Abstract
Diabetes mellitus is a chronic disease that is becoming a serious global health problem. Diabetes has been considered to be one of the major risks of cataract and retinopathy. Synthetic and natural product inhibitors of carbohydrate degrading enzymes are able to reduce type 2 diabetes and its complications. For a long time, potatoes have been portrayed as unhealthy for diabetic patients by some nutritionist due to their high starch content. However, purple and red potato cultivars have received considerable attention from consumers because they have high levels of polyphenolic compounds that have potent antioxidant activities. In this study, we screened the total phenolics (TP) and total anthocyanins (TA) and analyzed the phenolic and anthocyanin compounds in selected potato cultivars and advanced selections with distinct flesh colors (purple, red, yellow and white). Purple and red potato cultivars had higher levels of TP and TA than tubers with other flesh colors. Chlorogenic acid is the predominant phenolic acid, and major anthocyanin is composed of the derivatives of petunidin, peonidin, malvidin and pelargonidin. We tested the potential inhibitory effect of potato extracts on the activities of α-amylase and α-glucosidase, which were targeted to develop antidiabetic therapeutic agents. We also measured inhibitory effect of potato extracts on aldose reductase (AR) which is a key enzyme that has been a major drug target for the development of therapies to treat diabetic complications. Purple flesh tubers extract showed the most effective inhibition of α-amylase, α-glucosidase, and aldose reductase with IC50 values 25, 42, and 32 μg/ml, respectively. Kinetic studies showed that anthocyanins are noncompetitive inhibitors of these enzymes, whereas phenolic acids behaved as mixed inhibitors for α-amylase and α-glucosidase and noncompetitive inhibitors for AR. This study supports the development of a positive and healthful image of potatoes, which is an important issue for consumers.
Introduction
Diabetes mellitus (DM) is a chronic disease and is characterized by abnormal glucose tolerance and insulin resistance [1]. DM is associated with complications, such as metabolic syndrome, heart disease, renal function recession, and blindness. Post prandial hyperglycemia is a major risk factor in the development of type II diabetes [2]. One of the most effective methods to prevent diabetes and hyperglycemia is to control the glucose level in blood [3]. Sugars in blood originates from the hydrolysis of carbohydrates and is catalyzed by digestive enzymes, such as α-glucosidase and α- amylase. α-glucosidase is an intestinal cell membrane enzyme whose function is to hydrolyze polysaccharides. Similarly, α- amylase is an enzyme that is secreted by the pancreas and salivary glands that can hydrolyze starches and oligosaccharide into simple sugars. Inhibition of these enzymes can retard carbohydrate digestion, thus causing a reduction in the rate of glucose absorption into the blood. Therefore, inhibition of these enzyme activities in digestive organs is considered to be a therapeutic approach for managing diabetes [4–6]. Aldose reductase is a key enzyme in the polyol pathway. It catalyzes the reduction glucose to sorbitol and provides a common link in the onset of diabetic complications in various parts of the human body. Intracellular accumulation of sorbitol leads to the local hyperosmotic conditions that are responsible for the development of diabetic complications such as cataract, retinopathy, neuropathy, and nephropathy [7]. Therefore, aldose reductase has been an attractive drug target in the clinical management of these diabetic complications [8–10].
Some synthetic inhibitors of these enzymes, such as acarbose and voglibose, have been developed [11]. However, some side effects are seen with these inhibitors, such as flatulence and digestive and liver function disorders. Therefore, inhibitors that have no side effects and come from natural sources are preferred. Many studies have investigated the antidiabetic activities of these phytochemicals in vitro and in vivo [4–10]. Several research efforts have been reported for effective α-amylase and α-glucosidase and aldose reductase inhibition from natural sources to develop a physiological functional food or lead compounds for use in antidiabetic medications [4–8]. Among them, polyphenolic compounds are secondary plant metabolites and constitute the largest group of health-promoting phytochemicals. The compounds that are responsible for the inhibition of α-amylase, α-glucosidase, and aldose reductase include phenolic acid, flavonoids, flavonol and anthocyanins [4–10].
Diets rich in fruits and vegetables are associated with a lower risk of chronic diseases since fruits and vegetables are a good source of polyphenols. Potatoes are one of the major food crops, after rice, wheat, and maize. It has a favorable overall nutrient-to-price ratio compared with many other fruits and vegetables and are an affordable source of nutrition worldwide. Historically, potato plant breeders have focused on traits related to external quality, yield, durability and visual appeal, but rarely on nutritional quality. Developing new potato cultivars with higher levels of nutritional value and bioactive compounds is considered to be a realistic approach to increasing dietary nutritional and antioxidant intake. Breeders and geneticist worldwide are working to enhance the phytonutrient content of potatoes [12–14]. As a result, new potato cultivars with distinctive flesh and skin colors are being developed. Screening for genetic divergence, in terms of health and bioactive compounds among the wild relatives, is a useful tool for plant breeder for selecting an efficient choice of parents for breeding programs. Potato tubers with red and purple flesh are known to have high phenolic contents, significant antioxidant properties [15, 16] and anticancer activity [17]. Due to these potential health attributes, specialty/colored flesh potato consumption is continuously increasing over recent years. Still, potatoes are often maligned in nutrition circles because of their suspected cause to obesity and diabetics. There are no detailed reports on the potential role of potatoes in preventing diabetes and its complications. The study was aimed to investigate the potential role of potato polyphenolic compounds in the inhibition of α-amylase, α-glucosidase and aldose reductase activities for the implication of antidiabetic properties.
Materials and methods
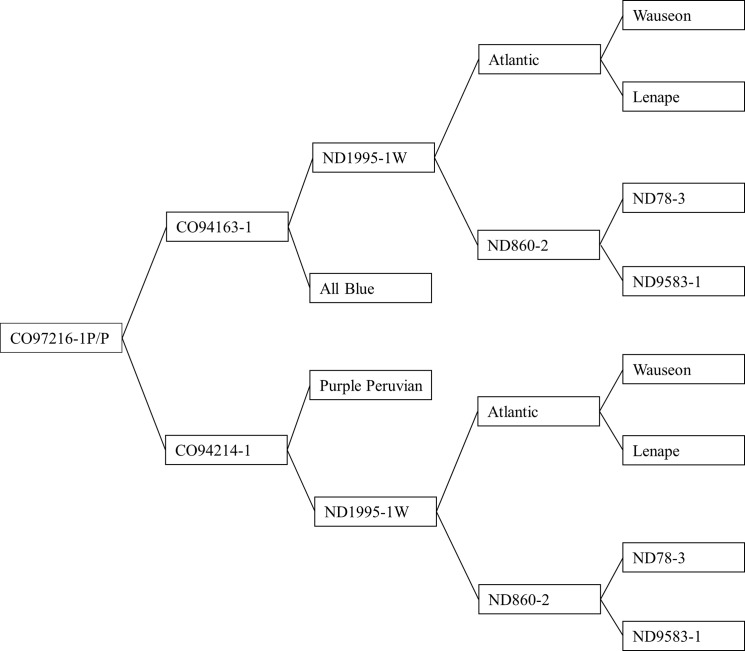
Purebred potato tubers Purple Majesty, CO97216-1P/P, CO97222-1R/R, CO97226-2R/R, Masquerade, Yukon Gold, Rio Grande Russet, and Russet Nugget with distinctive flesh colors used in this study are the product of Colorado potato breeding program at San Luis Valley Research Center (SLVRC), Colorado State University. They were resulted from the crosses made between specific male and female (Table 1) produced from earlier crosses. Fig 1 represents the pedigree for CO97216-1P/P. Twenty potato tubers of each clone were stored in a cooler at 10°C with above 90% humidity after harvesting from the field. All chemicals were purchased from Sigma Aldrich, St Louis, USA. Recombinant human aldose reductase was produced as previously described by Tarle et al. [18].
Table 1. Selected potato cultivars and physical appearance.
| clones | skin color | flesh color | parents (Female X Male) |
|---|---|---|---|
| CO97222-1R/R | Red | Red | CO94170-1 X Mountain Rose |
| CO97226-2R/R | Red | Red | Mountain Rose X CO94214-1 |
| Purple Majesty | Red | Purple | ND2008-2 X All Blue |
| CO97216-1P/P | Purple | Purple | CO94163-1 X CO94214-1 |
| Yukon Gold | White | Yellow | Norgleam X W5279-4 |
| Masquerade | White and purple | Yellow | Inka Gold X A91846-5R |
| Rio Grande Russet | White | White | Butte X A8469-5 |
Fig 1. Pedigree chart for the potato cultivar CO97216-1P/P.
