Abstract
IMPORTANCE
Alterations in ocular blood flow play an important role in the pathogenesis and progression of diabetic retinopathy (DR). However, the measurement of retinal blood flow in clinical studies has been challenging. En face Doppler optical coherence tomography (OCT) provides an effective method for measuring total retinal blood flow (TRBF) in the clinic.
OBJECTIVE
To investigate TRBF in eyes with DR of varying severity, with or without diabetic macular edema (DME), using en face Doppler OCT.
DESIGN, SETTING, AND PARTICIPANTS
This was a cross-sectional study conducted from May 23, 2014, to January 11, 2016, which analyzed 41 eyes with DR from 31 diabetic patients, 20 eyes without DR from 11 diabetic patients, and 16 eyes from 12 healthy age-matched controls, all at the New England Eye Center in Boston, Massachusetts.
MAIN OUTCOMES AND MEASURES
Participants were imaged with a high-speed, swept-source OCT prototype at 1050-nm wavelength using repeated en face Doppler OCT raster scans, comprising 600 × 80 axial scans and covering a 1.5 × 2-mm2 area centered at the optic disc. The TRBF was automatically calculated using custom Matlab software.
RESULTS
This study included 41 eyes with DR from 31 diabetic patients (mean [SD] age, 62.8 [13.4] years; 12 were female patients), 20 eyes without DR from 11 diabetic patients (mean [SD] age, 58.8 [10.1] years; 5 were female patients), and 16 eyes from 12 healthy age-matched controls (mean [SD] age, 57.9 [8.1] years; 8 were female participants). The mean (SD) TRBF was 28.0 (8.5) μL/min in the eyes with DME, 48.8 (13.4) μL/min in the eyes with DR but without DME, 40.1 (7.7) μL/min in the diabetic eyes without retinopathy, and 44.4 (8.3) μL/min in age-matched healthy eyes. A difference in TRBF between the eyes with DME that were treated and the eyes with DME that were not treated was not identified. The TRBF was consistently low in the eyes with DME regardless of DR severity. The eyes with moderate nonproliferative DR but without DME exhibited a wide range of TRBF from 31.1 to 75.0 μL/min, with the distribution being highly skewed.
CONCLUSIONS AND RELEVANCE
High-speed en face Doppler OCT can measure TRBF in healthy and diabetic eyes. Diabetic eyes with DME exhibited lower TRBF than healthy eyes (P ≤ .001). Further longitudinal studies of TRBF in eyes with DR would be helpful to determine whether reduced TRBF is a risk factor for DME.
Diabetic retinopathy (DR) is the leading cause of legal blindness among working-age adults in developed countries.1–3 Because of their important role in the development of DR, alterations of the retinal microvasculature in eyes with DR have been extensively studied in vivo using fluorescein angiography and indocyanine green angiography techniques and, more recently, optical coherence tomography (OCT) angiography. These investigations have detailed a variety of microvascular alterations, including microaneurysms, capillary dropout, and remodeling; interestingly, recent studies have also found these alterations to be present in some diabetic eyes without clinically apparent retinopathy.4–6 Despite the successes of fluorescein angiography/indocyanine green angiography and OCT angiography studies in identifying important diagnostic markers for DR, the underlying mechanisms of the pathogenesis and progression of DR are still incompletely understood.
The importance of retinal blood flow (RBF) in DR has also motivated investigations of total RBF (TRBF) in diabetic eyes. Researchers have used a variety of techniques to measure RBF changes in DR, including video fluorescein angiography,7,8 laser Doppler velocimetry,9–14 and color Doppler ultrasound imaging.15 Results from these studies provided a mixed picture of RBF in diabetic patients. In particular, while a majority of the studies noted increased flow in nonproliferative DR(NPDR),7–12 a few noted decreased flow.13,14,16 Results from studies in eyes with proliferative DR (PDR) were similarly mixed.8,13,15,16 When interpreting these conflicting results, it is important to understand the limitations of the techniques: video fluorescein angiography cannot measure blood flow velocity but detects the arteriovenous passage time; laser Doppler velocimetry measures maximum blood flow velocity in 1 vessel at a time and requires fundus photography–assisted vessel caliber measurements to determine TRBF; and color Doppler ultrasound imaging can detect flow velocity in the ophthalmic artery but lacks the spatial resolution necessary to measure TRBF. Because of these limitations, the term retinal blood flow is often used nonspecifically, describing alternate quantities, such as average flow velocity or arteriovenous passage time, which, while being surrogates, are not measures of true TRBF. For these reasons, more reliable and precise TRBF measurement techniques would likely be useful for investigating TRBF changes in DR.
