Abstract
Immune checkpoint inhibitors, particularly those targeting PD-1/PD-L1, produce durable responses in a subset of patients across cancer types. Although often well-tolerated, these agents can induce a broad spectrum of autoimmune-like complications that may affect any organ system. Treatment of these toxicities primarily consists of immune suppression with corticosteroids and other agents. This review will briefly discuss the mechanisms of immune-related adverse events (irAEs), overview the clinical and pathologic features of major toxicities caused by PD-1/PD-L1 blockade, and review their management.
Keywords: PD-1, toxicity, nivolumab, pembrolizumab, colitis, pneumonitis
Introduction
Immune checkpoint inhibitors have transformed the treatment of numerous cancers including melanoma, non-small cell lung cancer (NSCLC), urothelial cancer, renal cell carcinoma, Hodgkin lymphoma, and head and neck cancer.1 These agents include monoclonal antibodies that target the programmed death receptor-1 (PD-1) (pembrolizumab, nivolumab) and one of its ligands, PD-L1 (atezolizumab, durvalumab, avelumab), as well as the cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitor ipilimumab. The toxicity profile of these novel agents is quite distinct from conventional cytotoxic chemotherapy agents, and in general is somewhat more tolerable. While immune checkpoint inhibitors do not cause alopecia, myelosuppression, or have the emetogenic potential of other anti-cancer agents, they can induce immune-related adverse events (irAEs) which can affect almost any organ, and range from asymptomatic to fulminant (Figure 1).
Figure 1.
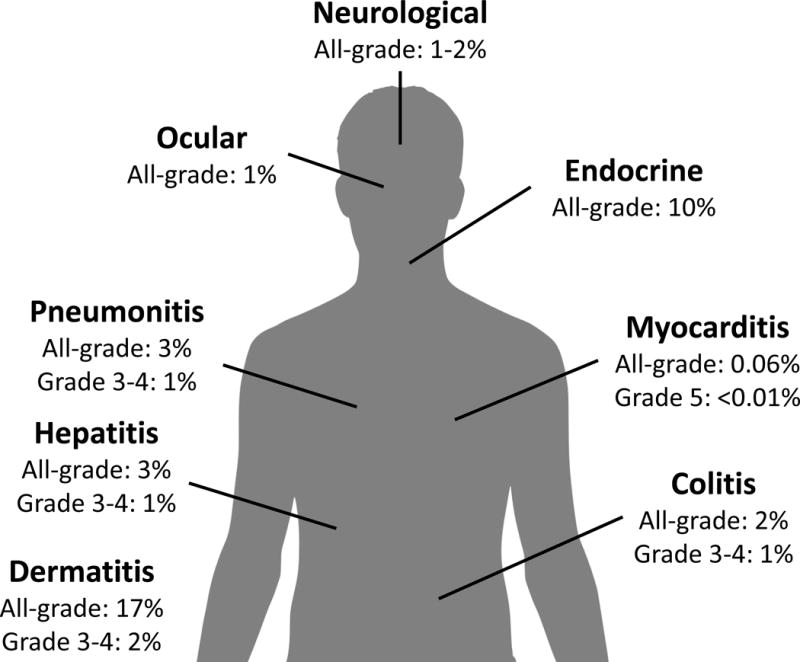
Approximate incidence of immune-related adverse events with anti-PD-1 monotherapy
The triggers of irAEs are incompletely catalogued, but appear related to the drugs’ mechanism of action. PD-1 is an immune inhibitory checkpoint expressed on the surface of activated T cells. When PD-1 engages its ligands, PD-L1 and PD-L2, T cells become functionally exhausted. This interaction is particularly important in the peripheral tissues and at sites of ongoing inflammation, including the tumor microenvironment.2 Anti-PD-1/PD-L1 agents block the PD-1/ligand interaction and reinvigorate these quiescent T cells. The resulting T cell response can effectively treat cancer but may also cause an autoimmune or inflammatory response in normal tissue resulting in irAEs. It remains unclear why particular patients or specific organs are affected in some patients and not in others. Hypotheses include genetic predisposition (similar to certain autoimmune disorders), environmental insults inducing subclinical inflammation, or shared antigens between tumor and affected tissue.3–5 Full discussion surrounding the pathogenesis of irAEs is beyond the scope of this review, and the remainder will focus on clinical aspects of immune toxicity. First, we will review the clinical and pathologic features of organ-specific irAEs, then follow with a discussion of treatment algorithms.
