Abstract
Cadmium, present locally in naturally high concentrations in the Northern Plains of the United States, is of concern because of its toxicity, carcinogenic properties, and potential for trophic transfer. Reports of natural concentrations in soils are dominated by dryland soils with agricultural land uses, but much less is known about cadmium in wetlands. Four wetland categories – prairie potholes, shallow lakes, riparian wetlands, and river sediments – were sampled comprising more than 300 wetlands across four states, the majority in North Dakota. Cd, Zn, P, and other elements were analyzed by ICP-MS, in addition to pH and organic matter (as loss-on-ignition). The overall cadmium content was similar to the general concentrations in the area’s soils, but distinct patterns occurred within categories. Cd in wetland soils is associated with underlying geology and hydrology, but also strongly with concentrations of P and Zn, suggesting a link with agricultural land use surrounding the wetlands.
Keywords: Prairie potholes, Riparian, Floodplains, Shallow lakes, Zinc
1. Introduction
Cadmium occurs locally in high concentrations in the Northern Plains of the United States due to the prevalence of shale-derived soils (Holmgren et al., 1993; Garrett, 1994; Hopkins et al., 1999). The metal accumulates in organisms and is of concern because of its relative toxicity and its carcinogenic properties (Waalkes, 2000; Goyer et al., 2004; Satarug et al., 2010), and has been banned in the European Union in a wide array of products (Communication Department of the European Union, 2011).
The Northern Plains region is highly productive in terms of agriculture and wildlife, and both may be impacted by Cd in the environment. Cadmium occurs in some foods, including grains and fish (Department of Health and Human Services, 2008; European Food Safety Authority, 2009). While there have been no direct reports of excessive concentrations of Cd in food from the Northern Plains, North Dakota sunflower seeds have the potential to exceed the maximum allowance designated by food safety regulators overseas (Comis, 1995). As for wildlife, the millions of small wetlands covering the Prairie Pothole Region support more than half of the important migratory bird species in the US (Kantrud et al., 1989), in addition to resident birds, animals, and plants. Although there have not been any confirmed negative impacts of cadmium on wildlife in the region, there is concern about low-level exposure to the metal and transfer to higher trophic levels, including humans (Li et al., 1997; Burger, 2008; Pillatzki et al., 2011; Rojas-Cifuentes et al., 2012).
Knowledge about the natural distribution of Cd in wetlands, the most productive of terrestrial ecosystems, has been sketchy at best despite the fact that its relative toxicity and carcinogenicity have been long known. Large-scale databases containing data on Cd distribution in soils are made available by government organizations, but these are substantially limited concerning wetland systems. The Geochemical Survey (US Geological Survey website) reports cadmium concentrations across the US, including some wetland soils and sediments, but mostly from drylands (we prefer the term ‘dryland’ to ‘upland’ as the opposite of ‘wetland’, because ‘upland’ is an ambiguous term – ‘uplands’ are elevated lands, such as hills and mountains, in British English), and with relatively high detection limits (2 mg kg−1). The EPA’s metadata analysis of Cd in soils (US EPA, 2003) reflects mostly dryland soils. The PLUTO database (US Geological Survey, 2001) includes soils as well as sediments from wet ecosystems, but the Cd detection limits are high; in many cases 2 mg kg−1 and sometimes even 20 mg kg−1, resulting in most samples with non-detectable concentrations of Cd. The Geochemical Atlas of Europe (Forum of European Geological Surveys website) reports Cd concentrations, to low detection limits (0.02 mg kg−1), in a variety of sediment types, including streams and floodplains. This database is useful for comparison with the United States.
Smaller-scale Cd distribution studies in our study area specific to wetlands are few and not very detailed. An early study on Cd and associated metals in riverine wetlands and prairie potholes by Martin and Hartman (1984) reported values from 13 Waterfowl Production Areas and National Wildlife Refuges across five states in the region. That study was valuable because it was probably the first report on Cd levels in wetlands in the area. However, the sampling density was very low and so the data did not sufficiently assess the variation in Cd in wetlands across the region, which comprises hundreds of thousands of wetlands. The same applies to their comparison of six riverine wetlands with five prairie potholes – the low number of observations across that huge region was simply not enough to come to meaningful comparisons between the two types of wetlands. The Minnesota Geological Survey sampled stream sediments in our sampling area, but not lakes (Lively and Thorleifson, 2009).
Our research group focuses predominantly on cycling of elements, most of which are metals, in wet ecosystems. Over recent years, we collected and analyzed the element composition of soils and sediments from wetlands across the Northern Plains in the US, mostly from North Dakota (ND) and Minnesota (MN), but also some from Montana (MT) and South Dakota (SD). Here we report the distribution of Cd and associated metals based on four separate studies on multi-element composition of (1) prairie wetland soils and (2) riparian floodplains in ND, MT, and SD; (3) shallow lake sediments in MN, and (4) sediments of two rivers of environmental concern in ND, the Souris and Turtle rivers. We included the latter, because sediments make up an important part of riparian floodplain soils. These studies combined provide an extensive dataset of Cd concentrations and other elements in wetland soils and sediments across the landscape.
The aims of this paper are to (1) understand the spatial distribution of Cd and associated elements in soils and sediments of wetlands across the region, (2) assess the associations of Cd with other elements, and (3) assess whether or not consistent differences exist between types of wetlands. Even though this study was carried out in the Northern Plains of the United States, the landscape and ecology are similar to, and the information we provide thus relevant to, other large areas of partly glaciated landscapes with prairie pothole-like wetlands and shallow lakes in the Northern Hemisphere (e.g. Canada, northern Europe, and northern Asia). Our reported limit of detection at 0.089 nmol g−1 (0.01 mg kg−1) was much lower than most of the existing databases. This is the first study over a wide geographic area with a large sample set that specifically addresses the distribution of Cd in wetlands.
