Abstract
Background and purpose
The frequency, clinical correlates, and risk factors of cerebral microbleeds (CMB) in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) are still poorly known. We aimed at determining the location and number of CMB and their relationship with clinical manifestations, vascular risk factors, drugs, and other neuroimaging features in CADASIL patients.
Methods
We collected clinical data by means of a structured proforma and centrally evaluated CMB on magnetic resonance gradient echo sequences applying the Microbleed Anatomical Rating Scale in CADASIL patients seen in 2 referral centers in Italy and United Kingdom.
Results
We evaluated 125 patients. CMB were present in 34% of patients and their presence was strongly influenced by the age. Twenty-nine percent of the patients had CMB in deep subcortical location, 22% in a lobar location, and 18% in infratentorial regions. After adjustment for age, factors significantly associated with a higher total number of CMB were hemorrhagic stroke, dementia, urge incontinence, and statins use (this latter not confirmed by multivariate analysis). Infratentorial and deep CMB were associated with dementia and urge incontinence, lobar CMB with hemorrhagic stroke, dementia, and statins use. Unexpectedly, patients with migraine, with or without aura, had a lower total, deep, and lobar number of CMB than patients without migraine.
Discussion
CMB formation in CADASIL seems to increase with age. History of hemorrhagic stroke, dementia, urge incontinence, and statins use are associated with a higher number of CMB. However, these findings need to be confirmed by longitudinal studies.
Introduction
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL; MIM 125310) is an inherited cerebral microangiopathy caused by NOTCH3 mutations [1]. The disease is characterized by migraine, frequently with aura, stroke, psychiatric and cognitive disturbances, usually with a progressive course leading to disability and dementia [2]. Usually, strokes are ischemic and of the lacunar type. However, CADASIL patients with intracerebral hemorrhage have also been described [3]. From the neuroimaging point of view, the most frequent features are a severe leukoencephalopathy involving the anterior temporal poles and external capsules and lacunar infarcts [4–8]. Cerebral microbleeds (CMB) on gradient echo MRI sequences have been reported in CADASIL [9–11], but their frequency, clinical correlates, and risk factors remain poorly defined.
The aims of this study were to investigate the location and number of CMB in CADASIL using an internationally proposed scale, the Microbleed Anatomical Rating Scale (MARS) [12], and to explore factors associated with their presence.
Methods
CADASIL patients seen in two referral centers for cerebrovascular diseases (both comprising a stroke unit and a clinic dedicated to patients with sporadic and genetic small vessel diseases) in Florence, Italy, and London, United Kingdom, were included. All patients had a typical NOTCH3 mutation which resulted in a change in a cysteine amino acid in one of the extra-cellular epidermal growth factor-like repeats, apart from one patient with a family history who had granular osmiophilic material (GOM) on electron microscopy examination of a skin biopsy. For all patients, information on clinical features, vascular risk factors, and use of antithrombotic drugs and statins was collected prospectively by means of a structured proforma. For this study, we reviewed each available brain MRI scan performed on 1.5 Tesla magnet for clinical purposes at the time of the clinical assessment. For each patient, MRI included at least axial and/or sagittal T1-weighted images, axial gradient echo T2*-weighted images, axial FLuid Attenuated Inversion Recovery (FLAIR) images, and axial or coronal T2-weighted fast spin-echo images. The study was approved by the local ethics committees (Comitato Etico Azienda Ospedaliero Universitaria Careggi and English Multicentre Research Ethics Committee) and all patients gave a written informed consent for genetic testing and participation in the study. No minor was enrolled in the study.
Definition of clinical variables
Stroke and TIA were defined according to current criteria [13,14]. Psychiatric disorders were recorded as present in the case of any of the following: 1) diagnosis of a psychiatric disease by a certified specialist (psychiatrist, geriatrician, or neurologist); 2) previous or current use of antipsychotic or antidepressant drugs or psychotherapy (the sole use of anxiolytics was not sufficient); 3) mood or behavior disorders referred by the patient or his/her family, not immediately related to bereavement, and that had interfered for at least 6 months with daily or work activities. Cognitive disorders were recorded as present if one of the following instances was present: 1) previous diagnosis of mild cognitive impairment or dementia by a certified specialist (geriatrician or neurologist); 2) overt cognitive impairment emerged during the first evaluation at our centers; 3) presence of cognitive decline from a previously normal status referred by a next of kin or by the patient and confirmed by neuropsychological testing. Neuropsychological cognitive performances were judged impaired if the patient scored 1.5 standard deviations below the age- and education-corrected means in at least one cognitive test. Headache and migraine with and without aura were defined according to the Headache Classification Committee of the International Headache Society [15]. Seizures were defined according to the International League Against Epilepsy Commission Report [16]. Acute reversible encephalopathy was defined as an episode of altered consciousness associated with seizures and/or hallucinations after exclusion of infectious, metabolic and paraneoplastic causes [17]. The presence of hypertension was ascertained based on a previous diagnosis or according to the World Health Organization Guidelines [18] as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg on multiple blood pressure measurements, taken on several separate occasions. Diabetes mellitus was defined according to the American Diabetes Association criteria [19]. Hypercholesterolemia included total cholesterol >200, low-density lipoprotein >130, and high-density lipoprotein <35 mg/dl (each value had to be found elevated in at least two measurements) [20]. Hyperhomocysteinemia was defined when elevated values were found according to local laboratory norms. Smoking was considered as present in case of current or previous history. Urge incontinence was clinically defined by each center investigators.
