Abstract
BACKGROUND
Distinguishing eosinophilic nasal polyps (NP) from noneosinophilic NP will impact prognosis and therapeutic responsiveness.
OBJECTIVE
To investigate the ability of clinical history and biomarkers to distinguish these conditions.
METHODS
A total of 74 consecutive patients undergoing surgery for NP were enrolled. Clinical presentations were evaluated using the 22-item sinonasal outcome test (SNOT-22). Biomarkers included absolute eosinophil count, IgE, and extent of tissue hyperplasia on sinus computed tomography scan. Tissue eosinophilia was quantified in 10 random hpf and data analyzed addressing both peak and average results.
RESULTS
No component of the SNOT-22 was predictive of tissue eosinophilia. Similarly, a medical history of allergic rhinitis, asthma, or aspirin-exacerbated respiratory disease was not predictive. An absolute eosinophil count of more than 300 was associated with NP tissue eosinophilia. In contrast, neither IgE nor extent of sinus computed tomography hyperplasia was predictive.
CONCLUSIONS
The ability to individualize therapies for NP is dependent on identifying clinical features or biomarkers of eosinophilia. However, with the exception of circulating eosinophilia, we could not identify a clinical feature or biomarker that robustly predicted the presence of tissue eosinophilia. Even more problematic, even the seeming “criterion standard” determination of tissue pathology was of limited value, as our cohort displayed a continuous spectrum of tissue eosinophil expression, making arbitrary any definitive cutoff distinguishing these conditions.
Keywords: Chronic rhinosinusitis, Nasal polyps, Eosinophils, Sinonasal outcome test, CT scan
The importance of properly distinguishing chronic rhinosinusitis (CRS) phenotypes is essential because of its impact on prognosis and therapeutic decisions. The presence of eosinophilic inflammation will define patients responsive to biotherapeutics that target eosinophils or type 2 cytokines. This is certainly understood in asthma where the presence of eosinophils is essential in not only defining subjects responsive to systemic and inhaled corticosteroids1–3 but also those who will respond to IL-5—targeting therapeutics.4–6 And, similarly, the presence of an IL-13high signature predicts response to its antagonists.7,8 Although topical and systemic corticosteroids (CCSs) are considered the mainstay of medical therapy for CRS,9–11 the requirement for infiltrating eosinophils has not been investigated as the basis for predicting their therapeutic responsiveness. But given the histological and immunological similarities of asthma and CRS, it is likely that this “eosinophil dependence” regarding steroid responsiveness in asthma will extend to CRS. Although not as well studied in CRS, at least 1 trial of an IL-5 antagonist in this condition reported efficacy in an IL-5high phenotype subgroup of patients.12
Current guidelines phenotype CRS according to the presence (chronic rhinosinusitis with nasal polyps [CRSwNP]) or absence (CRS without nasal polyps [NPs]) of NPs.13,14 This dichotomy was driven largely by the concept that CRSwNP is a disease more commonly characterized by prominent tissue eosinophilia along with a type 2 (IL-4high/IL-5high/IL-13high) cytokine profile. In contrast, CRS without NPs usually presents as an IL-5low non-eosinophilic disease. However, although the diagnosis of NPs strongly suggests the presence of a type 2 helper lymphocytehigh (TH2high)/IL-5high phenotype, this is far from absolute. In a recent comprehensive study investigating regional differences in CRS presentation, between 17% and 42% of patients from Europe, Australia, and China did not demonstrate a TH2high signature in their NPs and, similarly, anywhere from 20% to 75% of patients did not demonstrate an eosinophilhigh profile.15 Thus, although the presence of NPs has been used as presumptive evidence for an eosinophilic/TH2-driven process, given the frequency with which NPs can comprise a non-eosinophilic disease there is increasing recognition of the inadequacies of this approach.
With the importance of defining eosinophil status as the basis for determining individualized therapeutic approaches, a means of easily distinguishing noneosinophilic from eosinophilic NPs is required. The “criterion standard” determinant of inflammation would arguably be quantifying eosinophil number, and/or perhaps also eosinophil-derived mediators such as eosinophilic cationic protein (ECP), in tissue samples. However, this approach requires obtaining a surgical specimen and pathologists may not always be prepared to provide definitive reporting of eosinophilia or be able or to perform proper immunohistochemical analyses for eosinophil byproducts.
