Abstract
BACKGROUND
Postinfectious hydrocephalus in infants is a major health problem in sub-Saharan Africa. The conventional treatment is ventriculoperitoneal shunting, but surgeons are usually not immediately available to revise shunts when they fail. Endoscopic third ventriculostomy with choroid plexus cauterization (ETV–CPC) is an alternative treatment that is less subject to late failure but is also less likely than shunting to result in a reduction in ventricular size that might facilitate better brain growth and cognitive outcomes.
METHODS
We conducted a randomized trial to evaluate cognitive outcomes after ETV–CPC versus ventriculoperitoneal shunting in Ugandan infants with postinfectious hydrocephalus. The primary outcome was the Bayley Scales of Infant Development, Third Edition (BSID-3), cognitive scaled score 12 months after surgery (scores range from 1 to 19, with higher scores indicating better performance). The secondary outcomes were BSID-3 motor and language scores, treatment failure (defined as treatment-related death or the need for repeat surgery), and brain volume measured on computed tomography.
RESULTS
A total of 100 infants were enrolled; 51 were randomly assigned to undergo ETV–CPC, and 49 were assigned to undergo ventriculoperitoneal shunting. The median BSID-3 cognitive scores at 12 months did not differ significantly between the treatment groups (a score of 4 for ETV–CPC and 2 for ventriculoperitoneal shunting; Hodges–Lehmann estimated difference, 0; 95% confidence interval [CI], −2 to 0; P = 0.35). There was no significant difference between the ETV–CPC group and the ventriculoperitoneal-shunt group in BSID-3 motor or language scores, rates of treatment failure (35% and 24%, respectively; hazard ratio, 0.7; 95% CI, 0.3 to 1.5; P = 0.24), or brain volume (z score, −2.4 and −2.1, respectively; estimated difference, 0.3; 95% CI, −0.3 to 1.0; P = 0.12).
CONCLUSIONS
This single-center study involving Ugandan infants with postinfectious hydrocephalus showed no significant difference between endoscopic ETV–CPC and ventriculoperitoneal shunting with regard to cognitive outcomes at 12 months. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT01936272.)
The health burden of hydrocephalus in infants is substantial in sub-Saharan Africa, where it affects approximately 180,000 infants per year.1 Hydrocephalus in this region is most commonly postinfectious, occurring after neonatal ventriculitis.2 In resource-limited settings, there are economic, cultural, and geographic barriers to accessing treatment, even though the benefit–cost ratio for treatment has been shown to be favorable.3 The two available treatments are ventriculoperitoneal shunting4,5 and endoscopic third ventriculostomy with choroid plexus cauterization (ETV–CPC).6–11 The endoscopic procedure relieves hydrocephalus by diverting the flow of cerebrospinal fluid (CSF), and cauterization of the choroid plexus reduces CSF production, further reducing hydrocephalus.12 There is uncertainty regarding the relative benefits of these two procedures. Ventriculoperitoneal shunting is technically straightforward and has a lower rate of failure in the months immediately after surgery than does ETV–CPC, but it is more prone to failure over the long term, and patients with shunt-dependent hydrocephalus can require urgent shunt-revision surgery because of obstruction of the shunt tubing or valve.13 Shunt failure can be fatal in regions of the world where there is no access to immediate neurosurgical care. In contrast, ETV–CPC is technically more difficult than shunting, but it has the advantage that virtually all failures occur within 6 months,10,11,14 after which the risk of failure is low.13 This characteristic allows for correction during infancy, when the urgency of intervention is lower than it is later in life. Ventricular shunting, however, also generally results in a greater reduction of ventricle size than does ETV–CPC, potentially leading to better cognitive outcome than the endoscopic procedure; this difference between procedures has been shown inconsistently.15–23 We conducted a randomized trial to compare cognitive outcomes at 12 months between infants who underwent ETV–CPC and those who underwent ventriculoperitoneal shunting for postinfectious hydrocephalus in the resource-limited country of Uganda.
METHODS
TRIAL DESIGN AND OVERSIGHT
The trial was conducted at the CURE Children’s Hospital of Uganda (CCHU), a freestanding pediatric neurosurgical hospital in eastern Uganda that serves as a countrywide referral center for patients with hydrocephalus. Infants were eligible for participation in the trial if they were younger than 180 days of age, met established criteria for postinfectious hydrocephalus,2 had a mother who was at least 18 years of age, and lived in one of 31 districts in eastern Uganda that would facilitate access to follow-up at our center. Exclusion criteria included active CSF infection, computed tomographic (CT) evidence of congenital brain anomaly, and severe anatomical brain distortion or multiloculated hydrocephalus due to infection, any of which would make ventricular endoscopy difficult. Children were screened independently by two neurosurgeons, who determined whether either procedure would be appropriate to treat the hydrocephalus. Written informed consent was obtained from the mother of each patient in her home language; an English version of the consent form is provided with the protocol, available with the full text of this article at NEJM.org. All the authors vouch for the accuracy and completeness of the data, the reporting of adverse events, and the fidelity of the trial to the protocol.