Doppler OCT is an imaging technique that measures axial flow velocity, the velocity component aligned with the OCT beam.17 The first Doppler OCT TRBF measurements were performed using repeated pairs of circumpapillary scans to measure the axial flow velocity and the Doppler angle between the flow velocity and the OCT beam.18–20 A study21 using this technique in 5 eyes with PDR and 10 healthy eyes showed that the mean (SD) TRBF was significantly lower in the 5 eyes with PDR than in the 10 healthy eyes (15.8 [10.1] vs 47.6 [5.4] μL/min).22 A key limitation of this method is its susceptibility to the Doppler angle measurement errors—when the vessels are nearly perpendicular to the OCT beam, as is often the case at the optic disc margin, a small error in Doppler angle can cause a large error in TRBF. Owing to this limitation, the method requires reader input on Doppler angle and is difficult to fully automate. Recently, advances in high-speed OCT technology have enabled a new approach to Doppler OCT that obviates the need for the Doppler angle. This technique, known as en face Doppler OCT,23 measures blood flow in the plane perpendicular to the OCT beam (Figure 1). In particular, by scanning a small volume at the optic disc, one can compute the TRBF by integrating the axial blood flow velocity in the central retinal artery.24–26 With high-speed imaging sufficient to acquire the entire volumes, en face Doppler OCT enables a fully automatic measurement of TRBF, which is important for performing studies on larger cohorts. In a small study of 10 healthy participants, the en face Doppler demonstrated good repeatability, with a 6.1% median coefficient of variation.27
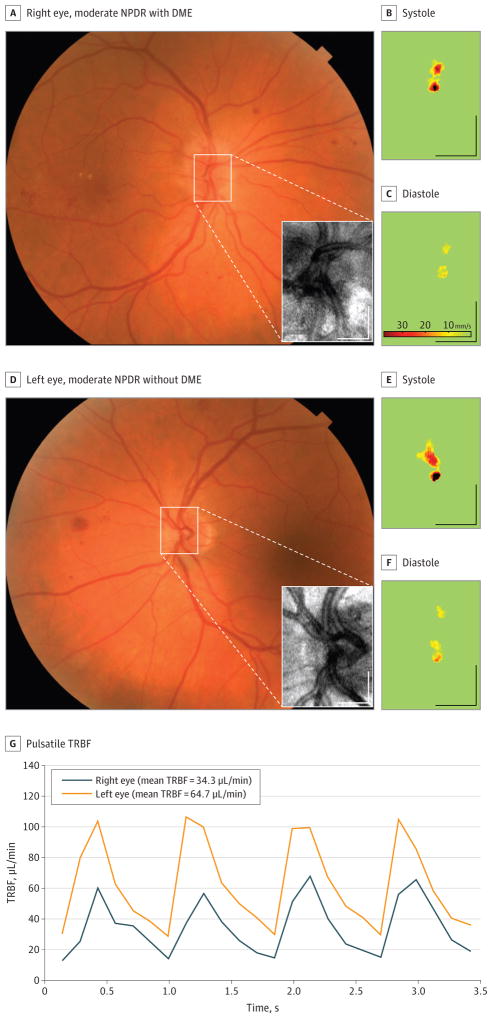
Figure 1. Measurement of Total Retinal Blood Flow (TRBF) in a Patient With Unilateral Diabetic Macular Edema (DME).
A and D, The area of the en face Doppler optical coherence tomographic scan (inset) is marked on the color fundus photograph. B, C, E, and F, Axial flow velocity en face profiles at systole and diastole. G, Pulsatile TRBF in the 2 eyes. Scale bars indicate 500 μm.
Methods
The study was a comparative analysis of TRBF in eyes from diabetic patients and age-matched healthy controls. The study was conducted at the New England Eye Center at Tufts Medical Center (Boston, Massachusetts) and was approved by the institutional review boards at Tufts Medical Center and the Committee on the Use of Humans as Experimental Subjects at the Massachusetts Institute of Technology (Cambridge, Massachusetts). The research adhered to the tenets of the Declaration of Helsinki28 and complied with the Health Insurance Portability and Accountability Act of 1996. Written informed consent was obtained before image acquisition. The collected OCT data were analyzed at the Massachusetts Institute of Technology.
Study Population
In this study, diabetic patients with or without DR and healthy controls were seen at the retina service of the New England Eye Center between May 23, 2014, and January 11, 2016, and were prospectively recruited to be imaged with the OCT prototype system. Blood pressure was measured during the OCT imaging session; intraocular pressure and axial eye length were recorded at the same visit. Ocular perfusion pressure was calculated as the difference between the mean blood pressure and intraocular pressure.
The inclusion criteria for the DR group were diabetic patients presenting vascular changes consistent with DR, with or without diabetic macular edema (DME), as diagnosed by a retina specialist based on a complete clinical examination, including a direct fundus examination and color fundus photographs. The presence and severity of DR were assessed using the Early Treatment of Diabetic Retinopathy Study: (1) mild NPDR (presence of microaneurysms in the retina); (2) moderate NPDR (more than mild but less than severe NPDR); (3) severe NPDR (>20 intraretinal hemorrhages in each of the 4 quadrants, venous beading in at least 2 quadrants, and intraretinal microvascular abnormalities in at least 1 quadrant in the absence of PDR); and (4) PDR (optic disc or retinal neovascularization).29 Exclusion criteria for the DR group were the presence of any concurrent ocular or systemic disease that would act as a confounding factor (age-related macular degeneration, hypertensive retinopathy, glaucoma, retinal vein or artery occlusion, a refractive error of > 6 diopters, or uveitis) and media opacity. Healthy controls were excluded if any ocular or systemic disease that potentially affects the vasculature was present.