We speculated that eosinophilic NPs and noneosinophilic NPs would present with distinct profiles in their medical history, symptom profile, or circulating biomarkers that would predict NP pathology. We therefore addressed the utility of the 22-item sinonasal outcome test (SNOT-22) to predict NP tissue diagnosis. In addition, we investigated whether the extent of hyperplastic changes in the sinuses, as assessed by Lund-Mackay score, along with the history of allergic rhinitis (AR), asthma, or aspirin-exacerbated respiratory disease (AERD) or circulating absolute eosinophil counts (AECs) or total IgE would support the diagnosis of eosinophilic CRS.
METHODS
Subjects
Our study group consisted of 74 subjects consecutively evaluated at the University of Virginia for CRSwNP and referred for functional endoscopic sinus surgery. Eligibility for surgery required a failure of medical therapy to control symptoms that included a course of oral followed by topical CCSs. Immediately preoperative oral steroids were not used. All subjects completed a SNOT-22 before surgery and, as part of their medical evaluation, AECs and total IgE concentrations were determined using standard clinical laboratory methodologies. The presence of AR was based on specific allergen testing or a strong clinical history of seasonal variation with sneezing and ocular complaints. Comorbid asthma was based on physician diagnosis and did not include remote and/or resolved childhood disease. AERD diagnosis was based on a compelling history of exacerbation of upper and/or lower airway symptoms after exposure to aspirin or other nonselective cyclooxygenase inhibitors. Finally, we quantified the extent of sinus hyperplasia via Lund-Mackay scoring of subjects’ sinus computed tomography (CT) scans. This study was performed with the approval of the University of Virginia Institutional Review Board.
Pathological scoring
A portion of each polyp was placed in 4% paraformaldehyde (Sigma, St Louis, Mo) overnight at 4°C. The next day specimens were washed in PBS and stored in 70% ethanol until paraffin embedding. Paraffin embedding, tissue sectioning, and hematoxylin-eosin staining were performed by the Histology Core Laboratory of the University of Virginia. NPs were scored for eosinophilia on the basis of the number of eosinophils in hematoxylin-eosin—stained sections. Sections were examined under 400× magnification in a blinded fashion and positive cells were counted in 10 random sections for each sample with the final number analyzed as both the peak and as the average number of cells per 10 hpf.
Surgical outcome
All patients were treated postoperatively with twice daily large volume nasal saline irrigation and topical nasal corticosteroid. Repeat SNOT-22 scoring was performed at a follow-up visit as close as feasible to 3 months postoperatively.
Statistical analyses
For complete details, see this article’s Online Repository at www.jaci-inpractice.org. Briefly, data were summarized by frequencies and percentages, and continuous scaled data were summarized by the mean and SD of the distribution. Spearman rank correlation analyses were conducted to examine the relationship of the preoperative SNOT-22 composite and individual component scores with “average” and “peak” tissue eosinophilia counts. Comparisons of average and peak tissue eosinophilia count in relationship to asthma, AR, smoking, and AERD status were conducted via the 2-sample Welch test. Blood biomarker (absolute eosinophilia count and total IgE) and the Lund-MacKay score relationships with tissue eosinophilia were examined by ANOVA by comparing the geometric means of the blood biomarker distributions between patients who were grouped according to the tertiles of the tissue average eosinophilia count. Finally, postoperative improvement in SNOT-22 scores was analyzed by Spearman rank correlation analyses as a function of eosinophil content of NP tissue. The Spotfire Splus version 8.2 statistical package (TIBCO Inc, Palo Alto, Calif) was used to conduct the aforementioned set of statistical analyses.
RESULTS
Subjects’ demographic characteristics
A total of 74 subjects with CRSwNP who underwent functional endoscopic sinus surgery were enrolled and their demographic features are summarized in Table I.
TABLE I.
Subjects’ demographic characteristics (total n = 74)
| Characteristic | n (%) |
|---|---|
| Sex: female | 35 (47.3) |
| Age (y), mean ± SD | 46.4 ± 14.9 |
| Asthma | 39 (53.4) |
| Allergies | 38 (51.4) |
| AERD | 16 (21.6) |
| Smoking | 12 (16.2) |
Tissue eosinophilia in NP samples
NPs were analyzed for tissue eosinophilia and data were analyzed as both peak (number/400× hpf) and average tissue eosinophil counts (eosinophil/400× hpf averaged over 10 regions). Distribution of subject numbers as a function of average and peak tissue eosinophil expression is displayed in Figure 1. When visualized as average expression, subjects can be identified with low and high expression of eosinophils. However, when viewed as peak number of tissue eosinophils, a continuous distribution of eosinophil expression is observed.
