reflects slow activity of the surface endo-arabinanases20) may have prevented transport into the periplasm of these organisms.
Figure 4. Cross-feeding of polysaccharide breakdown products between Bacteroides species.
a, Wild type B. thetaiotaomicron (WT) and mutants of the bacterium lacking the key surface degrading enzymes for each polysaccharide were mono-cultured and co-cultured with the wild type bacterium as indicated. Samples were taken at different time points. The colony forming units of these samples were determined by plating onto rich media (top panels) and the ratio of each bacterium in the culture (bottom panel) was determine by qPCR with primers unique to each strain. Error bars represent the s.e.m of biological replicates (n=3). b, B. ovatus (Bo), B. massiliensis (Bm) and B. uniformis were mono-cultured or co-cultured with wild type B. thetaiotaomicron (Bt) using the same experimental approach described in a.
The genetic basis of pectin utilization among the Bacteroides was investigted. Loci corresponding to B. thetaiotaomicron Gal-PUL in other Bacteroides species (Supplementary Fig. 15) contained an additional ORF, which, in the B. ovatus Gal-PUL, encodes a β-galactosidase (BACOVA_05493) with a retaining mechanism (Supplementary Fig. 16) that belongs to a previously unknown CAZy family (assigned GH147). The enzyme was particularly active against galactohexaose and galactan (Supplementary Fig. 6 and Supplementary Table 3). The importance of this enzyme is illustrated by the severe growth defect displayed by Δbacova_05493 on galactan (Fig. 2). Whole cell assays with galactan revealed the accumulation of galactose and not galactooligosaccharides, as occurs in B. thetaiotaomicron, suggesting that BACOVA_05493 is located on the bacterial surface. This was confirmed by whole cell assays of Δbacova_05493, which revealed no products were generated from galactan. This not only demonstrates that BACOVA_05493 is a surface enzyme but shows that the endo-galactanase, BACOVA_05488, consistent with its very low activity (Supplementary Fig. 2), did not contribute to galactan degradation.
We examined whether the B. thetaiotaomicron pectin PULs provide a genetic model for Bacteroides utilisation of these glycans. A 16S-based phylogenetic tree of the Bacteroides species was constructed and the organisms labelled for PUL conservation and growth on the respective oligo- and polysaccharides (Supplementary Fig. 14). There is ≥80% agreement between the presence of a PUL and growth of the bacterium on the corresponding poly- or oligosaccharide. Growth, however, was apparent in some organisms without an equivalent PUL, showing that bacteria can deploy alternative pathways to utilise a particular glycan. There were also examples of the presence of the cognate PUL without growth of the corresponding pectin. PUL conservation was also not always congruent with the 16S phylogeny (Supplementary Fig. 14 and Supplementary Fig. 15). Thus, B. ovatus, B. xylanisolvens and B. caccae form a monophyletic group yet only B. xylanisolvens has a galactan PUL, while only B. caccae has lost the RGI PUL (Supplementary Fig. 14). The arabinan PUL of B. ovatus is fragmented and that of B. caccae absent (Supplementary Fig. 15a). B. egghertii, B. stercoris and B. clarus, also form a monophyletic group, but only B. stercoris has conserved the galactan PUL, while B. eggerthii has an arabinan PUL but has lost its RGI PUL (Supplementary Fig. 15d). A final example involves B. thetaiotaomicron and B. xylanisolvens, which are closely related species, yet have different arabinan PULs. The arabinan PUL of B. thetaiotaomicron is identical to B. cellulosilyticus/oleiciplenus/intestinalis, while the arabinan PUL of B. xylanisolvens is similar to B. egghertii. Our observations suggest that in the course of evolution Bacteroides rapidly gain and lose PULs that target different pectin structures.
Discussion
Combining biochemical properties and cellular location of the enzymes that target pectins, with growth profiles of mutants containing gene deletions in the appropriate PULs, enabled models for the metabolism of each pectic substructure, showing how the individual pathways are coordinated by B. thetaiotaomicron (Fig 5). The data revealed that 30 GHs and PLs are required to degrade the major pectin domains. Given that large numbers of enzymes are also required to degrade starch and the hemicelluloses, it is evident that plant glycan metabolism explains the extremely large repertoire of CAZyme gene clusters in colonic Bacteroides species.
