Abstract
Transmission routes of the hepatitis E virus (HEV) are under debate. Here, we studied possible sexual transmission by comparing HEV prevalence in a Dutch sexual high-risk population (n = 1,482) with that in a general population (n = 1,487) while assessing sociodemographic and sexual risk factors. Overall prevalence of anti-HEV IgG of 18.9% (n = 562) was, adjusting for confounders, similar between the two populations (p = 0.44). Prevalence was higher with each year’s increase in age (adjusted OR: 1.03, 95%CI: 1.02–1.04, p<0.01), among men (adjusted OR: 1.24, 95%CI: 1.02–1.50, p = 0.03) and among individuals diagnosed with sexually transmitted infections (adjusted OR: 1.60, 95%CI: 1.02–2.49, p = 0.04). Our results only hint at the possibility of a sexual transmission route for HEV given higher rates in those with chlamydia and/or gonorrheal infections. Sexual transmission is not a dominant transmission route, as its prevalence was not higher for the sexual high-risk population than for the general population.
Introduction
Recently, the hepatitis E virus (HEV) has become a global public health concern, as an increase of autochthonous HEV infections was observed in developed countries. HEV is considered to be endemic in developing countries, where it is transmitted mainly through contaminated water and the faecal-oral route, causing epidemic outbreaks of genotypes 1 and 2 [1, 2]. Traditionally, HEV was considered to be a travel-related disease in developed countries, associated with genotypes 1 and 2. For genotypes 1 and 2, the mortality rate is usually low (0.07–0.6%); it is particularly severe among pregnant women, with mortality rates between 15–25% [3]. However, reported cases of patients that have not travelled to endemic areas—so-called autochthonous hepatitis infections—have increased in developed countries [4–10]. These infections are mainly caused by HEV genotype 3, symptomatic HEV genotype 3 infections are more common among middle-aged and older individuals as well as among men [10–12]. HEV infections are usually asymptomatic, but genotype 3 infections may cause chronic hepatitis in immunocompromised patients. This group includes patients who have received an organ transplant, patients receiving chemotherapy and HIV-infected individuals [13–16].
Transmission routes continue to be one of the most debated aspects of HEV. The most recent evidence suggest that HEV caused by genotypes 3 and 4 is transmitted zoonotically. In Europe, North America and Japan, HEV genotype 3 is widespread among pigs [17–19]. In these countries, domestic pigs and wild boars act as a reservoir [20]. It is proven that HEV is transmitted by ingesting uncooked or poorly cooked pork or game meat [21–23].
Studies are needed to identify all transmission routes of HEV, so appropriate preventive and control measures can be taken. The hepatitis A virus (HAV) and HEV genotypes 1 and 2 are both single-stranded RNA viruses with similar incubation periods and a faecal-oral transmission route. Sexual activity, including oral-anal contact, as the major transmission route among men who have sex with men (MSM) is another widely documented aspect of HAV [24, 25], but only a limited number of studies have focused on the possible sexual transmission route of HEV infections [26–30]. This study could contribute to policy on the prevention and control of HEV infections, to be targeted at vulnerable individuals who are at highest risk.
To explore the possible role of sexual transmission, we compared the HEV prevalence of a population with higher sexual risk to the general population. In addition, we assessed potential risk factors of sexual transmission through a cross-sectional study in the south of the Netherlands.
Methods
Study population
The STI clinic study and the GP study were approved by the Maastricht University Medical Centre Medical Ethical Committee (11-4-108 for the STI clinic cohort and 14-4-042 for the GP cohort). No informed consent was needed because of prevailing laws in the Netherlands, as it concerns an observational study using anonymous data only. Our study population consisted of two populations: a sexual high-risk population from a STI clinic cohort and the general population from a GP cohort. Venous blood samples were tested on HEV IgG. All individuals from the two study populations lived in urban areas in South Limburg. In the study period (December 2011 to November 2015), no clusters or outbreaks of HEV were reported in the study region.
First, the sexual high-risk study population (STI clinic cohort; n = 1,482) was compiled as a sample from a cohort of individuals aged 20–70, all of whom were tested for a sexually transmitted infection (STI) at the STI clinic between December 2011 and November 2015 (original cohort size about 24,500 individuals). This sample included women (n = 350); female swingers, heterosexuals who as a couple practise partner-swapping or group sex, or who visit sex clubs for couples [31] (n = 184); heterosexual men (n = 480); and men who have sex with men (MSM; n = 468). This STI clinic cohort represented a high-risk population, as 75.2% reported anal sex, had three or more sexual partners in the past six months or tested positive for Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), syphilis or HIV (see S1 Table).