FIGURE 1.
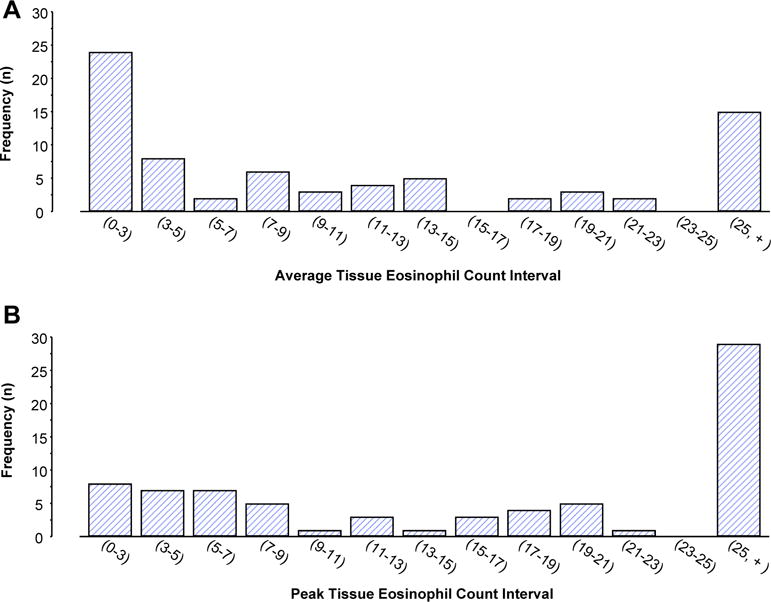
Tissue eosinophil distribution in NP samples. Eosinophils were counted in ten 400× hpfs. Distribution of the cohort as a function of average (A) and peak (B) expression is displayed. For the distribution of eosinophil numbers in Figure 1, A and B, (indicates inclusion of that number and) indicates less than that number.
SNOT-22 score as a function of tissue eosinophilia
We compared the total SNOT-22 score as a function of tissue eosinophil counts. As demonstrated in Figure 2, no correlation was observed whether analyzed as a function of peak (P= .195) or average (P = .189) eosinophil expression. For these analyses, the Spearman correlation was 0.16 (95% CI, −0.09 to 0.40) and 0.17 (95% CI, −0.09 to 0.40), respectively.
FIGURE 2.
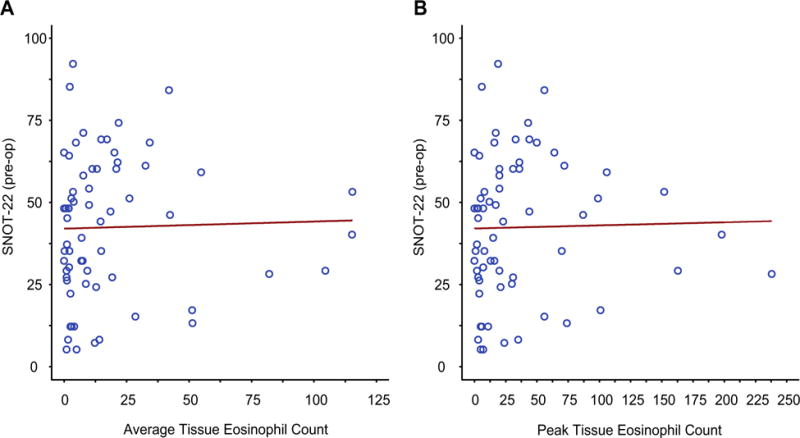
Correlation of total SNOT-22 scores with tissue eosinophilia. Correlation of total SNOT-22 scores with average (A) and peak (B) NP eosinophil counts is displayed. The Spearman rank correlation coefficient for the relationship in Figure 2, A, is 0.16 (95% CI, −0.09 to 0.40; P =.195) and the Spearman rank correlation coefficient for the relationship in Figure 2, B, is 0.17 (95% CI, −0.09 to 0.40; P = .189).
