Abstract
Purpose
Blood urea nitrogen (BUN) was reported to be associated with mortality in heart failure patients. We aimed to evaluate admission BUN concentration in a heterogeneous critically ill patient collective admitted to an intensive care unit (ICU) for prognostic relevance.
Methods
A total of 4176 medical patients (67±13 years) admitted to a German ICU between 2004 and 2009 were included. Follow-up of patients was performed retrospectively between May 2013 and November 2013. Association of admission BUN and both intra-hospital and long-term mortality were investigated by Cox regression. An optimal cut-off was calculated by means of the Youden-Index.
Results
Patients with higher admission BUN concentration were older, clinically sicker and had more pronounced laboratory signs of multi-organ failure including kidney failure. Admission BUN was associated with adverse long-term mortality (HR 1.013; 95%CI 1.012–1.014; p<0.001). An optimal cut-off was calculated at 28 mg/dL which was associated with adverse outcome even after correction for APACHE2 (HR 1.89; 95%CI 1.59–2.26; p<0.001), SAPS2 (HR 1.85; 95%CI 1.55–2.21; p<0.001) and several parameters including creatinine in an integrative model (HR 3.34; 95%CI 2.89–3.86; p<0.001). We matched 614 patients with admission BUN >28 mg/dL to case-controls ≤ 28mg/dL corrected for APACHE2 scores: BUN above 28 mg/dL remained associated with adverse outcome in a paired analysis with the difference being 5.85% (95%CI 1.23–10.47%; p = 0.02).
Conclusions
High BUN concentration at admission was robustly associated with adverse outcome in critically ill patients admitted to an ICU, even after correction for co-founders including renal failure. BUN might constitute an independent, easily available and important parameter for risk stratification in the critically ill.
Introduction
Parameters eligible for risk stratification in critically ill patients are of utmost interest to optimize patients`outcome. Elaborated scoring tools such as APACHE and SAPS scores are available and well validated–still, their complexity and reliance on volatile parameters such as heart rate limits their use especially in initial patients`risk stratification [1, 2].
Markers of renal function are known to be associated with mortality (not only) in cardiovascular disorders [3, 4]. Parameters used for evaluating patients’ renal function and fluid status are glomerular filtration rate (GFR), BUN (Blood Urea Nitrogen) and Creatinine (Cr) [5]. Although Cr is known to be predictive for adverse outcome, BUN was shown to have even additive value in heart failure patients [6, 7]. Further, BUN was shown to be an important marker for metabolic diseases and nutritional status of patients. BUN was evaluated for association with mortality in various settings: Liu et al [8] showed that BUN level predicts hospital mortality in acute aortic dissection patients. In another study, it was shown that elevated BUN constitutes an independent factor in risk stratification of acute necrotizing pancreatitis patients [9]. In patients suffering from peripheral artery disease, high BUN was associated with critical limb ischemia [10].
Both Cr and BUN are filtered from glomerulus due to their small molecular sizes. However, Cr is scarcely reabsorbed from tubules and excreted from the body with urine. On the other hand, urea is reabsorbed from the tubules and urea cycle also has an important physiological role in glomerular water balance. Therefore, BUN is a marker for both neurohumoral activity and renal function as urea reabsorption is altered not only by renal but also endocrine disturbances [11].
BUN is a key element reflecting the intricate interrelation between nutritional status, protein metabolism and renal situation of the patient. BUN might therefore constitute a useful parameter for predicting outcome in critically ill patients. In this study, we aimed to evaluate admission BUN level in critically ill patients admitted to an intensive care unit (ICU) for prognostic relevance.
Methods
Study subjects
4176 patients that were treated at the ICU of the Jena University Hospital between January 2004 and December 2009 were enrolled in this study. Main admission diagnoses were pneumonia (n = 533), pulmonary embolism (n = 153), acute coronary syndrome (n = 1909), sepsis (n = 544) and heart failure (n = 611). Inclusion criteria were admission to the medical ICU, and a documented BUN at admission. Standard laboratory values, patient’s medical history and clinical data were documented. All laboratory values were obtained from standard in-hospital laboratory. Follow-up of patients was performed retrospectively between May 2013 and November 2013. The primary endpoint of the study was all cause mortality. Mortality data were collected by review of medical records in our COPRA patient data management system (COPRA System GmbH, Berlin, Germany) and/or patient contact. Data on in-hospital mortality was available for 3847 patients and for long-term mortality on 4059 patients. The study has been approved by the local ethics committee of the University Hospital Jena and waived the requirement for written consent. All data were fully anonymized before evaluation. Minimal anonymized data set is made accessible without restrictions.