Second, the general study population (GP cohort; n = 1,487) was compiled as a sample from a cohort of individuals aged 40–70, all of whom were tested by general practitioners (GPs) for a study of the hepatitis B virus (HBV) and the hepatitis C virus (HCV) between September 2014 and April 2015 (original study size n = 3,434). The prevalence of past HCV infections was 0.27% (4/1,487). This is comparable to the prevalence in the general population [32–34]. No active HCV infections were diagnosed in the GP cohort. The prevalence of active and past HBV infections was 0.5% (7/1,487) and 8.9% (132/1,487) respectively. Overall, the prevalence of active and past HBV infections is 0.2–0.3% [35, 36] and 2.1% [36] in the Netherlands. This GP cohort represented a general population of both man and women from two urban areas, without any further exclusion criteria.
Data collection
Data on the test year, age, gender, ethnicity, HIV positivity and anti-HEV test results were available for all samples. In the STI clinic cohort, HIV and syphilis positivity was based on the testing of high-risk groups such as MSM. In the GP cohort, HIV positivity was based on self-reported data.
Additional data on sexual preference, reported anal sex, number of sexual partners in the past six months and diagnosis of CT/NG/syphilis were only available for the STI clinic cohort. Additional data on educational level, HBV and HCV positivity were only available for the GP cohort. Reporting anal sex, having three or more sexual partners in the past six months and being CT positive, NG positive, Syphilis positive or HIV positive were categorised as risk factors of sexual transmission.
Serological testing
Stored sera from the participants of the two study populations were tested for HEV IgG (recomWell hepatitis E virus IgG, Mikrogen GmbH, Neuried, Germany) at the Department of Medical Microbiology at Maastricht University Medical Center. HEV IgG was measured in U/ml: results below 20 U/ml were reported as negative, 20–24 U/ml (n = 41) were reported as grey areas (considered as negative in current analyses) and results above 24 U/ml were reported as positive. In a recent study, the Wantai assay detected more positives (prevalence of 48.2% compared to 17.3% with the Mikrogen assay) and had a lower detection limit. However, it is likely that this included more false positives and the Mikrogen assay had the highest correlation to the estimated overall IgG (prevalence of 18.7%). Therefore, we chose for the Mikrogen assay.
Statistical analysis
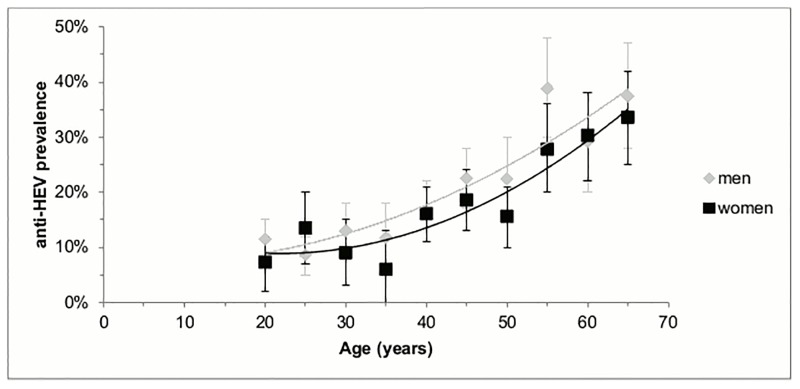
The prevalence of HEV was based on the number of positive HEV test results, divided by the total number of HEV tests and multiplied by 100. Three different models were constructed, including an overall model, a model for the STI clinic cohort and a model for the GP cohort. Univariate logistic regression analyses were used to assess the unadjusted associations between potential risk factors and HEV prevalence. All previously described variables (see the Data collection section) were included in the analyses as potential risk factors. However, we identified several known confounders on the basis of the literature, which led to adjustments in the multivariate logistic regression analyses. These confounders include the test year, age and gender. We used the Enter method to assess the OR and the 95% confidence intervals (p≤0.05). The age curves in Fig 1 were designed on the basis of a second-degree polynomial fit.
Fig 1. Anti-HEV IgG prevalence in five-year age groups (n = 2,969), south Netherlands, Dec 2011 to Nov 2015.
