Abstract
Objectives
The aim of this study was to investigate the potential of coronary ultrafast Doppler angiography (CUDA), a novel vascular imaging technique based on ultrafast ultrasound, to image noninvasively with high sensitivity the intramyocardial coronary vasculature and quantify the coronary blood flow dynamics.
Background
Noninvasive coronary imaging techniques are currently limited to the observation of the epicardial coronary arteries. However, many studies have highlighted the importance of the coronary microcirculation and microvascular disease.
Methods
CUDA was performed in vivo in open-chest procedures in 9 swine. Ultrafast plane-wave imaging at 2,000 frames/s was combined to an adaptive spatiotemporal filtering to achieve ultrahigh-sensitive imaging of the coronary blood flows. Quantification of the flow change was performed during hyperemia after a 30-s left anterior descending (LAD) artery occlusion followed by reperfusion and was compared to gold standard measurements provided by a flowmeter probe placed at a proximal location on the LAD (n = 5). Coronary flow reserve was assessed during intravenous perfusion of adenosine. Vascular damages were evaluated during a second set of experiments in which the LAD was occluded for 90 min, followed by 150 min of reperfusion to induce myocardial infarction (n = 3). Finally, the transthoracic feasibility of CUDA was assessed on 2 adult and 2 pediatric volunteers.
Results
Ultrahigh-sensitive cine loops of venous and arterial intramyocardial blood flows were obtained within 1 cardiac cycle. Quantification of the coronary flow changes during hyperemia was in good agreement with gold standard measurements (r2 = 0.89), as well as the assessment of coronary flow reserve (2.35 ± 0.65 vs. 2.28 ± 0.84; p = NS). On the infarcted animals, CUDA images revealed the presence of strong hyperemia and the appearance of abnormal coronary vessel structures in the reperfused LAD territory. Finally, the feasibility of transthoracic coronary vasculature imaging was shown on 4 human volunteers.
Conclusions
Ultrafast Doppler imaging can map the coronary vasculature with high sensitivity and quantify intramural coronary blood flow changes.
Key Words: angiography, blood flow, coronary, Doppler, imaging, ultrasound
Abbreviations and Acronyms: CFR, coronary flow reserve; CUDA, coronary ultrafast Doppler angiography; LAD, left anterior descending coronary artery; PVI, power-velocity integral
Graphical abstract
Coronary arteries secure the heart supply in blood and oxygen. Any dysfunction or disease of these vessels can lead in turn to adverse clinical outcomes. The coronary vasculature is organized in 3 compartments. The first is made of the epicardial coronary arteries, which run along the heart’s surface and exhibit diameters ranging from a few millimeters to 500 μm. The second includes the pre-arterioles, which penetrate the myocardium from the epicardium to the endocardium and exhibit diameters ranging from 500 μm to 100 μm. The third corresponds to the coronary microvasculature, which exhibits vessel diameters below 100 μm (1). To date, the epicardial coronary vasculature is the only compartment that can be imaged in vivo in humans (1) with current angiography techniques such as x-ray, computed tomography (CT) scan, or magnetic resonance imaging (2). As a consequence, cardiology practice has been centered on focal macroscopic coronary artery disease, that is, the functional assessment of epicardial stenoses based on fractional flow reserve and subsequent pharmacological or invasive treatment via percutaneous coronary interventions or surgery (3).
It is now recognized that coronary microvascular dysfunction, that is, including pre-arterioles, is another prognostic marker of myocardial ischemia 1, 4. Yet clinical guidelines in the management of stable ischemic heart disease only consider coronary microvascular dysfunction after excluding signs of epicardial disease (5). Therefore, there is a clear role for novel imaging tools capable of imaging and characterizing the pre-arteriolar coronary vasculature.
On the technology front, echocardiography capabilities are being redefined in the advent of ultrafast cardiac ultrasound imaging (6). Relying on plane-wave transmissions, ultrafast ultrasound enables the imaging of the heart at thousands of images per second, that is, 100 times faster than conventional clinical echocardiography (7). The major benefit of this technology lies in the acquisition of spatially synchronous, temporally highly resolved ultrasound datasets. Earlier studies have shown that post-processing of ultrafast ultrasound datasets can lead to a 30-fold increase in power Doppler sensitivity and generate rodent cerebrovascular atlases of 100 μm resolution 8, 9.
In the myocardium, 2-mm-thick cryomicrotome projections of swine coronary vasculature (10) reveal that dense vascular networks are being insonified during 2-dimensional echocardiography examinations (3- to 10-mm image slice thickness). In 2012, Osmanski et al. (11) presented a first sparse detection of intramyocardial blood flow in open-chest sheep experiments using directional ultrafast-power Doppler. More recently, we used ultrafast cardiac Doppler imaging to resolve left ventricle hemodynamics with millisecond accuracy (12). These advances exhibited the potential of ultrafast Doppler in mapping cardiac hemodynamics.