The trial was approved by the ethics boards of CCHU, Boston Children’s Hospital, the Hospital for Sick Children, and Pennsylvania State University. For administrative reasons, the trial was registered with ClinicalTrials.gov on July 29, 2013, which was 2 months after the first patient was screened (May 27, 2013) and after 6 of the 100 patients had been enrolled. Patients were randomly assigned in a nonstratified, simple 1:1 scheme generated at CCHU; assignments were concealed in sealed, opaque envelopes, which were opened in the preoperative holding area just before the patient entered the operating room.
SURGICAL TREATMENT OF HYDROCEPHALUS
ETV–CPC was performed through a unilateral frontal incision with a flexible endoscope.6 The floor of the third ventricle was opened to create a ventriculocisternostomy that produced a route for decompression of the ventricles into the perimesencephalic and peripontine CSF spaces. The choroid plexus in each lateral ventricle was cauterized. If required, the septum pellucidum was fenestrated to access the choroid plexus in the contralateral ventricle. If the floor of the third ventricle could not be opened or if there was intraoperative evidence of prepontine cistern scarring, which correlates with a high rate of later failure of endoscopic third ventriculostomy,14 a ventricular shunt was inserted.24 The ventricular shunt was a Chhabra shunt (Surgiwear), the standard type used at CCHU.4,5 All the patients received preoperative intravenous ceftriaxone prophylaxis, with an additional postoperative dose given after ventriculoperitoneal shunting, in accordance with standard CCHU treatment. Postoperative care, with a minimum 2-day hospital stay, was otherwise similar between the two groups.
Patients were examined at approximately 1, 3, 6, and 12 months after surgery. Home visits were conducted for patients who did not return for follow-up. Cranial CT was performed at 6 and 12 months.
OUTCOMES
The primary outcome was the Bayley Scales of Infant Development, Third Edition (BSID-3), cognitive scaled score at 12 months after surgery. Scores on the BSID-3, which is used to evaluate infants and toddlers 1 to 42 months of age, range from 1 to 19, with higher scores indicating better performance; the mean (±SD) score in the general population is 10±3. Secondary outcomes at 12 months included the BSID-3 expressive language, receptive language, general motor, and fine motor scaled scores. A trained evaluator under the supervision of a neuropsychologist, neither of whom was aware of the treatment assignments, used a culturally modified BSID-3 to evaluate all the children.22 The baseline BSID-3 assessment was performed within 1 week before surgery. In one case, the baseline assessment was performed on the first postoperative day. During the BSID-3 follow-up evaluations, the children wore hooded jackets to conceal the treatment assignment from the evaluators.
Other secondary outcomes were mortality, the rate of infection, and the time to treatment failure. Successful treatment was determined with the use of clinical and radiographic criteria2,4–6: the shift in the growth of head circumference to a normal rate, as plotted on a standard growth chart; decompression of the anterior fontanel; relief of symptoms of elevated intracranial pressure, such as irritability and vomiting; resolution of down-gaze or sixth cranial nerve palsy; and a decrease or arrest in ventriculomegaly as determined on ultrasonography or CT. Treatment failure was defined as treatment-related death or the need for a second operation for infection or for the recurrence of hydrocephalus.25
We used previously described, semiautomated image segmentation of CT scans to calculate brain and CSF volume, and normalized curves for brain growth that were derived from magnetic resonance imaging (MRI) scans in the National Institutes of Health Pediatric MRI Data Repository (https://pediatricmri.nih.gov/nihpd/info/index.html) to quantify the normative growth percentile for the age and sex of each patient.26 These assessments were performed by persons who were not aware of the patients’ BSID-3 scores but were aware of the treatment assignments.
Post hoc exploratory analyses included a comparison of cognitive scores between the treatment groups with adjustment for age at randomization and baseline BSID-3 score, comparison between the treatment groups of the change from baseline to 12 months in the BSID-3 score, the proportion of patients with substantial brain growth (an increase in the z score for brain volume of ≥1 from baseline to 12 months), and the proportion of patients with normal brain volume (i.e., a brain volume larger than 1 SD below the age-corrected and sex-corrected mean) at baseline and at 12 months.