The eyes of diabetic patients were classified as either with DR or without DR. The eyes with DR were stratified into 2 groups according to the presence or absence of DME. Each of these 2 groups was then further stratified into 3 groups according to disease severity: mild NPDR, moderate NPDR, and severe NPDR/PDR (Table 1).
Table 1.
Stratification of Diabetic Retinopathy and Treatment History
| Stratificationa | No. of Eyes | |||
|---|---|---|---|---|
| Mild NPDR | Moderate NPDR | Severe NPDR/PDR | Total | |
| No DME | 9 | 7 | 7 | 23 |
| DME | 1 | 12 | 5 | 18 |
| Total | 10 | 19 | 12 | 41 |
| Treatmentb | No Laser Treatment | Focal Laser | Laser PRP | Total |
| No anti-VEGF treatment | 7 | 4 | 0 | 11 |
| Anti-VEGF treatment | 2 | 3 | 1 | 6 |
| Total | 9 | 7 | 1 | 17 |
Abbreviations: DME, diabetic macular edema; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation; VEGF, vascular endothelial growth factor.
Sizes of the groups stratified by retinopathy severity and presence of DME.
Treatment history profile of the 17 patients with moderate or severe retinopathy and presence of DME.
High-Speed Swept-Source OCT Instrumentation
En face Doppler OCT was performed using a high-speed, swept-source/ Fourier-domain OCT prototype system, which has been described previously.30,31 The system used a 1050-nm vertical cavity surface-emitting laser with at unable light source enabling 400000 A-scans per second. When compared with the 840-nm systems used in commercial spectral/Fourier-domain OCT, the high-speed, 1050-nm wavelength, swept-source OCT system used in this study has 2 advantages. First, attenuation from light scattering is lower at longer wavelengths, improving the signal penetration into the large retinal vessels located at relatively deep depths into the optic nerve. Second, the longer wavelength, in conjunction with the higher imaging speed, reduces the amount of Doppler phase wrapping in the fast-flowing vessels, thereby simplifying measurement of axial flow velocity.
En face Doppler OCT was performed by repeatedly acquiring 600 × 80 A-scan volumes, each covering a 1.5 × 2-mm2 area at the optic disc. The B-scans were laterally over sampled to maintain a high correlation between the neighboring A-scans to ensure the quality of Doppler images. For each TRBF measurement, 24 volumes were acquired at a rate of 7.1 volumes per second, requiring a total acquisition time of 3.4 seconds. The image acquisition sequence was repeated 4 times to assess the repeatability of the TRBF measurement, although 1 measurement was randomly chosen for data analysis because some of the repeated measurements were invalid owing to low signal or excessive eye motion in some participants.
The OCT data processing proceeded in 2 independent phases. First, the raw data were converted into intensity and Doppler B-scans. Then, the volumetric blocks of intensity and Doppler images were loaded, and the TRBF in each en face plane was calculated in a continuous range of depths. A combination of image filters, histogram analyses, and morphologic operations were optimized for segmentation of the central retinal arterial area. To improve segmentation accuracy at diastoles when flow velocity is low, hysteresis thresholding was applied inside the segmented vessel area at the most recent systole. For this purpose, translational motion was corrected by registering each volume to the volume acquired at the most recent systole. After the TRBF measurements in all 24 volumes were computed, the mean TRBF was calculated as the average of the TRBF over an integer number of cardiac cycles.
Statistical Analysis
A linear mixed-effects model was used to test the significance of TRBF difference between 2 groups, in order to separately consider the variation among different participants and the variation between 2 eyes of the same participant. For comparison of other measurements such as blood pressure, intraocular pressure, and ocular perfusion pressure between 2 groups, the Mann-Whitney U test was used for simplicity. A 2-tailed P ≤ .05 was considered to be statistically significant.
Results
Forty-one eyes with DR from 31 patients (mean [SD] age, 62.8 [13.4] years; 12 were female patients), 20 eyes without DR from 11 patients (mean [SD] age, 58.8 [10.1] years; 5 were female patients), and 16 healthy eyes from 12 participants (mean [SD] age, 57.9 [8.1] years; 8 were female participants) were included in the analysis. The treatment history for the 17 eyes having DME and moderate or severe NDPR is given in Table 2.
Table 2.
Patient Demographics and Clinical Measurements
| Characteristic | Mean (SD) Value | ||
|---|---|---|---|
| Healthy Controls | Patients With DM but Without DR | Patients With DR | |
| Patients, No. | 12 | 11 | 31 |
| Eyes, No. | 16 | 20 | 41 |
| Age, y | 58.8 (10.1) | 57.9 (8.1) | 62.8 (13.4) |
| Blood pressure, mm Hg | |||
| Systolic | 115 (17) | 125 (7) | 121 (16) |
| Diastolic | 72 (7) | 81 (6) | 70 (13) |
| Mean | 87 (10) | 95 (8) | 87 (13) |
| Intraocular pressure, mm Hg | 13.0 (2.2) | 15.8 (2.4) | 14.9 (2.5) |
| Ocular perfusion pressure, mm Hg | 74 (9) | 79 (6) | 73 (12) |
| Axial eye length, mm | 23.5 (1.1) | 24.1 (1.4) | 24.0 (1.1) |
| Total retinal blood flow, μL/min | 44.4 (8.3) | 40.1 (7.7) | 39.6 (15.4) |
Abbreviations: DM, diabetes mellitus; DR, diabetic retinopathy.