Extraction of polyphenolic compounds
Phenolics and anthocyanins were extracted from potato tubers stored as mentioned above. Two-hundred grams of freeze-dried material was weighed into a 500-ml conical flask, and 250 ml of aqueous 80% methanol was added. The mixture was stirred overnight on a magnetic stirrer plate at 25°C. The mixtures were filtered through Whatman 100 filter paper. The residues were re-extracted under the same conditions. The combined filtrates were evaporated using a rotary evaporator until all of the methanol was evaporated. The final residue was lyophilized and kept at -80°C prior to analysis.
Analysis of total phenolics
A 20-μl aliquot of methanolic extract was mixed with 50 μl of distilled water in a 96-well flat-bottom assay plate. 50 μl of a commercial FCR solution (MP Biomedical, Solon, OH) was added and mixed well for 1 min in a plate reader (Power Wave XS2, BioTek Instruments, Winooski, VT). After 5 min, 80 μl of a 175 gml-1 sodium carbonate solution was added and immediately mixed with a pipette. The plates were incubated at 25°C in the dark with agitation at 150 rpm on an orbital shaker for two hours. The absorbances of the content were measured at 760 nm. The gallic acid in methanol was used as the standard, and the total phenolic values were quantified as micrograms of gallic acid equivalent per gram of dry weight material.
Analysis of total anthocyanin
The total monomeric anthocyanin was determined by the pH differential method. A 10-μl aliquot of the methanol extract of potato tubers was added separately to 290 μl of potassium chloride (pH 1.0) and sodium acetate (pH 4.5) buffers. The absorbance was measured at 515 nm and 700 nm for both sets of pH 1.0 and 4.5 solutions using water as a blank. The total anthocyanin content was calculated using the following equation:
where 449.2 and 26,900 are the molecular weight and molar absorptivity of cyanidine-3-glucoside (C3G), respectively, which was used as the standard; DF is the dilution factor, and l is the path length. The total μg/g dry weight of potato tuber anthocyanin was reported.
Analysis of chlorogenic acids
Three isomers of chlorogenic acid viz 5-caffeoylquinic acid (5CQA), 4-caffeoylquinic acid (4CQA), and 3-caffeoylquinc acid (3CQA) were quantified using Water 2695 HPLC spectrophotometer with a quaternary pump with the detector photodiode array and Nova Pak® C18 column. The mobile phase consisted 1% phosphoric acid (A) and acetonitrile (B) with a gradient 0–1 min 90% A and 10% B,1–20 min 30%A and 70%B and absorbance was monitored at 210–400 nm. The injected sample volume was 10 μl. 5CQA, 4CQA, and 3 CQA were quantified using the calibration curve for standard 5CQA, 4CQA and 3CQA.
Fractionation
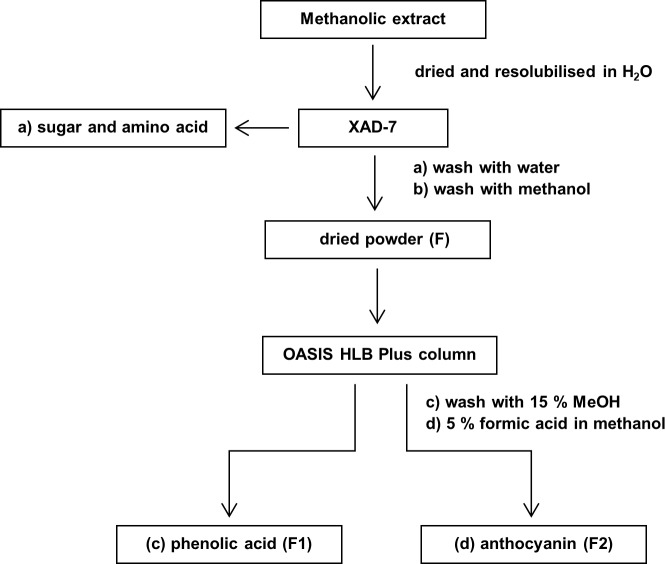
Five grams of the dried methanolic extract were dissolved in minimum water and then loaded onto an Amberlite XAD-7 column. The adsorbent was washed with water to remove sugars organic acids and salt. Phenolic compounds and anthocyanins retained in the adsorbent and eluted with methanol. The eluent was concentrated to dryness with a Büchi rotary evaporator connected to a high vacuum at 40°C. After evaporation, the remaining portion was placed in a freeze drier to remove the remaining water. Further, the XAD-7 extract was re-dissolved in a minimum volume of water and applied into OASIS HLB Plus cartridge. Two fractions: a) phenolic acid and b) anthocyanins were eluted with methanol and formic acid-methanol. Fig 2 represents the separation and isolation of these two fractions.
Fig 2. A flow diagram representing the purification and separation of methanolic extract of potato tubers.
LC-MS chromatogram
One milligram of each fraction (1 mg) were suspended in 1 ml of methanol-water (7:3). Five microliters of extract were injected into a Waters Acquity UPLC system and separated using a Waters Acquity UPLC HSS T3 column (1.8 μM, 1.0 x 100 mm) with a gradient from solvent A (water, 0.1% formic acid) to solvent B (Acetonitrile, 0.1% formic acid). Injections were made in 100% A, held at 100% A for 1 min, ramped to 98% B over 12 minutes, held at 98% B for 3 minutes, and then returned to the starting conditions over 0.05 minutes and allowed to re-equilibrate for 3.95 minutes, with a 200 μL/min constant flow rate. The column and samples were held at 50°C and 5°C, respectively. The column eluent was infused into a Waters Xevo G2 Q-TOF-MS with an electrospray source in either positive or negative ionization mode, scanning 50–1200 m/z at 0.2 seconds per scan, alternating between MS (6 V collision energy) and data-dependent MS/MS mode (15–30 V ramp). For positive ionization mode, the cone voltage and capillary voltages were set to 30 V and 2.2 kV, respectively. For negative ionization mode, the cone voltage and capillary voltages were set to 20 V and 2.0 kV, respectively. For both ionization modes, the source temp was 150°C and the nitrogen desolvation temperature was 350°C with a flow rate of 800 L/hr. Calibration was performed using sodium formate with a 1 ppm mass accuracy.
Inhibition of α-glucosidase, α-amylase and aldose reductase activities
α-glucosidase activity was assayed spectrophotometrically using PNPG as a substrate. The continuous production of p-nitrophenol was determined by measuring the absorbances at 405 nm in a reaction mixture containing 1U/ml glucosidase and 1mM PNPG incubated in pH 6.5 phosphate buffer. The initial reaction rate of PNPG hydrolysis in the absence of inhibitors, Vo was determined and was used as a control. The effect of a XAD-7 extract of each potato cultivar was assessed by adding different concentrations (10–200 μg/ml) in the glucosidase assay. The IC50 values were graphically determined as the half-maximal inhibitory concentration of the inhibitor species giving 50% inhibition. All assays were performed in triplicate.
α-amylase activity measurements were performed at 37°C using 2-chloro-4nitrophenyl α-D-maltotrioside (CNPG3) as a substrate. A total of 100 μl of α-amylase solution (1 μg/ml in phosphate buffer) was added to a reaction mixture containing 2.0 mM CNPG3, 200 mM sodium chloride, 5.0 mM calcium chloride, and 50 mM phosphate buffer at pH 6.5. The progress of the reaction was monitored by the absorbance at 405 nm for the production of chloro-nitrophenol in the presence of different concentrations of (10–200 μg/ml) sugar and organic acid free methanolic extract.
Aldose reductase activity was assayed using glyceraldehyde as a substrate and NADPH as a cofactor as described previously [19]. Inhibition assays contained a range of XAD-7 concentrations, keeping the final methanol concentration equivalent among reaction mixtures. Reaction rates were measured by monitoring the absorbance at 340 nm, and were determined in triplicate for each concentration of XAD-7. Reaction rates were compared to control assays not containing methanolic extract to determine inhibition.