Correlation of individual components of the SNOT-22 with tissue eosinophilia
We speculated that individual components of the SNOT-22 might be superior in predicting eosinophilia and therefore analyzed individual components of the SNOT-22. Again, only very poor correlations were observed (with Spearman rank correlation coefficients ranging from −0.22 to +0.24 for average and −0.20 to 0.26 for peak eosinophil counts) and none of these achieved statistical significance (see Figure E1 and Table E1 in this article’s Online Repository at www.jaci-inpractice.org).
Relationship of medical history, sinus CT, and blood biomarkers with tissue eosinophilia
Neither medical history of AR, asthma, or AERD or a personal history of smoking predicted tissue eosinophil count (Table II). Although tissue eosinophilia was slightly higher in the asthmatic cohort, these data did not approach statistical significance. Similarly, neither circulating total IgE concentration nor extent of hyperplasia observed on the sinus CT as quantified using the Lund-MacKay score predicted the extent of eosinophilia in the polyp sample (Table E1; Figure E1). In contrast, an elevated circulating eosinophil count (AEC > 300) was significantly associated with higher average (23.8; 95% CI, 13.2–34.3; P < .01) and peak (43.0; 95% CI, 26.1–60.0; P < .05) tissue eosinophil counts compared with subjects with an AEC of 300 or less (tissue eosinophil counts of 7.7 [3.5–12.0] and 19.3 [5.2–33.5], respectively). As an alternate means of analyzing the data, the cohort was divided into tertiles on the basis of average tissue eosinophilia. Again, only statistically insignificant positive correlations with AEC and Lund-Mackay score were observed whereas total IgE demonstrated an insignificant negative correlation (Figure 3).
TABLE II.
Asthma, AR, smoking, and AERD relation to CRS tissue eosinophilia
| Condition | Average tissue eosinophil count (95% CI) | P value | Peak tissue eosinophil count (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | |||
| Asthma | 18.1 (9.0–27.2) | 16.6 (8.2–25.0) | .801 | 37.8 (20.6–55.0) | 32.8 (17.6–48.1) | .664 |
| Allergies | 17.7 (7.4–28.1) | 21.4 (10.5–32.2) | .624 | 35.5 (17.5–52.5) | 56.5 (12.3–100.8) | .364 |
| AERD | 23.6 (6.9–40.3) | 18.4 (10.0–26.8) | .563 | 48.7 (16.0–81.4) | 44.6 (16.4–72.8) | .846 |
| Smoking history* | 16.7 (6.5–26.9) | 20.1 (11.4–28.7) | .597 | 36.6 (16.0–57.2) | 47.2 (20.0–74.4) | .522 |
| IgE ≥ 100 | 15.7 (5.5–25.9) | 22.1 (9.7–35.6) | .416 | 30.0 (14.7–45.2) | 41.5 (20.0–63.0) | .371 |
| LMS ≥ 10 | 17.1 (9.9–24.2) | 9.3 (0–26.7) | .318 | 32.8 (20.9–44.6) | 13.8 (0–39.0) | .118 |
| AEC > 300 | 23.8 (13.2–34.3) | 7.7 (3.5–12.0) | .006 | 43.0 (26.1–60.0) | 19.3 (5.2–33.5) | .031 |
LMS, Lund-Mackay score.
FIGURE 3.