Statistical analysis
Normally distributed data points are expressed as mean ± standard error of the mean. Differences between independent groups were calculated using ANOVA. Categorical data are expressed as numbers (percentage). Chi-square test was applied to calculate differences between groups. In-hospital mortality was assessed by both univariate and multivariate logistic regression. Univariate and multivariate Cox regression analysis was used to evaluate and to adjust for cofounding factors for long-term mortality. For the multivariate regression model, cofounders with a p-value <0.10 in the univariate analysis were included, then a backward variable elimination was performed. Elimination criterion was a p-value of more than 0.10. A p-value of <0.05 was considered statistically significant. We report 3 multivariate regression models. For case-control matching we matched patients in the in the BUN > 28mg/dL group vs the ≤ 28 mg/dL group on APACHE2 score at admission. We could match 614 patients based on these criteria. To compare in-hospital mortality between these paired groups we used McNemar’s test.
SPSS version 22.0 (IBM, USA) and MedCalc version 17.4.4 (MedCalc Software, USA) were used for all statistical analyses.
Calculation of SAPS2 and APACHE score
Initial Simplified Acute Physiology Score II (SAPS2) and Acute Physiology And Chronic Health Evaluation (APACHE) scores were calculated by the treating physician within 24 hours after admission as reported before.
Results
Study population
Baseline characteristics are shown in Table 1. Patients with higher admission BUN were older (70±12 years vs 62±14 years), clinically sicker as expressed by both higher SAPS2 (52±20 points vs 32±16 points; p<0.001) and APACHE2 (27±9 points vs 16±9 points; p<0.001) scores. Patients with higher BUN had more pronounced laboratory signs of multi-organ failure including liver (ALAT: 3.6±10.6 μmol/l*s vs 1.2±3.9 μmol/l*s; p<0.001; ASAT 7.2±24.9 μmol/l*s vs 3.0±6.6 μmol/l*s; p<0.001).
Table 1. Laboratory and clinical baseline characteristics.
4176 medical patients were split in three cohorts according to their BUN concentration at admission, comparison of means by ANOVA.