Kinetics of inhibition
The mode of enzyme inhibition by the potato extract compounds was determined by using the Michaelis-Menten and Lineweaver- Burk equations. PNP glycosides at concentrations ranging from 1 to 5 mM were used as substrates for α- glucosidase and α-amylase respectively. The enzyme activities were determined in the absence or presence of different concentrations of compound fractions. The concentrations of phenolic and anthocyanin compounds used for the inhibitory kinetics of amylase were 10, 20, 30 and 50 μg/ml for both enzymes. The kinetic parameters Vmax and Km were determined using the Lineweaver- Burk plot (LB plot) following the Michaelis-Menten equation.
Where Vmax is the maximal velocity and Km is the Michalis constant, and its value is equivalent to the substrate concentration at which the velocity is equal to one half of the velocity. Vmax and Km were obtained from the intercept and slope, respectively. The expression of the velocity of the reaction in the present case is given by
Where V is the velocity, [S] is the substrate concentration of PNPG and CNP-G3, [I] is the inhibitor concentration, Ki is the inhibitory constant for the competitive part, and Kii is the inhibitory constant for the noncompetitive part. The enzyme inhibitor and enzyme substrate inhibitor constants were calculated from secondary plots of the initial rate data by linear regression analysis. Plotting of slopes obtained from the LB plots vs inhibitor concentrations gave the Ki values, and intercepts vs inhibitor concentrations gave the Kii values from the x- intercepts.
Statistical analysis
All experiments were carried out in triplicate, and statistical analyses were performed by one-way analysis of variance (ANOVA) at a p ≤ 0.05 significance level using XLSTAT 2015 (Addinsoft USA, New York, NY). The error bars in the figures are based on the calculated Fishers LSD at α = 0.05 using the standard error for the LS means and were approximated at a T-value of 2.
Results and discussion
Total phenolic (TP) and total anthocyanin (TA) contents
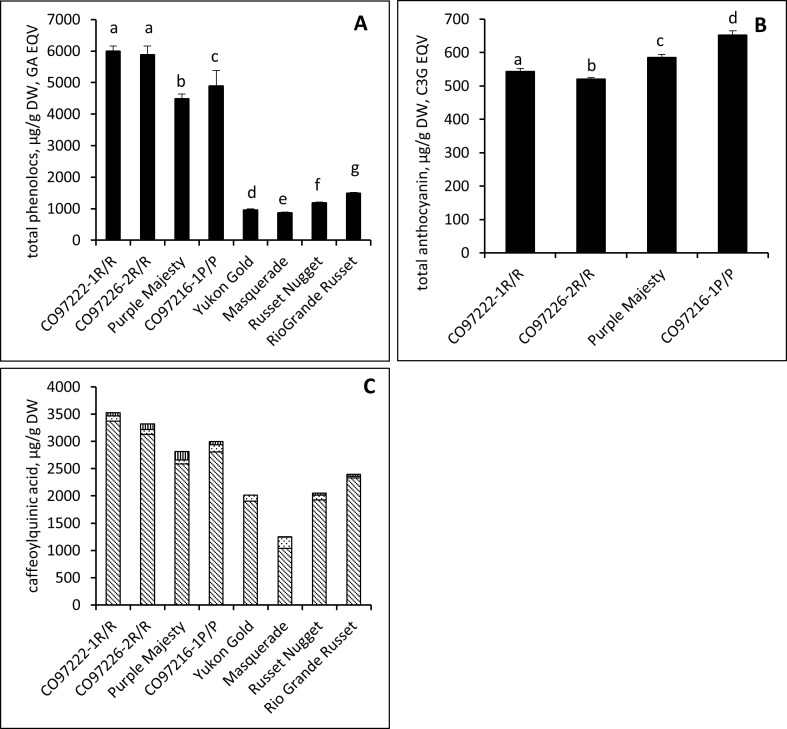
Selected potato tubers were evaluated for phenolics and anthocyanin as previous earlier reports had suggested that phenolics and anthocyanins are some of the major compound groups that constitute potato tubers and they impart extra health benefits. These groups of compounds were extracted with methanol as solvents. We measured TP and TA in these extracts and the results are shown in Fig 3 (S1 Table). The TP content was in the order CO97222-1R/R > CO97226-2R/R > CO97216-1P/P > Purple Majesty > Rio Grande Russet > Russet Nugget > Yukon Gold > Masquerade, with all of the values being significantly different at p < 0.05 except CO97222-1R/R and CO97226-2R/R. In general, red and purple potato tubers had higher phenolics than white and yellow potato tubers. Similar observations were reported that potato tubers with red flesh had a higher phenolic content than tubers with purple flesh [15]. Previous reports also indicated that red and purple-fleshed potato tubers had total phenolic contents comparable to phenolic-rich fruits and vegetables, such as berries and grapes [20, 21]. As chlorogenic acid constitute approximately 80% of phenolic acid and we have measured the levels of three isomeric forms of chlorogenic acid viz 5-caffeoylquinic acid (5CQA), 4-caffeoylquinic acid (4CQA), and 3-caffeoylquinic acid (3CQA) in the selected potato cultivars and results are shown in Fig 3C. 5-caffeoylquinc acid levels were comparatively higher than the other two isomers. The order of chlorogenic acids in the potato cultivars are CO97222-1R/R > CO97226-2R/R > CO97216-1P/P > Purple Majesty > Rio Grande Russet > Russet Nugget > Yukon Gold > Masquerade.
Fig 3. Amount of total phenolic (TP) and total anthocyanins (TA) in potato cultivars.
Error bars represent the calculated LSD for the least square means given at α = 0.05. Significant differences are denoted by different letters, same or shared letters indicate that they are not significant to each other.
Anthocyanins are reported to have significant antioxidant activity and antidiabetic properties and highly abundant in fruits and vegetables [22]. The presence of these compounds is the main contributor to the red and purple color of the potato tubers. The total anthocyanin contents were found to be higher in purple potato tubers than red potato tubers in the order CO97216-1P/P > Purple Majesty > CO97222-1R/R > CO97226-2R/R (S1 Table). All of the values were significantly different at p < 0.05. In the literature, very few reports are available on the anthocyanin content potato tubers with colored flesh [13]. Recently, we demonstrated that purple flesh potato tubers have an anthocyanin content comparable to that of blueberries and pomegranate [21].
Fractionation and characterization
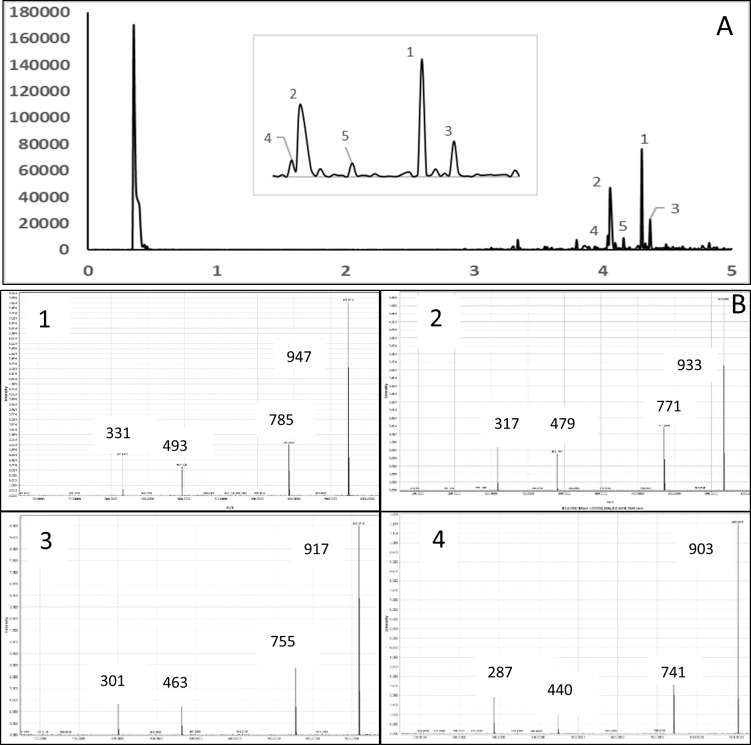
Our previous and other studies reported that purple and red flesh potato tubers have a high level of polyphenolic compounds [12, 13, 15, 21]. However, most of these studies were characterization and analysis methanolic crude extract and their potential antioxidant activities. In this study, we were interested in separating and isolating phenolic acids and anthocyanins. As shown in the flow diagram in Fig 2, sugars, amino acids and other water soluble nonphenolic compounds were removed using an Amberlite XAD-7 column. The compounds remained in the adsorbent were eluted with methanol and then further purified by an Oasis HLB plus column. Eluting the column with 15% MeOH and 5% formic acid in methanol separated into phenolic acids (F1) and anthocyanins (F2), respectively. Moreover, a group of compounds present in F1 and F2 were characterized by LCMS in ESI–ve and +ve mode, respectively and results are shown in the Table 2 taking CO97216-1P/P and CO97222-1R/R as representative potato cultivars. The TIC of F1 and F2 and selective spectra from CO97216-1P/P are shown in Figs 4 and 5 respectively.