Definition of neuroimaging variables
MRI scans were reviewed by a single trained observer (SN). CMB were defined as small, round, well-defined lesions, hypointense and associated with blooming effect on MR gradient echo T2* sequences, 2–10 mm in diameter [12,21]. They were further evaluated by applying the MARS [12]. This scale classifies CMB into infratentorial (including brainstem and cerebellum), deep (basal ganglia, thalamus, external and internal capsules, corpus callosum, deep and periventricular white matter), and lobar (frontal, parietal, temporal, occipital, and insula, including cortical and subcortical regions with U fibers). According to the MARS, we classified CMB in definite, as previously defined, and possible, when the above mentioned characteristics were less clear [12]; only definite CMB were considered in our analyses. Presence and severity of white matter changes on FLAIR or T2-weighted images were evaluated according to a modified Fazekas scale [22]: grade 1: single lesions <10 mm; areas of ‘grouped’ lesions <20 mm in any diameter; grade 2: single lesions between 10 and 20 mm; areas of ‘grouped’ lesions more than 20 mm in any diameter; no more than ‘connecting bridges’ between individual lesions; grade 3: single lesions or confluent areas of hyperintensity >20 mm in any diameter. The presence of hyperintense lesions on FLAIR and T2-weighted images in the anterior temporal lobe white matter, external capsule, and pons was also recorded. Lacunar infarcts were defined as focal hyperintensities on T2-weighted images, 3–15 mm in diameter, and with a corresponding hypointensity on T1-weighted images [21].
Statistical analysis
Intra-rater agreement for number and location of CMB was determined. Twenty-eight anonymized scans were reviewed by the same observer 6 months after the first evaluation blinded to clinical information. Agreement was calculated using the intraclass correlation coefficient. Reliability was high for each MARS category: 0.888 for total CMB, 0.904 for infratentorial, 0.943 for deep, and 0.810 for lobar CMB. Descriptive analyses were used to characterize the baseline sample in terms of demographic, clinical and neuroimaging features. Analysis of variance (ANOVA) or Pearson correlation were applied to test for associations of clinical and neuroimaging variables with the number and location of CMB. Analyses were adjusted for age using UNIANOVA (IBM, SPSS Statistics version 24). Significantly associated variables were included in a stepwise linear regression to identify independent predictors of number and location of CMB. Because of the small number of CMB in each site specified in the MARS, we grouped them in the 3 location categories defined in the scale (deep subcortical, lobar, infratentorial).
Results
One hundred and twenty-five CADASIL patients (91 probands and 34 relatives) were included in the analysis. Most patients had NOTCH3 mutations on exon 4, followed by exons 11, 3, and 20. Demographic, clinical and neuroimaging features are summarized in Table 1. The mean (SD) age at assessment was 50.6 (14.2) years and 56 patients (45%) were males. The mean (SD) age at onset of the disease was 33.4 (17.1) years. The most frequent symptom at onset was migraine with or without aura, followed by stroke and psychiatric disturbances. Eight patients (3 probands and 5 relatives) were asymptomatic at the time of assessment having had presymptomatic genetic testing. All of these patients had the MRI abnormalities typical of CADASIL: 1 proband and 2 relatives had leukoencephalopathy involving the temporal pole and the external capsule, while 2 probands and 3 relatives had leukoencephalopathy extended only to the temporal pole.