STATISTICAL ANALYSIS
On the basis of previously obtained data,22 we expected that this population of children would have a BSID-3 cognitive scaled score of 6±4 after relief of hydrocephalus. We estimated that a clinically meaningful adjusted difference between the treatment groups would be 3 (approximately three quarters of an SD) on this scale. Assuming 20% mortality and 5% loss to follow-up, we estimated that 50 infants per group (100 in total) would need to be recruited in order for the trial to have 90% power to detect a clinically meaningful between-group difference in the BSID-3 cognitive scaled score at a two-sided alpha level of 0.05.
The primary analysis, which was performed in the modified intention-to-treat population (i.e., all patients who underwent randomization and for whom data were available at 12 months), was a comparison of 12-month scaled BSID-3 cognitive scores between the groups. The analysis was conducted with the use of the Mann–Whitney test and Hodges–Lehmann estimate of confidence intervals for medians. An analysis of covariance was also performed, with adjustment for age at randomization and baseline BSID-3 cognitive score. The secondary outcomes of 12-month scaled BSID-3 expressive language, receptive language, general motor, and fine motor scores were compared between the treatment groups with the use of the Mann–Whitney test. The Kaplan–Meier method with a log-rank test was used to estimate the between-group difference in the probability of treatment failure over time. Cox regression analysis was used to estimate the hazard ratio for treatment failure, with adjustment for age at randomization. The proportional-hazards assumption was confirmed by the nonsignificance of treatment as an interaction with time. Brain volumes were converted to z scores on the basis of previously described age-adjusted and sex-adjusted volume distributions,26 and these normalized brain and CSF volumes were compared between the treatment groups with the use of the Mann–Whitney test. For the results of the post hoc exploratory analyses, raw results and 95% confidence intervals are provided.
RESULTS
PATIENTS
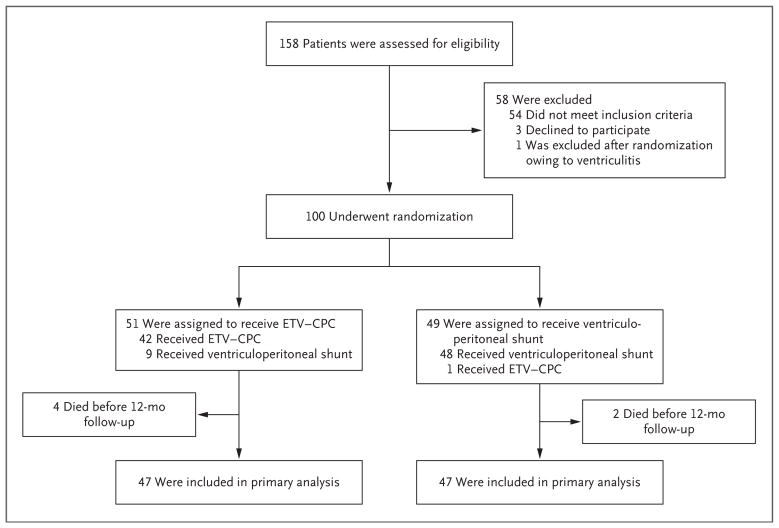
Between May 2013 and April 2015, a total of 158 patients were screened (Fig. 1), and 100 patients were enrolled; 51 were assigned to the endoscopic-ventriculostomy group, and 49 were assigned to the ventriculoperitoneal-shunt group. The reasons for the exclusion of 57 of the patients were distorted ventricular anatomy that would have precluded the ability to safely perform third ventriculostomy (26 patients), ventricular loculations that would have required either endoscopic fenestrations or more than one ventriculoperitoneal-shunt catheter (17), preexisting congenital anomalies (4), active CSF infections (4), absent cortical mantle (3), and the mother’s decision to decline to provide consent (3). To account for the exclusion (due to active ventriculitis) of 1 patient after randomization, an additional patient was recruited. The baseline characteristics of the patients are shown in Table 1. Of the 51 patients who were randomly assigned to the ETV–CPC group, 42 underwent ETV–CPC. Of the 49 patients who were randomly assigned to the ventriculoperitoneal-shunt group, 48 received a ventriculoperitoneal shunt. Head circumference and CSF volume were significantly greater at baseline in the ventriculoperitoneal-shunt group than in the ETV–CPC group, but there were no other significant imbalances between the groups. Outcome data were missing for 6 patients (4 in the ETV–CPC group and 2 in the ventriculoperitoneal-shunt group), all of whom died before the 12-month follow-up; these patients were not included in the modified intention-to-treat population but are included in the intention-to-treat survival analysis.
Figure 1.