Figure 5. Model of pectin utilization by B. thetaiotaomicron.
Chemical structures of the sugars in the major pectins (a). Models for degradation of galactan (b, blue), arabinan (c, purple), homogalacturonan (d, green) and rhamnogalacturonan I (e, peach) are displayed. The black arrows indicate the linkage cleaved by the various enzymes, while the grey arrows show the direction of the degradative pathway.
In contrast to several Bacteroides glycan degrading systems where the surface GHs act slowly and target infrequent linkages8,26,27, the equivalent enzymes of B. thetaiotaomicron that cleave galactan and the backbone of HG and RGI rapidly degrade their target polysaccharide. This likely reflects substrate accessibility to enzyme attack, and thus organisms with efficient surface enzymes that target accessible carbohydrates would be more competitive than bacteria in which the corresponding GHs or PLs were inefficient. This model (Fig. 5), however, does not apply to arabinan degradation where low activity of the surface enzymes was evident. This observation underpins the distinct mechanisms, distributive or selfish, by which glycans are metabolized by Bacteroides spp.
The RGI-PUL, in addition to orchestrating RGI backbone depolymerisation, removes remnants of linked polysaccharides and single sugar sidechains (Fig. 5). In contrast, PULs that mediate degradation of other branched glycans8,9,26,27 depolymerize both the respective side chains and backbone structures. We propose that B. thetaiotaomicron does not necessarily target intact pectin structures but are able to utilise pectin domains generated by other organisms in the HGM. The RGI backbone exposed through symbiotic relationships with other intestinal microorganisms, or upstream processing by other PULs of B. thetaiotaomicron, is likely to contain additional pectin remnants explaining the complexity of enzymes encoded by the RGI-PUL.
The cross-feeding experiments demonstrate that galactooligosaccharides released by B. thetaiotaomicron are used by other organisms. The utilization of other pectin-derived PBPs, however, is more restricted. These data illustrate how glycans are made available to the general community by primary degraders. Such cross-feeding has been observed between strains of Bacteroides cultured on fructans and soluble starch28, with the recipient organism providing a benefit to the glycan degrading bacterium29. Possible non-Bacteroides beneficiaries of pectin-derived cross-feeding within the HGM are Bifidobacterium species, which generally utilize PBPs rather than the polysaccharide27. Contrasting oligosaccharide utilisation profiles observed among Bacteroides spp. may allow for co-existence of species within the same niche targeting different components of the same glycans without competition.
The critical role played by a surface exo-β-galactosidase in galactan metabolism in some Bacteroides species is intriguing. This contrasts with all other Bacteroides glycan degrading systems described to date, which deploy endo-acting CAZymes3,8,9,27,30. These organisms may target galactooligosaccharides, albeit with a high DP, released by other organisms within the HGM, obviating that need for endo-cleavage. This indicates that different Bacteroides target galactans in distinct nutritional niches within the gut. The data also illustrate the risk associated with generating models for glycan degradation based solely on prediction of enzyme function through CAZy family assignment. To fully understand glycan metabolism a molecular genetics approach informed by biochemical and transcriptional data in harness with bioinformatics predictions is required.
This report provides a model for how the pectic network is metabolized by a Bacteroides species in the HGM. Surprising variations in selective glycan metabolism and the constitution of individual pathways were apparent. This contrasts with the extensive conservation of other PULs8–10,27. This suggests that organisms have adopted a variety of strategies to metabolise dietary pectins. A salient feature of pectin utilization is the elaboration of enzymes in the RGI-PUL, reflecting the requirement to remove remnants from other pectic glycans and the extraordinary number of enzymes deployed in depolymerizing the disaccharide backbone. Dissecting the mechanism of pectin degradation contributes to our understanding of the foodweb within the HGM.