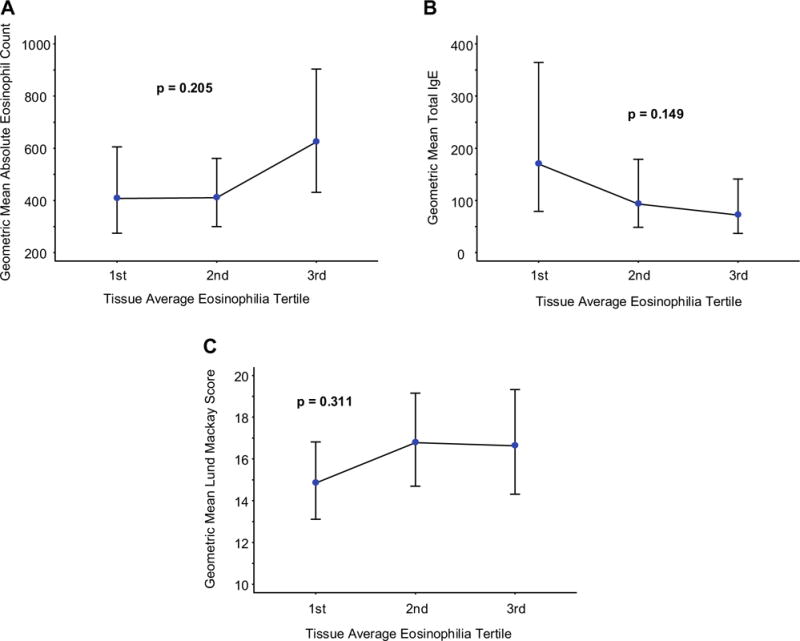
Correlation of clinical biomarkers with tissue eosinophilia. AEC (A), total serum IgE (B), and LMS (C) as a function of tissue average eosinophil tertile. Blue circles identify the geometric mean (GM), and vertical lines identify the GM 95% CI. P values are for the null hypothesis test that there are no tertile-to-tertile differences in the GM of the distribution. LMS, Lund-Mackay score.
Postoperative medical outcome
After functional endoscopic sinus surgery, all patients were treated with a regimen that included twice daily large volume saline irrigation of the sinuses and topical CCSs. SNOT-22 scores were reassessed as close as feasible to 3 months after the surgery and improvement in SNOT-22 scores analyzed as a function of eosinophil content of NP tissue. Clinically meaningful statistically significant improvements were seen in both high and low eosinophil cohorts, but eosinophil status did not influence the extent of that improvement (Figure 4).
FIGURE 4.
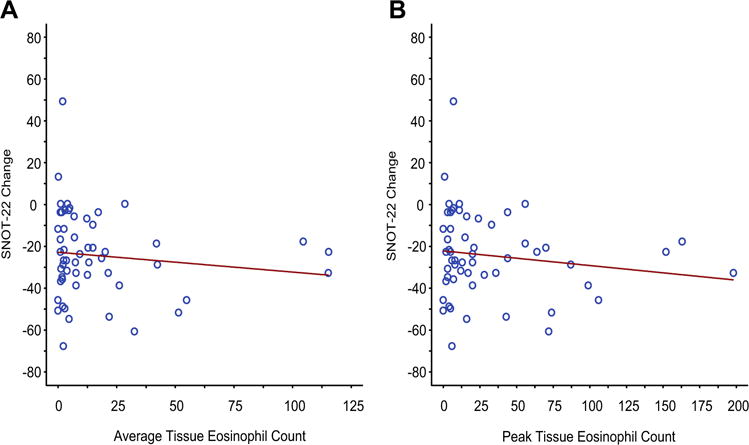
Correlation of postoperative improvement with tissue eosinophilia. Postoperative improvement in SNOT-22 scores as a function of average (A; rs = −0.13; 95% CI, −0.39 to 0.15; P =.345) and peak (B; rs = −0.12; 95% CI, −0.37 to 0.16; P =.394) tissue eosinophilia.
DISCUSSION
CRS with or without nasal polyps comprises numerous distinct conditions based on unique immunologic- and pathology-based characteristics16–20 and the responsiveness to therapeutic interventions such as CCSs and immune modulators will almost certainly depend on individualized determination of each patient’s distinct endotype. CRS often coexists with asthma and shares numerous pathogenic features.21–24 In asthma, it is well established that responsiveness to CCSs is dependent on the presence of an eosinophilic phenotype2,25 and similarly, TH2high asthma predicts responsiveness to biologics such as anti-IgE and anti—IL-5, and the IL-4/IL-13—targeting agents.4–6,25,26 Although not as well studied as asthma, there is evidence that CRS responsiveness to anti—IL-5 is dependent on an IL-5high signature.12,27 And although steroids both topically and systemically are known to be effective for NPs,9–11 the extent to which this is equally true in eosinophilic and noneosinophilic forms of the disease has not been explored.