Clinical and Pathologic Features of irAEs
Pneumonitis
Pneumonitis is defined as focal or diffuse inflammation of the lung parenchyma. Serious events (grade 3 or higher) occur in less than 1% of patients treated with anti-PD-1/L1, but may occasionally lead to deaths. In a large meta-analysis of nearly 4500 patients treated with anti-PD-1 agents, all-grade pneumonitis was reported more commonly in renal cell carcinoma (4.1%) and NSCLC (4.1%) patients than in melanoma patients (1.6%). It also appeared more frequently in patients receiving combination therapy with either anti-CTLA-4 or peptide agents in addition to anti-PD-1 (6.6%) compared to anti-PD-1 monotherapy (1.6%). Pneumonitis related deaths were rare, but did occur in 4 NSCLC patients receiving anti-PD-1 monotherapy and 1 melanoma patients receiving combined PD-1/CTLA-4 blockade.6 The seemingly increased potential for pneumonitis in NSCLC patients may be due to underlying lung disease, smoking history, or tumor burden, and providers should monitor patients closely regardless of tumor histology.
The median time to onset of pneumonitis is 2.8 months after initiating PD-1 therapy but ranges quite broadly from 9 days to 19.2 months7. Pneumonitis has a variable presentation in terms of both clinical and radiographic findings, making diagnosis challenging. The most common patient symptoms are cough and dyspnea although some patients are completely asymptomatic at diagnosis. Other causes of cough and dyspnea must be considered including infection, pulmonary embolism, and exacerbation of underlying lung disease (e.g. progression of malignancy, worsening of chronic obstructive pulmonary disease, etc).
Computed tomography (CT) is generally needed to establish the diagnosis. A spectrum of radiographic features has been described, including ground glass opacities (GGO), reticular opacities, cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS), nonspecific interstitial pneumonia (NSIP) and hypersensitivity pneumonitis (HP). GGO and COP are the most commonly described CT findings. The radiographic pattern has also been associated with the grade of pneumonitis. For patients with the NSIP/HP, COP, and AIP/ARDS patterns, the median of grades of pneumonitis were 1, 2, and 3, respectively. CT involvement of pneumonitis has also been described as more extensive in patients with NSCLC than in patients with melanoma or lymphoma8.
Given the potential severity of pneumonitis, patients should be closely monitored for symptoms of cough or dyspnea with a low threshold for cross sectional chest imaging. Following treatment (See Treatment section below), re-challenge with anti-PD-1 is generally not recommended. However, if the initial episode was low grade (grade 1–2), reinitiating therapy could be considered after a detailed discussion with the patient. Further investigation is needed to identify risk factors and to better define diagnostic criteria for pneumonitis.
Diarrhea/Colitis
Diarrhea and colitis are the most common severe irAEs experienced with immune checkpoint inhibitors, particularly with anti-CTLA-4 containing regimens. Diarrhea, which is defined as an increase in stool frequency, has overlapping features with colitis but is clinically distinct. Colitis specifically refers to the inflammation of the colon which is manifested clinically as abdominal pain, diarrhea and bloody stools. Regardless, the management of these adverse events is essentially identical. For this review, the use of the terms colitis and diarrhea will be interchangeable except in reference to clinical trial definitions.
A key component in the management of colitis is patient counselling prior to therapy initiation. Patients should report colitis symptoms rapidly, as delay in diagnosis and treatment often increases the risk for potentially morbid and or even fatal toxicity. Upon presentation with diarrhea or other symptoms of colitis, a differential diagnosis of other potential etiologies must also be considered, which should include infections such as Clostridium difficile and other bacterial/viral pathogens. If other symptoms such as fever, chills, nausea or vomiting suggest an infectious or alternative cause, they should be ruled out before, or concurrently while initiating treatment for immune-related colitis.