Table 1. Demographic, clinical and neuroimaging characteristics of the 125 CADASIL patients enrolled in the study.
| Age at assessment, years (mean ± SD) | 50.6 ± 14.2 |
| Age at onset, years (mean ± SD) | 33.4 ± 17.1 |
| Age at first stroke, years (mean ± SD) | 49.7 ± 12.8 |
| Male gender, n (%) | 56/125 (45) |
| Ischemic stroke, n (%) | 49/125 (39) |
| TIA, n (%) | 19/125 (15) |
| Recurrent ischemic events, n (%) | 30/125 (24) |
| Hemorrhagic stroke, n (%) | 3/125 (2) |
| Cognitive impairment, n (%) | 46/125 (37) |
| Dementia, n (%) | 9/123 (7) |
| Psychiatric disturbances, n (%) | 54/125 (43) |
| Migraine, n (%) | 84/125 (67) |
| Migraine with aura, n (%) | 60/125 (48) |
| Prolonged aura, n (%) | 16/120 (13) |
| Seizures, n (%) | 11/125 (9) |
| Encephalopathy, n (%) | 15/125 (12) |
| Urge incontinence, n (%) | 28/116 (24) |
| Hypertension, n (%) | 37/125 (30) |
| Diabetes, n (%) | 7/125 (6) |
| Hypercholesterolemia, n (%) | 70/121 (58) |
| History of smoking, n (%) | 59/124 (48) |
| Hyperhomocysteinemia, n (%) | 26/99 (26) |
| Antithrombotic treatment, n (%) | 83/119 (70) |
| Statins use, n (%) | 49/122 (40) |
| Fazekas grade 1, n (%) | 22/125 (18) |
| Fazekas grade 2, n (%) | 18/125 (14) |
| Fazekas grade 3, n (%) | 82/125 (66) |
| Pontine leukoaraiosis, n (%) | 65/122 (53) |
| Temporal pole involvement, n (%) | 110/125 (88) |
| External capsule involvement, n (%) | 96/125 (77) |
| Lacunar infarcts, n (%) | 80/125 (64) |
CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; TIA: transient ischemic attack
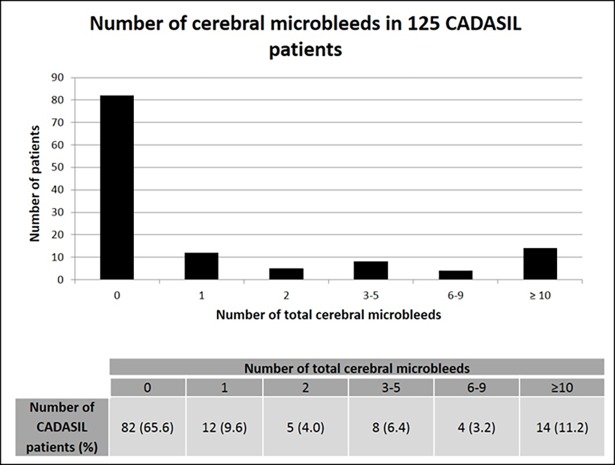
Forty-three patients (34%) had at least one CMB (range 1–73) (Fig 1). Twenty-nine percent had CMB in a deep subcortical location (range 1–24), most frequently in the thalamus, 22% had CMB in a lobar location (1–37), mainly temporal, and 18% in an infratentorial location (1–12) (Fig 2). The associations of number of total, infratentorial, deep, and lobar CMB with demographic, clinical and imaging variables are shown in Tables 2 and 3.
Fig 1. Total number of cerebral microbleeds in 125 CADASIL patients.
Proportions of patients with different numbers of CMB are reported in the bar graph and in the table.
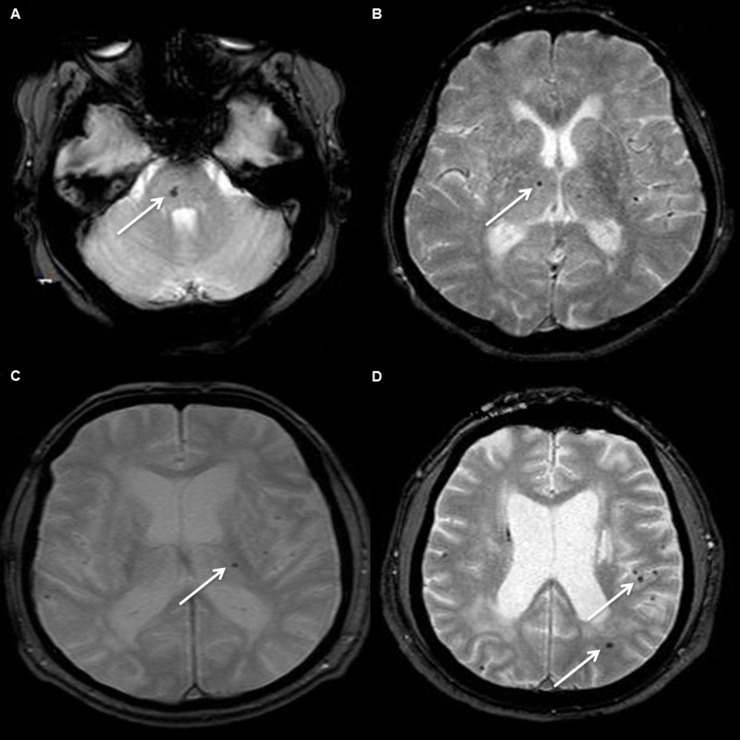
Fig 2. Examples of different locations of cerebral microbleeds in our sample of CADASIL patients.