Results
A total of 2,969 individuals were tested on HEV IgG antibodies: 1,482 from the STI clinic cohort and 1,487 from the GP cohort. The study population had a median age of 44 (IQR 30–54), 54.9% were men (n = 1,630) and 11.0% (n = 326) had a non-western ethnicity (see S1 Table). Anti-HEV IgG was detected in 562 samples (18.9%, 95%CI: 17.5–20.4).
Univariate risk factors
In univariate analyses, the overall HEV prevalence was higher with each year’s increase in age (p<0.01) as well as for individuals from the GP cohort (p<0.01; see Table 1). For the STI clinic cohort, HEV prevalence was higher with each year’s increase in age (p<0.01), for MSM (p = 0.04), for individuals who reported anal sex (p = 0.02), for individuals who had three or more sexual partners (p = 0.05) and with a diagnosis of CT or NG (p = 0.05). For the GP cohort, the HEV prevalence was higher with each year’s increase in age (p<0.01) and borderline significant for men (p = 0.10).
Table 1. Determinants of anti-HEV IgG prevalence, south Netherlands, Dec 2011 to Nov 2015.
| Total study population (n = 2,969) | Univariate anti-HEV IgG prevalence | Multivariate OR (95%CI) adjusted for test yeara, age, gender | |
| Age (median = 44, IQR = 30–54) | *** | 1.03*** (1.02–1.04) | |
| Gender | |||
| - Men | 309/1,630 | 19.0% | 1.24* (1.02–1.50) |
| - Women | 253/1,339 | 18.9% (ref) | 1.00 |
| Population | |||
| - High sexual risk (STI clinic cohort) | 176/1,482 | 11.9% (ref) | 1.00 |
| - General (GP cohort) | 386/1,487 | 26.0%*** | 1.19 (0.76–1.87) |
| STI clinic cohort (n = 1,482) | adjusted for test yeara, age, sexual preference | ||
| Age (median = 30, IQR = 24–39) | *** | 1.02** (1.01–1.04) | |
| Gender | |||
| - Men | 118/948 | 12.4% | 1.23 (0.88–1.73) |
| - Women | 58/534 | 10.9% (ref) | 1.00 |
| Sexual preference | |||
| - Women | 33/350 | 9.4% (ref) | 1.00 |
| - Female swinger | 25/184 | 13.6% | 1.17 (0.65–2.11) |
| - Heterosexual men | 52/480 | 10.8% | 1.19 (0.75–1.89) |
| - MSM | 66/468 | 14.1%* | 1.41 (0.90–2.23) |
| Reported anal sex | |||
| - Yes | 87/629 | 13.8%* | 1.27 (0.86–1.88) |
| - No | 81/812 | 10.0% (ref) | 1.00 |
| - Unknown | 8/41 | 19.5%# | 2.11# (0.92–4.83) |
| Number of sexual partners (median = 3, IQR = 2–5) | |||
| - <3 in past 6 months | 61/617 | 9.9% (ref) | 1.00 |
| - ≥3 in past 6 months | 114/856 | 13.3%* | 1.31 (0.93–1.85) |
| - Unknown | 1/9 | 11.1% | 1.09 (0.13–9.03) |
| Chlamydia and/or gonorrhoeae positive | |||
| - Yes | 28/171 | 16.4%* | 1.60* (1.02–2.49) |
| - Nob | 148/1,311 | 11.3% (ref) | 1.00 |
| Syphilis positive | |||
| - Yes | 1/4 | 25.0% | 1.75 (0.16–18.72) |
| - No | 14/114 | 12.3% (ref) | 1.00 |
| - Untested | 161/1,364 | 11.8% | 0.88 (0.46–1.68) |
| HIV positive | |||
| - Yes | 6/37 | 16.2% | 1.08 (0.43–2.74) |
| - No | 167/1,427 | 11.7% (ref) | 1.00 |
| - Untested | 3/18 | 16.7% | 1.48 (0.42–5.22) |
| GP cohort (n = 1,487) | adjusted for test yeara, age, gender | ||
| Age (median = 53, IQR = 46–62) | *** | 1.04*** (1.03–1.05) | |
| Gender | |||
| - Men | 191/682 | 28.0%# | 1.25# (0.99–1.58) |
| - Women | 195/805 | 24.2% (ref) | 1.00 |
| Educational level | |||
| - Low | 185/676 | 27.4% | 1.12 (0.82–1.51) |
| - Medium | 94/371 | 25.3% | 1.11 (0.80–1.55) |
| - High | 102/427 | 23.9%(ref) | 1.00 |
| - Unknown | 5/13 | 38.5% | 1.43 (0.45–4.55) |
| HIV positive | |||
| - Yes | 1/5 | 20.0% (ref) | 1.00 |
| - No | 352/1,374 | 25.6% | 1.46 (0.16–13.42) |
| - Untested | 33/108 | 30.6% | 1.91 (0.20–18.21) |
| HBV positive | |||
| - Yes (active or past infection) | 43/139 | 30.9% | 1.15 (0.78–1.70) |
| - No (no infection or vaccinated) | 343/1348 | 25.4% (ref) | 1.00 |
| HCV positive | |||
| - Yes (past infection) | 3/4 | 75.0%# | 8.77# (0.89–86.40) |
| - No (no infection) | 383/1483 | 25.8% (ref) | 1.00 |
a Test year of 2012 includes two tests from December 2011.