In this paper, we report noninvasive ultrasound imaging of the coronary vasculature and assessment of the coronary flow reserve (CFR) using a new technique we call coronary ultrafast Doppler angiography (CUDA). This technique involves acquiring ultrasound images of the beating heart at 2,000 frames per second and processing them adaptively to account for myocardial wall motion and retrieve Doppler signals from tissue clutter throughout the heart cycle. We present ultrasound images of the epicardial and pre-arteriolar (diameter 500 to 100 μm) coronary compartments in a series of in vivo open-chest swine experiments and assess pre-arteriolar CFR using a metric that is proportional to blood flow, referred to as power-velocity integrals (13). Finally, we demonstrate contrast agent–free, transthoracic ultrasound images of the human coronary vasculature.
Methods
Animal experiment protocol
Eight 2.5-month-old female domestic swine (Sus scrofa domesticus) weighing 20 to 25 kg were anesthetized with isoflurane 2%, intubated, and ventilated. After sternotomy, a coronary flow probe (Transonic, Ithaca, New York) was placed around the left anterior descending coronary artery (LAD). During a first set of experiments, the LAD was transiently occluded for 30 s to induce reactive hyperemia (n = 5). Then the swine received intravenous adenosine infusion (0.5 mg/kg/min) to induce major pharmacological coronary artery vasodilation. During a second set of experiments, the LAD was transiently occluded for 90 min, followed by 150 min of reperfusion to induce myocardial infarction (n = 3). After the animals were euthanized, the heart was excised and cut in slices that were incubated with triphenyltetrazolium chloride to reveal infarcted areas in white and viable tissues in red. The animal procedure was approved by the Institutional Animal Care and Use Committee of Ecole Veterinaire de Maison-Alfort (ComEth ANSES-ENVA-UPEC) according to the European Commission guiding principles (2010/63/EU).
Human application
In this set of transthoracic experiments in humans, we used the same ultrafast ultrasound scanner and ultrasound probe as in open-chest experiments. A trained sonographer positioned the probe in real time using B-mode imaging. Subsequently, the ultrafast sequence was launched, and ultrafast ultrasound datasets were acquired. The processing was identical to the animal experiments. This study had been approved by the proper ethics committee (Comité de Protection des Personnes–Ile-de-France VI, study identifications: 2015-A00438-41 and 2015-A00187-42), and informed consent was signed by the adults or by the parents of the children.
Ultrafast ultrasound imaging sequence
All experiments were conducted with a linear 6-MHz ultrasound probe (Vermon, Tours, France) and a programmable ultrafast ultrasound scanner (Aixplorer, SuperSonic Imagine, Aix-en-Provence, France). High-sensitivity Doppler imaging was achieved by use of a dedicated ultrafast plane-wave imaging sequence. We designed a plane-wave ultrasound sequence consisting of 8 tilted plane waves to perform 1 image (14), which enabled imaging of the myocardial wall at a frame rate of 2,000 images/s (Figure 1B) at a depth of up to 45 mm. Considering the 6-MHz central frequency, this allowed us to sample coronary blood flows below 24 cm/s (15). Each ultrafast Doppler image consisted of a 0.5-s ultrafast acquisition (or 1,080 images), which fully covered the diastolic phase of the heart cycle in swine and in humans under normal physiological conditions.
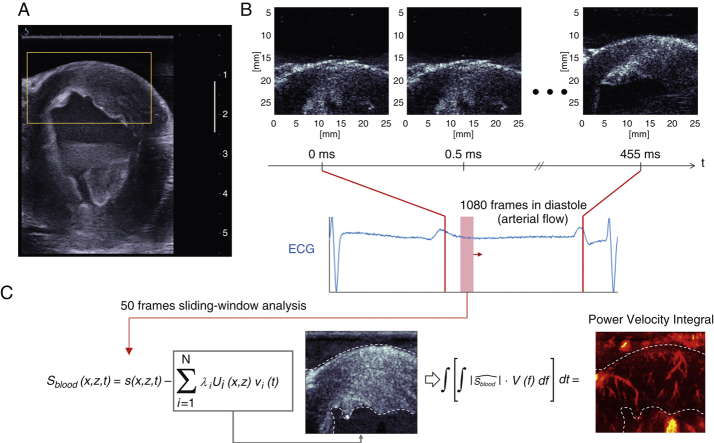
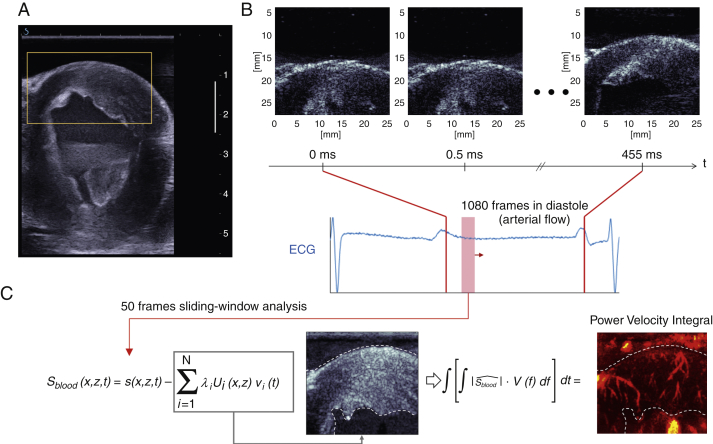
Figure 1.