Table 2. Phenolic and anthocyanin compounds present in purple and red potato tubers.
| Potato cultivar | phenolic acid and anthocyanins | Molecular ion, (m/z) | MS/MS(m/z) | % composition |
|---|---|---|---|---|
| CO97216-1P/P | chlorogenic acid | 353 | 191, 179, 173, 135 | - |
| petunidin-3-coumaroylrutinoside-5-glucoside | 933 | 479,771, 317 | 28.24 | |
| Peonidin-3-coumaroyllrutinoside-5-glucoside | 917 | 755, 463, 301 | 13.82 | |
| malvidin-3-coumaroylrutinoside-5-glucoside | 947 | 785, 493, 331 | 45.91 | |
| cyanidin-3-coumaroylrutinoside-5-glucoside | 903 | 741, 433, 287 | 6.61 | |
| CO97222-1R/R | Chlorogenic acid | 353 | 191, 179, 173, 135 | - |
| Pelarogonidin-3-coumaroylrutinoside-5-glucoside | 887 | 725, 433, 271 | 88.67 | |
| Pelarogonidin-3-caffeoylrutinoside-5-glucoside | 903 | 741, 433, 271 | 6.82 | |
| Pelarogondin-3-feruloylrutinoside-5-glucoside | 917 | 755, 433, 271 | 4.5 |
Fig 4.
Total ion chromatogram (TIC) of F1fraction from CO97216-1P/P tubers in ESI—ve mode (A). MS/MS spectra of peaks 1,2, and 5 (B).
Fig 5.
Total ion chromatogram (TIC) of F2 fraction from CO97216-1P/P tubers in ESI + ve mode (A). Inset: enlarged chromatogram of the selected area.MS/MS spectra of each peak (B).
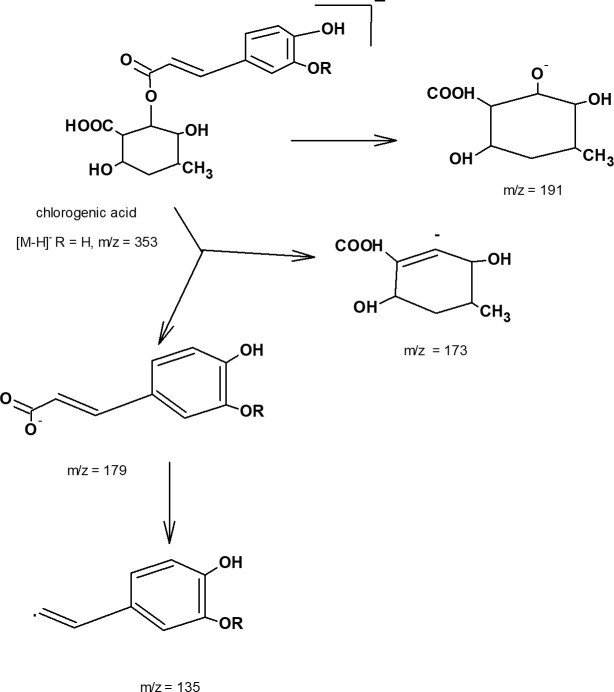
In ESI negative ion mode F1 fraction had major compounds 1–8 and had [M-1]- ion with m/z 353 in accord with the molecular formula for chlorogenic acid C16H18O9 with different retention times (Fig 4A). In MS/MS it gave the fragments with m/z 191, 179 and 135 with varying intensities which are the characteristic fragments of chlorogenic acid isomers. Fig 4B represents the typical spectrum of peaks 1, 2 and 5 from the TIC chromatogram. The relative intensities of the characteristic fragments of each the peak are given in Table 3. Chlorogenic acids are the esterified form of caffeic acid and quinic acid. Fragmentation of chlorogenic acid in negative mode under specific LCMS experimental condition lead to cleavage of intact caffeoyl (m/z 179 and 173) and quinic acid (m/z 191) fragments (Fig 6). 3-caffeoylqunic acid, 4-caffeoylquinic acid, and 5-caffeoylquinic acid are the common isomers of chlorogenic acid and highly abundant in various fruits and vegetables. It has been reported that exposing UV light, chemical and biochemical treatment of tissues and electric field during MS data acquisition leads to isomeric transformation of chlorogenic acid [23]. Therefore, with their cis, trans stereoiosomeric form chlorogenic acid yields various number of isomers. Fang et al identified six different isomers of chlorogenic acid in dried plums based on the nature and intensities of fragmented ions in LC/MS/MS spectra [24]. In a similar study Ncube et al. reported the profiling of 10 isomers of chlorogenic acid in Nicotiana tabacum tissues [25]. In our current study major phenolic acid compound present in red and purple fleshed potato tubers were chlorogenic acid and its isomers. Previous studies on the characterization of phenolic acid compounds in potato tubers also reported that chlorogenic acid constituted with its different isomers [13]. In this study, we have identified eight isomers and quantified 3 major isomers of chlorogenic acid in the F1 fraction of CO97216-1P/P.
Table 3. Phenolic and anthocyanin compounds present in purple and red potato tubers Relative intensities of characteristic fragment of chlorogenic acid in ESI MS/MS spectra.
| Fragment ion intensities | |||||
|---|---|---|---|---|---|
| Compounds | 353 | 191 | 179 | 173 | 135 |
| 1 | 73 | 9400 | 820 | 1000 | 41 |
| 2 | 130 | 2100 | 980 | 26 | 390 |
| 3 | 250 | 1600 | 680 | 120 | 130 |
| 4 | 51 | 6400 | 120 | 260 | 110 |
| 5 | 7600 | 4700 | 120 | 88 | 40 |
| 6 | 7600 | 5300 | 180 | 150 | 0 |
| 7 | 25 | 1800 | 100 | 270 | 34 |
| 8 | 34 | 1400 | 120 | 30 | 38 |
Fig 6. Fragment pattern for chlorogenic acid in MS/MS.
Analysis of F2 fraction in positive mode by LC-MS revealed the presence of acylated anthocyanins. From the ESI-MS/MS TIC (Fig 5) we found that major anthocyanins present in the purple fleshed tuber CO97216-1P/P were petunidin-3-coumaroylrutinoside-5-glucoside (28.24%), peonidin-3-coumaroylrutinoside-5glucoside (13.82%), malvidin-3-(p-coumarylrutinoside)-5-glucoside (45.91%) and cyanidin-3-coumaroylrutinoside -5 glucoside (6.61%) with M+ values of 933, 917, 947, and 903. These anthocyanins were identified based on their relative nature and intensities of characteristic fragmented ions as given in Table 2. Relative abundancy was calculated based on peak intensities. The MS/MS spectra of each peak are shown in the Fig 5B. Red fleshed cultivar CO97222-1R/R contained mainly pelarogonidin-3-coumaroylrutinoside-5-glucoside (88.67%) with m/z for M+ 887 and fragments 725, 433 and 271. Other anthocyanins found were pelargonidin caffeoylrutinoside-5-glucoside (6.82%) and pelargonidin-3-ferroylrutiniside-5-glucoside (4.5%). Some previous reports described the presence of such group anthocyanin compounds in red and purple potatoes [13, 26–28]. However, the availability and the relative abundancy of these compounds depends on cultivars and agronomic conditions [13, 26–28].