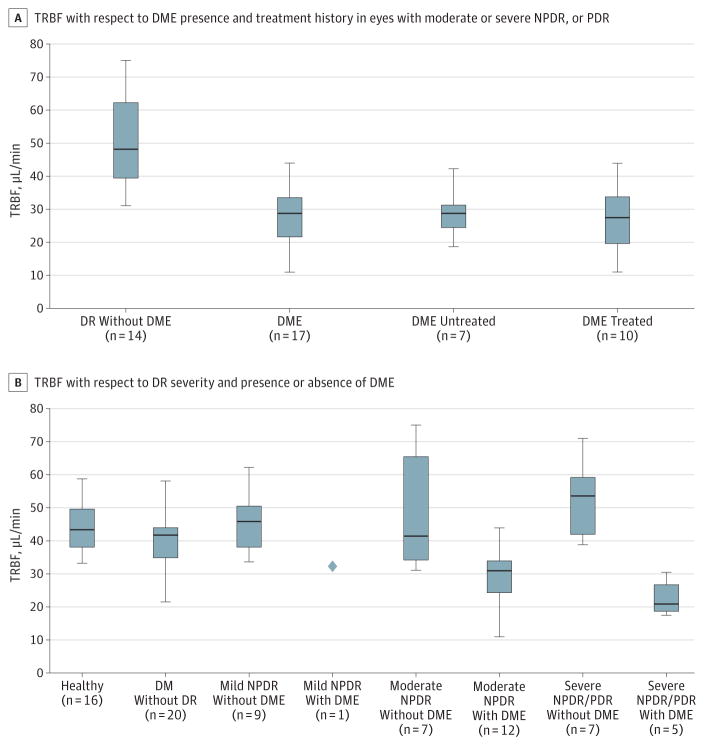
There was no significant difference in mean blood pressure or mean ocular perfusion pressure between control and DR groups. As a group, diabetic patients without DR exhibited a significantly higher mean blood pressure (P ≤ .05) and a higher mean ocular perfusion pressure (P ≤ .05) than the healthy participants. The mean (SD) TRBF was 44.4 (8.3) μL/min in the healthy eyes, 40.1 (7.7) μL/min in the eyes of diabetic patients without DR, 48.8 (13.4) μL/min in the eyes with DR but without DME, and 28.0 (8.5) μL/min in the eyes with DR and DME. As shown in Figure 2A, TRBF was significantly decreased in the eyes with DR and DME compared with the healthy eyes (P ≤ .001, determined by use of the linear mixed-effects model) and the eyes with DR but without DME (P ≤ .001, determined by use of the linear mixed-effects model). Note that the eyes with mild NPDR were excluded for this comparison to prevent disease severity from being a confounding factor. Comparison of TRBF between the treatment-naive (28.7 [7.6] μL/min) and treated (27.0 [9.8] μL/min) DME groups showed no significant separation between the 2 groups (P > .50). For this series of statistical tests, the eyes with mild NPDR were excluded in order to match retinopathy severity between the eyes with DR but without DME and the eyes with DR and DME. Figure 2B illustrates that the eyes with DME had a significantly lower TRBF than did the healthy eyes, regardless of retinopathy severity.
Figure 2. Boxplots of Measurements of Total Retinal Blood Flow (TRBF).
The horizontal line in the middle of each box indicates the median, while the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the minimum and maximum. DM indicates diabetes mellitus; DME, diabetic macular edema; DR, diabetic retinopathy; NPDR, nonproliferative DR; PDR, proliferative DR.
Among eyes with DME, the mean (SD) TRBF was 30.7 (10.2) μL/min (P ≤ .002) for the mild/moderate NPDR group and 22.8 (5.6) μL/min (P ≤ .002) for the severe NPDR/PDR group, both being significantly lower than in healthy controls. In contrast, TRBF in eyes without DME was not lower compared with the healthy controls. The eyes with mild NPDR but without DME had a mean (SD) TRBF of 45.4 (9.2) μL/min, which was similar to the TRBF in the healthy eyes. In the eyes with severe NPDR or PDR, but without DME, the mean (SD) TRBF was 52.2 (12.2) μL/min, which is higher than the TRBF in the healthy eyes, but the difference was not statistically significant (P > .05). Finally, in eyes with moderate NPDR but without DME, the mean (SD) TRBF was 49.5 (19.1) μL/min, covering a wide range from 31.1 to 75.0 μL/min.