Coronary Ultrafast Doppler Angiography Acquisition Sequence and Processing
(A) Conventional ultrasound imaging in real time was used to select the heart section of interest (mid parasternal short-axis view here). The region of interest of the anterior wall is outlined in yellow. (B) Ultrafast acquisition of the anterior wall region of interest and corresponding electrocardiogram. A total of 1,000 frames were typically acquired in diastole over 0.5 s. (C) An adaptive spatiotemporal filter was used to separate coronary flow from tissue clutter through diastole using a sliding window analysis. Using a singular value decomposition on sliding short ensembles of frames (<50), we separated tissue signal (gray-scale image) contained in the highest eigenvectors from blood signal contained in the lowest eigenvectors (red-scale image). Our filtering method relies on the fact that tissue is highly echogenic and exhibits cohesive motion throughout the image plane, whereas coronary blood signal is weakly echogenic and has a random motion nature, because erythrocytes are freely floating within vessels.
CUDA vasculature detection
Post-processing was performed after the acquisition of the ultrasound dataset. We performed a sliding spatiotemporal analysis of short ensembles of frames (typically 50 frames covering 25 ms) throughout the ultrafast ultrasound datasets. For each ensemble of frames, an adaptive spatiotemporal temporal clutter filter retrieved Doppler signals from the coronary vasculature (Figure 1C, Online Appendix). Signed power Doppler maps were generated and superimposed to the ultrafast B-mode images. To establish the ability of CUDA to provide a quantitative readout of myocardial perfusion, we analyzed CUDA signals using power-velocity integrals (PVI), a metric proportional to flow rate (13).
Statistical analysis
Continuous variables are presented as mean ± SD. Comparisons of flow changes were made with the Student 2-tailed paired t test. A Bartlett test was used to check the homogeneity of variances. The level of significance was set at an alpha level of ≤0.05. Linear regression and Bland-Altman plots were used for correlation of LAD flow variations between a flowmeter probe and ultrasound Doppler imaging. Analyses were conducted using MedCalc software (MedCalc Software, Mariakerke, Belgium).
Results
Coronary ultrafast ultrasound Doppler angiography unveils the intramural coronary vasculature in vivo
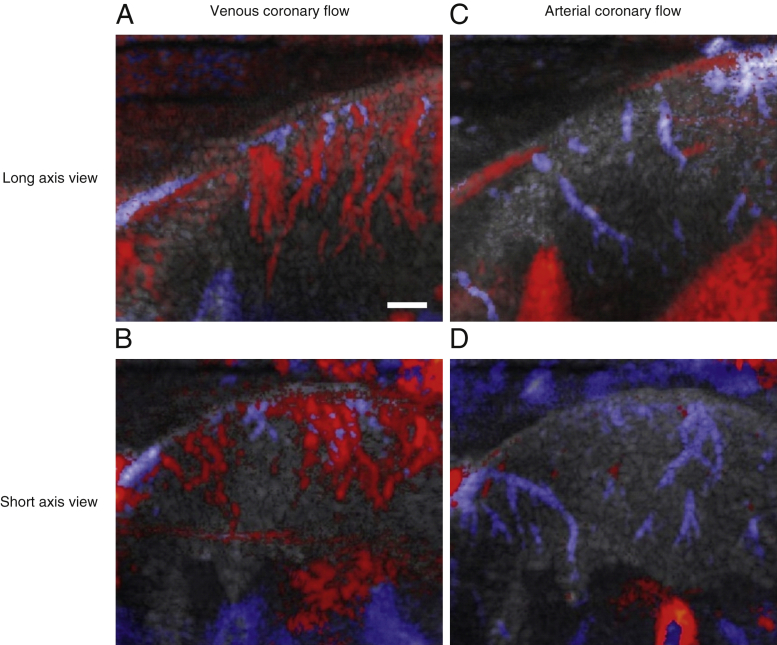
To show the capability of the CUDA imaging method to visualize surface and subsurface coronary vasculature, we conducted a series of open-chest swine experiments (n = 9). We present here long-axis and short-axis images of the coronary vasculature overlaid on B-mode anatomic images of the myocardium (Figure 2, Online Video 1). Vessels were color-coded using the echocardiography color Doppler conventions: upward flows in red and downward flows in blue. In Figures 2A and 2B, acquired in end systole before the transition to arterial flow, blood flows upward in intramural coronary veins from the endocardium to the epicardium and downward in the epicardial vein. In Figures 2C and 2D, acquired in protodiastole after the transition to arterial flow, the opposite takes place: blood flows up in the epicedial artery and down in the pre-arteriolar compartment in the direction of the endocardium. Note that in Figure 2D, the epicardial artery appears in cross section, which is more likely to happen in a short-axis view. These results demonstrate the feasibility of anterior wall color Doppler coronary angiography using ultrafast ultrasound.
Figure 2.
Ultrafast Color Doppler Imaging of Intramural Coronary Vasculature in Open-Chest Swine Experiments
Red indicates blood flows moving upward, and blue indicates blood flows moving downward. (Top) An example of long-axis results in 1 animal: (A) venous coronary flow in mid-systole and (C) arterial coronary flow in mid-diastole. (Bottom) Results in a mid-level short-axis view showing (B) venous and (D) arterial flow in the same animal. Inversion of venous and arterial flow patterns is clearly visible: in systole, venous blood flow moves upward from the endocardium to the epicardium before moving downward in the epicardial veins. In diastole, arterial blood flow goes up in the epicardial vessels before flowing down in the myocardium. The epicardial and arteriolar compartments are successfully detected. Vessels below 100 μm remain below resolution. See Online Video 1. Scale bar = 3 mm.