T2*-weighted magnetic resonance images showing cerebral microbleeds (white arrows) in infratentorial (A), deep subcortical (B, C) and lobar locations (D).
Table 2. Correlation between the total number of cerebral microbleeds and demographics, drugs, clinical and other neuroimaging features in 125 CADASIL patients.
| Number of CMB mean (SD) |
p* | p** | |
|---|---|---|---|
| Male gender (yes vs no) | 3.8 (9.9) vs 3.8 (10.9) | 0.981 | 0.676 |
| Ischemic stroke (yes vs no) | 5.5 (11.3) vs 2.7 (9.7) | 0.144 | 0.980 |
| TIA (yes vs no) | 7.8 (17.1) vs 3.0 (8.6) | 0.065 | 0.662 |
| Recurrent ischemic events (yes vs no) | 5.6 (9.8) vs 3.2 (10.6) | 0.286 | 0.501 |
| Hemorrhagic stroke (yes vs no) | 25.3 (21.8) vs 3.3 (9.5) | <0.001 | 0.001 |
| Cognitive impairment (yes vs no) | 8.8 (15.3) vs 0.8 (3.6) | <0.001 | 0.140 |
| Dementia (yes vs no) | 22.4 (24.2) vs 2.4 (6.9) | <0.001 | <0.001 |
| Psychiatric disturbances (yes vs no) | 4.0 (8.9) vs 3.6 (11.5) | 0.833 | 0.575 |
| Migraine (yes vs no) | 1.3 (4.4) vs 8.8 (16.0) | <0.001 | <0.001 |
| Migraine with aura (yes vs no) | 1.1 (4.7) vs 6.3 (13.3) | 0.005 | 0.004 |
| Prolonged aura (yes vs no) | 0.4 (1.0) vs 4.1 (10.9) | 0.169 | 0.141 |
| Seizures (yes vs no) | 9.6 (15.0) vs 3.2 (9.7) | 0.051 | 0.265 |
| Acute reversible encephalopathy (yes vs no) | 1.2 (2.8) vs 4.1 (11.0) | 0.306 | 0.246 |
| Urge incontinence (yes vs no) | 12.0 (17.8) vs 1.4 (5.2) | <0.001 | 0.008 |
| Hypertension (yes vs no) | 8.0 (15.7) vs 2.0 (6.4) | 0.003 | 0.650 |
| Diabetes (yes vs no) | 7.3 (15.8) vs 3.6 (10.0) | 0.363 | 0.870 |
| Hypercholesterolemia (yes vs no) | 5.0 (10.0) vs 2.5 (11.2) | 0.199 | 0.721 |
| History of smoking (yes vs no) | 3.4 (9.1) vs 4.2 (11.6) | 0.680 | 0.821 |
| Hyperhomocysteinemia (yes vs no) | 6.4 (15.8) vs 3.4 (8.9) | 0.236 | 0.386 |
| Antithrombotic treatment (yes vs no) | 5.0 (12.0) vs 1.6 (6.1) | 0.111 | 0.450 |
| Statins use (yes vs no) | 3.8 (7.5) vs 3.9 (12.2) | 0.967 | 0.009 |
| Fazekas grade 1 (yes vs no) | 0.0 (0.0) vs 4.6 (11.3) | 0.036 | 0.429 |
| Fazekas grade 2 (yes vs no) | 0.2 (0.5) vs 4.4 (11.3) | ||
| Fazekas grade 3 (yes vs no) | 5.7 (12.4) vs 0.1 (0.3) | ||
| Pontine leukoaraiosis (yes vs no) | 5.9 (13.0) vs 0.9 (3.9) | 0.007 | 0.918 |
| Temporal pole involvement (yes vs no) | 4.0 (10.7) vs 2.5 (8.2) | 0.619 | 0.968 |
| External capsule involvement (yes vs no) | 4.9 (11.6) vs 0.0 (0.2) | 0.026 | 0.106 |
| Lacunar infarcts (yes vs no) | 5.8 (12.5) vs 0.2 (1.2) | 0.003 | 0.916 |
CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; CMB: cerebral microbleeds; SD: standard deviations; TIA: transient ischemic attack
* ANOVA
** ANOVA adjusted for age
p values reported in bold are statistically significant
Table 3. Correlation between the number of infratentorial, deep, and lobar cerebral microbleeds and drugs, clinical and other neuroimaging features in 125 CADASIL patients.