Discussion
Perhaps the most noteworthy finding of this study is that the eyes with DME exhibit markedly reduced TRBF irrespective of treatment history or DR severity. A study by Guan et al32 investigated RBF in the superior temporal arterioles of eyes with DR using the Canon Laser Blood Flowmeter. The ratio between the maximum and minimum flow velocities, often referred to as vascular resistivity, was suggested to be a risk factor for DME. However, significant differences were not detected in the vessel diameters, the flow velocity, or the TRBF.32 Our results showing reduced TRBF in DME are not in agreement with these findings. However, it is important to note that the repeatability of the single-vessel blood flow measurement using the Canon Laser Blood Flowmeter is relatively poor—the coefficient of variation in healthy participants was reported to range from 4.8%to 39.7%, and the median was 19.9%.33 In contrast, in a study of healthy eyes, the en face Doppler OCT method was reported to have a coefficient of variation ranging from 0.8% to 10.9%, with a median of 6.1%.27 In the present study, the coefficient of variation in the 23 eyes with DR with more than 3 valid TRBF measurements fell in between 1.8% and 11.0%, and its median was 7.0%.
With regard to a potential causal relationship between reduced TRBF and DME, some groups have commented that the increased tissue hydrostatic pressure associated with DME may cause displacement and disruption of the smaller capillaries and may result in reduced TRBF. Conversely, recent studies have suggested that leukostasis, the accumulation of leukocytes on the luminal surface of the retinal capillaries, could be a major contributor to the dysfunction of the blood-retinal barrier and the development of capillary nonperfusion in diabetic patients.34 Histopathologic studies have shown that microaneurysms in DR may be engorged with leukocytes, red blood cell breakdown products, and eventually fibers, impeding flow in the retinal microvasculature.35 It may be that ischemia, as measured by reduced TRBF, creates an environment rich in vascular endothelial growth factor (VEGF) that precipitates DME.36 There is now evidence in the OCT angiography literature demonstrating that loss of capillary perfusion is correlated with DR severity37–39 and that DME is accompanied by loss of perfusion in the retinal capillaries, especially in the deep capillary plexus, and that these changes may precede the development of edema.40–42 Interestingly, in the eyes with moderate NPDR but without DME, a strongly skewed distribution covering a wide range of TRBF measurements was observed (Figure 3). This distribution suggests that there may be 2 clusters within the group: the subgroup with a higher TRBF could exhibit a similar TRBF as in the DME-absent severe NPDR/PDR group, and the subgroup with a lower TRBF could have TRBF values close to those in the groups with DME. However, the present study is very preliminary, and it is beyond the scope of this study to draw any definitive conclusions on how DME and reduced TRBF are related.
Figure 3.
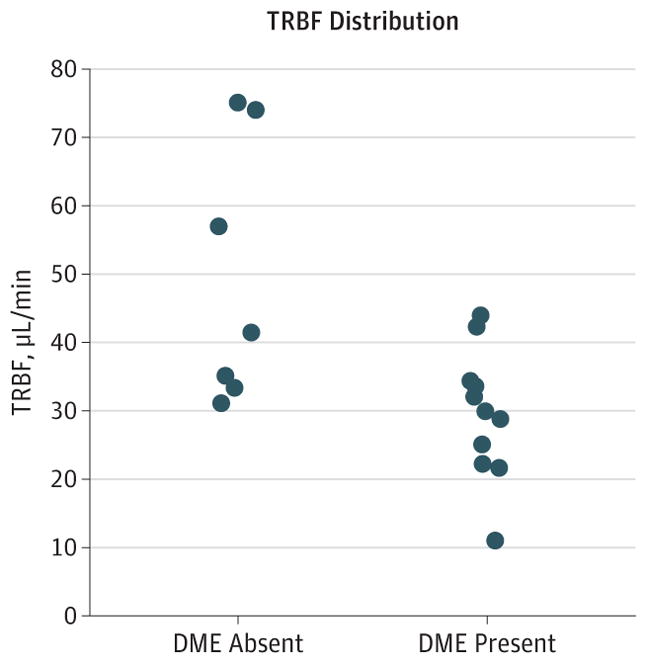
Scatterplot of Total Retinal Blood Flow (TRBF) vs the Presence or Absence of Diabetic Macular Edema (DME) in Eyes With Moderate Nonproliferative Diabetic Retinopathy
According to the prevailing theory in the laser Doppler velocimetry literature, the TRBF in eyes with NPDR is expected to be higher than normal and increasing with increasing disease severity.7–12,43,44 Our measurements in the DME-absent eyes with severe NPDR or PDR suggest that there could be a slight increase in TRBF in these groups, although statistical significance was not demonstrated, perhaps owing to the small group sizes. Investigators have postulated that an increased glucose level in a patient with diabetes may be responsible for increased TRBF. Laser Doppler velocimetry studies on type 1 diabetes45 and type 2 diabetes46 without clinically pronounced retinopathy showed that a decrease in insulin-induced blood glucose level caused a transient decrease in RBF. Meanwhile, one of these studies also showed that the diabetic patients without retinopathy presented with a lower temporal RBF than was found in healthy controls. These results must be interpreted with caution because insulin reduces hyperglycemia and dilates the blood vessels at the same time. It is difficult to speculate on any pathophysiological causes considering the small size of this study.