Insofar as accurate phenotyping of NPs will prove important in determining appropriate management strategies, we evaluated the ability of clinical presentation, CT scan, and blood biomarkers to distinguish eosinophilic from noneosinophilic presentations of NPs. Neither the composite SNOT-22 score nor any individual component symptom was predictive of tissue pathology (Figures 2 and E1). We were surprised by this result because one of our premises was that noneosinophilic (often a neutrophil-predominant process) would be more likely to be associated with pain/pressure, similar to what is observed in acute rhinosinusitis. In contrast, the reported reduced expression of prostaglandin E2 in E-CRS28,29 led us to predict less sensation of pain/pressure in this presentation. Also, it has been suggested that the neuropathy associated with eosinophilia could contribute to the anosmia frequently observed in patients with NPs, but again, we found no linkage of loss of smell to an eosinophilic phenotype.
Similarly, no component of the medical history including a diagnosis of AR or asthma was predictive of eosinophil status. In retrospect, the current result is reasonable because asthma itself can be an eosinophilic or noneosinophilic disease and AR can predispose to both eosinophilic and noneosinophilic presentations of NPs.30 As with a history of AR, total IgE had no predictive value (Table II and Figure 3). Among the biomarkers we studied, only circulating eosinophilia was predictive of tissue eosinophil status (Table II). We also speculated that E-NPs would act as a self-propagating TH2-driven disease and that this would predict greater hyperplasia on Lund-Mackay scoring, but that was not observed.
Thus, with the exception of AEC, nothing in the medical history, symptom presentation, or biomarker profile predicts tissue eosinophilia. It is however possible that utilization of only polyp, as opposed to uncinate or other ethmoid tissue, may have limited our ability to explore tissue eosinophilia. These limitations contrast with the advantage of using the presence or absence of NPs to define phenotypes, given the ability to use rhinoscopy to rapidly and efficiently make a definitive designation. However, although generally predictive of a TH2high/IL-5high eosinophilic process, the mere presence of NPs cannot be used to assume the presence of eosinophilia. There is wide regionally driven variability in the extent to which NPs are associated with the expression of IL-5 ranging from as high as 83% in Benelux to a low of 20% in Chengdu, China.15 And, similarly, using a cutoff of an ECP/myeloperoxidase ratio of more than 1, the expression of an eosinophil signature in NPs ranged from 20% to 75%.
The “criterion standard” determinant of inflammation would seem to have been quantifying eosinophil number (or perhaps also eosinophil-derived mediators such as ECP) in tissue samples. In our study, although it was possible to readily define some subjects as having “low” and “high” numbers of eosinophils in their tissue, largely, a continuous distribution was observed (Figure 1, A and B) and, as with an ECP/myeloperoxidase ratio of more than 1, presumably any definitive cutoff would be arbitrary. A further challenge in defining “eosinophilic” NPs is recognizing that eosinophils are not randomly distributed throughout the polyps but can be clustered in different areas. It is, again, arbitrary to determine whether a single focus of intense eosinophilia should be sufficient to determine phenotype as opposed to demanding a more consistent widespread distribution of eosinophilia. In our analyses, we used both peak and average eosinophil counts. It is important to note that these approaches did not produce meaningfully divergent results, suggesting that either approach is appropriate.
Finally, all patients were treated with large volume nasal saline irrigation and topical CCSs postoperatively. We speculated that, similar to asthma, the presence of eosinophilia might predict CCS responsiveness and produce a superior clinical outcome. However, this was not observed, which reflects the robust clinical improvement observed across both cohorts, confirming the beneficial effects of both surgical and maximal medical therapy.31
In summary, we speculate that—similar to asthma—therapeutic responsiveness to CCSs and type 2—targeting immune modulators in patients with NPs will be dependent on the presence of an eosinophilic pathology. The ability to individualize therapies therefore will require identifying clinical features or biomarkers for eosinophilic NPs. However, with the exception of circulating eosinophilia, we could not identify a clinical feature or biomarker that robustly predicted the presence of polyp tissue eosinophilia. Even more problematic, the “criterion standard” determination of tissue pathology was of limited value indicating that if tissue eosinophilia is predictive of disease phenotype, polyp tissue is likely suboptimal for this purpose. Although individuals could be identified who were unambiguously eosinophilic or noneosinophilic, a large portion of our cohort presented across a continuous spectrum of tissue eosinophil expression, rendering arbitrary any definitive cutoff distinguishing these conditions.
Supplementary Material
What is already known about this topic?
Studies have demonstrated that nasal polyps can be eosinophilic or noneosinophilic and this knowledge can direct treatment; however, there is no way to distinguish these conditions other than by histology.