| Infratentorial | Deep | Lobar | ||||
|---|---|---|---|---|---|---|
| Number of CMB mean (SD) |
p* | Number of CMB mean (SD) |
p* | Number of CMB mean (SD) |
p* | |
| Male gender (yes vs no) | 0.4 (1.1) vs 0.6 (1.9) | 0.486 | 1.3 (2.8) vs 1.9 (4.8) | 0.578 | 2.1 (6.8) vs 1.2 (4.8) | 0.193 |
| Ischemic stroke (yes vs no) | 0.7 (1.6) vs 0.4 (1.6) | 0.915 | 2.0 (3.9) vs 1.4 (4.1) | 0.482 | 2.7 (7.3) vs 0.9 (4.5) | 0.687 |
| TIA (yes vs no) | 1.3 (2.8) vs 0.4 (1.3) | 0.261 | 3.5 (6.3) vs 1.3 (3.4) | 0.375 | 3.0 (8.7) vs 1.4 (5.1) | 0.873 |
| Recurrent ischemic events (yes vs no) | 0.9 (1.7) vs 0.4 (1.6) | 0.857 | 2.8 (4.9) vs 1.3 (3.7) | 0.869 | 1.9 (4.8) vs 1.5 (6.1) | 0.182 |
| Hemorrhagic stroke (yes vs no) | 1.7 (2.9) vs 0.5 (1.6) | 0.534 | 6.7 (6.1) vs 1.5 (3.9) | 0.127 | 17.0 (17.1) vs 1.3 (4.9) | <0.001 |
| Cognitive impairment (yes vs no) | 1.2 (2.3) vs 0.1 (0.7) | 0.105 | 3.7 (5.7) vs 0.4 (1.9) | 0.073 | 3.9 (9.0) vs 0.3 (1.5) | 0.374 |
| Dementia (yes vs no) | 3.2 (3.8) vs 0.3 (1.1) | <0.001 | 8.8 (8.2) vs 1.1 (3.0) | <0.001 | 10.4 (13.6) vs 1.0 (4.1) | <0.001 |
| Psychiatric disturbances (yes vs no) | 0.6 (1.4) vs 0.5 (1.7) | 0.859 | 1.9 (4.0) vs 1.4 (4.1) | 0.978 | 1.5 (5.2) vs 1.7 (6.2) | 0.346 |
| Migraine (yes vs no) | 0.3 (1.1) vs 0.9 (2.2) | 0.125 | 0.8 (2.7) vs 3.3 (5.6) | 0.001 | 0.2 (0.9) vs 4.5 (9.5) | <0.001 |
| Migraine with aura (yes vs no) | 0.3 (1.1) vs 0.8 (1.9) | 0.106 | 0.6 (2.8) vs 2.5 (4.8) | 0.007 | 0.2 (0.9) vs 3.0 (7.8) | 0.008 |
| Prolonged aura (yes vs no) | 0.2 (0.7) vs 0.5 (1.6) | 0.409 | 0.1 (0.3) vs 1.7 (4.0) | 0.084 | 0.1 (0.2) vs 1.9 (6.3) | 0.236 |
| Seizures (yes vs no) | 0.7 (1.5) vs 0.5 (1.6) | 0.709 | 3.4 (5.0) vs 1.4 (3.9) | 0.498 | 5.4 (10.7) vs 1.3 (5.0) | 0.113 |
| Acute reversible encephalopathy (yes vs no) | 0.3 (0.6) vs 0.6 (1.7) | 0.472 | 0.8 (2.1) vs 1.7 (4.2) | 0.346 | 0.1 (0.3) vs 1.8 (6.1) | 0.242 |
| Urge incontinence (yes vs no) | 1.6 (2.8) vs 0.2 (0.8) | 0.011 | 5.0 (6.8) vs 0.7 (2.1) | 0.003 | 5.3 (10.2) vs 0.6 (3.1) | 0.062 |
| Hypertension (yes vs no) | 1.0 (2.3) vs 0.3 (1.1) | 0.909 | 3.1 (5.6) vs 1.0 (3.1) | 0.910 | 3.9 (9.2) vs 0.7 (3.2) | 0.497 |
| Diabetes (yes vs no) | 0.0 (0.0) vs 0.5 (1.6) | 0.057 | 2.0 (2.8) vs 1.6 (4.1) | 0.360 | 5.3 (13.1) vs 1.4 (5.1) | 0.384 |
| Hypercholesterolemia (yes vs no) | 0.7 (1.6) vs 0.3 (1.7) | 0.973 | 2.3 (4.3) vs 0.8 (3.6) | 0.699 | 2.0 (5.8) vs 1.3 (6.0) | 0.383 |
| History of smoking (yes vs no) | 0.4 (1.4) vs 0.6 (1.8) | 0.518 | 1.4 (3.7) vs 1.9 (4.4) | 0.593 | 1.6 (5.5) vs 1.7 (6.1) | 0.878 |
| Hyperhomocysteinemia (yes vs no) | 1.1 (2.6) vs 0.4 (1.3) | 0.164 | 2.6 (5.7) vs 1.5 (3.8) | 0.422 | 2.7 (7.7) vs 1.5 (5.4) | 0.571 |
| Antithrombotic treatment (yes vs no) | 0.7 (1.9) vs 0.2 (0.9) | 0.721 | 2.1 (4.5) vs 0.7 (2.9) | 0.547 | 2.2 (6.9) vs 0.7 (2.6) | 0.425 |
| Statins use (yes vs no) | 0.7 (1.5) vs 0.4 (1.7) | 0.375 | 2.1 (4.3) vs 1.3 (3.9) | 0.211 | 1.0 (2.3) vs 2.1 (7.3) | 0.001 |
| Fazekas grade 1 (yes vs no) | 0.0 (0.0) vs 0.6 (1.7) | 0.731 | 0.0 (0.0) vs 1.9 (4.4) | 0.529 | 0.0 (0.0) vs 2.0 (6.3) | 0.427 |