CUDA quantitatively assesses coronary flow variations during reactive hyperemia induced by brief coronary artery occlusion
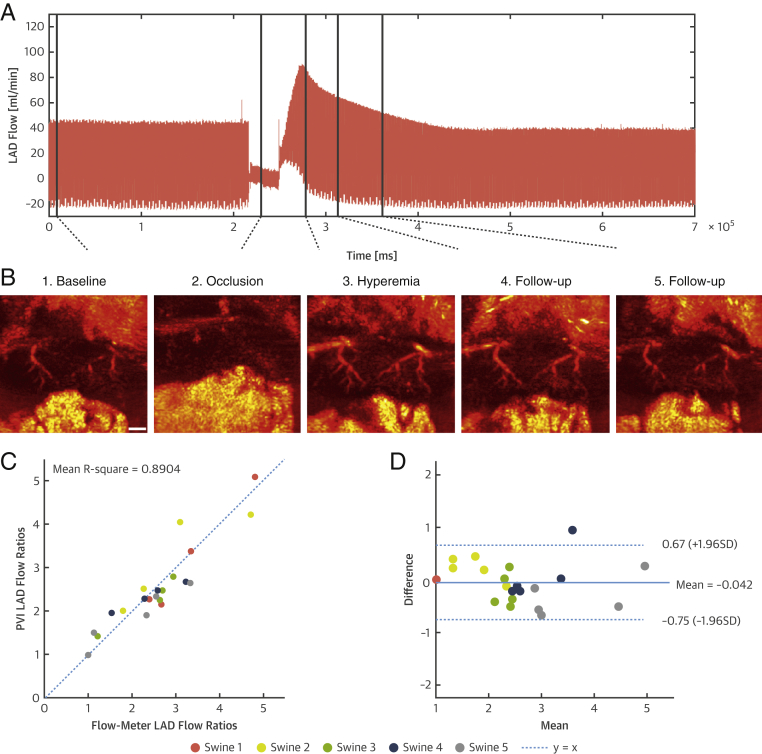
We evaluated the capacity of our imaging method to assess physiological coronary flow variations in a set of experiments with brief episodes of coronary artery occlusion followed by reperfusion, that is, inducing reactive hyperemia (n = 5). We positioned a flow probe on the LAD proximal to the ultrasound mid-ventricle parasternal short-axis view to compare flow variations assessed by ultrafast Doppler against flow variations measured by the flow probe used as a gold standard. Figure 3A presents the coronary blood flow recording acquired with a flowmeter probe on the LAD, which underwent a 30-s total occlusion. We acquired ultrafast Doppler images of the coronary microvasculature at specific time points, for instance, baseline, occlusion, peak of hyperemia, and during follow-up. In the reported example (Figure 3B), 2 branches of the LAD vasculature can be observed. The branches disappear during occlusion as blood flow is restricted in the epicardial artery. At the peak of hyperemia, the vasculature reappears with a higher-power Doppler intensity because the flow has increased and vessels are dilated. Power Doppler intensity subsequently decays during follow-up of the hyperemia event toward baseline levels. This result demonstrates that ultrafast-power Doppler intensities correlated with the coronary flow.
Figure 3.
Quantitative CUDA Assessment of Coronary Flow Variations
(A) Flow rate in the epicardial LAD assessed with an invasive flow probe positioned proximal to the image plane. A baseline phase is followed by a 30-s occlusion and subsequent reperfusion, inducing hyperemia. (B) Corresponding ultrafast Doppler images in 1 animal at baseline and during occlusion, peak hyperemia, and decay of the hyperemia event. The intramural coronary vasculature disappears during occlusion before reappearing brighter during hyperemia, which demonstrates that ultrafast Doppler imaging is sensitive to blood flow variations. Scale bar = 3 mm. (C) LAD blood flow variations derived from PVIs and correlation with flowmeter-derived flow variations in 5 animals. (D) Corresponding Bland-Altman plot of the data. CUDA = coronary ultrafast Doppler angiography; LAD = left anterior descending coronary artery; PVI = power-velocity integral.
To establish the ability of CUDA to provide a quantitative readout of myocardial perfusion, we present in Figure 3C the correlation of ultrafast Doppler–derived PVI flow variations against coronary blood flow variations measured with the flow probe in 5 swine. We found a strong correlation between this metric and invasive flow probe measurements (mean r2 = 0.89 for 5 swine), corroborated by a Bland-Altman plot (Figure 3D). These results demonstrate that quantitative hyperemia-to-baseline ratios can be derived using ultrafast Doppler imaging.