It is worth noting the possible influence of anti-VEGF treatment on TRBF in the eyes with DME because anti-VEGF agents have been linked to transient reductions in RBF.47–51 For the 6 eyes that received anti-VEGF treatment, the injections were given based on a treat-and-extend protocol. Therefore, the timing of the injections was different for all 6 eyes. Three eyes were treated with anti-VEGF no more than 60 days prior to the en face Doppler imaging, and their mean (SD) TRBF was 25.3 (9.2) μL/min. The other 3 eyes had not received any anti-VEGF treatment within 120 days before the en face Doppler measurement, and their mean (SD) TRBF was 29.4 (5.2) μL/min. The similarity of the TRBF measurements in the 2 groups is consistent with OCT angiography findings in the retinal capillaries.52 Studies with larger numbers of patients are required to confirm that anti-VEGF treatment is not a major factor for the reduced blood flow observed in the present study.
Limitations
Because of its high speed, the en face Doppler volumetric scanning technique used in this study has 1 key limitation: when there are reflections from hyperreflective layers, such as the retinal nerve fiber layer, the inner-segment/outer-segment ellipsoid, and the retinal pigment epithelium, a nonzero Doppler phase can be detected even without any flow. Although our processing software can reduce these artifacts, it is difficult to completely remove the artifacts because the same axial flow velocity corresponds to a smaller Doppler phase at higher imaging speeds. These artifacts, especially in participants with low TRBF, can cause an overestimation or an underestimation of blood flow depending on the segmentation threshold. Manual inspection of the segmented flow velocity in the en face images is required in such cases. A solution for these artifacts is to increase the interscan time between the 2A-scans paired for calculating Doppler phase. This can be achieved by doubling the density of the A-scans per unit length and pairing alternate, rather than subsequent, A-scans. This approach may require cardiac gating27 because the volume sampling rate of 3.5 volumes per second may not be enough to accurately capture flow pulsatility. The other limitations of the study include its observational nature. Because our recruitment was not guided by a priori information from a pilot study, there are cohorts that have excessively small group sizes for statistical testing. However, this study itself may serve as a pilot study for future en face Doppler OCT investigations of TRBF in DR.
Conclusions
This study used high-speed en face Doppler OCT to investigate TRBF in diabetic eyes with or without DR and in healthy eyes. The TRBF was found to be significantly lower in the eyes with DME than in both the healthy eyes and the eyes with DR but without DME, regardless of retinopathy severity. In the eyes with moderate NPDR but without DME, we observed a strongly skewed TRBF distribution.
Key Points.
Question
What is the role of retinal blood flow (RBF) in the pathogenesis and progression of diabetic retinopathy (DR)?
Findings
A cross-sectional investigation of total RBF (TRBF) using en face Doppler optical coherence tomography (OCT) demonstrated that the TRBF was lower in eyes with diabetic macular edema (DME) (mean [SD], 28.0 [8.5] μL/min) than in either eyes with DR but without DME (48.8 [13.4] μL/min) or healthy eyes (44.4 [8.3] μL/min).
Meaning
The measurements of TRBF suggest that ischemia may be associated with DME, although the influence of anti–vascular endothelial growth factor treatments must be investigated.
Acknowledgments
Funding/Support: This work was supported by the National Institute of Health contracts R01-EY011289-30, R44-EY022864-03, and R01-CA075289-19, the Air Force Office of Scientific Research contracts FA-9550-15-1-0473 and FA9550-12-1-0499, the Macula Vision Research Foundation, the Champalimaud Foundation, and the Massachusetts Lions Club. Mr Lee was partially supported by Samsung Scholarship from Seoul, South Korea. Dr Novais was supported by the Coordination for the Improvement of Higher Education Personnel Foundation within the Ministry of Education of Brazil, Brasilia, Distrito Federal, Brazil.
Additional Contributions: We thank Benjamin M. Potsaid, PhD, Massachusetts Institute of Technology and Thorlabs Inc, and Vijaysekhar Jarayaman, PhD, Praevium Research Inc, for their scientific contributions, and Lori L. Price, MAS, Tufts University School of Medicine, for her help with the statistical analysis. No compensation was received from funders.
Footnotes
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentation: This paper was presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology; May 5, 2016; Seattle, Washington.
Author Contributions: Dr Fujimoto and Mr Lee had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Novais and Mr Lee are joint first authors and contributed equally to the article.
Study concept and design: Lee, Novais, Waheed, Adhi, Moult, Choi, Baumal, Fujimoto.
Acquisition, analysis, or interpretation of data: Lee, Novais, Waheed, Adhi, de Carlo, Cole, Moult, Lane, Baumal, Duker.
Drafting of the manuscript: Lee, Novais, Waheed, Moult, Lane.
Critical revision of the manuscript for important intellectual content: Lee, Waheed, Adhi, de Carlo, Cole, Moult, Choi, Baumal, Duker, Fujimoto.
Statistical analysis: Lee, Moult.
Obtained funding: Waheed, Fujimoto.
Administrative, technical, or material support: Cole, Choi, Lane, Duker, Fujimoto.