| BUN 0–20 mg/dL | BUN 20–40 mg/dL | BUN >40mg/dL | overall cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 1820 | n = 1296 | n = 1060 | n = 4176 | ||||||
| mean | SEM | mean | SEM | mean | SEM | mean | SEM | p-value | |
| age | 62.2 | 13.8 | 70.1 | 11.7 | 69.9 | 12.2 | 66.6 | 13.4 | <0.001 |
| SAPS2 (pts) | 31.7 | 16.0 | 44.3 | 19.3 | 51.8 | 18.8 | 42.2 | 19.9 | <0.001 |
| APACHE2 (pts) | 16.2 | 8.6 | 22.2 | 8.9 | 26.6 | 8.5 | 21.5 | 9.6 | <0.001 |
| lactate (mmol/L) | 2.0 | 3.4 | 2.8 | 3.1 | 3.3 | 4.0 | 2.6 | 3.5 | <0.001 |
| PCT (mmol/L) | 5.6 | 15.6 | 13.4 | 38.5 | 14.5 | 33.8 | 12.0 | 32.5 | 0.001 |
| glucose (mmol/L) | 9.0 | 3.4 | 10.6 | 4.1 | 10.9 | 3.9 | 10.0 | 3.9 | <0.001 |
| haemoglobine (mmol/L) | 8.2 | 3.6 | 7.6 | 1.1 | 7.3 | 4.6 | 7.8 | 3.4 | <0.001 |
| ASAT (μmol/l*s) | 3.0 | 6.6 | 5.0 | 18.1 | 7.2 | 24.9 | 4.9 | 17.5 | <0.001 |
| ALAT (μmol/l*s) | 1.2 | 3.9 | 2.4 | 7.4 | 3.6 | 10.6 | 2.3 | 7.6 | <0.001 |
| max_Gamma_GT_μmol | 1.4 | 2.5 | 1.7 | 2.2 | 2.1 | 2.3 | 1.7 | 2.4 | <0.001 |
| max_Bilirubin_ges_μmol | 16.7 | 19.4 | 19.3 | 26.7 | 31.2 | 56.6 | 21.3 | 35.5 | <0.001 |
| leucocytes (G/L) | 10.8 | 5.1 | 12.5 | 9.0 | 14.2 | 15.8 | 12.2 | 10.1 | <0.001 |
| BUN (mg/dL) | 13.8 | 3.7 | 27.9 | 5.6 | 72.6 | 32.4 | 33.1 | 29.1 | <0.001 |
| creatinine (mg/dL) | 84.0 | 26.6 | 135.9 | 81.0 | 307.4 | 215.7 | 156.8 | 149.5 | <0.001 |
| sodium (mmol/L) | 139.5 | 4.3 | 140.7 | 5.1 | 140.6 | 7.1 | 140.1 | 5.4 | <0.001 |
| potassium (mmol/L) | 4.0 | 0.4 | 4.1 | 0.6 | 4.3 | 0.7 | 4.1 | 0.6 | <0.001 |
Survival data
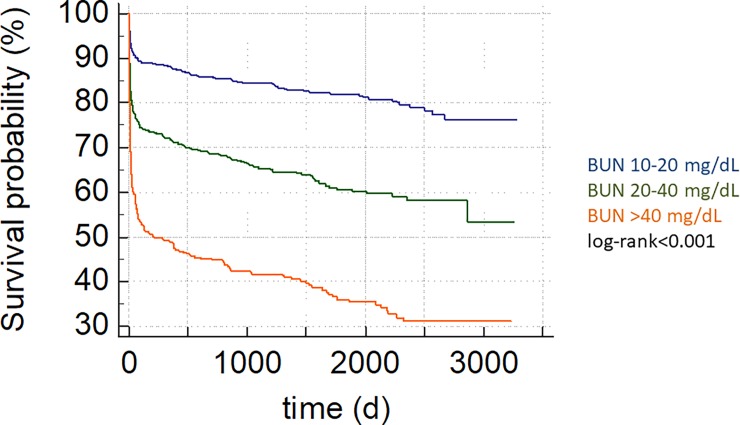
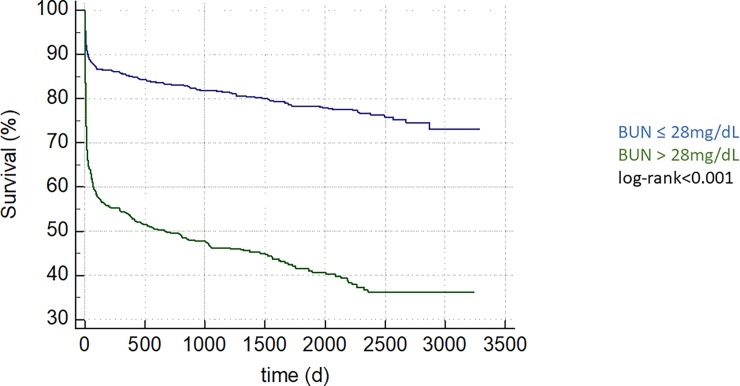
Patients with BUN above 40 mg/dL evidenced adverse in-hospital outcome compared to the 10–20 mg/dL group (HR 6.05; 95%CI 4.63–7.90; p<0.001; Table 2, Fig 1). Admission BUN was associated with adverse long-term mortality (HR 1.013; 95%CI 1.012–1.014; p<0.001) regardless of admission diagnosis (Table 3). We performed ROC-analysis for BUN (AUC 0.67 95%CI 0.65–0.69) and compared it to SAPS2 (AUC 0.76 95%CI 0.74–0.78; p<0.0001 vs BUN) and APACHE2 (AUC 0.74 95%CI 0.72–0.76; p<0.001 vs BUN). Further, an optimal admission BUN cut-off was calculated at 28 mg/dL. This cut-off was associated with both in-hospital (HR 4.16; 95%CI 3.39–5.10; p<0.001) as well as with long-term mortality (HR 3.74; 95%CI 3.29–4.25; p<0.001, Fig 2).