Noninvasive measurement of intramural coronary flow reserve with adenosine
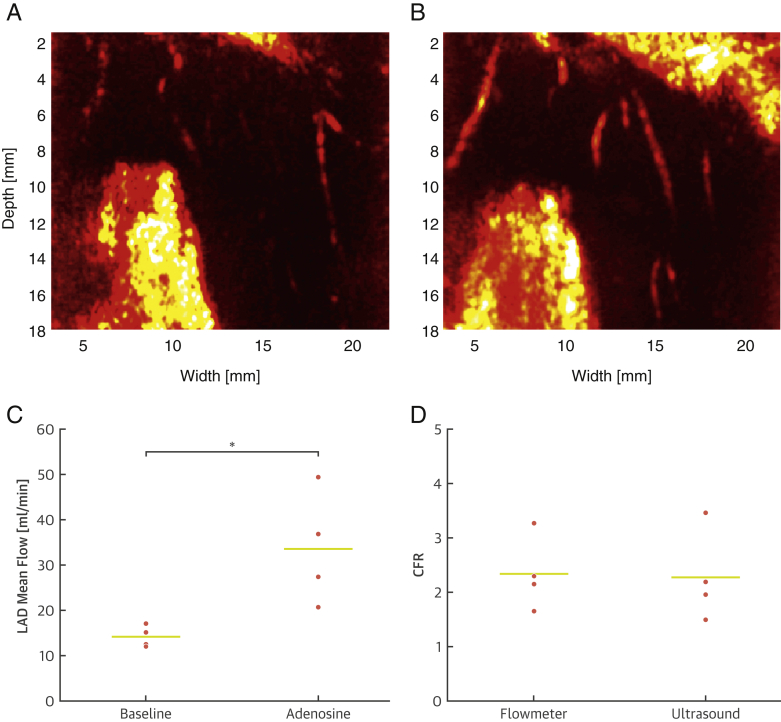
CFR, the ratio of coronary flow during maximal coronary vasodilatation to coronary flow at baseline, is a prognostic physiological parameter widely used to diagnose stable ischemic heart disease (5). To assess whether we could determine this ratio using CUDA, we induced maximal coronary vasodilatation using adenosine while imaging or measuring blood flow using an LAD flowmeter. Figure 4A presents ultrafast Doppler images of the coronary vasculature at baseline and during adenosine-induced vasodilatation, revealing a clear Doppler signal increase under adenosine administration. Figure 4B shows statistically significant mean blood flow variations assessed with a LAD flowmeter proximal to the imaging plane of interest, which evolved from 14.2 ml/min at baseline to 33.6 ml/min under adenosine administration (p = 0.04). Figure 4C shows a comparison of CFR values derived from PVI measurements at the pre-arteriolar level to CFR values derived from flowmeter measurements. We performed 16 measurements in 4 swine: 4 at baseline and 4 under adenosine using ultrasound coregistered with 4 measurements at baseline and 4 under adenosine using flowmeter. The mean ultrasound-derived CFR was 2.28 ± 0.84, whereas the flowmeter CFR was 2.35 ± 0.65 (p = NS). Values were in excellent agreement and demonstrated the capacity of ultrafast Doppler ultrasound imaging to assess CFR noninvasively.
Figure 4.
CFR Assessment With CUDA
(A) Comparison of ultrafast Doppler images of the anterior wall coronary artery vasculature at baseline and (B) with intravenous adenosine administration (0.5 mg/kg/min) in 1 animal. The increase in intramural vessel intensity denotes an increase in blood volume, which reveals the vasodilatative action of adenosine. (C) Statistical significance of vasodilatation induced by adenosine compared with baseline assessed with a flow probe connected to a flowmeter in 4 animals. LAD mean flow was more than doubled under the action of adenosine (p < 0.05). (D) CFR assessed with power-velocity integrals in intramural coronary arteries versus CFR assessed by flowmeter (gold standard) in 4 animals. Both metrics showed excellent agreement, demonstrating the capability of ultrafast Doppler imaging to assess CFR noninvasively. CFR = coronary flow reserve; LAD = left anterior descending coronary artery.
Exploring the coronary vasculature during myocardial infarction using CUDA
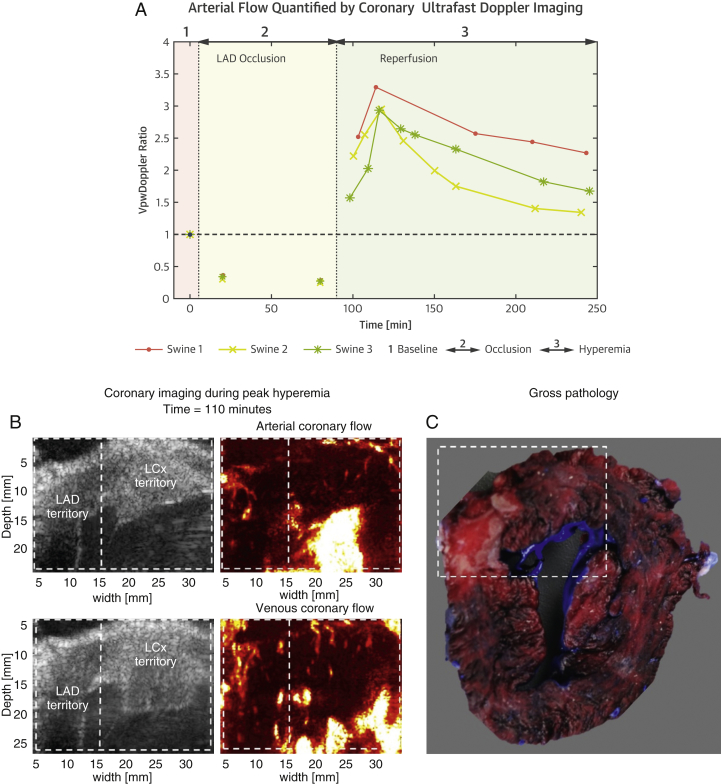
After validating the quantitative assessment of blood flow variations using CUDA, we explored whether our imaging method could monitor coronary hemodynamics during myocardial infarction. To induce myocardial infarction, the LAD was fully occluded for 90 min and then reperfused (experiment performed in 3 swine). We monitored the anterior wall coronary vasculature with ultrafast Doppler at baseline, during occlusion, and for 250 min after reperfusion. In all 3 experiments, coronary blood flow peaked 20 min after reperfusion (Figure 5A). Subsequent heart sections stained with triphenyltetrazolium chloride revealed the presence of large infarcted LAD regions, as well as viable reperfused regions (Figure 5C). Although anatomic B-mode images of infarcted regions did not provide specific insights on tissue damage, ultrafast Doppler images revealed the presence of dilated vessels and strong hyperemia in the reperfused region, which peaked at 20 min after reperfusion and decreased slowly during the following 2 h. In addition to hyperemia, we observed the appearance of abnormal coronary vessel structures in the reperfused LAD territory. An example of abnormal arterial flows is shown in Figure 5B: the arterial network in the LAD territory ends with a larger arterial flow region within the myocardial wall, which could be related to vascular damage and hemorrhagic spots. Additionally, abnormal venous flows were observed with large dilated and disorganized vessels. Similar patterns were observed in all 3 animals.