Effect on α- amylase, α-glucosidase, and aldose reductase activity
IC50 values for α- amylase, α-glucosidase, and aldose reductase activity
The effect of the XAD-7 methanolic extract on the activity of α- amylase, and α-glucosidase was investigated using PNPG and CNPG-3substrates. The inhibitory effect on aldose reductase was investigated by using glyceraldehyde as a substrate and NADPH as a cofactor. Each extract inhibited the activities of α-amylase, α-glucosidase and aldose reductase in a dose-dependent manner. Acarbose was used as positive control for inhibition of α-glucosidase and α-amylase whereas quercetin was used against aldose reductase. To quantify the inhibitory potential of the extracts, we determined the half-maximal inhibitory concentration (IC50) for each fraction that gave rise to 50% suppression of the original enzyme activity. On the basis of IC50 values it was found that purple and red flesh potato tubers have significantly more potential for inhibiting these enzyme activities (Table 4 and S1 Table) and with the following order of potency CO97216-1P/P > Purple Majesty > CO97222-1R/R > CO97226-2R/R > Rio Grande Russet > Russet Nugget > Masquerade > Yukon Gold.
Table 4. IC50 values of the XAD-7methanolic extract for inhibition of and α-glucosidase, α-amylase and aldose reductase.
The values represent the three sets of data with standard deviation. Significant differences are denoted by different letters, same or shared letters indicate that they are not significant to each other.
| Potato clones | α-amylase (μg/ml) | α-glucosidase (μg/ml) | aldose reductase (μg/ml) |
|---|---|---|---|
| CO97222-1R/R | 30.82 ± 1.34a,c | 45.98 ± 0.53a | 39.55 ± 0.95d |
| CO97226-2R/R | 33.81 ± 0.83c | 47.74 ± 0.65c | 46.06 ± 0.64c |
| Purple Majesty | 28.99 ± 1.35a | 45.2 ± 0.60a | 35.57 ± 0.98a |
| CO97216-1P/P | 25.52 ± 0.79b | 42.42 ± 0.94b | 32.48 ± 0.78b |
| Yukon Gold | 58.48 ± 1.11e | 62.79 ± 0.39e | 58.72 ± 0.47f |
| Masquerade | 55.50 ± 0.59d | 58.12 ± 0.68d | 49.16 ± 0.52e |
| Russet Nugget | 41.34 ± 0.89g | 78.65 ± 0.48g | 126.31 ± 5.01h |
| Rio Grande Russet | 44.86 ± 1.19f | 75.11 ± 0.21f | 172.76 ± 3.89g |
| Acarbose | 12.86 | 15.65 | - |
| Quercetin | - | - | 13.87 |
There have been many reports of natural compounds as α-glucosidase inhibitors [4–10]. Secondary plant metabolites such phenols, polyphenols, and anthocyanin attributed potential health benefits through their antidiabetic, anti-carcinogenic, anti-inflammatory properties. We hypothesized that presence of phenolic acids and anthocyanins in purple and red flesh purple potato tubers would have significant inhibitory effect on α-glucosidase, α- amylase, and aldose reductase activities. In support of this hypothesis, we observed that methanolic extract of the selected potato tubers had significant properties with IC50 from 25 to 172 μg/ml towards inhibition of the activities of these enzymes. It was observed that the extent of inhibition correlated with the levels of chlorogenic acid and anthocyanins. Purple and red flesh potato cultivars had a higher level of chlorogenic acids than the white and yellow ones, and subsequently, they have higher activity towards the inhibition of these enzyme’s activities. Moreover, purple flesh potato tubers had higher levels of anthocyanin compared to other color flesh potato tubers. The combination of phenolic acid and anthocyanin in the extracts of CO97216-1P/P made the strongest inhibition of α-glucosidase, α- amylase, and aldose reductase activities. A positive correlation between the polyphenolic content of the natural extract and inhibition of glucosidase and amylase activity were also seen in some previous reports [4–6].
Mode of inhibition on α- amylase, α-glucosidase, and aldose reductase activity
Most of the previous studies on inhibitory activities of natural compounds on α-amylase, α-glucosidase and aldose reductase were tested with the crude extracts and their IC50 values [4–10]. While IC50 values showed the potency of a natural compounds, more valuable information were obtained by the kinetics of inhibition by group or individual compounds isolated form natural extract. A number of bioactive compounds such as chlorogenic acid, caffeic acid, kaempferol, luteolin, (+)-catechin/(-)-epicatechin, betaglucogallin have been reported as active inhibitors of α-amylase, α-glucosidase and aldose reductase [4–10]. Inhibitory activities with their mode of the kinetics of inhibition have also been reported for natural product containing a mixture of compounds. For instance the methanolic extract of finger millet showed strong inhibition towards glucosidase and pancreatic amylase with noncompetitive inhibition [29], polyphenol extracts of Maqui (Aristotelia chilensis) leaves acted as mixed inhibitors of α-amylase and noncompetitive inhibitors of α-glucosidase [30], Pine bark extract (PBE) non-competitively inhibited human salivary and porcine pancreatic amylases [31], Gymnema montanum (GLEt) leaves extract showed competitive inhibition [32] and Muscadine anthocyanin extract inhibited α-glucosidase in competitive nature with Ki and IC50 as low as 0.5 mg/ml and 1.5 mg/ml [33]. In our study we have demonstrated the mode of inhibition by phenolic acids and anthocyanin compounds isolated from potato extract.
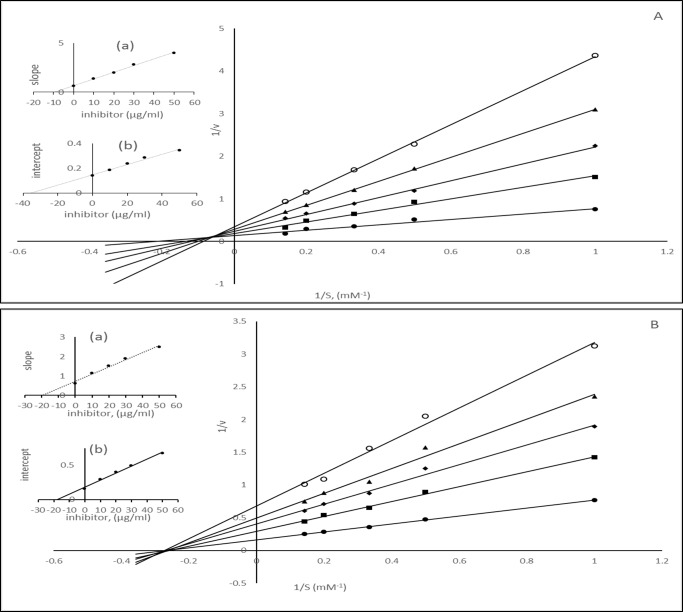
An inhibitor can interact with an enzyme in various ways. Studies on the kinetics of inhibition are a major tool that enables us to distinguish between inhibitory mechanisms. Among the polyphenolic compounds tested for the inhibition of α-amylase α-glucosidase and AR so far were found to be competitive, noncompetitive and mixed inhibitors [4–10]. Such a variation in inhibitor potency, as well as the mode of enzyme inhibition, is not unusual since the inhibitor potency of polyphenolic compounds depends on several factors, such as the structure and stability of the inhibitors. As aforementioned XAD-7 methanolic extract composed of phenolic acid and anthocyanin, we tested the nature of the inhibition by F1 and F2 fractions of CO97216-1P/P. The initial velocity ‘v’ of the hydrolysis reactions catalyzed by α-glucosidase, pancreatic α- amylase, and aldose reductase were measured at various substrate concentrations [S] (1–5 mM) in presence and absence of F1 and F2 inhibitors[I] (10, 20, 30 and 50 μg/ml). We noted significant changes in the Km and Vmax in absence as well as the presence of these inhibitors. Double reciprocal plots for α-glucosidase and α-amylase respectively in the presence of extract inhibitors showed that straight lines were obtained with PNPG and CNPG3 substrates (Figs 7 and 8 and S1 Table). In the assay α-glucosidase had a Km of 4.44 mM for PNP-glycoside and Vmax value of 7.027 μmoles. In the presence of 10, 20, 30 and 50 μg/ml of phenolic acid compounds apparent Vmax values were found to be 5.33, 4.18, 3.50 and 2.89 μmoles and Km values changed to 7.29, 8.0 10.0, and 11.49 respectively (Fig 7A). In the presence of 10, 20, 30 and 50 μg/ml of anthocyanin compounds Km values remained constant whereas Vmax values changed to 3.05, 2.44, 1.88 and 1.32 μmoles respectively (Fig 7B). As 5-caffeoylquinic acid was found to be major phenolic acid, we tested the kinetics of α-glucosidase activity in its presence which showed changes in both Km and Vmax values (S1 Fig).