Methods
Producing recombinant proteins
DNA fragments encoding predicted CAZymes and binding proteins were amplified without signal sequence by PCR using appropriate primers. The resultant DNA was then cloned into pET21a or pET28a/b linearized using appropriate restriction enzymes. The expressed protein included a His6-tag fusion at the N-terminus. Escherichia coli strains BL21(DE3) or TUNER were transformed with the plasmids and grown to mid-exponential phase before induction with 1 mM (BL21(DE3)) or 0.2 mM (TUNER) isopropyl β-D-galactopyranoside (IPTG), and the culture was grown for a further 5 h at 37 °C or 16 h at 16 °C, respectively. The recombinant proteins were purified to >90% electrophoretic purity by immobilized metal ion affinity chromatography (IMAC) using Talon, a cobalt-based matrix, with bound proteins eluted with 100 mM imidazole, describe previously 10. To generate seleno-methionine (Se-Met) proteins for structure resolution, E. coli cells were cultured as described previously 10, and the proteins were purified using IMAC as described above. For crystallization, the Se-Met proteins were further purified by size exclusion chromatography. After IMAC, fractions containing the purified proteins were buffer-exchanged, using PD-10 Sephadex G-25M gel-filtration columns (GE Healthcare), into 10 mM Na-HEPES buffer, pH 7.5, containing 150 mM NaCl and were then subjected to gel filtration using a HiLoad 16/60 Superdex 75 column (GE Healthcare) at a flow rate of 1 ml min−1. For crystallization trials, purified proteins were concentrated using an Amicon 10-kDa molecular mass centrifugal concentrator and washed three times with 5 mM DTT (for the Se-Met proteins) or water (for native proteins).
Site-directed mutagenesis
Site-directed mutagenesis was carried out employing a PCR-based NZY-Mutagenesis kit (NZYTech Ltd) using the plasmids encoding the appropriate enzymes as the template. The mutated DNA clones were sequenced to ensure that only the appropriate DNA change was introduced after the PCR.
Purification of oligosaccharides
Galactooligosaccharides were generated by incubation of 3 g of galactan with 100 mM HCl incubated for 3 h at 100 °C and neutralised by NaOH titration. The oligosaccharide mixture was freeze dried and resuspended in water before being applied to a P2-BioGel (BioRad) column with a 0.22 ml/min flow rate. Fractions were evaluated for oligosaccharide content and purity by TLC. Pure fractions of defined oligosaccharides were pooled and concentrated. Oligosaccharide size was confirmed by Mass Spectrometry and HPAEC. Crude oligosaccharide mixtures were generated by partial digestion with appropriate enzymes; BT0360 and BT0367 (arabinan), BT4668 (galactan), BT4170 (P-RGI/AM-RGI) and BT4116 (HG). Reactions were boiled and filter sterilised to remove precipitate before being evaluated by TLC.
Preparation of RGI-AM
Arabisopsis thaliana seeds were resuspended in distilled water (1 g/ml) and incubated at 4 °C for 16 h while stirring. The solution was centrifuged and supernatant filtered through G1 glass filter (15-40 µm pore size). This was then dialysed against 2 x 40 volumes of water before freeze drying. Typical yield was 1 g from 80 g seeds.