b Including 14 individuals not tested for CT and 13 individuals not tested for NG.
*p≤0.05
**p≤0.01
***p≤0.001
#0.05≤p≤0.10
Independent risk factors
In multivariate analyses, the overall HEV prevalence was higher with each year’s increase in age (adjusted OR: 1.03, 95%CI: 1.02–1.04, p<0.01) and for men as compared to women (adjusted OR: 1.24, 95%CI: 1.02–1.50, p = 0.03; see Table 1 and Fig 1). HEV prevalence was similar among the STI clinic cohort and the GP cohort, after adjusting for confounding factors (see Table 1).
For the STI clinic cohort, most univariate sexual risk factors attenuated and became statistically non-significant after adjusting for test year, age and sexual preference. Independent predictors for HEV prevalence were older age (adjusted OR: 1.02, 95%CI: 1.01–1.04, p<0.01) and a diagnosis of CT or NG (adjusted OR: 1.60, 95%CI: 1.02–2.49, p = 0.04).
For the GP cohort, HEV prevalence was higher with each year’s increase in age (adjusted OR: 1.04, 95%CI: 1.03–1.05, p<0.01).
Discussion
No firm conclusions can be drawn about sexual transmission of HEV from this study given that the overall prevalence of anti-HEV IgG was the same between the sexual high-risk population and the general population. Our results only hint at the possibility of sexual transmission of HEV as having a sexually transmitted infection (CT and/or NG) was found to be associated with a higher HEV prevalence. Though this may lead to occasional HEV outbreaks, as is the case for HAV, sexual HEV transmission is unlikely to influence the prevalence at the population level. More research is needed on possible sexual transmission of HEV.
Anti-HEV IgG was detected in 18.9% of the 2,969 blood samples from individuals either attending an STI clinic or belonging to the general population. The adjusted HEV prevalence was similar among the two cohorts. This observed prevalence is similar to the figure of 17% found in a German study among persons aged 18–79 years, which used the same HEV assay [37]. The observed prevalence is lower than a study among Dutch blood donors with a prevalence of 27% [11]. This difference in HEV prevalence is unlikely only explained by the use of the Wantai assay with a slightly higher sensitivity [38], it is more likely explained by the older study population. The identification of age and gender factors with relation to HEV prevalence confirms the results from several previous studies [10–12, 39]. HEV prevalence is thought to be related to age, because of the cumulative effect of lifetime exposure. However, no clear and thorough explanation has as yet been found for its connection with gender.
Interestingly, HEV prevalence was higher for individuals who tested positive for CT and/or NG. This higher prevalence is unlikely explained by the zoonotic transmission route of HEV. The ingestion of HEV-infected food is not believed to be more common among individuals who tested positive for CT and/or NG. Furthermore, a similar trend is seen in other risk factors of sexual transmission such as being an MSM, reporting anal sex, having three or more sexual partners in the past six months and being tested positive for syphilis. This may suggest that sex may be a possible transmission route for HEV. Whether HEV could be transmitted via the faecal-oral route, via blood contact during risky sexual behaviour or via person-to-person contact remains unknown and should be the subject of further study.