Table 2. Higher admission BUN levels are associated with adverse in-hospital outcome.
Hazard ratios (HR) were obtained by logistic regression analysis.
| BUN | HR | 95%CI | p-value |
|---|---|---|---|
| 10–20 mg/dL | 1.00 | ||
| 20–40 mg/dL | 2.32 | 1.75–3.08 | <0.001 |
| >40 mg/dL | 6.05 | 4.63–7.90 | <0.001 |
| >28 mg/dL | 4.16 | 3.39–5.10 | <0.001 |
Fig 1. Higher admission BUN levels are associated with adverse long-term outcome, depicted as Kaplan-Meier curve, group comparison by log-rank test, p-value <0.001.
Table 3. Admission BUN concentration is associated with long-term mortality regardless of admission diagnosis [pneumonia (n = 533), pulmonary embolism (n = 153), acute coronary syndrome (ACS; n = 1909), sepsis (n = 544) and heart failure (AHF, n = 611)].
Hazard ratios (HR) were obtained by Cox regression analysis.
| admissio diagnosis | HR | 95%CI | p-value |
|---|---|---|---|
| overall cohort | 1.013 | 1.012–1.014 | <0.001 |
| sepsis | 1.005 | 1.002–1.008 | <0.001 |
| AHF | 1.008 | 1.004–1.012 | <0.001 |
| pneumonia | 1.008 | 1.004–1.011 | <0.001 |
| pulmonary embolism | 1.034 | 1.024–1.044 | <0.001 |
| ACS | 1.029 | 1.026–1.033 | <0.001 |
Fig 2. An admission BUN concentration above 28mg/dL, the optimal cut-off calculated by Youden Index, is associated with long term mortality, depicted as Kaplan-Meier curve, group comparison by log-rank test, p-value <0.001.
An admission BUN >28 mg/dL was associated with long-term mortality regardless of admission diagnosis (Table 4). The association of admission BUN >28 mg/dL with long-term mortality remained even after correction for APACHE2 (HR 1.89; 95%CI 1.59–2.26; p<0.001), SAPS2 (HR 1.85; 95%CI 1.55–2.21; p<0.001) and several parameters including creatinine in an integrative model (HR 3.34; 95%CI 2.89–3.86; p<0.001; Table 5).
Table 4. An admission BUN concentration above 28mg/dL is associated with long-term mortality regardless of admission diagnosis [pneumonia (n = 533), pulmonary embolism (n = 153), acute coronary syndrome (ACS; n = 1909), sepsis (n = 544) and heart failure (AHF, n = 611)].
Hazard ratios (HR) were obtained by Cox regression analysis.
| admission diagnosis | HR | 95%CI | p-value |
|---|---|---|---|
| overall cohort | 3.74 | 3.29–4.25 | <0.001 |
| sepsis | 1.77 | 1.33–2.36 | <0.001 |
| AHF | 1.76 | 1.33–2.32 | <0.001 |
| pneumonia | 1.59 | 1.21–2.10 | 0.002 |
| pulmonary embolism | 5.8 | 3.27–10.26 | <0.001 |
| ACS | 5.41 | 4.25–6.90 | <0.001 |
Table 5. An admission BUN concentration above 28mg/dL is associated with mortality after correction for several cofounders in a multivariate analysis.
Hazard ratios (HR) were obtained by Cox regression analysis, for the multivariate regression models, a backward variable elimination was performed. Elimination criterion was a p-value of more than 0.10.