CAZyme Assays
Spectrophotometric quantitative assays for the α-l-rhamnosidase BT4145, L-arabinofuranosidases (BT0349, BT0348 and BT0368), β-d-galactosidases (BT4667, BT4151, BT4156, BT4160 and BACOVA_05493) and carbohydrate esterase (BT4158) were monitored by the formation of NADH, at A340 nm using an extinction coefficient of 6,230 M−1 cm−1, with an appropriately linked enzyme assay system. The assays were adapted from purchased Megazyme International assay kits. These kits were as follows: the l-rhamnose assay kit (K-RHAMNOSE); l-arabinose/d-galactose assay kit (K-ARGA); acetic acid detection kit (K-ACET). Activity of pectic lyases (BT4170, BT4175, BT4115, BT4116) were measured at A235nm. Activity on 4-nitophenyl-glycosides was monitored at A400nm. The activity of BT4668 to hydrolyse galactan was determined in 20 mM sodium phosphate buffer, pH 7.5 at 37 °C containing an appropriate concentration of the polysaccharide and 1 mg ml−1 BSA. Reactions were incubated at 37 °C and at regular time intervals 500 μl aliquots were removed and the amount of reducing sugar was quantified using the dinitrosalicylic acid reagent 31 and a standard curve of xylose in the reaction conditions used. Substrate depletion assays were performed as described previously8 to determine BT4668 activity on galactooligosaccharides while production of d-galactose was used to assay BT4160 activity on galactooligosaccharides. The mode of action of enzymes were determined using HPAEC or TLC, as appropriate. In brief, aliquots of the enzyme reactions were removed at regular intervals and, after boiling for 10 min to inactivate the enzyme and centrifugation at 13,000g, the amount of substrate remaining or product produced was quantified by HPAEC using standard methodology. The reaction substrates and products were bound to a Dionex CarboPac PA100 (Galactooligosaccharides/Arabinooligosaccharides), PA1 (Monosaccharides) or PA20 (Polygalacturonic acid oligosaccharides) column and glycans eluted with an initial isocratic flow of 100 mM NaOH then a 0–200 mM sodium acetate gradient in 100 mM NaOH at a flow rate of 1.0 ml min−1, using pulsed amperometric detection. Linked assays were checked to make sure that the relevant enzyme being analysed was rate limiting by increasing its concentration and ensuring a corresponding increase in rate was observed. A single substrate concentration was used to calculate catalytic efficiency (kcat/KM), and was checked to be markedly less than KM by halving and doubling the substrate concentration and observing an appropriate increase or decrease in rate. The equation V0 = (kcat/KM)[S][E] was used to calculate kcat/KM unless substrate depletion was used then the calculation was as follows ln(kcat/KM) = (S0/St)/[E], in which [E] and [S] are enzyme and substrate concentration, respectively. All reactions were carried out in 20 mM sodium phosphate buffer, pH 7.0, with 150 mM NaCl (defined as standard conditions) and performed in at least technical triplicates.
Isothermal Titration Calorimetry
The binding of proteins to their glycan ligands was quantified by isothermal titration calorimetry (ITC), as described previously27. Titrations were carried out in 50 mM Na-HEPES buffer, pH 7.5 at 25 °C. The reaction cell contained protein at 50–100 μM, while the syringe contained either the oligosaccharide at 1–10 mM or the polysaccharide at 3–10 mg ml−1. Integrated heats were fitted to a single-site model using Microcal Origin v7.0 to derive n, Ka, and ΔH values. ΔG and ΔS were calculated from the equation –RTlnKa = -ΔG = ΔH -TΔS where R is the gas constant and T temperature in Kelvins.
Electrospray ionisation mass spectrometry (ESI-MS)
The molecular mass of purified oligosaccharides (in 10 mM ammonium acetate, pH 7.0) were analysed via negative ion mode infusion/offline ESI-MS following dilution (typically 1:1 (v/v)) with 5% trimethylamine in acetonitrile.
Electrospray data was acquired using an LTQ-FT mass spectrometer (Thermo) with a FT-MS resolution setting of 100,000 at m/z = 400 and an injection target value of 1,000,000. Infusion spray analyses were performed on 5–10 μl of samples using medium ‘nanoES‘ spray capillaries (Thermo) for offline nanospray mass spectrometry in negative ion mode at 1 kV.
1H-NMR determination of catalytic mechanism
Enzymes BT4145 and BACOVA_05493 were freeze dried in 20 mM Tris-HCl, 500 mM NaCl, pH 7.5 as were substrates α-L-Rha-α1,4-D-GalA and (β1,4-Galp-)3, respectively and resuspended in deuterium oxide. Prior to addition of enzyme an initial 1H-NMR spectra was obtained. Enzyme was added and spectra recorded at appropriate time intervals. The ratio of α- and β- monosaccharide products was determined to deduce catalytic mechanism.
2D NMR of arabinotetraose before and after treatment with BT0349
NMR spectra were recorded at 298 K in D2O with a Bruker AVANCE III spectrometer operating at 600 MHz equipped with a TCI CryoProbe. Two-dimensional 1H-1H TOCSY, ROESY, DQFCOSY, 13C HSQC and HSQC-TOCSY experiments were performed, using established methods32; the mixing times were 70 ms and 200 ms for the TOCSY and ROESY experiments, respectively. Chemical shifts were measured relative to internal acetone (δH =2.225, δC=31.07 ppm). Data were processed using the Azara suite of programs (v. 2.8, copyright 1993-2017, Wayne Boucher and Department of Biochemistry, University of Cambridge, unpublished) and chemical-shift assignment was performed using Analysis v2.433.