To our knowledge, this is the first study to explore the possible sexual transmission of HEV and its impact at the population level in the Netherlands. However, the following limitations must be considered. First, no data on sexual risk behaviour were available for individuals from the GP cohort and data on parenteral risk behaviour, injective drug use, were not included due to its low prevalence. However, we compared the sexual risk behaviour of the STI clinic cohort to the general population in the Netherlands. In our study 40% of the individuals reported anal sex in the past six months compared to 7–18% in the general population [40, 41]. Overall more sexual risk behaviour was reported in the STI clinic cohort compared to the general population. Second, the age distribution differs between the STI clinic cohort and the GP cohort. Age was included as confounder in the multivariate logistic regression analyses. Additionally, we assessed the HEV prevalence in the 40–50 year olds. No difference was detected between the STI clinic cohort and the GP cohort in this age group too, p = 0.678. Matching cases and controls on age and/or sex is desirable for future studies[26, 27]. Third, the comparison to HEV prevalence in other studies is difficult to make, as sensitivity and specificity are highly variable between the different serological assays [42, 43]. Fourth, no data on occupational exposure to swine, as a known risk factor of HEV, were available. Finally, using this cross-sectional design we were unable to assess causal associations, a longitudinal study including genotyping would have been the ideal design. Our results only hint at the possibility of a sexual transmission route for HEV given higher rates in those with chlamydia and/or gonorrheal infections. Sexual transmission is not a dominant transmission route, as its prevalence was not higher for the STI clinic cohort than for the GP cohort. Whether sexual transmission of HEV occurs, and whether it could consequently lead to outbreaks in the same vein as HAV, should preferably be studied at the individual level in future prospective research. Considering the limited clinical impact of HEV infections, we do not recommend increased HEV testing in sexual high-risk clinical settings, with the possible exception of patients who are immunocompromised.
Supporting information
(DOCX)
Data Availability
Data are unsuitable for public deposition due to ethical restrictions and privacy of participant data (wet bescherming personengegevens Wbp or Personal Data Protection Act: http://wetten.overheid.nl/BWBR0011468/geldigheidsdatum_13-07-2015). Interested researchers who meet the criteria for access to confidential data may contact the head of the data-archiving (Helen Sijstermans: Helen.Sijstermans@ggdzl.nl) to request the data.
Funding Statement
The authors received no specific funding for this work, this is an investigator-initiated study.
References
- 1.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20(1):23–31. Epub 1983/01/01. . [DOI] [PubMed] [Google Scholar]
- 2.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48(3):494–503. Epub 2008/01/15. doi: 10.1016/j.jhep.2007.12.008 . [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15(1):9–20. Epub 2000/03/17. . [DOI] [PubMed] [Google Scholar]
- 4.Zanetti AR, Schlauder GG, Romano L, Tanzi E, Fabris P, Dawson GJ, et al. Identification of a novel variant of hepatitis E virus in Italy. J Med Virol. 1999;57(4):356–60. Epub 1999/03/24. doi: 10.1002/(SICI)1096-9071(199904)57:4<356::AID-JMV5>3.0.CO;2-D . [DOI] [PubMed] [Google Scholar]
- 5.Garbuglia AR, Scognamiglio P, Petrosillo N, Mastroianni CM, Sordillo P, Gentile D, et al. Hepatitis E virus genotype 4 outbreak, Italy, 2011. Emerg Infect Dis. 2012;19(1):110–4. Epub 2012/12/25. doi: 10.3201/eid1901.120983 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20(8):784–90. Epub 2008/07/12. doi: 10.1097/MEG.0b013e3282f5195a . [DOI] [PubMed] [Google Scholar]
- 7.Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, et al. Hepatitis E in new zealand. J Gastroenterol Hepatol. 2007;22(8):1236–40. Epub 2007/05/11. doi: 10.1111/j.1440-1746.2007.04894.x . [DOI] [PubMed] [Google Scholar]