Study supervision: Novais, Waheed, Baumal, Duker, Fujimoto.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Waheed reports receiving grants from the Macula Vision Research Foundation during the conduct of the study, nonfinancial support from Carl Zeiss Meditec, and personal fees from Optovue, Nidek, Regeneron, Genentech, Janssen, and Ocudyne and nonfinancial support from Topcon outside the submitted work. Dr Waheed also reports receiving grants (to the New England Eye Center) from Carl Zeiss Meditec and Optovue and serving as a consultant to Regeneron and Genentech. Dr Baumal reports serving as a speaker for Allergan and Genetech, as well as having received compensations from Optovue. Dr Duker reports receiving grants from Carl Zeiss Meditec and Optovue while also serving as a consultant to Carl Zeiss Meditec and Optovue. Dr Duker also reports serving as a consultant to Allergan, Aura Biosciences, Lumenis, Omeros, Santen, Thrombogenics, and Ocudyne; being a stock holder in Hemera Biosciences and Ophthotech; and being on the board of directors of Eleven Biotherapeutics and pSivida Corporation. Dr Fujimoto reports having a personal financial interest in Optovue and also receiving royalties from a patent owned by the Massachusetts Institute of Technology and licensed to Optovue. Dr Fujimoto and Mr Moult report receiving grants from the National Institutes of Health and the Air Force Office of Scientific Research during the conduct of the study. Dr Choi reports receiving grants from the National Institutes of Health during the conduct of the study. No other disclosures are reported.
References
- 1.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 2.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Thylefors B. A global initiative for the elimination of avoidable blindness. Community Eye Health. 1998;11(25):1–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 5.Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35(11):2371–2376. doi: 10.1097/IAE.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Carlo TE, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364–2370. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 7.Kohner EM, Hamilton AM, Saunders SJ, Sutcliffe BA, Bulpitt CJ. The retinal blood flow in diabetes. Diabetologia. 1975;11(1):27–33. doi: 10.1007/BF00422814. [DOI] [PubMed] [Google Scholar]
- 8.Cunha-Vaz JG, Fonseca JR, de Abreu JR, Lima JJ. Studies on retinal blood flow: II, diabetic retinopathy. Arch Ophthalmol. 1978;96(5):809–811. doi: 10.1001/archopht.1978.03910050415001. [DOI] [PubMed] [Google Scholar]
- 9.Feke GT, Tagawa H, Yoshida A, et al. Retinal circulatory changes related to retinopathy progression in insulin-dependent diabetes mellitus. Ophthalmology. 1985;92(11):1517–1522. doi: 10.1016/s0161-6420(85)33827-7. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida A, Feke GT, Morales-Stoppello J, Collas GD, Goger DG, McMeel JW. Retinal blood flow alterations during progression of diabetic retinopathy. Arch Ophthalmol. 1983;101(2):225–227. doi: 10.1001/archopht.1983.01040010227008. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald JE, Riva CE, Baine J, Brucker AJ. Total retinal volumetric blood flow rate in diabetic patients with poor glycemic control. Invest Ophthalmol Vis Sci. 1992;33(2):356–363. [PubMed] [Google Scholar]
- 12.Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. BMJ. 1992;305(6855):678–683. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair NP, Feke GT, Morales-Stoppello J, et al. Prolongation of the retinal mean circulation time in diabetes. Arch Ophthalmol. 1982;100(5):764–768. doi: 10.1001/archopht.1982.01030030768009. [DOI] [PubMed] [Google Scholar]
- 14.Burgansky-Eliash Z, Nelson DA, Bar-Tal OP, Lowenstein A, Grinvald A, Barak A. Reduced retinal blood flow velocity in diabetic retinopathy. Retina. 2010;30(5):765–773. doi: 10.1097/IAE.0b013e3181c596c6. [DOI] [PubMed] [Google Scholar]
- 15.Mendívil A, Cuartero V, Mendívil MP. Ocular blood flow velocities in patients with proliferative diabetic retinopathy and healthy volunteers: a prospective study. Br J Ophthalmol. 1995;79(5):413–416. doi: 10.1136/bjo.79.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon TJ, Chowiencyzk P, Kohner EM. Measurement of retinal blood flow in diabetes by the blue-light entoptic phenomenon. Br J Ophthalmol. 1986;70(1):43–46. doi: 10.1136/bjo.70.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitgeb R, Schmetterer L, Drexler W, Fercher A, Zawadzki R, Bajraszewski T. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt Express. 2003;11(23):3116–3121. doi: 10.1364/oe.11.003116. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Bower BA, Izatt JA, Tan O, Huang D. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007;12(4):041215. doi: 10.1117/1.2772871. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008;13(6):064003. doi: 10.1117/1.2998480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009;93(5):634–637. doi: 10.1136/bjo.2008.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52(2):840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JC, Wong BJ, Tan O, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(9):6104–6111. doi: 10.1167/iovs.13-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan VJ, Sakadzić S, Gorczynska I, et al. Quantitative cerebral blood flow with optical coherence tomography. Opt Express. 2010;18(3):2477–2494. doi: 10.1364/OE.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann B, Potsaid B, Kraus MF, et al. Total retinal blood flow measurement with ultrahigh speed swept source/Fourier domain OCT. Biomed Opt Express. 