In general, the diagnosis of immune-related colitis is largely clinical. If there is uncertainty with the diagnosis, a colonoscopy can be useful to direct management. Immune-related colitis can exhibit endoscopic findings ranging from normal to mild erythema to severe inflammation with biopsy findings including lamina propria expansion and neutrophilic cryptitis.9 Histologically, findings between ipilimumab and anti-PD-1 related colitis have no clear distinctive features. Radiographic imaging such as computed tomography will often capture changes associated with colitis such as diffuse or segmental bowel wall thickening, but is not routinely indicated, nor specific for diagnosis.10
The incidence and timing of colitis/diarrhea varies between the specific immune checkpoint inhibitor (ICI) used. Patients treated with anti-CTLA-4 agents, such as ipilimumab, experience severe (grade 3–4) colitis/diarrhea much more frequently (up to 10% vs 1–2%), and in a dose dependent manner compared to patients who receive anti-PD-1 agents.11–13 With dual blockade of CTLA-4 and PD-1 with ipilimumab and nivolumab, 9% of patients experienced severe diarrhea.14 Although only ~10% of patients reported grade 3–4 diarrhea/colitis, approximately 16% of patients discontinued combination therapy due to these symptoms. The timing of colitis is somewhat stereotypical with ipilimumab; onset is generally within 6–9 weeks of therapy initiation.15 However, the incidence of diarrhea/colitis becomes less predictable with anti-PD-1 agents. Retrospective studies by our group suggest that colitis related to anti-PD-1 generally occurs later, but is widely variable.16 Combined nivolumab/ipilimumab related colitis occurs with a more similar trajectory as ipilimumab alone, but may occur even after a single dose.
Hepatitis
Hepatotoxicity related to immune therapy most frequently manifests as abnormal laboratory findings with rare instances of clinically significant symptoms. Immune-related hepatitis is diagnosed most commonly during routine laboratory checks, demonstrating elevations in serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) with rare occurrences of elevation in bilirubin, (usually concurrently when present). Patients will seldom have fevers, vomiting, or jaundice associated with hepatic inflammation. Imaging is unnecessary for diagnosis, but radiographic findings such as mild hepatomegaly, periportal edema, and periportal lymphadenopathy have been reported.17 Liver biopsies demonstrate features such as severe panlobular hepatitis with prominent perivenular infiltrate with endothelialitis and primary biliary pattern with mild portal mononuclear infiltrate around proliferated bile ductules.17,18 As with colitis, appropriate suspicion for alternative etiologies should be maintained, including viral hepatitis, liver metastases, or other medication induced hepatotoxicity (e.g. acetaminophen or statins).
The incidence of hepatitis varies with the ICI agent. Any grade of hepatitis occurred in up to 10% of patients with anti-CTLA-4 agents,11,13,19 up to 7% with anti-PD1 agents,20 and up to 33% with combination anti-CTLA-4 and anti-PD-1.14 Timing of immune-related hepatitis is fairly characteristic with anti-CTLA-4 containing regimens, occurring most commonly 8 to 12 weeks after starting therapy, although onset can be variable in anti-PD-1 treated patients.21 Given that immune-related hepatitis is most commonly diagnosed through laboratory findings, routine liver function labs should be collected prior to each dose of ICI.
Dermatologic
Dermatologic toxicities are among the most common irAEs noted in patients treated with ICIs22. With anti-PD-1 agents, 30–40% of patients will experience a dermatologic irAE of some grade during therapy.22 These toxicities can include rash, pruritis, and vitiligo which are fairly similar to those observed in patients treated with anti-CTLA-4 agents.
The incidence of all-grade rash in patients treated with anti-PD-1 agents is 14-17% although the incidence of severe rash is <2%. Onset of rash generally occurs within one month of starting treatment but can occasionally arise much later into therapy. The rash most commonly occurs on the trunk or extremities and is characterized by erythematous macules, papules, and plaques. Pruritis is frequently also present23. Papulopustular rashes, lichenoid dermatitis, bullous pemphigoid, Sweet’s syndrome, Stevens Johnson syndrome, and toxic epidermal necrolysis have all been associated with anti-PD-1 therapy as well but are very rare.22 Histologically, the most common finding is interface dermatitis.22 For fulminant rashes, those refractory to steroids, or blistering rashes, biopsy is indicated to rule out more severe etiologies.
Other notable dermatologic irAEs include pruritis and vitiligo. Pruritis occurs in 13–20% of patients with high-grade pruritis occurring in less than 2% of patients. Vitiligo occurs in up to 8% of patients, primarily those with melanoma.23 Ultimately, most dermatologic toxicities are mild and resolve with topical treatment; however, refractory symptoms warrant evaluation by a dermatologist.