Fig 7. Lineweaver-Burk plot for the activities of α-glucosidase in the presence of various concentration of substrates (1–5 mM) and inhibitors.
A) different concentrations of phenolic acids (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml) B) different concentrations of anthocyanins (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml).
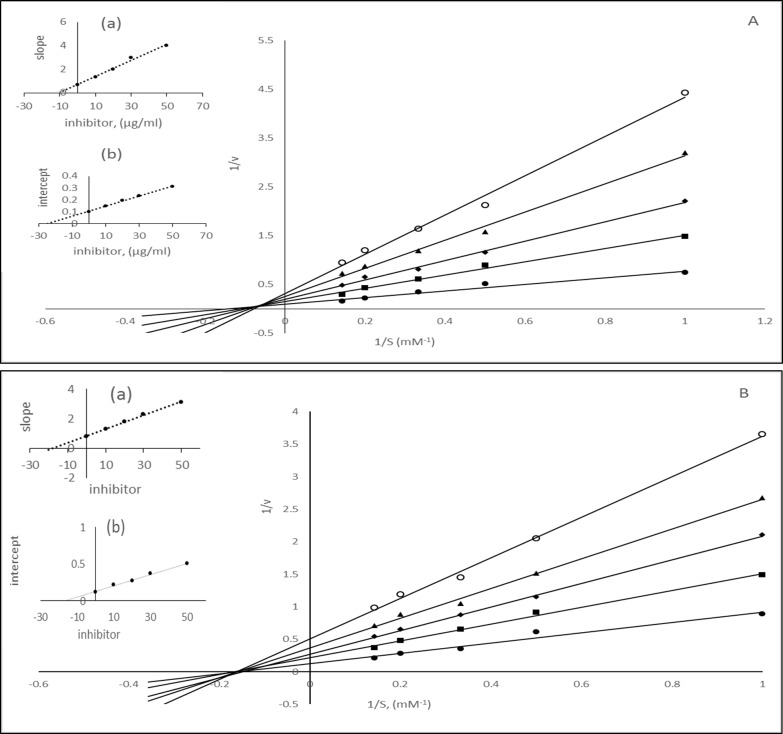
Fig 8. Lineweaver-Burk plot for the activities of α-amylase in the presence of various concentration of substrates (1–5 mM) and inhibitors.
A) different concentration of phenolic acids, (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml) B) different concentration of anthocyanins (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml).
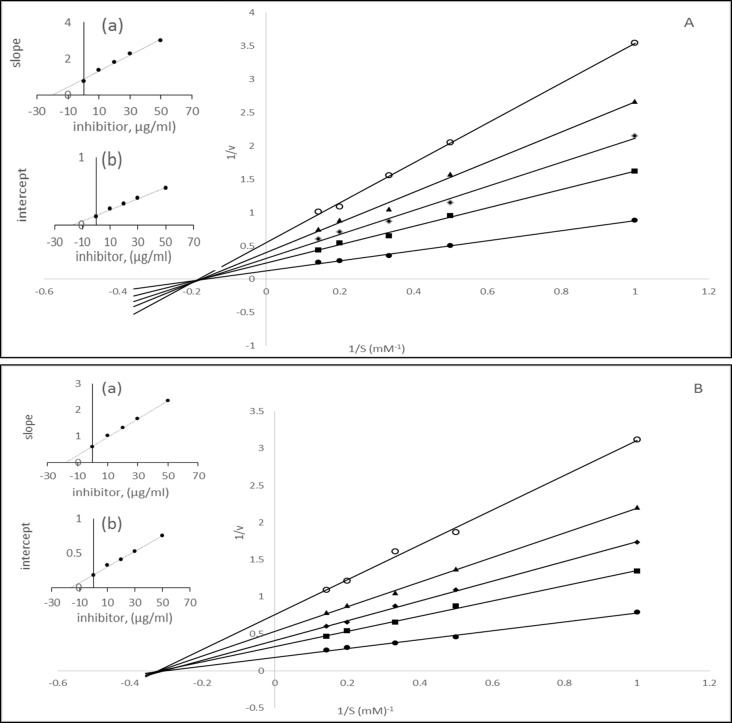
In another assay for α-amylase Km values increases and Vmax values decreases with increase in the inhibitor concentration (Fig 8A and 8B). Thus in both assays for α-amylase and α-glucosidase we found that there was an increase in Km and decrease in Vmax values with the increase in the concentration of phenolic acid fraction (F1). However, in the presence of anthocyanin fraction (F2) for α-glucosidase and α-amylase assay the Km values remained constant and Vmax values decrease. 5CQA also resulted in the increase in Km and decreased in Vmax values. Thus it is apparent that phenolic acid fraction (F1) behaved as mixed inhibitors for α-glucosidase and α-amylase whereas anthocyanin compounds acted as noncompetitive inhibitors. 5-caffeoylquinic acid acted as a mixed inhibitor of α-glucosidase and α-amylase. The results from aldose reductase assays in the presence of F1 and F2 are shown in Fig 9 (S1 Table). These results indicated that the binding of the phenolic compounds affected the velocity of the reaction catalyzed by aldose reductase proportionately to the concentration of the phenolic acid compounds in the reaction mixture, without affecting the Km (Fig 9A). In our assay, we found the values of Km and Vmax 3.03 mM and 9.70 μmoles/min respectively. The values of Vmax changed to 6.13, 4.50, 3.70 and 3.14 in the presence of 10, 20, 30 and 50 μg/ml phenolic compounds respectively. The anthocyanin fraction F2 also showed a similar set of straight lines which meet at a single point in the concentration axis (Fig 9B). 5CQA resulted in the changes in the Vmax values, but km remained constant (S1 Fig). Thus it was demonstrated that both phenolic and anthocyanin compounds acted as noncompetitive inhibitors of aldose reductase. The extent of changes in the Km and Vmax values under different fractions depends on the nature of the compounds. The affinity of the enzymes for the inhibitors varies and can be seen from the inhibition constants. The dissociation constant for competitive inhibition Ki was determined from the secondary plot of the slope of the straight lines of 1/v vs. 1/S against the inhibitor concentration. The intercept on the inhibitor axis of this plot gives the value of Ki. The value of Kii, the inhibitor constant for noncompetitive inhibition, was obtained from a linear secondary plot of 1/Vmax against the inhibitor concentration. The intercept on the inhibitor axis gives the Kii values. The kinetic data for inhibition of α-amylase, α-glucosidase, and aldose reductase catalyzed hydrolysis reaction are shown in Table 5. For anthocyanin fraction F2, the value of Ki was equal to Kii in the assays for α-amylase, α-glucosidase and aldose reductase, which are typical noncompetitive inhibitors. For phenolic compounds fraction F1, Kii > Ki for α-glucosidase and α-amylase, as is the case of the mixed inhibitor with a major mode of inhibition being the competitive type. Similarly, chlorogenic acid acted mixed inhibitor against α-amylase and α-glucosidase. However, Kii = Ki for aldose reductase as for noncompetitive inhibitors.
Fig 9. Lineweaver-Burk plot for the activities of aldose reductase in the presence of various concentration of substrates (1–5 mM) and inhibitors.
A) different concentration of phenolic acids, (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml) B) different concentration of anthocyanins (● 0, ▪ 10, ◊ 20 Δ 30, ○50 μg/ml).