- 8.Mansuy JM, Peron JM, Abravanel F, Poirson H, Dubois M, Miedouge M, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74(3):419–24. Epub 2004/09/16. doi: 10.1002/jmv.20206 . [DOI] [PubMed] [Google Scholar]
- 9.Drobeniuc J, Greene-Montfort T, Le NT, Mixson-Hayden TR, Ganova-Raeva L, Dong C, et al. Laboratory-based surveillance for hepatitis E virus infection, United States, 2005–2012. Emerg Infect Dis. 2013;19(2):218–22; quiz 353. Epub 2013/01/26. doi: 10.3201/eid1902.120961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koot H, Hogema BM, Koot M, Molier M, Zaaijer HL. Frequent hepatitis E in the Netherlands without traveling or immunosuppression. J Clin Virol. 2015;62:38–40. doi: 10.1016/j.jcv.2014.11.020 . [DOI] [PubMed] [Google Scholar]
- 11.Slot E, Hogema BM, Riezebos-Brilman A, Kok TM, Molier M, Zaaijer HL. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013;18(31). Epub 2013/08/10. . [DOI] [PubMed] [Google Scholar]
- 12.Dalton HR, Bendall RP, Rashid M, Ellis V, Ali R, Ramnarace R, et al. Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol. 2011;23(12):1200–5. Epub 2011/09/24. doi: 10.1097/MEG.0b013e32834ca4da . [DOI] [PubMed] [Google Scholar]
- 13.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358(8):811–7. Epub 2008/02/22. doi: 10.1056/NEJMoa0706992 . [DOI] [PubMed] [Google Scholar]
- 14.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361(10):1025–7. Epub 2009/09/04. doi: 10.1056/NEJMc0903778 . [DOI] [PubMed] [Google Scholar]
- 15.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481–9. Epub 2011/03/01. doi: 10.1053/j.gastro.2011.02.050 . [DOI] [PubMed] [Google Scholar]
- 16.Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis. 2012;18(5):869–72. Epub 2012/04/21. doi: 10.3201/eid1805.111712 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutjes SA, Lodder WJ, Bouwknegt M, de Roda Husman AM. Increased hepatitis E virus prevalence on Dutch pig farms from 33 to 55% by using appropriate internal quality controls for RT-PCR. J Virol Methods. 2007;143(1):112–6. Epub 2007/02/27. doi: 10.1016/j.jviromet.2007.01.030 . [DOI] [PubMed] [Google Scholar]
- 18.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8(11):698–709. Epub 2008/11/11. doi: 10.1016/S1473-3099(08)70255-X . [DOI] [PubMed] [Google Scholar]
- 19.Van Der Poel WH, Koopmans M, De Roda Husman AM. Hepatitis E virus in Nederland. Inf Bulletin. 2002;13(8):299–303. [Google Scholar]
- 20.Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34–41. doi: 10.1016/j.coviro.2014.12.006 . [DOI] [PubMed] [Google Scholar]
- 21.Wichmann O, Schimanski S, Koch J, Kohler M, Rothe C, Plentz A, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198(12):1732–41. Epub 2008/11/06. doi: 10.1086/593211 . [DOI] [PubMed] [Google Scholar]
- 22.Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202(6):825–34. Epub 2010/08/11. doi: 10.1086/655898 . [DOI] [PubMed] [Google Scholar]
- 23.Hazards EPanel oB, Ricci A, Allende A, Bolton D, Chemaly M, Davies R, et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA Journal. 2017;15(7):e04886–n/a. doi: 10.2903/j.efsa.2017.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorgos L. Sexual transmission of viral hepatitis. Infect Dis Clin North Am. 2013;27(4):811–36. doi: 10.1016/j.idc.2013.08.002 . [DOI] [PubMed] [Google Scholar]
- 25.Freidl GS, Sonder GJ, Bovee LP, Friesema IH, van Rijckevorsel GG, Ruijs WL, et al. Hepatitis A outbreak among men who have sex with men (MSM) predominantly linked with the EuroPride, the Netherlands, July 2016 to February 2017. Euro Surveill. 2017;22(8). doi: 10.2807/1560-7917.ES.2017.22.8.30468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sazzad HMS, Luby SP, Labrique AB, Kamili S, Hayden TM, Kamili NA, et al. Risk Factors Associated with Blood Exposure for Sporadic Hepatitis E in Dhaka, Bangladesh. Am J Trop Med Hyg. 2017. doi: 10.4269/ajtmh.17-0261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abravanel F, Lhomme S, Fougere M, Saune K, Alvarez M, Peron JM, et al. HEV infection in French HIV-infected patients. J Infect. 2017;74(3):310–3. doi: 10.1016/j.jinf.2016.12.004 . [DOI] [PubMed] [Google Scholar]