Although dermatologic symptoms can negatively influence patient quality of life, there is an association between developing a dermatologic toxicity from anti-PD-1 treatment and favorable patient outcomes. A similar finding was observed for targeted agents (sunitinib, erlotinib), where developing a rash portends a better prognosis. Several studies have suggested that developing either a rash or vitiligo while being treated with an anti-PD-1 agent is associated with improved progression free and overall survival24,25. Interestingly, vitiligo appears almost exclusively in melanoma patients, suggesting that T cells may be cross-reactive to pigment producing cells in both tumor and normal skin. Further investigation is needed to understand the relationship between dermatologic toxicity and response to treatment.
Endocrine
Approximately 1–2% of patients experience high-grade endocrinopathies that are immunotherapy-related. The pituitary, thyroid, and adrenal glands can all be affected by checkpoint inhibitors, and with PD-1 inhibitors, thyroid dysfunction is the most common. Unlike other irAEs, endocrinopathies are generally irreversible. These events can also be difficult to diagnose because of non-specific symptoms including fatigue, nausea, headache, and weakness. Evaluation can include vital signs and laboratory analysis with thyroid-stimulating hormone (TSH), free thyroxine (T4), adrenocorticotropic hormone (ACTH), cortisol, follicle stimulating hormone (FSH), luteinizing hormone (LH), growth hormone (GH) and a basic metabolic panel.
Diagnosing thyroid dysfunction from ICI may be somewhat complex. Hypothyroidism is the most common complication but may be preceded by a mild, subclinical thyroiditis. On occasion, a more clinically significant and symptomatic hyperthyroid state can occur that usually resolves with observation, but may occasionally require beta blockade or methimazole. Thyroid function should be assessed frequently, at least every other treatment. Primary vs. secondary hypothyroidism should also be distinguished. Elevated TSH with a low free T4 is consistent with primary hypothyroidism. When TSH and free T4 are both low, this is consistent with a secondary process such as pituitary insufficiency. Hyperthyroidism, characterized by a low TSH and elevated free T4, can be seen transiently in thyroiditis, or durably when associated with ICI-induced Graves’ disease.26
Hypophysitis is a potentially dangerous event that is characterized by headache, fatigue, and weakness. Laboratory evaluation reveals low levels of some or all hormones made by the pituitary including TSH, ACTH, LH, FSH, and GH. Brain magnetic resonance imaging (MRI) may reveal pituitary inflammation or enlargement.27 Hypophysitis is more common with anti-CTLA-4 agents alone or in combination with anti-PD-1 agents (1–17%) than with anti-PD-1 monotherapy (1–6%)22,28. Time of onset is generally 8–12 weeks after ICI.28 Treatment with steroids may reverse the acute inflammation; however, for most cases, long-term hormone replacement is necessary. Adrenal insufficiency (AI) is another rare but life-threatening complication of ICI. This can be due to secondary AI in the setting of hypohysitis or less commonly primary AI. Signs of AI include hypotension, tachycardia, hyponatremia, and hyperkalemia. Stress dose steroids are given, and urgent evaluation by an endocrinologist is needed for highly symptomatic or unstable patients.
Rare toxicities
Neurological toxicities
A wide range of neurologic symptoms have been contributed to anti-PD-1 therapy ranging from mild neuropathies to progressive debilitating syndromes.29 The median onset of these neurological events has been reported fairly early, at approximately 6-7 weeks after treatment initiation, with resolution in 75% of patients after a median time of 4–5 weeks. Increased awareness and recognition by oncologists and neurologists of these potentially life-threatening irAEs is needed. In particular, progressive neuropathies (e.g. Guillain Barre syndrome), aseptic meningitis/encephalitis, and neuromuscular conditions (e.g. Myasthenia Gravis) may cause highly morbid or fatal complications.30,31 Fortunately, the incidence of these events is low, up to 1–2% based on various studies.29,32 Consideration of alternative etiologies (e.g. spinal cord compression, brain metastases, cerebrovascular accident) is also imperative.