Growth of Bacteorides and generation of mutants
Bacteroides mutants were generated by deletion or replacement of the target gene with an inactive version by counter selectable allelic exchange using the pExchange-tdk plasmid. The full method is described in34. Mutants generated in this study are distinguished by the locus tag of the gene deleted/inactivated (Δbtxxx or ΔBACOVAxxxxx).
Bacteroides spp. were routinely cultured under anaerobic conditions at 37 °C using an anaerobic cabinet (Whitley A35 Workstation; Don Whitley) in culture volumes of 0.2, 2 or 5 ml) of TYG (tryptone-yeast extract-glucose medium) or minimal medium (MM) containing 0.5-1% of an appropriate carbon source and 1.2 mg ml−1 porcine haematin (Sigma-Aldrich) as previously described9. The growth of the cultures were routinely monitored at OD600 nm using a Biochrom WPA cell density meter for the 5 ml cultures or a Gen5 v2.0 Microplate Reader (Biotek) for the 0.2 and 2 ml cultures.
Protein cellular localisation
Cellular localization of proteins was carried out as described previously8. In brief, B. thetaiotaomicron cultures were grown overnight (OD600 nm value of 2.0) in 5 ml MM containing 0.5 % potato rhamnogalacturonan I (P-RGI) or homogalacturonan. The next day, cells were collected by centrifugation at 5,000g for 10 min and resuspended in 2 ml PBS. Proteinase K (0.5 mg ml−1 final concentration) was added to 1 ml of the suspension and the other half left untreated (control). Both samples were incubated at 37 °C overnight followed by centrifugation (5,000g for 10 min) to collect cells. To eliminate residual proteinase K activity, cell pellets were resuspended in 1 ml of 1.5 M trichloroacetic acid and incubated on ice for 30 min. Precipitated mixtures were then centrifuged (5,000g, 10 min) and washed twice in 1 ml ice-cold acetone (99.8%). The resulting pellets were allowed to dry in a 40 °C heat block for 5 min and dissolved in 250 μl Laemmli buffer. Samples were heated for 5 min at 98 °C and mixed by pipetting several times before resolving by SDS–PAGE using 12% gels. Electrophoresed proteins were transferred to nitrocellulose membranes by Western blotting followed by immunochemical detection using primary rabbit polyclonal antibodies (Eurogentec) generated against various proteins and secondary goat anti-rabbit antibodies (Santa Cruz Biotechnology). For BT4119 the anti-sera failed to produce the desired reactivity. Thus, a C-terminal Flag peptide (DYKDDDDK) was incorporated at the C-terminals of the native proteins expressed by B. thetaiotaomicron through counter-selectable allelic exchange34. This allowed for their detection using rabbit anti-Flag antibodies (Sigma) as primary antibodies. In the case of BT4668, BT0360 and BT0367 mutations (that lead to the inactivation of the encoded enzymes) were made in each gene within the B. thetaiotaomicron genome to generate the mutants Δbt4668, Δbt0360, Δbt0367 and Δbt0360/Δbt0367. Cells were grown in MM containing 0.5% arabino- or galacto-oligosaccharides to activate the target PULs. The cells were harvested from mid-log phase 5 ml cultures and concentrated in 0.5 ml PBS. The resuspended cells were incubated with the target glycans in an aerobic environment, conditions in which only the activity of only the surface enzymes can be monitored. The appropriate time intervals samples were taken, subjected to HPAEC analysis. The data were compared to that of wild type B. thetaiotaomicron to explore whether the loss in enzyme activity occurred at the bacterial surface.