Figure 5.
Imaging and Pathology Before, During, and After Reperfusion
(A) PVI ratios are shown for 3 swine at baseline, during 90-min LAD occlusion, and during reperfusion. The hyperemia peak is observed after 20 min of reperfusion. (B) Strong local hyperemia and dilated arteries and veins presenting abnormal structures are observed with ultrafast Doppler imaging during reperfusion in the LAD territory (time = 110 min). (C) Corresponding heart section stained with triphenyltetrazolium chloride. Infarcted tissue appears white. White dotted lines outline regions that contain infarcted areas of interests in the heart section, and corresponding B-mode and Doppler images. LAD = left anterior descending coronary artery; LCx = left circumflex artery; VpwDoppler ratio = power velocity integral ratio.
Noninvasive transthoracic imaging of the coronary vasculature in humans
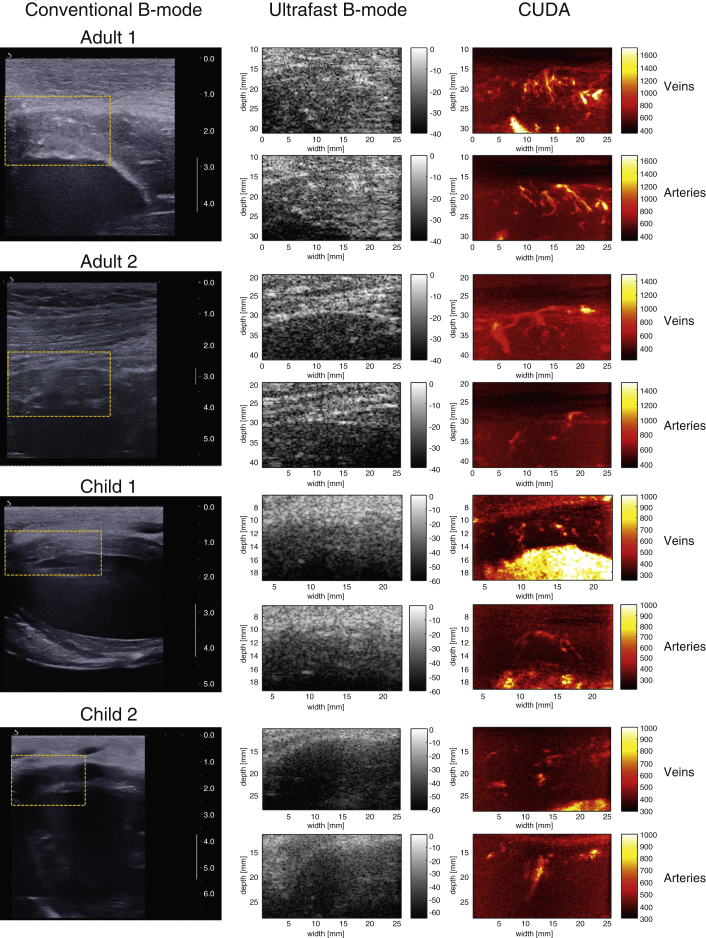
In a step toward translation to clinical practice, we evaluated the capability of our method to detect the coronary vasculature transthoracically in humans. We imaged the coronary vasculature in 2 children and 2 adults. In Figure 6, we present the first transthoracic images of coronary vasculature in humans that were obtained with CUDA during the examination of 2 adults (29 and 39 years of age) and 2 children (4.5 and 9.7 years of age). These results demonstrate that it is possible to detect intramural coronary veins and arteries in a transthoracic clinical in vivo setting.
Figure 6.