| Fazekas grade 2 (yes vs no) | 0.0 (0.0) vs 0.6 (1.7) | 0.1 (0.2) vs 1.9 (4.3) | 0.1 (0.3) vs 1.9 (6.2) | |||
| Fazekas grade 3 (yes vs no) | 0.8 (1.9) vs 0.0 (0.0) | 2.5 (4.8) vs 0.0 (0.2) | 2.5 (7.0) vs 0.0 (0.2) | |||
| Pontine leukoaraiosis (yes vs no) | 0.7 (1.8) vs 0.1 (0.7) | 0.984 | 2.4 (4.6) vs 0.4 (2.1) | 0.771 | 2.7 (7.8) vs 0.3 (1.2) | 0.998 |
| Temporal pole involvement (yes vs no) | 0.5 (1.6) vs 0.3 (1.3) | 0.886 | 1.7 (4.2) vs 0.9 (3.1) | 0.785 | 1.7 (6.0) vs 1.3 (3.8) | 0.876 |
| External capsule involvement (yes vs no) | 0.7 (1.8) vs 0.0 (0.0) | 0.400 | 2.1 (4.5) vs 0.0 (0.0) | 0.218 | 2.1 (6.5) vs 0.0 (0.2) | 0.083 |
| Lacunar infarcts (yes vs no) | 0.8 (1.9) vs 0.0 (0.0) | 0.597 | 2.4 (4.8) vs 0.2 (1.2) | 0.988 | 2.6 (7.1) vs 0.0 (0.0) | 0.747 |
CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; CMB: cerebral microbleeds; SD: standard deviations; TIA: transient ischemic attack
* ANOVA adjusted for age
p values reported in bold are statistically significant
Among patients on antithrombotic treatment, only one was receiving anticoagulant treatment. Three unrelated patients (39, 54, and 67 years) experienced hemorrhagic strokes: 2 patients had a thalamo-capsular hemorrhage and one of these had also a hemispheric cerebellar hemorrhage, while the third patient suffered from an interpeduncular cistern subarachnoid hemorrhage. None of them was receiving antiplatelets, anticoagulants or statins at the time of hemorrhage, but all were affected by hypertension. CMB were present in all of these patients both in lobar and deep subcortical locations.
For each location, the presence and number of CMB was strongly associated with age (Pearson correlation, p<0.001). Regarding the total number of CMB, Pearson correlation coefficients were the following: 0.478 for age at time of assessment, 0.450 for age at disease onset, and 0.318 for age at first stroke. Therefore, in Tables 2 and 3 age-adjusted p values are presented. After adjustment for age, a history of hemorrhagic stroke, dementia, urge incontinence, and statins use were factors significantly associated with a higher total number of CMB (Table 2). Considering location, infratentorial and deep CMB were significantly associated with dementia and urge incontinence, while lobar CMB with hemorrhagic stroke, dementia, and statins use (Table 3). Patients with migraine, with or without aura, had significantly fewer total CMB than patients who did not suffer from migraine (1.4 ± 4.5 versus 8.5 ± 15.9, p<0.001) (Table 2). This negative association was found also considering separately deep and lobar locations and remained statistically significant after correction for age (Tables 2 and 3). On the multivariate analysis, each variable that was statistically significant at the univariate analysis maintained an independent predictive value with the exception of the statins use. No correlation between other neuroimaging characteristics typical of CADASIL and number and location of CMB was found after adjustment for age in our cohort.