Cross-feeding and competition assays
Prior to co-culture each Bacteroides spp. was grown in TYG and washed in PBS before being used to inoculate MM containing 0.5% glycan. Samples of 0.5 ml were taken at regular intervals during growth, which were serially diluted and plated onto Brain-Heart Infusion (BHI, Sigma-Aldrich) with agar and porcine hematin for determination of total CFU/ml of the culture. Genomic DNA was purified from the remainder of the sample (Bacterial genomic DNA purification kit, Sigma-Aldrich). Quantitate PCR was used to determine the ratio of different Bacteroides spp. or mutants in the sample using primers specific for unique regions in each Bacteroides sp. genome or tag introduced into one of two att sites. The Ratio of each species/mutant was used to calculate the CFU/ml of each organisms in the culture.
Quantitative RT-PCR (RT-qPCR)
Comparison of the levels of transcription of susC homologues (susCH) from each of the pectin PULs was performed by RT-qPCR. Previous studies have shown susCH genes are a good proxy for expression of their cognate PUL35. B. ovatus was cultured in 5 ml of MM containing 0.5% (w/v) carbon source, as described above. Triplicate bacterial cultures were harvested at mid-log phase (OD600 ~0.8) and placed in RNAprotect (Qiagen), then stored at −80 °C overnight, before purification with RNeasy kit (Qiagen). RNA purity was assessed spectrophotometrically, and 1 μg of RNA was used immediately for reverse transcription (QuantiTect Reverse Transcription kit, Qiagen). RT-qPCR was performed in a 96-well plate on a LightCycler 480 System (Roche) with FastStart Essential DNA Green Master (Roche) using the standard primer. Reactions were carried out in 10 μl, consisting of 5 μl SYBR Green mix, 20 ng of cDNA, and 1 μM (susCH genes) or 0.125 μM (16 S rRNA) primer mix. Reaction conditions were 95 °C 600 s, followed by 45 cycles of 95 °C for 10 s, 55 °C for 10 s, 72 °C for 10 s. Cq values were calculated using LightCycler 480 SW 1.5. Data were normalized to 16 S rRNA transcript levels, and change in expression level calculated as fold-change compared with minimal media, glucose cultures.
Crystal structure determination
Crystallisation
All proteins were concentrated to 10 mg/ml. BT4170 native crystallised in 20 mM sodium/potassium phosphate 20% (w/v) polyethylene glycol (PEG) 3350. BT4170 co-crystallised with 10 mM of oligosaccharide reaction products generated by BT4170 (defined as ligand) in 100 mM succinic acid, sodium phosphate glycine buffer at pH 6.0 and 25 % (w/v) PEG 1500. BT4170 inactive mutant K285A was co-crystalized with 30 mM ligand in 200 mM potassium chloride and 20% PEG 3350. Selenomethionine-containing BT4155 crystalized in 200 mM sodium chloride, 100 mM Bis-Tris buffer pH 5.5 and 25% PEG 3350. BT0349 with 500 mM L-arabinose was crystallised in 20 % PEG 3350 and 200 mM ammonium formate. All samples were cryo-protected by supplementing the mother liquor with 20% PEG 400.
Data collection and processing
BT0349, BT4170 and BT4170 K285A ligand data were indexed and integrated with the automated pipeline Xia2 (3da protocol)36. BT4170 in complex with ligand and BT4155 were indexed and integrated with XDS37. The data were scaled with either XDS or Aimless38. Space group determination was confirmed with Pointless39. The phase problem for BT0349 and BT4155 was solved by SeMet-SAD using hkl2map40 and the shelx pipeline41. BT4170 native apo data were solved by molecular replacement with the pipeline Balbes42 with the PDB model 1RU4 as search model. Initial models of BT0349, BT4155 and BT4170 were improved by successive runs of automated model building program arp_warp43 and buccaneer44. BT4170 TRI SCACCHARIDE and BT4170 inactive mutant K285A data were solved using the 4170 native apo model. All models were refined and improved using successive cycle of Refmac45 and manual model building with Coot46. All models were validated using Coot46 and molprobity47. Five percent of the observations were randomly selected for the Rfree set. The data processing, refinement statistics and protein database (PDB) codes are reported in Supplementary Table 9.