Transthoracic CUDA Feasibility in Human Volunteers
(Left) Conventional ultrasound image showing the region of interest selected for coronary ultrafast Doppler angiography (CUDA) processing. This real-time imaging mode of the ultrasound scanner was used for positioning (the scale bar is in centimeters). (Middle) Ultrafast B-mode images of the region of interest depicted in yellow. The resolution is lower, but the edges of the myocardial wall can be detected. Note that contrary to the open-chest experiments, parenchyma echogenicity is present above the myocardium, because it is a transthoracic measurement. (Right) CUDA images of coronary veins in systole and coronary arteries in diastole.
Discussion
These results establish that ultrafast ultrasound combined with adaptive coronary Doppler processing is capable of visualizing the epicardial and intramural coronary vasculature, a vascular compartment currently uncharted in vivo in humans by existing clinical angiography techniques. Furthermore, this imaging technique allows the determination of PVI, a metric proportional to coronary flow, providing a quantitative measurement of coronary flow variations and CFR. This opens new possibilities for the noninvasive characterization of intramural CFR in patients with microvascular angina. Ultrafast Doppler imaging of the coronary vasculature could also play a role in cardiovascular research, because it can monitor physiological changes and anatomic changes of epicardial and intramural vessels in animal models of infarction, including abnormal coronary vessel structures. Moreover, the capability of CUDA to image both venous and arterial flows could also be useful for better understanding of the venous myocardial drainage, which remains somewhat unknown. In a final effort toward clinical translation, we demonstrated that our method could be readily used to image intramural coronary flow in pediatric cardiology.
Clinical application
Estimating CFR is critical to diagnose patients with stable coronary artery disease 16, 17. It remains difficult, however, to assess CFR noninvasively. Fractional flow reserve is the current gold standard (18) according to European Society of Cardiology and American College of Cardiology guidelines but is an invasive, catheter-based method. Current clinical guidelines recommend coronary CT and stress-rest imaging (encompassing echocardiography, magnetic resonance imaging, positron emission tomography, and single-photon emission CT) (5). New tools are appearing, in particular CT-derived fractional flow reserve 19, 20, but require further clinical validation. In this context, CUDA may become a novel major tool for measuring the CFR noninvasively with a nonionizing and portable imaging modality that can be used at the patient’s bedside. Another cumbersome situation in clinical practice is the evaluation of angina due to microvascular disease, or microvascular angina. European Society of Cardiology guidelines (5) refer to this condition as angina with normal coronary arteries. This patient population currently represents a challenge, because their condition cannot be diagnosed with existing imaging tools, which cannot visualize intramural coronary vasculature. Finally, the technology could immediately play a role in interventional cardiology, for example, to assess reperfusion after a bypass surgery or a percutaneous coronary intervention.
Study limitations
In this study, CUDA did not provide an absolute quantification of flow rate because of the angle dependency of Doppler imaging and could only quantify the flow rate changes during hyperemia or adenosine infusion thanks to the PVI. The feasibility of transthoracic imaging was shown only on 4 normal human hearts (2 adults and 2 children), and the clinical interest of this technology remains to be demonstrated on patients with coronary diseases. Another limitation of the study is the use of a linear transducer array, which is not suitable for transthoracic imaging in adult patients. A maximal imaging depth of 45 mm was achieved in this study due to the limited penetration of our linear transducer array. Imaging at a larger depth will require the use of a lower frequency, which implies a decrease of the spatial resolution. Therefore, the performance of CUDA at large depths remains to be investigated in further study. Future development of ultrafast Doppler imaging is ongoing, and the application of this technology to the human adult heart is emerging. The feasibility of transthoracic ultrafast imaging has already been demonstrated in adults (21) and was used to map intracardiac blood flows (12), electromechanical coupling (22), and myocardial stiffness 23, 24. Therefore, CUDA could be translated to the human adult heart in the near future in both a transthoracic setup and in transesophageal echocardiography. Moreover, we have recently introduced ultrafast imaging in 3 dimensions (3D) and have shown the feasibility of imaging in 3D the human adult heart at a volume rate of 2,500 volumes/s using a prototype of a 3D ultrafast imaging scanner (25). CUDA imaging will highly benefit from this volumetric imaging, enabling the mapping of the entire complex vascular anatomy.
Conclusions
In this study, intramyocardial coronary vasculature of open-chest swine were imaged at ultrahigh sensitivity using CUDA. The change in coronary flow rate was quantified by CUDA during hyperemia and infusion of adenosine and validated by invasive flowmeter measurements. The transthoracic feasibility of this approach was shown in 4 human cases. These results could open new possibilities for the noninvasive characterization of intramural CFR in patients with microvascular angina.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Estimating CFR is critical to diagnose patients with stable coronary artery disease and is valuable for the evaluation of any ischemic heart disease. However, it remains difficult to assess CFR noninvasively. The results of this study show that noninvasive assessment of CFR could be performed using ultrafast echocardiography. This may provide a nonionizing, contrast-free, and portable technique for noninvasive CFR assessment.
TRANSLATIONAL OUTLOOK: The major impact of this study is the finding that ultrafast ultrasound can visualize intramural coronary vasculature in a beating heart and quantify the change in coronary blood flow.
Footnotes
This work was supported by the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC grant agreement 311025 and the ANR-10-IDEX-0001-02 PSL Research University. Dr. Tanter is a cofounder of SuperSonic Imagine. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
David Maresca, Email: dmaresca@caltech.edu.