Ocular toxicities
Immune toxicity of the eyes often present as intraocular inflammation, or uveitis. This entity occurs in 1% of patients treated with anti-PD-1/L1 therapy, but can have significant comorbidity if not treated.33,34 A consultation with ophthalmology is recommended to establish the diagnosis and facilitate prompt treatment with topical or oral corticosteroids.
Cardiac toxicities
Clinically significant cardiovascular toxicities from anti-PD-1 monotherapy have ranged widely from cases of pericarditis, hypertension, atrial and ventricular arrhythmias, autoimmune myocarditis and myocardial infarction with an incidence around 1%.35 The onset ranged widely from 2 to 17 weeks and most cases improved or resolved with corticosteroids or supportive medication/care.
The combination of anti-PD-1 and CTLA-4 blockade has led to several reported cases of fatal immune-related myocarditis.5 Pharmacovigilance studies revealed that the incidence of myocarditis is higher (0.27% vs 0.06%) and more severe (0.17% vs <0.01% fatal cases) in patients treated with combination of ipilimumab and nivolumab versus nivolumab alone. These cases were characterized by rapidly progressive and severe arrhythmias (including complete heart block and ventricular fibrillation). Thus, prompt recognition is necessary with urgent cardiology consultation and transfer to a cardiac care unit if available. We and others have also begun to screen patients weekly for troponin elevations during the first 3-6 weeks of combination PD-1/CTLA-4 therapy. Troponin appears to be the most sensitive marker of myocarditis, and elevated levels should be worked up with ECG, echocardiogram, and cardiology consult.
Treatment Algorithms
The general guidelines for treatment of immune related adverse events of anti-PD1/L1 therapies are largely based on clinical trial experience with ipilimumab, and requires familiarity with Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf).36 As a general principle, mild cases (grade 1) of irAEs generally require symptomatic management or monitoring, with no interruption of therapy (Figure 2). For patients that experience moderate irAEs (grade 2), treatment should be held until symptoms or toxicities improve to grade 1 or less. In some instances, low-dose corticosteroids (prednisone 0.5 mg/kg/day or equivalent) can be started if symptoms are bothersome, or fail to improve within a week. For patients with more severe (grade 3 or 4) irAEs, treatment should usually be permanently discontinued. High-dose corticosteroids (prednisone 1–2 mg/kg/day or equivalent) should be commenced rapidly. Ambulatory, non-toxic patients can be considered for oral therapy, but more ill-appearing or dehydrated patients should be admitted for intravenous steroid administration. Once symptoms improve to grade 1 or less, a gradual taper of corticosteroids over at least a month should be initiated. If symptoms do not improve after 3 days with corticosteroids (especially intravenous), alternative immununosuppressants such as infliximab or mycophenolate mofetil should be strongly considered. Repeat dosing of such immununosuppressants may be necessary if symptoms persist. While the above information is a generalization, some important site specific management for severe irAE are detailed below.
Figure 2.
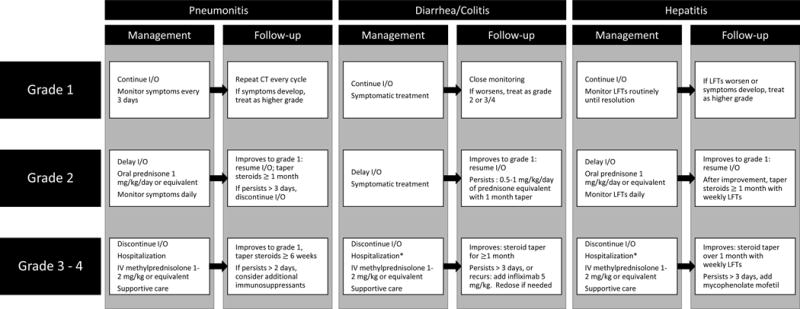
Algorithm for management of common irAEs (pneumonitis, diarrhea/colitis, hepatitis).
*Hospitalization may not be required for ambulatory, well-hydrated patients available for close outpatient follow-up
Pneumonitis
Grade 1 pneumonitis (imaging findings only) should be monitored closely. Grade 2 pneumonitis (mild symptoms), however, represents a departure from the aforementioned, general algorithms, in that prednisone 1–2mg/kg should be initiated and pulmonary consultation should be considered. For patients with grade 3–4 pneumonitis, a bronchoscopy should be considered to rule out infectious causes prior to (or concurrently with) starting immunosuppression. Patients should be promptly hospitalized and started on high-dose corticosteroids (prednisone 1–2 mg/kg/daily or equivalent). Additional immunosuppression such as infliximab, mycophenolate mofetil, and cyclophosphamide should be considered. Once symptoms improve to grade 1 or less, a steroid taper of 4–6 weeks should be started.