Table 5. Kinetic data on the inhibition of amylase, glucosidase and aldose reductase by phenolic and anthocyanin compounds.
| enzyme | inhibitor | Ki | Kii | Kii/Ki | type of inhibition |
|---|---|---|---|---|---|
| α-amylase | F1 | 8.7 | 22.5 | 2.58 | Mixed inhibition |
| F2 | 17.5 | 17.2 | 0.98 | Non-competitive | |
| 5CQA | 19.0 | 36.0 | 1.89 | Mixed inhibition | |
| α-glucosidase | F1 | 9.5 | 35.2 | 3.70 | Mixed inhibition |
| F2 | 18.5 | 18.2 | 0.98 | Non-competitive | |
| 5CQA | 13.1 | 18.4 | 1.40 | Mixed inhibition | |
| aldose reductase | F1 | 12.6 | 12.1 | 1.04 | Non-competitive |
| F2 | 28.1 | 28.4 | 1.01 | Non-competitive | |
| 5CQA | 10.1 | 10.3 | 1.01 | Non-competitive |
Some previous report on inhibitory potency of chlorogenic acid demonstrated that the binding position of caffeic acid to the quinic acid in chlorogenic acid has played an important role towards the inhibition of glucosidase activity. Xu et al. found that position of caffeoyl unit to the quinic acid affect the rate and mode of inhibition α-glucosidase with an order of 5CQA> 4CQA>3CQA [34]. In that study, they also found that dicaffeoylquinic acid was stronger than monocaffeoylquinic acid and acted as noncompetitive with a closure mixed type inhibition. From the kinetic data in our assay, we observed that the phenolic acid group containing caffeoylquinic acids and its isomers acted as mixed type inhibitors against α-glucosidase. In a separate study of 5-caffeoylquinc acid, it was seen to be a mixed inhibitor (S1 Fig) of α-glucosidase. Several anthocyanin extracts have been reported as competitive, mixed and noncompetitive inhibitors α-glucosidase. You et al. found that cyanidin derivative of Musscadine acted as noncompetitive inhibition of glucosidase [33]. Pelargonidin derivatives acted as a noncompetitive inhibitor against α-glucosidase. The anthocyanin extract of purple fleshed potato cultivar CO97216-1P/P containing petunidin, cyanidin, and malvidin derivatives showed a noncompetitive inhibition in our study. Narita et al. reported that chlorogenic acid acted as a mixed inhibitor in a kinetic study of inhibition of pancreatic α-amylase [35]. Similar to α-glucosidase the structural difference in caffeoylquinic acid also influences the inhibitory activity of amylase. 5CQA had higher inhibition activity compared to 4CQA and 3CQA. A detail kinetic of inhibition by young apple polyphenols (YAP) containing chlorogenic acid showed mixed type inhibition of α-amylase [36]. In a similar study the methanolic extract of finger millet, which consists of various phenolic compounds, such as gallic acid, caffeic acid, ferulic acid, showed strong inhibition towards glucosidase and pancreatic amylase with a noncompetitive inhibition [29]. In a similar way, the phenolic acids containing caffeoylquinic acid acted as a mixed inhibitor of α-amylase in our assay. Various anthocyanin compounds have been reported as effective inhibitors of α-amylase. Delphinidin, cyanidin, Malvidin, peonidin aglycon derivatives from cherries showed competitive inhibition against α-amylase [37]. The anthocyanins present in the CO97216-1P/P showed a noncompetitive inhibition. Several reports are available on aldose reductase inhibition by natural products from medicinal plants, food, and vegetables [38]. The major group of compounds comprised phenolic compounds such as flavonoids, phenolic acids, anthocyanins and other bioactive compounds. In a study of inhibition behavior of some phenolic acids (tannic acid, chlorogenic acid, and p-coumaric acid) reported that chlorogenic acid and other phenolic acid acted as mixed and noncompetitive inhibitors of rat kidney aldose reductase [39]. Chlorogenic acid extract from Wrightia tinctoria was found to be an uncompetitive inhibitor of aldose reductase [40]. Finger millet phenolic acid compounds consisting gallic acid, p-coumaric acid, ferulic acid showed a noncompetitive inhibition towards aldose reductase [41]. Yoshimoto et al. reported that methanolic extract of sugarcane composed of quinic acid, caffeic acid and its derivative chlorogenic acid is a potent inhibitor of aldose reductase with IC50 values 0.14 mg/ml [42]. Yawadio et al. reported that black and brown rice with anthocyanin compounds cyandin -3-glucoside, peonidin-3-glucoside, ferulic acid, and tocopherol are found to be a potent inhibitor of aldose reductase with an IC50 value of 8.7 to 27.5 μg/ml against human recombinant AR [43]. Although various anthocyanin extracts have been reported as effective inhibitors of aldose reductase data on inhibition kinetics are scanty. The anthocyanin compounds from purple fleshed potato tubers CO97216-1P/P showed a noncompetitive inhibition towards aldose reductase.
Conclusions
Colorado State University postharvest program have been screening the potato cultivars and advanced selections for improved nutrition and health benefits. This study indicated that colored flesh potato tubers contain a mixture of several phenolic compounds ranging from phenolic acid to anthocyanins. We have separated, isolated and characterized the group of phenolic acid and anthocyanin compounds in colored flesh potatoes. It is interesting to note that these group of compounds had significant inhibitory activities on α-glucosidase, α-amylase, and aldose reductase. These phenolic compounds acted effective non-competitive inhibitors and mixed with carbohydrate-hydrolyzing enzymes such as α-amylase and α-glucosidase. Thus polyphenol rich potato tubers have the potential to interfere with or delay absorption of dietary carbohydrates in the small intestine, leading to suppression of postprandial blood glucose sugars. However, there are reports of a problem on the direct intestinal absorption of anthocyanins and phenolic compounds. It was mentioned that about 1% or less of ingested anthocyanin is estimated to be incorporated into plasma. There is a possibility of biotransformation of these phenolic compounds after consumption. Food-grade phenolic inhibitors from potatoes are potentially safer, and therefore may be preferred alternatives for inhibition of carbohydrate breakdown and control of glycemic index. Additionally, the significant inhibitory effect on aldose reductase suggests that phenolic fractions in potato tubers can possess constituents with antidiabetic and inhibitory effects on diabetic complications.
Supporting information
Lineweaver-Burk plot for the activities of α-glucosidase (A), α-amylase (B), and aldose reductase (C) in the presence of various concentration of substrates (1–5 mM) and inhibitors. different concentration of 5-caffeoylquinic acid, (● 0, ▪ 10, ◊ 20 Δ 30, μg/ml), and a) and b) are the secondary plots for Ki and Kii.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially supported by Colorado Potato Administrative Committee Area II funded Postharvest Program of SSJ; the USDA-National Institute of Food and Agriculture Special Research Grants Program – Potato Breeding Research Grant No. 2015-34141-23965 (DGH); National Institute of Health Grants EY005856, EY021498, and EY028147 and a Challenge Grant to the Department of Ophthalmology from Research to Prevent Blindness (JMP and DL), New York, NY.