Comparative genomics analysis
PULs similar to the RGI, galactan, arabinan and homogalacturonan PULs were searched in HGM Bacteroidetes genomes. The identification of similar PULs was based on PUL alignments. Gene composition and order of Bacteroidetes PULs were computed using the PUL predictor described in PULDB48. Then, in a manner similar to amino acid sequence alignments, the predicted PULs were aligned to the appropriate pectin PULs according to their modularity as proposed in the RADS/RAMPAGE method49. Modules taken into account include CAZy families, sensor-regulators and suscd-like genes. Finally, PUL boundaries and limit cases were refined by BLASTP-based analysis. The previously unknown glycoside hydrolase families discovered in this study are listed in the main text.
Supplementary Material
Acknowledgements
This work was supported in part by an Advanced Grant from the European Research Council (Grant No. 322820) awarded to H.J.G. and B.H. supporting A.S.L., D.N., A.C. and N.T., a Wellcome Trust Senior Investigator Award to HJG (grant No. WT097907MA) that supported J.B. and E.C.L. a European Union Seventh Framework Initial Training Network Programme entitled the “WallTraC project” (Grant Agreement number 263916) awarded to M-C.R. and H.J.G, which supported X.Z. and J.S. The Biotechnology and Biological Research Council project “Ricefuel” (grant numbers BB/K020358/1) awarded to H.J.G. supported A.L. We thank Diamond Light Source for access to beamline I02, I04-1 and I24 (mx1960, mx7854 and mx9948) that contributed to the results presented here, and to Joes Gray at Newcastle University for assistance with the mass spectrometry.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article
Author contributions
Enzyme characterisation was carried out by A.S.L., J.B., X.Z., A.L., I.V. R.M., K.C., B.F., J.S. The generation of oligosaccharide products by M-C.R., X.Z., A.S.L., A.C. and D.N. Gene deletion strains were constructed by A.S.L., D.N., R.M., B.F., J.B. and D.W.A. Co-culturing experiments were carried out by J.B. and A.S.L. Phylogenetic reconstruction and metagenomic analysis was by N.T. and B.H. Bacterial growth and transcriptomic experiments: Y.X., E.C.L. and E.C.M. X-ray protein crystallography was by A.B., A.C., A.S.L. and J.B. N.M.R. experiments was by A.S.L. and K.S. Experiments were designed by D.W.A., H.J.G. and E.C.L., S.C.M. and H.J.G. The manuscript was written by H.J.G. with substantial contributions from D.W.A. E.C.L., N.T. and B.H. Figures were prepared by E.C.L. and A.S.L.
Data availability. The data that support the findings of this study are available from the corresponding author upon request. The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information. Complete western blot images are provided in Supplementary Fig. 1. The crystal structure datasets generated (coordinate files and structure factors) have been deposited in the Protein Data Bank (PDB) and are listed in Supplementary Table 9 together with the PDB accession codes.
References
- 1.Caffall KH, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert JA, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai MS, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, et al. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science. 2015;350:aac5992. doi: 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartmell A, et al. How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans. Proc Natl Acad Sci U S A. 2017;114:7037–7042. doi: 10.1073/pnas.1704367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuskin F, et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517:165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsbrink J, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndeh D, et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544:65–70. doi: 10.1038/nature21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert HJ. The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol. 2010;153:444–455. doi: 10.1104/pp.110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau JM, Mcneil M, Darvill AG, Albersheim P. Treatment of Rhamnogalacturonan-I with Lithium in Ethylenediamine. Carbohyd Res. 1987;168:245–274. [Google Scholar]
- 14.Coenen GJ, Bakx EJ, Verhoef RP, Schols HA, Voragen AGJ. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohyd Polym. 2007;70:224–235. [Google Scholar]
- 15.Bonnin E, Garnier C, Ralet MC. Pectin-modifying enzymes and pectin-derived materials: applications and impacts. Appl Microbiol Biotechnol. 2014;98:519–532. doi: 10.1007/s00253-013-5388-6. [DOI] [PubMed] [Google Scholar]