What does this article add to our knowledge?
No clinical biomarker other than absolute eosinophil count was able to help distinguish patients with eosinophilic polyps from patients with noneosinophilic polyps.
How does this study impact current management guidelines?
This study emphasizes the importance of histologic examination of surgically obtained tissue to direct treatment.
Acknowledgments
This study was supported by the National Institutes of Health (grant nos. RO1 AI1057438, UO1 AI100799, and R56 AI120055).
Abbreviations
- AEC
Absolute eosinophil count
- AERD
Aspirin-exacerbated respiratory disease
- AR
Allergic rhinitis
- CCS
Corticosteroids
- CRS
Chronic rhinosinusitis
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- CT
Computed tomography
- ECP
Eosinophil cationic protein
- NP
Nasal polyp
- SNOT
Sinonasal outcome test
Footnotes
Conflicts of interest: J. W. Steinke has received research support from the National Institutes of Health; has received lecture fees from the American Academy of Allergy, Asthma & Immunology; and receives royalties for the LUVA cell line. S. Payne has received consultancy fees from Acclarent, Medtronic, and Cook; has provided expert testimony for various legal firms; has received research support from Allakos and Knopp Bioscience; and receives royalties from Jaypee Publishing. L. Borish has received research support from the National Institutes of Health and is on the American Board of Allergy and Immunology. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Brown HM. Treatment of chronic asthma with prednisolone: significance of eosinophils in the sputum. Lancet. 1958;2:1245–7. doi: 10.1016/s0140-6736(58)91385-0. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65:384–90. doi: 10.1136/thx.2009.126722. [DOI] [PubMed] [Google Scholar]
- 4.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 5.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–66. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–41. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 7.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 8.Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–56. doi: 10.1136/thoraxjnl-2014-206719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartwig S, Linden M, Laurent C, Vargo AK, Lindqvist N. Budesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial) J Laryngol Otol. 1988;102:148–51. doi: 10.1017/s0022215100104372. [DOI] [PubMed] [Google Scholar]
- 10.Steinke JW, Payne SC, Tessier E, Borish L, Han JK, Borish L. Pilot study of budesonide inhalent suspension irrigations for chronic eosinophilic sinusitis. J Allergy Clin Immunol. 2009;124:1352–4. doi: 10.1016/j.jaci.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med. 2011;154:293–302. doi: 10.7326/0003-4819-154-5-201103010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995.e1-e8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Slavin RG, Spector SL, Berstein IL, Kaliner MA, Kennedy DW, Virant FS, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S13–47. doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 14.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012: a summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–53. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 17.Berger G, Kattan A, Bernheim J, Ophir D. Polypoid mucosa with eosinophilia and glandular hyperplasia in chronic sinusitis: a histopathological and immunohistchemical study. Laryngoscope. 2002;112:738–45. doi: 10.1097/00005537-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007;137:925–30. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121:2262–7. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Gao Z, Wang H, Li T, He W, Lv W, et al. Transcriptome analysis reveals distinct gene expression profiles in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. Sci Rep. 2016;6:26604. doi: 10.1038/srep26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 22.ten Brinke A, Grootendorst DC, Schmidt JT, de Bruine FT, van Buchem MA, Sterk PJ, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–6. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 23.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Gaffey TA, Tarara JE, et al. Features of airway remodeling eosinophilic inflammation in chronic rhinosinusitis: is the histopathology similar to asthma. J Allergy Clin Immunol. 2003;112:877–82. doi: 10.1016/j.jaci.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Mascia K, Borish L, Patrie J, Hunt J, Phillips CD, Steinke JW. Chronic hyperplastic eosiniphilic sinusistis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–7. doi: 10.1016/S1081-1206(10)61323-3. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 27.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Schmid M, Gode U, Schafer D, Wigand ME. Arachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmatics. Acta Otolaryngol. 1999;119:277–80. doi: 10.1080/00016489950181819. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Novo CA, Watelet JB, Claeys C, van Cauwenberge P, Bachert C. Pros-taglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–96. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Steinke JW, Borish L. Chronic rhinosinusitis phenotypes and endotypes. Ann Allergy Asthma Immunol. 2016;117:234–40. doi: 10.1016/j.anai.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol. 2013;111:246–251.e2. doi: 10.1016/j.anai.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