2011;2(6):1539–1552. doi: 10.1364/BOE.2.001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi W, Baumann B, Liu JJ, et al. Measurement of pulsatile total blood flow in the human and rat retina with ultrahigh speed spectral/Fourier domain OCT. Biomed Opt Express. 2012;3(5):1047–1061. doi: 10.1364/BOE.3.001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi W, Potsaid B, Jayaraman V, et al. Phase-sensitive swept-source optical coherence tomography imaging of the human retina with a vertical cavity surface-emitting laser light source. Opt Lett. 2013;38(3):338–340. doi: 10.1364/OL.38.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B, Choi W, Liu JJ, et al. Cardiac-gated en face Doppler measurement of retinal blood flow using swept-source optical coherence tomography at 100,000 axial scans per second. Invest Ophthalmol Vis Sci. 2015;56(4):2522–2530. doi: 10.1167/iovs.14-16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 29.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5 suppl):786–806. [PubMed] [Google Scholar]
- 30.Choi W, Moult EM, Waheed NK, et al. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology. 2015;122(12):2532–2544. doi: 10.1016/j.ophtha.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moult E, Choi W, Waheed NK, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan K, Hudson C, Wong T, et al. Retinal hemodynamics in early diabetic macular edema. Diabetes. 2006;55(3):813–818. doi: 10.2337/diabetes.55.03.06.db05-0937. [DOI] [PubMed] [Google Scholar]
- 33.Guan K, Hudson C, Flanagan JG. Variability and repeatability of retinal blood flow measurements using the Canon Laser Blood Flowmeter. Microvasc Res. 2003;65(3):145–151. doi: 10.1016/s0026-2862(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zeng H, Bao S, Wang N, Gillies MC. Diabetic macular edema: new concepts in pathophysiology and treatment. Cell Biosci. 2014;4:27. doi: 10.1186/2045-3701-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol. 1995;79(4):362–367. doi: 10.1136/bjo.79.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45(2):617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 37.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353–2363. doi: 10.1097/IAE.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 38.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362–OCT370. doi: 10.1167/iovs.15-18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhanushali D, Anegondi N, Gadde SG, et al. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT519–OCT525. doi: 10.1167/iovs.15-18901. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y. New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci. 2016;57(9):OCT348–OCT355. doi: 10.1167/iovs.15-18782. [DOI] [PubMed] [Google Scholar]
- 41.de Carlo TE, Chin AT, Joseph T, et al. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47(2):108–114. doi: 10.3928/23258160-20160126-02. [DOI] [PubMed] [Google Scholar]
- 42.Spaide RF. Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 2015;160(6):1200–1210. doi: 10.1016/j.ajo.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Pemp B, Polska E, Garhofer G, Bayerle-Eder M, Kautzky-Willer A, Schmetterer L. Retinal blood flow in type 1 diabetic patients with no or mild diabetic retinopathy during euglycemic clamp. Diabetes Care. 2010;33(9):2038–2042. doi: 10.2337/dc10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen HT, van Duinkerken E, Verbraak FD, et al. Retinal blood flow is increased in type 1 diabetes mellitus patients with advanced stages of retinopathy. BMC Endocr Disord. 2016;16(1):25. doi: 10.1186/s12902-016-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- 46.Grunwald JE, Riva CE, Martin DB, Quint AR, Epstein PA. Effect of an insulin-induced decrease in blood glucose on the human diabetic retinal circulation. Ophthalmology. 1987;94(12):1614–1620. doi: 10.1016/s0161-6420(87)33257-9. [DOI] [PubMed] [Google Scholar]
- 47.Bonnin P, Pournaras JA, Lazrak Z, et al. Ultrasound assessment of short-term ocular vascular effects of intravitreal injection of bevacizumab (Avastin) in neovascular age-related macular degeneration. Acta Ophthalmol. 2010;88(6):641–645. doi: 10.1111/j.1755-3768.2009.01526.x. [DOI] [PubMed] [Google Scholar]
- 48.Mete A, Saygili O, Mete A, Gungor K, Bayram M, Bekir N. Does ranibizumab (Lucentis) change retrobulbar blood flow in patients with neovascular age-related macular degeneration? Ophthalmic Res. 2012;47(3):141–145. doi: 10.1159/000330509. [DOI] [PubMed] [Google Scholar]
- 49.Hosseini H, Lotfi M, Esfahani MH, et al. Effect of intravitreal bevacizumab on retrobulbar blood flow in injected and uninjected fellow eyes of patients with neovascular age-related macular degeneration. Retina. 2012;32(5):967–971. doi: 10.1097/IAE.0b013e31822c28d6. [DOI] [PubMed] [Google Scholar]
- 50.Sakalar YB, Senturk S, Yildirim M, Keklikci U, Alakus MF, Unlu K. Evaluation of retrobulbar blood flow by color Doppler ultrasonography after intravitreal ranibizumab injection in patients with neovascular age-related macular degeneration. J Clin Ultrasound. 2013;41(1):32–37. doi: 10.1002/jcu.21989. [DOI] [PubMed] [Google Scholar]
- 51.Bonnin P, Pournaras JA, Makowiecka K, et al. Ultrasound assessment of ocular vascular effects of repeated intravitreal injections of ranibizumab for wet age-related macular degeneration. Acta Ophthalmol. 2014;92(5):e382–e387. doi: 10.1111/aos.12356. [DOI] [PubMed] [Google Scholar]
- 52.Mané V, Dupas B, Gaudric A, et al. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography [published online September 8, 2016] Retina. doi: 10.1097/IAE.0000000000001289. [DOI] [PubMed] [Google Scholar]