| multivariate model 1 | ||||||
| univariate | multivariate | |||||
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| BUN >28 mg/dL | 3.74 | 3.29–4.25 | <0.001 | 1.89 | 1.59–2.26 | <0.001 |
| APACHE2 | 1.07 | 1.07–1.08 | <0.001 | 1.07 | 1.06–1.08 | <0.001 |
| multivariate model 2 | ||||||
| univariate | multivariate | |||||
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| BUN >28 mg/dL | 3.74 | 3.29–4.25 | <0.001 | 1.85 | 1.55–2.21 | <0.001 |
| SAPS2 | 1.04 | 1.03–1.04 | <0.001 | 1.03 | 1.03–1.04 | <0.001 |
| multivariate model 3 | ||||||
| univariate | multivariate | |||||
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| BUN >28 mg/dl | 3.74 | 3.29–4.25 | <0.001 | 3.34 | 2.89–3.86 | <0.001 |
| creatinine (mg/dL) | 1.001 | 1.001–1.001 | <0.001 | 1.02 | 0.98–1.05 | 0.44 |
| age (y) | 1.02 | 1.02–1.03 | <0.001 | 1.02 | 1.01–1.02 | <0.001 |
| sex (m/w) | 0.97 | 0.86–1.08 | 0.55 | 0.9 | 0.79–1.03 | 0.9 |
| lactate (mmol/L) | 1.06 | 1.06–1.07 | <0.001 | 1.07 | 1.06–1.07 | <0.001 |
This was true for the association of admission BUN > 28 mg/dL with intra-ICU mortality, which remained associated with mortality after correction for SAPS2 (HR 1.70 95%CI 1.27–1.27; p<0.001) as well as APACHE2 (HR 1.78 95%CI 1.34–2.38; p<0.001).
Matched-control analysis
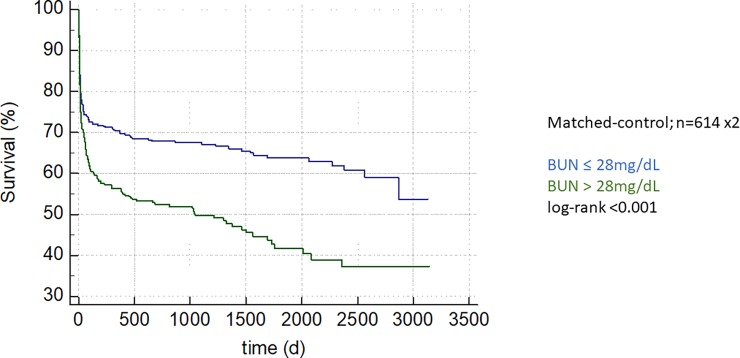
We matched 614 patients with admission BUN >28 mg/dL to case-controls evidencing admission BUN < 28mg/dL corrected for APACHE2 scores: The BUN > 28mg/dL group evidenced adverse intra-hospital outcome in a paired analysis with the difference being 5.85% (95%CI 1.23–10.47%; p = 0.02). Regarding long-term mortality, again, patients in the > 28mg/dL group evidenced adverse outcome (Fig 3; log-rank <0.001).
Fig 3. An admission BUN concentration above 28mg/dL, the optimal cut-off calculated by Youden Index, is associated with long term mortality in a matched-control analysis of 614 patients matched on APACHE2 scores, depicted as Kaplan-Meier curve, group comparison by log-rank test, p-value <0.001.
Discussion
Identifying patients at high risk for death as soon as possible is of utmost interest in critically ill patients. In our study BUN was independently associated with mortality in critically ill patients admitted to a medical ICU. This association existed regardless of admission diagnosis and remained statistically significant even after correction for relevant cofounders in several multivariate analyses.
This association of BUN with mortality is in accordance to literature: Regardless of admission diagnosis such as acute pulmonary embolism [12], acute pancreatitis [13], acute decompensated heart failure [14], upper gastrointestinal bleeding [15, 16] or ventilation failure which requires mechanical aid [17] high admission BUN levels were reported to be associated adverse outcome. Our study supports this notion in critically ill medical patients.
ROC analysis in this study revealed an optimal admission BUN cut-off of 28 mg/dL which is relatively low, especially in critically ill patients. But, similarly Filippatos et al [7] also found highest 60-day mortality in patients suffering from heart failure in the highest BUN quartile (BUN>40 mg/dL). Even subtle BUN changes comparing patients evidencing BUN concentration above 25 mg/dL against BUN levels between 20 to 25 mg/dL were reported to be predictive for mortality [18]. Therefore, our results support existing literature in this aspect and add substantial knowledge in the heterogeneous patient collective admitted to an ICU.