Skin
Supportive measures are usually sufficient for low-grade rashes, and include topical emollients or low potency corticosteroids, as well as oral antihistamines. If low-grade rashes remain persistent and bothersome, a short burst of low dose steroids may improve symptoms (e.g. prednisone 20–40mg for 5 days). For grade 3–4 rash, high-dose oral corticosteroids should be started with consideration of alternative immunosuppressants such as infliximab, mycophenolate mofetil or cyclophosphamide if symptoms do not begin to resolve. In rare instances, Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.37 In such cases, prompt hospitalization and consultation by dermatology and critical care are necessary. Management relies heavily on supportive care with the consideration of increasing corticosteroids to prednisone equivalent of 2 mg/kg/daily.
Diarrhea/colitis
Anti-diarrheals can be prescribed for low grade diarrhea, but patients should be monitored and closely for worsening. For grade 3–4 colitis, along with high-dose corticosteroids, addition of infliximab (5 mg/kg) should be strongly considered if symptoms do not improve promptly (within 3 days). If symptoms persist after 2 weeks, another dose of infliximab at the same dose should be considered. For truly refractory cases, anti-integrin therapy (vedolizumab), complete bowel rest with total parenteral nutrition, or even colectomy have been reported, although close consultation with gastroenterologists and surgeons should be maintained.38,39 Once symptoms improve to grade 1 or less, a steroid taper of 4–6 weeks should be started. During the steroid taper, colitis flares can occur. In these instances, increasing steroids or consideration of infliximab may be indicated.
Hepatitis
Patients with grade 3–4 hepatitis should receive high-dose corticosteroids. If liver function does not improve or relapses during a taper, mycophenolate mofetil (500–1000 mg every 12 hours) or tacrolimus should be started. Infliximab is contraindicated in cases of hepatitis due to its hepatotoxic nature. Once liver functions begin to improve, a gradual taper should begin with a taper of mycophenolate mofetil until prednisone is tapered to 10 mg per day. Rarely, patients may have steroid and mycophenolate refractory hepatitis. Improvement with the addition of anti-thomymocyte globulin at 1.5 mg/kg for 2 consecutive days with steroids and mycophenolate has been reported.40
Cardiac
The optimal treatment for immune-myocarditis is not clear. Treatment regimens should certainly include high-dose corticosteroids. Alternative immunosuppressants such as infliximab, anti-thymocyte globulin, mycophenolate mofetil and tacrolimus should be considered in patients that do not respond to corticosteroids or have severe disease. Supportive management for arrhythmias and potentially decreased ejection fraction is also a cornerstone of management.
Neurologic
Treatment for neurologic toxicities should be initiated promptly and typically includes high-dose systemic corticosteroids. Neurologic consultation should also be obtained to aid in clarifying the diagnosis. Condition-specific treatments may also be beneficial, including intravenous immunoglobulin or plasmapheresis.41
Conclusions
In summary, PD-1/PD-L1 inhibitors have rapidly changed treatment algorithms for numerous malignancies. Despite their impressive response rates and relative tolerability, checkpoint inhibitors can result in both unique and severe toxicities. It is important that providers are aware of the range of irAEs that patients can experience as well as their presentation, natural history, and treatment. Multidisciplinary management with endocrine, gastrointestinal, or pulmonary specialists may be indicated in many cases. Further study is needed to determine risk factors that predispose patients to toxicity and how to optimally manage irAEs.
Acknowledgments
Funding: DBJ is supported by the NIH/NCI (K23 CA204726) and the James C. Bradford Jr. Melanoma Fund.
Footnotes
Conflicts of interest: DBJ serves on advisory boards for BMS, Genoptix, Incyte, Merck, and Novartis, and receives research-related funding from Incyte.