Enrollment, Randomization, Treatment, and Follow-up.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Overall (N = 100) | ETV–CPC Group (N = 51) | Ventriculoperitoneal-Shunt Group (N = 49) |
|---|---|---|---|
| Median age at randomization (IQR) — mo | 3.1 (2.6 to 4.1) | 3.1 (2.2 to 4.2) | 3.1 (2.7 to 3.9) |
| Female sex — no. (%) | 39 (39) | 21 (41) | 18 (37) |
| Median weight (IQR) — kg | 6.0 (5.0 to 7.0) | 5.6 (4.5 to 7.0) | 6.2 (5.4 to 7.2) |
| Median head circumference (IQR) — cm | 48.5 (45.2 to 50.8) | 48.1 (44.5 to 50.0) | 48.7 (45.8 to 53.5) |
| Median BSID-3 scaled score (IQR)† | |||
| Cognitive | 1 (1 to 5) | 1 (1 to 5) | 1 (1 to 5) |
| Expressive language | 3 (1 to 6) | 3 (1 to 7) | 2 (1 to 6) |
| Receptive language | 6 (1 to 8) | 6 (2 to 9) | 5 (1 to 8) |
| General motor | 3 (1 to 5) | 3 (1 to 6) | 2 (1 to 4) |
| Fine motor | 3 (1 to 5) | 4 (1 to 6) | 3 (1 to 4) |
| Median CSF volume (IQR) — cm3 | 783 (570 to 1050) | 741 (542 to 992) | 824 (639 to 1345) |
| Median z score for brain volume (IQR) | −2.6 (−3.2 to −1.9) | −2.6 (−3.1 to −2.1) | −2.4 (−3.4 to −1.8) |
| Normal brain volume — no./total no. (%) | 10/99 (10) | 5/50 (10) | 5/49 (10) |
Data on brain volume and cerebrospinal fluid (CSF) volume were available for 50 infants in the endoscopic third ventriculostomy with choroid plexus cauterization (ETV–CPC) group and 49 infants in the ventriculoperitoneal-shunt group. There were significant between-group differences in head circumference (P = 0.03 by Mann–Whitney test) and CSF volume (P = 0.05 by Mann–Whitney test). IQR denotes interquartile range.
Bayley Scales of Infant Development, Third Edition (BSID-3), scaled scores range from 1 to 19, with higher scores indicating better performance. The scores are scaled to a mean (±SD) of 10±3 in the general population.
OUTCOMES
Primary outcome data at 12 months were available for 47 patients in each treatment group (Fig. 1). There was no significant between-group difference at 12 months in the median BSID-3 cognitive score, which was 4 in the ETV–CPC group and 2 in the ventriculoperitoneal-shunt group (estimated difference, 0; 95% confidence interval [CI], −2 to 0; P=0.35) (Table 2). The results were similar when adjusted for age at randomization and baseline BSID-3 cognitive score (P=0.44).
Table 2.
Outcomes at 12 Months.*
| Outcome | Overall (N = 100) | ETV–CPC Group (N = 51) | Ventriculoperitoneal-Shunt Group (N = 49) | Estimated Difference (95% CI)† | P Value‡ |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Median BSID-3 cognitive score (IQR) | 3 (1 to 6) | 4 (1 to 7) | 2 (1 to 6) | 0 (−2 to 0) | 0.35 |
| Secondary outcomes | |||||
| Median BSID-3 expressive language score (IQR) | 2 (1 to 5) | 3 (2 to 4) | 2 (1 to 5) | 0 (−1 to 1) | 0.84 |
| Median BSID-3 receptive language score (IQR) | 4 (3 to 6) | 5 (3 to 6) | 4 (2 to 6) | 0 (−1 to 0) | 0.34 |
| Median BSID-3 general motor score (IQR) | 1 (1 to 3) | 1 (1 to 4) | 1 (1 to 2) | 0 (0 to 0) | 0.14 |
| Median BSID-3 fine motor score (IQR) | 4 (1 to 7) | 4 (1 to 7) | 3 (1 to 6) | 0 (−2 to 0) | 0.25 |
| Median CSF volume (IQR) — cm3 | 311 (97 to 526) | 410 (236 to 668) | 171 (80 to 366) | −221 (−325 to −131) | <0.001 |
| Median brain volume (IQR) — z score | −2.3 (−3.3 to −1.0) | −2.4 (−3.5 to −1.4) | −2.1 (−3.2 to −0.9) | 0.3 (−0.3 to 1.0) | 0.12 |
| Treatment failure — no. (%) | 30 (30) | 18 (35) | 12 (24) | −10.8 (−28.6 to 7.0) | 0.28 |
| Infection — no. (%) | 4 (4) | 2 (4) | 2 (4) | 0.2 (−7.5 to 7.8) | 1.00 |
| All-cause mortality — no. (%) | 6 (6) | 4 (8) | 2 (4) | −3.8 (−13.0 to 5.5) | 0.68 |
Data at 12 months for BSID-3 scores and volume measurements were available for 47 patients in each treatment group (94 patients in total).