Mathieu Pernot, Email: mathieu.pernot@inserm.fr.
Appendix
References
- 1.Camici P.G., d’Amati G., Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 2.Fihn S.D., Blankenship J.C., Alexander K.P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez E.J., Lee K.L., Jones R.H., for the STICHES Investigators Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Hoef T.P., Siebes M., Spaan J.A.E., Piek J.J. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36 doi: 10.1093/eurheartj/ehv235. 3312–19a. [DOI] [PubMed] [Google Scholar]
- 5.Task Force Members. Montalescot G., Sechtem U., Achenbach S. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology [published correction appears in Eur Heart J 2014;35:2260–1] Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 6.Cikes M., Tong L., Sutherland G.R., D’hooge J. Ultrafast cardiac ultrasound imaging: technical principles, applications, and clinical benefits. J Am Coll Cardiol Img. 2014;7:812–823. doi: 10.1016/j.jcmg.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Tanter M., Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:102–119. doi: 10.1109/TUFFC.2014.6689779. [DOI] [PubMed] [Google Scholar]
- 8.Demené C., Tiran E., Sieu L.-A. 4D microvascular imaging based on ultrafast Doppler tomography. Neuroimage. 2016;127:472–483. doi: 10.1016/j.neuroimage.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Macé E., Montaldo G., Cohen I., Baulac M., Fink M., Tanter M. Functional ultrasound imaging of the brain. Nat Methods. 2011;8:662–664. doi: 10.1038/nmeth.1641. [DOI] [PubMed] [Google Scholar]
- 10.Spaan J., Kolyva C., van den Wijngaard J. Coronary structure and perfusion in health and disease. Philos Trans A Math Phys Eng Sci. 2008;366:3137–3153. doi: 10.1098/rsta.2008.0075. [DOI] [PubMed] [Google Scholar]
- 11.Osmanski B.-F., Pernot M., Montaldo G., Bel A., Messas E., Tanter M. Ultrafast Doppler imaging of blood flow dynamics in the myocardium. IEEE Trans Med Imaging. 2012;31:1661–1668. doi: 10.1109/TMI.2012.2203316. [DOI] [PubMed] [Google Scholar]
- 12.Osmanski B.-F., Maresca D., Messas E., Tanter M., Pernot M. Transthoracic ultrafast Doppler imaging of human left ventricular hemodynamic function. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:1268–1275. doi: 10.1109/TUFFC.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck T., Mucci R.A., Guerrero J.L., Holmvang G., Handschumacher M.D., Levine R.A. The power-velocity integral at the vena contracta: a new method for direct quantification of regurgitant volume flow. Circulation. 2000;102:1053–1061. doi: 10.1161/01.cir.102.9.1053. [DOI] [PubMed] [Google Scholar]
- 14.Montaldo G., Tanter M., Bercoff J., Benech N., Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:489–506. doi: 10.1109/TUFFC.2009.1067. [DOI] [PubMed] [Google Scholar]
- 15.Bercoff J., Montaldo G., Loupas T. Ultrafast compound Doppler imaging: providing full blood flow characterization. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:134–147. doi: 10.1109/TUFFC.2011.1780. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi A., Stone G.W., Leipsic J. Prognostic determinants of coronary atherosclerosis in stable ischemic heart disease: anatomy, physiology, or morphology? Circ Res. 2016;119:317–329. doi: 10.1161/CIRCRESAHA.116.308952. [DOI] [PubMed] [Google Scholar]
- 17.De Bruyne B., Fearon W.F., Pijls N.H.J., for the FAME 2 Trial Investigators Fractional flow reserve-guided PCI for stable coronary artery disease [published correction appears in N Engl J Med 2014;371:1465] N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 18.Takx R.A.P., Blomberg B.A., El Aidi H. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8:e002666. doi: 10.1161/CIRCIMAGING.114.002666. [DOI] [PubMed] [Google Scholar]
- 19.Nørgaard B.L., Leipsic J., Gaur S., for the NXT Trial Study Group Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C.A., Fonte T.A., Min J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 21.Papadacci C., Pernot M., Couade M., Fink M., Tanter M. High-contrast ultrafast imaging of the heart. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:288–301. doi: 10.1109/TUFFC.2014.6722614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provost J., Gambhir A., Vest J., Garan H., Konofagou E.E. A clinical feasibility study of atrial and ventricular electromechanical wave imaging. Heart Rhythm. 2013;10:856–862. doi: 10.1016/j.hrthm.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correia M., Provost J., Chatelin S., Villemain O., Tanter M., Pernot M. Ultrafast harmonic coherent compound (UHCC) imaging for high frame rate echocardiography and shear-wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:420–431. doi: 10.1109/TUFFC.2016.2530408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song P., Bi X., Mellema D.C. Quantitative assessment of left ventricular diastolic stiffness using cardiac shear wave elastography. J Ultrasound Med. 2016;35:1419–1427. doi: 10.7863/ultra.15.08053. [DOI] [PubMed] [Google Scholar]
- 25.Provost J., Papadacci C., Arango J.E. 3D ultrafast ultrasound imaging in vivo. Phys Med Biol. 2014;59:L1–L13. doi: 10.1088/0031-9155/59/19/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.