- 16.Martens EC, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem. 2009;284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenwright AJ, et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 2017;541:407–411. doi: 10.1038/nature20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 20.Cartmell A, et al. The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases. J Biol Chem. 2011;286:15483–15495. doi: 10.1074/jbc.M110.215962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickersgill R, Smith D, Worboys K, Jenkins J. Crystal structure of polygalacturonase from Erwinia carotovora ssp. carotovora. J Biol Chem. 1998;273:24660–24664. doi: 10.1074/jbc.273.38.24660. [DOI] [PubMed] [Google Scholar]
- 22.van Santen Y, et al. 1.68-angstrom crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J Biol Chem. 1999;274:30474–30480. doi: 10.1074/jbc.274.43.30474. [DOI] [PubMed] [Google Scholar]
- 23.Sengkhamparn N, et al. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohydr Res. 2009;344:1842–1851. doi: 10.1016/j.carres.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Renard CM, Crepeau MJ, Thibault JF. Glucuronic acid directly linked to galacturonic acid in the rhamnogalacturonan backbone of beet pectins. Eur J Biochem. 1999;266:566–574. doi: 10.1046/j.1432-1327.1999.00896.x. [DOI] [PubMed] [Google Scholar]
- 25.Raghavan V, Lowe EC, Townsend GE, 2nd, Bolam DN, Groisman EA. Tuning transcription of nutrient utilization genes to catabolic rate promotes growth in a gut bacterium. Mol Microbiol. 2014;93:1010–1025. doi: 10.1111/mmi.12714. [DOI] [PubMed] [Google Scholar]
- 26.Bagenholm V, et al. Galactomannan catabolism conferred by a polysaccharide utilization locus of Bacteroides ovatus: enzyme synergy and crystal structure of a beta-mannanase. J Biol Chem. 2017;292:229–243. doi: 10.1074/jbc.M116.746438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogowski A, et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 2015;6:7481. doi: 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol. 2014;24:40–49. doi: 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Foster KR, Comstock LE. The evolution of cooperation within the gut microbiota. Nature. 2016;533:255–259. doi: 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foley MH, Cockburn DW, Koropatkin NM. The Sus operon: a model system for starch uptake by the human gut Bacteroidetes. Cell Mol Life Sci. 2016;73:2603–2617. doi: 10.1007/s00018-016-2242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 32.Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ. Protein NMR Spectroscopy: Principles and Practice. Academic Press; 1996. [Google Scholar]
- 33.Vranken WF, et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 34.Koropatkin NM, Martens EC, Gordon JI, Smith TJ. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure. 2008;16:1105–1115. doi: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Despres J, et al. Unraveling the pectinolytic function of Bacteroides xylanisolvens using a RNA-seq approach and mutagenesis. BMC Genomics. 2016;17:147. doi: 10.1186/s12864-016-2472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter G. xia2: an expert system for macromolecular crystallography data reduction. J Appl Crystallogr. 2010;43:186–190. [Google Scholar]
- 37.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans PR, Murshudov GN. How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans PR. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr D Biol Crystallogr. 2011;67:282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pape T, Schneider TR. HKL2MAP: a graphical user interface for macromolecular phasing with SHELX programs. J Appl Crystallogr. 2004;37:843–844. [Google Scholar]
- 41.Sheldrick GM. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr D Biol Crystallogr. 2010;66:479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long F, Vagin AA, Young P, Murshudov GN. BALBES: a molecular-replacement pipeline. Acta Crystallogr D Biol Crystallogr. 2008;64:125–132. doi: 10.1107/S0907444907050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langer G, Cohen SX, Lamzin VS, Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc. 2008;3:1171–1179. doi: 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowtan K. Fitting molecular fragments into electron density. Acta Crystallogr D Biol Crystallogr. 2008;64:83–89. doi: 10.1107/S0907444907033938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vagin AA, et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol Crystallogr. 2004;60:2184–2195. doi: 10.1107/S0907444904023510. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 47.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terrapon N, Lombard V, Gilbert HJ, Henrissat B. Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformatics. 2015;31:647–655. doi: 10.1093/bioinformatics/btu716. [DOI] [PubMed] [Google Scholar]
- 49.Terrapon N, Weiner J, Grath S, Moore AD, Bornberg-Bauer E. Rapid similarity search of proteins using alignments of domain arrangements. Bioinformatics. 2014;30:274–281. doi: 10.1093/bioinformatics/btt379. [DOI] [PubMed] [Google Scholar]
- 50.Varki A, et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 2015;25:1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.