Hodges–Lehmann estimates and 95% confidence intervals were used for differences in medians and normal approximation for differences in proportions. Because of the use of the Hodges–Lehmann estimator, the estimated difference is not the crude difference between the medians.
P values were based on a Mann–Whitney test or Fisher’s exact test.
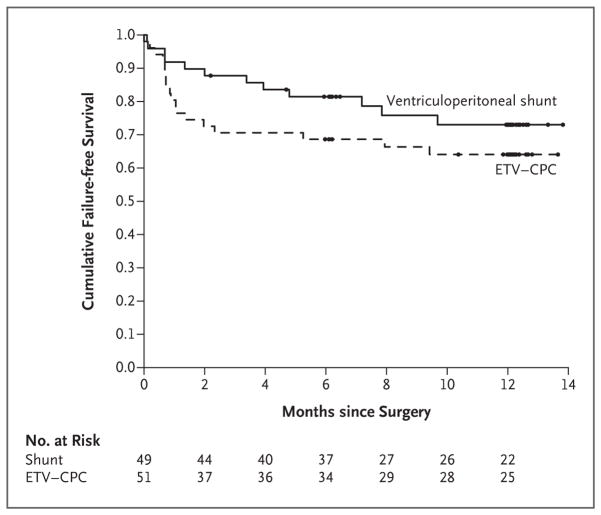
There was no significant difference between the treatment groups at 12 months in the BSID-3 expressive language score (estimated difference, 0; 95% CI, −1 to 1; P = 0.84), receptive language score (estimated difference, 0; 95% CI, −1 to 0; P = 0.34), general motor score (estimated difference, 0; 95% CI, 0 to 0; P = 0.14), or fine motor score (estimated difference, 0; 95% CI, −2 to 0; P = 0.25) or in brain volume at 12 months (estimated difference in median z score, 0.3; 95% CI, −0.3 to 1.0; P = 0.12) (Table 2). The median CSF volume at 12 months was significantly lower in the ventriculoperitoneal-shunt group than in the ETV–CPC group (estimated difference, −221 cm3; 95% CI, −325 to −131; P<0.001). There were 18 treatment failures (35%) in the ETV–CPC group and 12 (24%) in the ventriculoperitoneal-shunt group (P=0.28) (Table 2 and Fig. 2). The estimated rates of failure-free survival did not differ significantly between the groups (adjusted hazard ratio for treatment failure, 0.7; 95% CI, 0.3 to 1.5; P = 0.24 by log-rank test).
Figure 2. Kaplan–Meier Survival Curves for Time to First Treatment Failure in the Intention-to-Treat Population.
ETV–CPC denotes endoscopic third ventriculostomy with choroid plexus cauterization.
Two deaths were judged by the investigators to be related to the trial treatment: one in a patient who was randomly assigned to the ETV–CPC group and underwent ETV–CPC, in whom death was attributed to treatment failure 8 months after surgery; and one in a patient who was assigned to the ETV–CPC group and crossed over to ventriculoperitoneal shunting, in whom ventriculitis and subdural empyema developed 5.5 months after surgery. Nine other patients died from unrelated causes, 4 of whom died during the first 12 months after surgery: 4 patients died from acute gastroenteritis (at 7, 12, 24, and 29 months), 3 from malnutrition (at 12, 12, and 21 months), 1 from pneumonia (at 22 months), and 1 from measles (at 24 months). There were four procedure-related infections (one fatal, as described above), all of which occurred after ventriculoperitoneal shunting was performed (two as assigned and two crossovers). Total mortality and rates of infection did not differ significantly between the treatment groups (Table 2).