Discussion
In this binational series of CADASIL patients, we found that one third of patients had CMB and that these ones occurred throughout subcortical, infratentorial and lobar regions. We also found that history of hemorrhagic stroke, dementia, and urge incontinence were independently associated with a higher number of CMB, while patients with migraine had a lower burden of CMB in comparison with non-migraineurs. Regarding many aspects, our study confirmed the results obtained in previous studies focused on CMB in CADASIL. In particular, the prevalence of CMB in this CADASIL cohort is in keeping with other CADASIL series [9,11] that reported prevalence ranging between 25% and 69% [7,10]. In spite of the absence of a clearly predominant location of CMB in our sample, the most frequent one was thalamic, followed by the lobar one, mainly temporal, as also described in other series [7,9,10,23]. Finally, the number of CMB was strongly positively correlated with age in our patients. A strict correlation between CMB and increasing age has been widely described in the literature, both in sporadic cerebral microangiopathy [24–26] and in other CADASIL samples [7,9,10,23,27]. In CADASIL patients, one could speculate that this strong correlation of CMB with age might be at least partially explained by the disease progression and a higher prevalence of hypertension in more advanced ages.
In our study, dementia was associated with CMB in each analyzed location and this association was also confirmed after a multivariate analysis, in contrast with data reported by others [11]. An association between number of CMB and cognitive decline in specific domains, such as memory and executive function, was clearly reported by Liem and colleagues [28]. Of note, urge incontinence was more frequent in CADASIL patients with a higher number of CMB in infratentorial and deep locations, independently from the age. An association between urinary urgency and cerebral small vessel disease has been reported in the literature, but mainly related with severe white matter changes in patients with sporadic microangiopathy [29]. Little is known about a possible correlation between CMB and urinary disturbances in subjects with sporadic small vessel disease and no data are available about other CADASIL populations.
No correlation was found between the use of antithrombotic drugs and CMB after adjustment for age. On a first-step of analysis, we found that statins use was associated with a higher number of total and lobar CMB. In different populations, such as patients suffering from acute cerebrovascular events, an association between high-dose atorvastatin and a higher number of CMB was found [30]. However, in our sample, the association between statins use and CMB disappeared on a multivariate analysis.
A quite surprising result was the association between migraine, both with and without aura, and a lower number of CMB, particularly in deep and lobar location. To the best of our knowledge, this is the first study investigating the correlation between CMB and migraine. This finding is not of immediate interpretation from a pathogenic point of view and needs to be confirmed in other patient series.
We did not find a relation between CMB and other features of cerebral small vessel disease. At present, we do not have a precise explanation for this. The hypothesis could be that the pathophysiological mechanisms underlying CMB in CADASIL are partially different from those responsible for leukoaraiosis and lacunar infarct. However, we had no mean to test this hypothesis in this study.
The study has a number of strengths. First, it included a considerable number of patients taking into account that CADASIL is a rare disease. Second, the assessment of CMB was performed by applying a structured scale, allowing quantification and localization. Third, this is the first study to investigate the relationship of considerable number of clinical variables with CMB in CADASIL patients. In the literature, only data about cognitive profile and some psychiatric disturbances are reported [23,28,31,32], but data are missing about many other clinical features typical of CADASIL. Fourth, our sample is composed of patients from different countries and this could make our results more generalizable to the CADASIL patient community.
This study has also limitations. It is a cross-sectional study and therefore it is not possible to draw definitive conclusions about the real predictors of the location and number of CMB in CADASIL patients. Longitudinal studies are needed to confirm our observations. Another possible limitation is the low prevalence in our sample of some of the examined clinical manifestations that resulted associated with a higher number of CMB (in particular, only 3 patients had hemorrhagic stroke and only 9 patients had dementia).
In summary, our study shows that CMB are common in CADASIL and may have a number of clinical correlates. It is possible that their use may help in optimal planning of treatments such as anti-platelet agents but assessing this will require prospective longitudinal studies and randomized controlled trials.