References
- 1.Wolchok JD. PD-1 Blockers. Cell. 2015;162:937. doi: 10.1016/j.cell.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra45. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 4.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, Pamer EG, Wolchok JD. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1607–16. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35:709–17. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res. 2016;22:6051–60. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM, Shi C. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology. 2017;70:558–67. doi: 10.1111/his.13118. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, Ibrahim N. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200:W468–74. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–82. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 16.Wang DY, Kim DW, Shah NJ, Conry RM, Mehta RJ, Silk AW, Zhou A, Voorhees AL, McKee SB, Norrell J, Mehnert JM, Puzanov I, Gibney GT, Rapisuwon S, Eroglu Z, Johnson DB. Clinical presentation of immune-related colitis associated with PD-1 inhibitor monotherapy (MONO) and combination PD-1/CTLA-4 inhibitors (COMBO) in melanoma. Journal of Clinical Oncology. 2017;35:9566. [Google Scholar]
- 17.Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, Ibrahim N. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071–7. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci. 2012;57:2233–40. doi: 10.1007/s10620-012-2140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardo SG, Moskalenko M, Pan M, Shah S, Sidhu HK, Sicular S, Harcharik S, Chang R, Friedlander P, Saenger YM. Elevated rates of transaminitis during ipilimumab therapy for metastatic melanoma. Melanoma Res. 2013;23:47–54. doi: 10.1097/CMR.0b013e32835c7e68. [DOI] [PubMed] [Google Scholar]
- 20.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 22.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, Lacouture ME. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015;151:1206–12. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 26.Gan EH, Mitchell AL, Plummer R, Pearce S, Perros P. Tremelimumab-Induced Graves Hyperthyroidism. Eur Thyroid J. 2017;6:167–70. doi: 10.1159/000464285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson DB, Mudigonda TV, Sosman JA. Melanoma and a Headache. Diagnosis: Hypophysitis. JAMA Oncol. 2015;1:1167–8. doi: 10.1001/jamaoncol.2015.2537. [DOI] [PubMed] [Google Scholar]
- 28.Konda B, Nabhan F, Shah MH. Endocrine dysfunction following immune checkpoint inhibitor therapy. Curr Opin Endocrinol Diabetes Obes. 2017 doi: 10.1097/MED.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 29.Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, Suijkerbuijk KPM, Azevedo S, Li H, Reshef D, Avila A, Reardon DA. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncologist. 2017;22:709–18. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DB, Saranga-Perry V, Lavin PJ, et al. Myasthenia gravis induced by ipilimumab in metastatic melanoma patients. J Clin Oncol. 2013 doi: 10.1200/JCO.2013.51.1683. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29:806–12. doi: 10.1097/WCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 32.Spain L, Walls G, Julve M, O’Meara K, Schmid T, Kalaitzaki E, Turajlic S, Gore M, Rees J, Larkin J. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28:377–85. doi: 10.1093/annonc/mdw558. [DOI] [PubMed] [Google Scholar]
- 33.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 34.Diem S, Keller F, Ruesch R, Maillard SA, Speiser DE, Dummer R, Siano M, Urner-Bloch U, Goldinger SM, Flatz L. Pembrolizumab-triggered Uveitis: An Additional Surrogate Marker for Responders in Melanoma Immunotherapy? J Immunother. 2016;39:379–82. doi: 10.1097/CJI.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 35.Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular Toxicities Associated with Cancer Immunotherapies. Curr Cardiol Rep. 2017;19:21. doi: 10.1007/s11886-017-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SERVICES USDOHAH, editor. CTCAE_4.0. Common Terminology Criteria for Adverse Events (CTCAE) 2009. Version 4.0. [Google Scholar]
- 37.Pollack M, Betof AS, Rappazzo K, Valentine I, Eroglu Z, Johnson DB, Shoushtari AN. Safety of resuming anti-PD-1 (aPD1) in patients (pts) with immune-related adverse events (irAEs) during combined anti-CTLA-4 (aCTLA4) and aPD1 in metastatic melanoma (MM) Journal of Clinical Oncology. 2017;35:9544. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–92. doi: 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781–5. doi: 10.1097/MCG.0b013e31828f1d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chmiel KD, Suan D, Liddle C, Nankivell B, Ibrahim R, Bautista C, Thompson J, Fulcher D, Kefford R. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol. 2011;29:e237–40. doi: 10.1200/JCO.2010.32.2206. [DOI] [PubMed] [Google Scholar]
- 41.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]