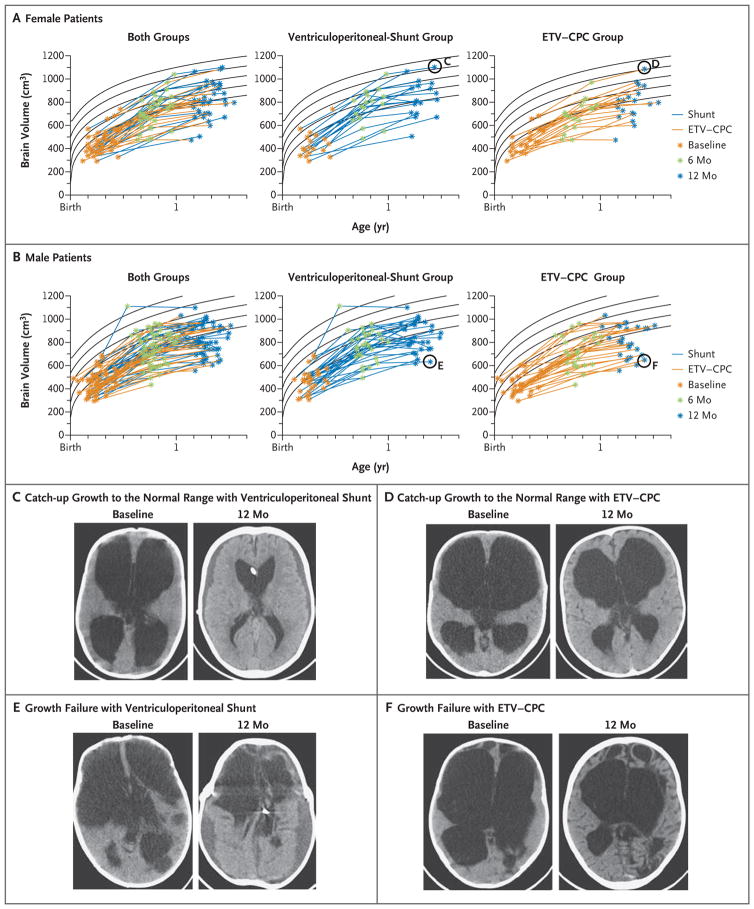
Post hoc analyses comparing the change in BSID-3 scores from baseline to 12 months between the treatment groups are shown in Table S1 in the Supplementary Appendix, available at NEJM.org. The percentage of patients in whom a normal brain volume was achieved was 21% in the ETV–CPC group and 28% in the ventriculoperitoneal-shunt group, and the percentage of patients with substantial brain growth was 17% in the ETV–CPC group and 36% in the ventriculoperitoneal-shunt group (Fig. 3, and Table S1 in the Supplementary Appendix). Overall, 10% of infants had normal brain volume at baseline, but 27% had an increase in brain volume of more than 1 SD, with 24% of patients having a normal volume at 12 months. In the ventriculoperitone-al-shunt group, head circumference at 12 months was numerically smaller than in the ETV–CPC group (estimated difference, −1.6 cm; 95% CI, −3.0 to −0.2), and there was a numerically larger reduction in head circumference from baseline in this group (estimated difference, −4.0 cm; 95% CI, −5.5 to −2.5). These analyses were repeated according to the treatment as received, and the results were similar (Tables S2 and S3 in the Supplementary Appendix). The results of the remainder of the post hoc analyses are provided in Table S4 in the Supplementary Appendix.
Figure 3. Brain Volume.
Panels A and B show brain-volume trajectories for female and male patients, determined by examination of preoperative, 6-month postoperative, and 12-month postoperative scans. Curve fits (Weibull distribution) to normative data26 are indicated with black lines representing the age-adjusted mean and 1 and 2 SD above and below the mean. The scans represented by the circled data points in Panel A are shown in Panels C and D (for a patient in each treatment group who had catch-up growth to the normal range), and the scans represented by circled data points in Panel B are shown in Panels E and F (for a patient in each treatment group who had growth failure). In Panels C through F, the preoperative (baseline) scan is shown on the left, and the corresponding 12-month postoperative scan is shown on the right.
DISCUSSION
For infants younger than 6 months of age with postinfectious hydrocephalus in Uganda, our randomized trial did not show a significant difference between ETV–CPC and ventriculoperitoneal shunting with regard to the primary outcome of cognitive development. Previous studies4–6,21,22,27,28 provided equipoise for our trial, which showed that the between-group differences in median BSID-3 outcomes were not significant and that the estimates of differences in the effects of the two treatments were no greater than 1 point on the BSID-3 scale, with 95% confidence intervals of no greater than 2 points in either direction (Table 2).
Because of the severity of the initial brain injury from infection,28 the overall developmental outcome was relatively poor for both treatments. Nearly all the patients (90%) began the trial with an abnormally low brain volume, but nearly a quarter (24%) of the entire group had a normal brain volume by 1 year (Fig. 3, and Table S1 in the Supplementary Appendix). Ventriculoperitoneal shunting resulted in a significantly greater reduction in CSF volume and a numerically higher proportion of patients with substantial brain growth. The proportion of children who ultimately had a normal brain volume, however, was numerically similar in the two treatment groups, which suggests that similar growth was achieved with both treatments.