References
- 1.King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025; prevalence, numercal estimates, and projections. Diabetes care 21:144–1431. [DOI] [PubMed] [Google Scholar]
- 2.Lebovitz HE (2001) Effect of the posprandial state on the nontraditional risk factors. American Journal of Cardiology 88:204–205 [DOI] [PubMed] [Google Scholar]
- 3.Rines AK, Sharabi K, Tavares CDJ, Puigserver (2016) targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nature Reviews Drug Discovery 15: 786–804. doi: 10.1038/nrd.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, More P, Derle A, Patil AB, Markad P et al. (2014) Novel hit for the treatment of type II diabetes Mellitus with inhibitory activity against α-amylase and α-glucosidase. PlosOne 9: e106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Y, Sang W, Zhou M, Ren G (2010) Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J Agric Food Chem 58: 770–774. doi: 10.1021/jf903234c [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Du YD, Song H (2010) α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem 123: 6–13. [Google Scholar]
- 7.Williamson JR, Kilo C, Tilton RG, In:Rudeman N, Williamson JR, Brownlee M, editors, Hyperglycemia, diabetes and vacular disease. New York:Oxford University Press; 1992. p. 691–714. [Google Scholar]
- 8.Oates PJ (2008) Aldose reductase, still a compelling target for diabetic neuropathy. Current Drug Targets 9:14–36. [DOI] [PubMed] [Google Scholar]
- 9.Puppala M, Ponder J, Suryanarayana P, Reddy GB, Petrash M, Labarbera DV (2012) The isolation and characcterisation of β-glucogallin as a novel aldose reductase inhibitor form Emblica Officinalis PlosOne 4:e31399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita JH and Nishimura C (1988) The involvement of aldose reductase in diabetic complications. Diabetes-Metabolism Reviews 4:323–337. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Choi DS, Lee MK, LeeH YW, Park TS (2014) Comparison of Acarbose and Voglibose in Diabetes Patients Who Are Inadequately Controlled with Basal Insulin Treatment: Randomized, Parallel, Open-Label, Active-Controlled Study. J Korean Med Sci 29: 90–97. doi: 10.3346/jkms.2014.29.1.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stushnoff C, Holm D, Thompson MD, Jiang W, Thompson HJ, Joyce NI, Wilson P (2008) Antioxidant properties of cultivars and selections from the Colorado potato breeding program. American Journal of Potato Research 85: 267–276. [Google Scholar]
- 13.Pillai SS, Navarre Du RA, Bamberg J (2013) Analysis of polyphenols, anthocyanins, and carotenoids in tubers from Solanumtuberosum group phureja, stenotomum and andigena. American Journal of Potato Research 90: 440–450. [Google Scholar]
- 14.Lachman J, Hamouz K (2005) Red and purple colored potatoes as a significant antioxidant source of human nutrition–a review. Plant, Soil and Environment 51: 477–482. [Google Scholar]
- 15.Perla V, Holm DG, Jayanty SS (2012) Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Science and Technology 45: 161–171. [Google Scholar]
- 16.Brown CR (2005) Antioxidants in potato. American Journal of Potato Research 82: 163–172. [Google Scholar]
- 17.Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, Vanamala JK (2015) Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J NutrBiochem 26:1641–9. [DOI] [PubMed] [Google Scholar]
- 18.Tarle I, Borhani DW, Wilson DK, Quiocho FA, Petrash JM (1993) Probing the active site of human aldose reductase. Site-directed mutagenesis of Asp-43, Tyr-48, Lys-77, and His-110. J BiolChem 268: 25687. [PubMed] [Google Scholar]
- 19.Nakano T, Petrash JM (1996) Kinetic and spectroscopic evidence for active site inhibition of human aldose reductase. Biochemistry 27:11196. [DOI] [PubMed] [Google Scholar]
- 20.Madiwale GP, Reddivari L, Stone M, Holm DG, Vanamala J (2012) Combined effects of storage and processing on the bioactive compounds and pro-apoptotic properties of color-fleshed potatoes in human colon cancer cells. Journal of Agricultural and Food Chemistry 60: 11088–11096. doi: 10.1021/jf303528p [DOI] [PubMed] [Google Scholar]
- 21.Kalita D, Jayanty S (2014). Comparison of Polyphenol Content and Antioxidant Capacity of Colored Potato Tubers, Pomegranate, and Blueberries. J Food Proc Tech 5: 1–7. [Google Scholar]
- 22.Ghosh D, Konishi T (2007) Anthocyanins and anthocyanin-rich extracts:role in diabetes and eye function. Asia pac J clinNutr 16:200–208. [PubMed] [Google Scholar]
- 23.Xie C, Yu K, Zhong D, Yuan T, Ye F, Jarrel JA, Millar A, Chen X (2011) Investigation of isomeric transformations of chlorogenic acid in buffers ad biological matrix by Ultra Performance Liquid Chromatography coupled with hybrid quadrapole/ion mobility/orthogonal acceleration time-of-flight mass spectrometry. J Agri Food Chem 59:11078–11087. [DOI] [PubMed] [Google Scholar]
- 24.Fang N, Yu Shangong, Prior RL (2002) LC/MS/MS characterization of phenolic constituents in dried plums. J Agr Food Chem 50:3579–3585. [DOI] [PubMed] [Google Scholar]
- 25.Ncube EN, Mhlongo MI, Piater LA, Steenkamp PA, Dubery IA, Madala NE (2014) Analysis of chlorogenic acid and related cinnamic acid derivatives from Nicotianata bacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissolution method Cem Cent J 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giusti MM, Polit Feranda, Ayvaz H, D, Manrique Tay (2014) Characterization and quantitation of anthocyanins and other phenolics in native Andean potatoes J Agric Food Chem 62: 4408–4416. doi: 10.1021/jf500655n [DOI] [PubMed] [Google Scholar]
- 27.Eichhorn S, Winterhalter 92005) Anthocyanin from pigmented potato (Solanum tuberosum L.) varieties. Food Res Int 38:943–948. [Google Scholar]
- 28.Kita A, Bakowska-Baczak A, hamouz K, Kulakowska K, Lisinska G (2013) The effect of frying on anthocyanin stability and antioxidant activity of crisp from red and purple-fleshed potatoes. J Food Comp and Analysis 32:169–175. [Google Scholar]
- 29.Shobana S, Sreerama YN, Malleshi NG (2009) Composition and enzyme inhibitory properties of finger millet (Elusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem 115: 1268–1273. [Google Scholar]
- 30.Rubilar M, Jara C, Poo Y, Acevedo F,Guitterez C, Sineiro J, Shene C (2011) Extracts of Maqui (Aristotelia chilensis) and Murta (Ugnimolinae turcz): sources of antioxidant compounds and α-glucosidaseα/α-amylase inhibitors. J Agric Food Chem 59: 1630–1637. doi: 10.1021/jf103461k [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI (2005) Inhibitory effect of pine extract on alpha-glucosidase activity and postprandial hyperglycemia. Nutrition. 21:756–61 doi: 10.1016/j.nut.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Ramkumar KM, Thayumanavam, Plavannan T, Rajaguru P (2010) Inhibitory effect of Gymnema montanum leaves on α-glucosidase activity and α-amylase activity and their relationship with polyphenolic content. Med Chem Res 19: 948–961. [Google Scholar]
- 33.You Q, Chen F, Wang X, Luo P G, Jiang Y (2011) Inhibitory effects of Muscadine anthocyanins on α-glucosidase and pancreatic lipase activities. J Agric Food Chem. 59: 9506–9511. doi: 10.1021/jf201452v [DOI] [PubMed] [Google Scholar]
- 34.Xu D, Wang Q, Zhang W, Hu B, Zhou L, Zeng X, Sun Y (2015) Inhibitory activities of caffeoylquinic acid derivatives from llexkudingcha C.J Tseng on α-glucosidase from Saccharomyces cerevisiae. J Agric Food Chem 63: 3694–3703. doi: 10.1021/acs.jafc.5b00420 [DOI] [PubMed] [Google Scholar]
- 35.Narita Y, Inouye K (2009) Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas α-amylase isozymes I and II. J Agric Food Chem 57: 9218–9225. doi: 10.1021/jf9017383 [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Chen W, Meng Y, Yang X, Yuan L, Guo Y (2016) Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem 208:51–60. doi: 10.1016/j.foodchem.2016.03.093 [DOI] [PubMed] [Google Scholar]
- 37.Homoki JR, Nemes A, Fazekas E, Gyemant G, Balogh P, Gal F, Al-Asri J, Mortier J, Wolber G, Babinszky Remenyik (2016) Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitory activity of different Hungarian sour cherry varieties (Prunus cerasus L.) Food Chem 194: 222–229. doi: 10.1016/j.foodchem.2015.07.130 [DOI] [PubMed] [Google Scholar]
- 38.Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB. (2008) Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pac J Clin Nutr 17:558–65. [PubMed] [Google Scholar]
- 39.Alim Z, Kilin Ҫ, Şengϋl, Beydemir Ş, B (2017) Inhibition behaviors of some phenolic acids on rat kidney aldose reductase enzyme;in vitro study. J Enz Inhib Med Chem 32, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunapajaru N (2011) in Isolation and characterization of aldose reductase inhibitors from Wrightia tinoctora R Br seeds. Thesis, UMI dissertation Publishing 3483405, i-ii.
- 41.Chethan S, Dharmesh SM, Malleshi NG (2008) Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Biororg Med Chem 16: 10085–10090. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimoto M, Kurata R, Takagaki K, et al. (2007) Pharmaceuticals for prophylactics or therapeutic treatment of diabetes or its complications, and food compositions containing them JP2007119346A. [Google Scholar]
- 43.Yawadio R, S Tanimori S Morita N (2007) Identification of phenolic compounds isolated from pigmented rice and their aldose reductase inhibitory activities 101:1616–1625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lineweaver-Burk plot for the activities of α-glucosidase (A), α-amylase (B), and aldose reductase (C) in the presence of various concentration of substrates (1–5 mM) and inhibitors. different concentration of 5-caffeoylquinic acid, (● 0, ▪ 10, ◊ 20 Δ 30, μg/ml), and a) and b) are the secondary plots for Ki and Kii.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.