BUN is known to increase with age and BUN has to be interpreted with caution in elderly [18]. Still in our study cohort, even after correction for age in a multivariate analysis BUN remained predictive for mortality.
In a clinical setting, BUN is mostly interpreted and evaluated with special emphasis on renal failure and fluid balance. On the other hand, BUN is known to be influenced by neurohumoral activation in heart failure and by protein metabolism. In this study, we could show in a multivariate analysis, that BUN was robustly associated with mortality even after correction for renal function–indicating an additive role of BUN to Cr. Patients admitted to an ICU constitute a heterogeneous patient collective. Still, in our study BUN was associated with adverse outcome regardless of admission diagnosis, whereas a mechanistic analysis why and how BUN adds information useful for risk stratification is beyond the scope of this study.
There are sophisticated tools available for risk stratification such as APACHE 2 and SAPS 2 scores which are widely used for risk stratification in critically ill patients. Still, these scores a relatively complex, take some time to calculate and take both volatile parameters—such as heart rate—and parameters which are not always available upon admission—such as concomitant diseases—into account: Therefore, there certainly is a need for a simple tool for quick initial risk stratification. BUN concentration is easy to obtain and a straightforward cut-off such 28 mg/dL can easily be interpreted. This value is also similar to the cut-off used in the tried and tested CURB-65 score for pneumonia severity and might be combined with simple clinical assessments such as initial blood pressure or jugular venous pressure to quickly and easily assess critically ill patient when admitted to an ICU or even an emergency department [19]. At least in our study cohort, high BUN concentration upon admission remained associated with adverse outcome even after correction for both SAPS2 and APACHE2 in a multivariate analysis. As it was even predictive for mortality in a matched-control analysis, we think BUN might constitute a simple and powerful tool robustly associated with mortality for early risk stratification in critically ill patients. In future studies, our BUN cut-off of 28 mg/dL should be prospectively evaluated in the critically ill. One could speculate about combining two simple and cheap modalities such as e.g. BUN concentration and clinical evaluation of fluid status e.g. using jugular vein pressure.
Conclusion
High BUN concentrations upon ICU admission correctly identify patients who are clinically sicker and evidence more pronounced laboratory signs of multi-organ failure. Admission BUN concentrations were robustly associated with both, intra-hospital and long-term mortality in our cohort study. As this remained true both after correction for several relevant co-founders in a multivariate analysis as well is in matched-control analysis, we think that BUN might constitute an independent risk factor, with additive value to renal failure, possibly indicating neurohumoral activation and disturbed protein metabolism. BUN is easy to measure and might therefore be a potent tool for risk stratification of critically ill patients.
Supporting information
(XLSX)
Acknowledgments
We would like to thank Katharina Bannier and Julian Gonschorrek for their support in collecting the patients’ follow-up.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Okazaki H, Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, et al. New scoring system (APACHE-HF) for predicting adverse outcomes in patients with acute heart failure: evaluation of the APACHE II and Modified APACHE II scoring systems. J Cardiol. 2014;64(6):441–9. doi: 10.1016/j.jjcc.2014.03.002 . [DOI] [PubMed] [Google Scholar]
- 2.Previsdomini M, Cerutti B, Merlani P, Kaufmann M, van Gessel E, Rothen HU, et al. SwissScoring—a nationwide survey of SAPS II assessing practices and its accuracy. Swiss Med Wkly. 2014;144:w14090 doi: 10.4414/smw.2014.14090 . [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455–69. doi: 10.1093/eurheartj/eht386 . [DOI] [PubMed] [Google Scholar]