The incidence of complications, including infection and treatment-related death, was similar in the two groups. There was a numerically but not significantly higher rate of early treatment failure rate in the ETV–CPC group (Fig. 2). However, previous reports have suggested the failure rates associated with ETV–CPC are lower thereafter.10,11,13,14 The duration of our trial was not sufficient to explore the rate of late ETV–CPC failure beyond 1 year.
Our trial addressed only one type of hydrocephalus, the postinfectious variety, which is often associated with substantial additional brain injury.28 It is possible that results might differ for higher-functioning infants with less primary brain injury than those in this trial. The trial was performed at a single center with established expertise in ETV–CPC, which limits the generalizability of the results. The endoscopic procedure, however, has been successfully taught and applied in other low-income and middle-income countries (http://cure.org/hydrocephalus), including Nigeria and Zambia.29,30 Our trial lacked sufficient power to rule out small differences in outcomes, including treatment failure and brain volume. Although the evaluators of the primary outcome were not aware of the treatment assignments, the caregivers and the medical team were, and this could have biased the assessments of some of the secondary outcomes. An interval of 1 year is relatively short, and assessing cognitive development in young infants, especially those with poor functioning, is challenging. Although the BSID-3 is a standardized and frequently used tool, longer-term follow-up with other assessment tools is needed.
In conclusion, in Ugandan infants with postinfectious hydrocephalus, this single-center, randomized trial did not show a significant difference in cognitive outcomes at 12 months between infants who underwent ETV–CPC and those who underwent ventriculoperitoneal-shunt placement.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Fogarty International Center (project numbers R21TW009612 and R01HD085853). No industry funding was received for this trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Warf BC. Educate one to save a few, educate a few to save many. World Neurosurg. 2013;79(2 Suppl):S15.e15–S15.e18. doi: 10.1016/j.wneu.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg. 2005;102:1–15. doi: 10.3171/ped.2005.102.1.0001. [DOI] [PubMed] [Google Scholar]
- 3.Warf BC, Alkire BC, Bhai S, et al. Costs and benefits of neurosurgical intervention for infant hydrocephalus in sub-Saharan Africa. J Neurosurg Pediatr. 2011;8:509–21. doi: 10.3171/2011.8.PEDS11163. [DOI] [PubMed] [Google Scholar]
- 4.Warf BC. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J Neurosurg. 2005;102:358–62. doi: 10.3171/ped.2005.102.4.0358. [DOI] [PubMed] [Google Scholar]
- 5.Lane JD, Mugamba J, Ssenyonga P, Warf BC. Effectiveness of the Bactiseal Universal Shunt for reducing shunt infection in a sub-Saharan African context: a retrospective cohort study in 160 Ugandan children. J Neurosurg Pediatr. 2014;13:140–4. doi: 10.3171/2013.11.PEDS13394. [DOI] [PubMed] [Google Scholar]
- 6.Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg. 2005;103:475–81. doi: 10.3171/ped.2005.103.6.0475. [DOI] [PubMed] [Google Scholar]
- 7.Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr. 2008;2:310–6. doi: 10.3171/PED.2008.2.11.310. [DOI] [PubMed] [Google Scholar]
- 8.Warf BC, Stagno V, Mugamba J. Encephalocele in Uganda: ethnic distinctions in lesion location, endoscopic management of hydrocephalus, and survival in 110 consecutive children. J Neurosurg Pediatr. 2011;7:88–93. doi: 10.3171/2010.9.PEDS10326. [DOI] [PubMed] [Google Scholar]
- 9.Warf BC, Dewan M, Mugamba J. Management of Dandy-Walker complex-associated infant hydrocephalus by combined endoscopic third ventriculostomy and choroid plexus cauterization. J Neurosurg Pediatr. 2011;8:377–83. doi: 10.3171/2011.7.PEDS1198. [DOI] [PubMed] [Google Scholar]
- 10.Warf BC, Tracy S, Mugamba J. Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants. J Neurosurg Pediatr. 2012;10:108–11. doi: 10.3171/2012.4.PEDS1253. [DOI] [PubMed] [Google Scholar]
- 11.Warf BC. Congenital idiopathic hydrocephalus of infancy: the results of treatment by endoscopic third ventriculostomy with or without choroid plexus cauterization and suggestions for how it works. Childs Nerv Syst. 2013;29:935–40. doi: 10.1007/s00381-013-2072-1. [DOI] [PubMed] [Google Scholar]