Supporting information
(XLS)
Acknowledgments
The publication costs were supported by the non-profit organization Associazione per la Ricerca sulle Demenze (ARD) ONLUS, Department of Neurology, Luigi Sacco Hospital, Milan, Italy.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssière C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- 2.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9 [DOI] [PubMed] [Google Scholar]
- 3.Rinnoci V, Nannucci S, Valenti R, Donnini I, Bianchi S, Pescini F, et al. Cerebral hemorrhages in CADASIL: report of four cases and a brief review. J Neurol Sci. 2013;330:45–51. doi: 10.1016/j.jns.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Auer DP, Pütz B, Gössl C, Elbel G, Gasser T, Dichgans M. Differential lesion patterns in CADASIL and sporadic subcortical arteriosclerotic encephalopathy: MR imaging study with statistical parametric group comparison. Radiology. 2001;218:443–451. doi: 10.1148/radiology.218.2.r01fe24443 [DOI] [PubMed] [Google Scholar]
- 5.Markus HS, Martin RJ, Simpson MA, Dong YB, Ali N, Crosby AH, et al. Diagnostic strategies in CADASIL. Neurology. 2002;59:1134–1138. [DOI] [PubMed] [Google Scholar]
- 6.van Den Boom R, Lesnik Oberstein SA, van Duinen SG, Bornebroek M, Ferrari MD, Haan J, et al. Subcortical lacunar lesions: an MR imaging finding in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Radiology. 2002;224:791–796. doi: 10.1148/radiol.2243011123 [DOI] [PubMed] [Google Scholar]
- 7.van den Boom R, Lesnik Oberstein SA, Ferrari MD, Haan J, van Buchem MA. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR imaging findings at different ages—3rd-6th decades. Radiology. 2003;229:683–690. doi: 10.1148/radiol.2293021354 [DOI] [PubMed] [Google Scholar]
- 8.Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 9.Lesnik Oberstein SA, van den Boom R, van Buchem MA, van Houwelingen HC, Bakker E, Vollebregt E, et al. Cerebral microbleeds in CADASIL. Neurology. 2001;57:1066–1070. [DOI] [PubMed] [Google Scholar]
- 10.Dichgans M, Holtmannspötter M, Herzog J, Peters N, Bergmann M, Yousry TA. Cerebral microbleeds in CADASIL: a gradient-echo magnetic resonance imaging and autopsy study. Stroke. 2002;33:67–71. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006;129:2375–2383. doi: 10.1093/brain/awl177 [DOI] [PubMed] [Google Scholar]
- 12.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d [DOI] [PubMed] [Google Scholar]
- 13.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 14.Ad hoc Committee established by the Advisory Council for the National Institute of Neurological and Communicative Disorders and Stroke. A classification and outline of cerebrovascular diseases II. Stroke. 1975;6:564–616. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1–96. [PubMed] [Google Scholar]
- 16.Blume WT, Lϋders HO, Mizrahi E, Tassinari C, Van Emde Boas W, Engel J Jr. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–1218. [DOI] [PubMed] [Google Scholar]
- 17.Schon F, Martin RJ, Prevett M, Clough C, Enevoldson TP, Markus HS. "CADASIL coma": an underdiagnosed acute encephalopathy. J Neurol Neurosurg Psychiatry. 2003;74:249–252. doi: 10.1136/jnnp.74.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. International Society of Hypertension Guidelines for the Management of Hypertension: guidelines Subcommittee. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 19.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in adults (Adult treatment panel II). Circulation. 1994;89:1333–1445. [DOI] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability—the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050 [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O’Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007; 69:172–179. doi: 10.1212/01.wnl.0000265221.05610.70 [DOI] [PubMed] [Google Scholar]
- 24.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550–555. doi: 10.1161/01.STR.0000199847.96188.12 [DOI] [PubMed] [Google Scholar]
- 26.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9 [DOI] [PubMed] [Google Scholar]
- 27.Yao M, Jouvent E, During M, Godin O, Hervé D, Guichard JP, et al. Extensive white matter hyperintensities may increase brain volume in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2012;43:3252–3257. doi: 10.1161/STROKEAHA.112.664854 [DOI] [PubMed] [Google Scholar]
- 28.Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, et al. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007;38:923–928. doi: 10.1161/01.STR.0000257968.24015.bf [DOI] [PubMed] [Google Scholar]
- 29.Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, et al. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x [DOI] [PubMed] [Google Scholar]
- 30.Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 31.Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009;72:143–148. doi: 10.1212/01.wnl.0000339038.65508.96 [DOI] [PubMed] [Google Scholar]
- 32.Noh SM, Chung SJ, Kim KK, Kang DW, Lim YM, Kwon SU, et al. Emotional disturbance in CADASIL: its impact on quality of life and caregiver burden. Cerebrovasc Dis. 2014;37:188–194. doi: 10.1159/000357798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.