- 4.van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J. 2016;37(33):2577–85. doi: 10.1093/eurheartj/ehv588 . [DOI] [PubMed] [Google Scholar]
- 5.Dalcomune DM, Terrao J, Porto ML, Vasquez EC, Baldo MP, Pereira TM. Predictive value of cystatin C for the identification of illness severity in adult patients in a mixed intensive care unit. Clin Biochem. 2016;49(10–11):762–7. doi: 10.1016/j.clinbiochem.2016.04.004 . [DOI] [PubMed] [Google Scholar]
- 6.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, et al. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94(7):957–60. doi: 10.1016/j.amjcard.2004.06.041 . [DOI] [PubMed] [Google Scholar]
- 7.Filippatos G, Rossi J, Lloyd-Jones DM, Stough WG, Ouyang J, Shin DD, et al. Prognostic value of blood urea nitrogen in patients hospitalized with worsening heart failure: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) study. J Card Fail. 2007;13(5):360–4. doi: 10.1016/j.cardfail.2007.02.005 . [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Sun LL, Wang J, Ji G. Blood urea nitrogen in the prediction of in-hospital mortality of patients with acute aortic dissection. Cardiol J. 2017. doi: 10.5603/CJ.a2017.0075 . [DOI] [PubMed] [Google Scholar]
- 9.Faisst M, Wellner UF, Utzolino S, Hopt UT, Keck T. Elevated blood urea nitrogen is an independent risk factor of prolonged intensive care unit stay due to acute necrotizing pancreatitis. J Crit Care. 2010;25(1):105–11. doi: 10.1016/j.jcrc.2009.02.002 . [DOI] [PubMed] [Google Scholar]
- 10.Gary T, Pichler M, Schilcher G, Hafner F, Hackl G, Rief P, et al. Elevated Blood Urea Nitrogen is Associated With Critical Limb Ischemia in Peripheral Arterial Disease Patients. Medicine. 2015;94(24):e948 doi: 10.1097/MD.0000000000000948 ; PubMed Central PMCID: PMCPMC4616554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med. 2004;116(7):466–73. doi: 10.1016/j.amjmed.2003.11.014 . [DOI] [PubMed] [Google Scholar]
- 12.Tatlisu MA, Kaya A, Keskin M, Avsar S, Bozbay M, Tatlisu K, et al. The association of blood urea nitrogen levels with mortality in acute pulmonary embolism. J Crit Care. 2017;39:248–53. doi: 10.1016/j.jcrc.2016.12.019 . [DOI] [PubMed] [Google Scholar]
- 13.Wu BU, Bakker OJ, Papachristou GI, Besselink MG, Repas K, van Santvoort HC, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med. 2011;171(7):669–76. doi: 10.1001/archinternmed.2011.126 . [DOI] [PubMed] [Google Scholar]
- 14.Kajimoto K, Minami Y, Sato N, Takano T, Investigators of the Acute Decompensated Heart Failure Syndromes r. Serum sodium concentration, blood urea nitrogen, and outcomes in patients hospitalized for acute decompensated heart failure. Int J Cardiol. 2016;222:195–201. doi: 10.1016/j.ijcard.2016.07.255 . [DOI] [PubMed] [Google Scholar]
- 15.Kumar NL, Claggett BL, Cohen AJ, Nayor J, Saltzman JR. Association between an increase in blood urea nitrogen at 24 hours and worse outcomes in acute nonvariceal upper GI bleeding. Gastrointest Endosc. 2017;86(6):1022–7 e1. doi: 10.1016/j.gie.2017.03.1533 . [DOI] [PubMed] [Google Scholar]
- 16.Al-Naamani K, Alzadjali N, Barkun AN, Fallone CA. Does blood urea nitrogen level predict severity and high-risk endoscopic lesions in patients with nonvariceal upper gastrointestinal bleeding? Can J Gastroenterol. 2008;22(4):399–403. ; PubMed Central PMCID: PMCPMC2662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan SW, Kao HK, Yu WK, Lien TC, Chen YW, Wang JH, et al. Synergistic impact of low serum albumin on intensive care unit admission and high blood urea nitrogen during intensive care unit stay on post-intensive care unit mortality in critically ill elderly patients requiring mechanical ventilation. Geriatr Gerontol Int. 2013;13(1):107–15. doi: 10.1111/j.1447-0594.2012.00869.x . [DOI] [PubMed] [Google Scholar]
- 18.Kirtane AJ, Leder DM, Waikar SS, Chertow GM, Ray KK, Pinto DS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol. 2005;45(11):1781–6. doi: 10.1016/j.jacc.2005.02.068 . [DOI] [PubMed] [Google Scholar]
- 19.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. doi: 10.1136/thorax.58.5.377 ; PubMed Central PMCID: PMCPMC1746657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.