- 12.Kahle KT, Kulkarni AV, Limbrick DD, Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788–99. doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni AV, Drake JM, Kestle JR, et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery. 2010;67:588–93. doi: 10.1227/01.NEU.0000373199.79462.21. [DOI] [PubMed] [Google Scholar]
- 14.Warf BC, Kulkarni AV. Intraoperative assessment of cerebral aqueduct patency and cisternal scarring: impact on success of endoscopic third ventriculostomy in 403 African children. J Neurosurg Pediatr. 2010;5:204–9. doi: 10.3171/2009.9.PEDS09304. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher JM, Bohan TP, Brandt ME, et al. Cerebral white matter and cognition in hydrocephalic children. Arch Neurol. 1992;49:818–24. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JM, Bohan TP, Brandt ME, et al. Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Childs Nerv Syst. 1996;12:192–9. doi: 10.1007/BF00301250. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher JM, McCauley SR, Brandt ME, et al. Regional brain tissue composition in children with hydrocephalus: relationships with cognitive development. Arch Neurol. 1996;53:549–57. doi: 10.1001/archneur.1996.00550060093022. [DOI] [PubMed] [Google Scholar]
- 18.Azab WA, Mijalcic RM, Nakhi SB, Mohammad MH. Ventricular volume and neurocognitive outcome after endoscopic third ventriculostomy: is shunting a better option? A review. Childs Nerv Syst. 2016;32:775–80. doi: 10.1007/s00381-016-3032-3. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AV, Hui S, Shams I, Donnelly R. Quality of life in obstructive hydrocephalus: endoscopic third ventriculostomy compared to cerebrospinal fluid shunt. Childs Nerv Syst. 2010;26:75–9. doi: 10.1007/s00381-009-0983-7. [DOI] [PubMed] [Google Scholar]
- 20.Larysz P, Larysz D, Mandera M. Radiological findings in relation to the neurodevelopmental outcome in hydrocephalic children treated with shunt insertion or endoscopic third ventriculostomy. Childs Nerv Syst. 2014;30:99–104. doi: 10.1007/s00381-013-2200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandell JG, Kulkarni AV, Warf BC, Schiff SJ. Volumetric brain analysis in neurosurgery: Part 2. Brain and CSF volumes discriminate neurocognitive outcomes in hydrocephalus. J Neurosurg Pediatr. 2015;15:125–32. doi: 10.3171/2014.9.PEDS12427. [DOI] [PubMed] [Google Scholar]
- 22.Warf B, Ondoma S, Kulkarni A, et al. Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr. 2009;4:564–70. doi: 10.3171/2009.7.PEDS09136. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni AV, Donnelly R, Mabbott DJ, Widjaja E. Relationship between ventricular size, white matter injury, and neurocognition in children with stable, treated hydrocephalus. J Neurosurg Pediatr. 2015;16:267–74. doi: 10.3171/2015.1.PEDS14597. [DOI] [PubMed] [Google Scholar]
- 24.Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization (ETV/CPC) for hydrocephalus in infants and children with special emphasis on the developing world. 2. Tuttlingen, Germany: Endo-Press; 2015. [Google Scholar]
- 25.Marano PJ, Stone SS, Mugamba J, Ssenyonga P, Warf EB, Warf BC. Reopening of an obstructed third ventriculostomy: long-term success and factors affecting outcome in 215 infants. J Neurosurg Pediatr. 2015;15:399–405. doi: 10.3171/2014.10.PEDS14250. [DOI] [PubMed] [Google Scholar]
- 26.Mandell JG, Langelaan JW, Webb AG, Schiff SJ. Volumetric brain analysis in neurosurgery: Part 1. Particle filter segmentation of brain and cerebrospinal fluid growth dynamics from MRI and CT images. J Neurosurg Pediatr. 2015;15:113–24. doi: 10.3171/2014.9.PEDS12426. [DOI] [PubMed] [Google Scholar]
- 27.Warf BC, Wright EJ, Kulkarni AV. Factors affecting survival of infants with myelomeningocele in southeastern Uganda. J Neurosurg Pediatr. 2011;7:127–33. doi: 10.3171/2010.11.PEDS10428. [DOI] [PubMed] [Google Scholar]
- 28.Warf BC, Dagi AR, Kaaya BN, Schiff SJ. Five-year survival and outcome of treatment for postinfectious hydrocephalus in Ugandan infants. J Neurosurg Pediatr. 2011;8:502–8. doi: 10.3171/2011.8.PEDS11221. [DOI] [PubMed] [Google Scholar]
- 29.Bankole OB, Ojo OA, Nnadi MN, Kanu OO, Olatosi JO. Early outcome of combined endoscopic third ventriculostomy and choroid plexus cauterization in childhood hydrocephalus. J Neurosurg Pediatr. 2015;15:524–8. doi: 10.3171/2014.10.PEDS14228. [DOI] [PubMed] [Google Scholar]
- 30.Mweshi M, Amosun S, Ngoma M, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in childhood hydrocephalus in Zambia. Med J Zambia. 2010;37(4):e246–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.