- 28.Eker A, Tansel O, Kunduracilar H, Tokuc B, Yulugkural Z, Yuksel P. [Hepatitis E virus epidemiology in adult population in Edirne province, Turkey]. Mikrobiyol Bul. 2009;43(2):251–8. . [PubMed] [Google Scholar]
- 29.Christensen PB, Engle RE, Jacobsen SE, Krarup HB, Georgsen J, Purcell RH. High prevalence of hepatitis E antibodies among Danish prisoners and drug users. J Med Virol. 2002;66(1):49–55. . [DOI] [PubMed] [Google Scholar]
- 30.Thomas DL, Yarbough PO, Vlahov D, Tsarev SA, Nelson KE, Saah AJ, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35(5):1244–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dukers-Muijrers NH, Niekamp AM, Brouwers EE, Hoebe CJ. Older and swinging; need to identify hidden and emerging risk groups at STI clinics. Sex Transm Infect. 2010;86(4):315–7. doi: 10.1136/sti.2009.041954 . [DOI] [PubMed] [Google Scholar]
- 32.Kok A, Zuure FR, Weegink CJ. Hepatitis C in Nederland: schaarse gegevens over actuele prevalentie en de noodzaak van epidemiologisch onderzoek en innovatieve opsporingsmethoden. Nederlands Tijdschrift voor Geneeskunde 2007;151:2367–71. [PubMed] [Google Scholar]
- 33.Slavenburg S, Verduyn-Lunel FM, Hermsen JT, Melchers WJ, te Morsche RH, Drenth JP. Prevalence of hepatitis C in the general population in the Netherlands. Neth J Med. 2008;66(1):13–7. Epub 2008/01/26. . [PubMed] [Google Scholar]
- 34.Vriend HJ, Op de Coul EL, van de Laar TJ, Urbanus AT, van der Klis FR, Boot HJ. Hepatitis C virus seroprevalence in the Netherlands. Eur J Public Health. 2012;22(6):819–21. doi: 10.1093/eurpub/cks013 . [DOI] [PubMed] [Google Scholar]
- 35.Marschall T, Kretzschmar M, Mangen MJ, Schalm S. High impact of migration on the prevalence of chronic hepatitis B in the Netherlands. Eur J Gastroenterol Hepatol. 2008;20(12):1214–25. Epub 2008/10/24. doi: 10.1097/MEG.0b013e32830e289e . [DOI] [PubMed] [Google Scholar]
- 36.van Marrewijk CM, Velshuijzen IK, Conyn-van Spaendonck MAE, Kooy H, van den Hof S, Dorigo-Zetsma JW. Prevalence of hepatitis B viral markers in the Dutch population: a population-based serosurveillance study (Pienter project) Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu; 1999 [updated 1999-03-11]. http://www.rivm.nl/bibliotheek/rapporten/243680001.html.
- 37.Faber MS, Wenzel JJ, Jilg W, Thamm M, Hohle M, Stark K. Hepatitis E virus seroprevalence among adults, Germany. Emerg Infect Dis. 2012;18(10):1654–7. doi: 10.3201/eid1810.111756 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pas SD, Streefkerk RH, Pronk M, de Man RA, Beersma MF, Osterhaus AD, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58(4):629–34. doi: 10.1016/j.jcv.2013.10.010 . [DOI] [PubMed] [Google Scholar]
- 39.RIVM. LCI-richtlijn Hepatitis E. 2007.
- 40.Heijne JC, van Liere GA, Hoebe CJ, Bogaards JA, van Benthem BH, Dukers-Muijrers NH. What explains anorectal chlamydia infection in women? Implications of a mathematical model for test and treatment strategies. Sex Transm Infect. 2016. doi: 10.1136/sextrans-2016-052786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Survey of Sexual Attitudes and Lifestyles (Natsal-3). http://www.natsal.ac.uk/media/3935/natsal-3-reference-tables.pdf.
- 42.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82(5):799–805. doi: 10.1002/jmv.21656 . [DOI] [PubMed] [Google Scholar]
- 43.Rossi-Tamisier M, Moal V, Gerolami R, Colson P. Discrepancy between anti-hepatitis E virus immunoglobulin G prevalence assessed by two assays in kidney and liver transplant recipients. J Clin Virol. 2013;56(1):62–4. doi: 10.1016/j.jcv.2012.09.010 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and privacy of participant data (wet bescherming personengegevens Wbp or Personal Data Protection Act: http://wetten.overheid.nl/BWBR0011468/geldigheidsdatum_13-07-2015). Interested researchers who meet the criteria for access to confidential data may contact the head of the data-archiving (Helen Sijstermans: Helen.Sijstermans@ggdzl.nl) to request the data.