The biogeochemistry of metals in the environment, particularly in wetlands, is a major influence on distribution and movement in ecological systems. Cadmium concentrations can be affected by wetland conditions in several ways, including sediment transport, oxidation/reduction, pH, and organic matter content. Franzen et al. (2006) reported higher values of DTPA-extractable Cd concentrations in ‘depressional’ landscape positions compared to slope or ‘upland’ landscape positions. These observations may mean that the total amounts of Cd per weight unit were higher in depressional landscape positions due to downward movement of Cd from upslope to downslope, or the chemistry at depressional landscape positions rendered more Cd extractable at similar total amounts. If we assume that the extractability of Cd did not change much across landscape positions, then wetlands, particularly prairie potholes, being situated in depressions in the landscape, would be expected to act as sinks of metals. This might result in generally higher levels of metal concentrations compared to dryland conditions. However, we did not sample drylands adjacent to the wetlands in our studies, nor do such data exist in enough detail from other studies to make in-depth comparisons.
A typical characteristic of flooded wetland soils is the development of anoxic, reduced conditions. This results in accumulation of organic matter in the soil, formation of metal sulfides, and commonly near-neutral pH. Cd can remain immobilized in wetland soils under these conditions (Gambrell, 1994; Jacob and Otte, 2003). We thus expected a correlation between sediment Cd concentrations and organic matter content due to binding/adsorption (Salomons and Förstner, 1984; Gambrell, 1994; Spurgeon et al., 2008). Other factors affecting Cd concentrations include the particle size distribution of the soils/sediments – most metals bind predominantly smaller particles, particularly the fraction smaller than 63 μm – and pH (Salomons and Förstner, 1984). Because our samples were sieved through either 63 μm or 180 μm sieves before analysis, and did not otherwise assess particle size distributions on the samples, we are unable to assess the relationship between particle size of soils/sediments and Cd concentrations for our data. Similarly, differences between the data sets in determination of pH limit our ability to make comparisons across the entire data set.
It was further expected that Cd concentrations would correlate with Pb, Zn, and perhaps Ag, As, and S concentrations, because the chemistry of the metals is known to be similar and associated with S chemistry (Salomons and Förstner, 1984; Chaney, 2010). As Chaney (2010) pointed out, Cd and Zn should always be considered together, because they have similar biogeochemical behavior in soils and accumulation in organisms. However, as Schultz et al. (1980) noted, they can be decoupled and have low overall correlation in Pierre Shale. In addition, there can be anthropogenic additions of Cd and Zn to soils and sediments from the use of mineral P fertilizers (Mortvedt, 1996).
We also expected relationships between Cd concentrations and the underlying geology, with higher Cd concentrations in wetlands occurring where the surface geology, and thus the soil via pedogenesis, was higher in Cd. In the central and eastern portion of the region, multiple glacial advances from a north-northwesterly direction deposited shale-bearing tills, most recently during late Wisconsin time. In eastern North Dakota, these tills overlie bedrock that itself is shale, typically the Cretaceous Pierre Shale. Schultz et al. (1980) studied the geochemistry of the Pierre Shale across the western U.S. The presence of shale as bedrock, and as a lithologic component of the substrate on which wetlands were formed, is an important influence on soil and wetland chemistry in the region. For example, we expected higher Cd values for the sediments of the Turtle River in ND and for the wetlands in the northeast corner of ND, which are adjacent to the Pembina escarpment. There the Pierre Shale outcrops and the Pierre aquifer is near the surface. These units are known for their relatively high concentrations of metals including Cd (Hopkins et al., 1999). The Pierre aquifer partly supplies water to the Turtle River (Dalrymple and Dwelle, 2012).
2. Materials and methods
Four different data sets were collated and will be referred to as ‘Potholes’, ‘Shallow Lakes’, ‘Riparian Floodplains’, and ‘River Sediments’. In the ‘Potholes’ project, the majority of the wetlands sampled were located in regions that had been formed by glaciation, and thus are depressional wetlands, or potholes. Some of the ‘pothole-like’ wetlands included in this study were in soils unaffected by the most recent glaciation events, but for ease of description, they are included in the ‘Potholes’ group. For all data sets, samples were taken (typically at least 5 replicates per wetland) from a region stretching about 1000 km from Montana in the northwest to southern Minnesota in the southeast (Fig. 1).
Fig. 1.
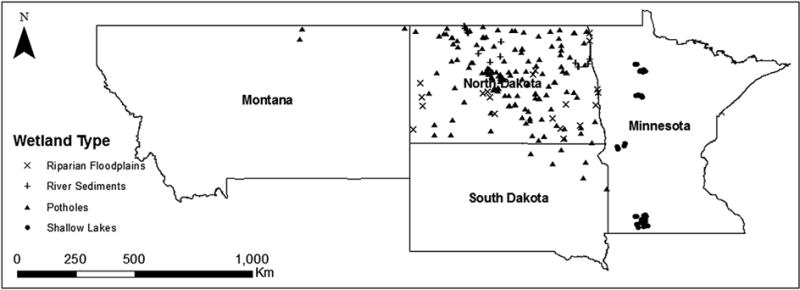
Geographic distribution of wetlands in this study.
Details of all sampling methods are available online (Supplementary File A). In short, grab samples were collected from the top 0–10 cm using either inverted plastic bags or PVC corers (shallow lakes only) and transported to our laboratory. Samples were dried, homogenized, sieved (either −180 μm or −63 μm), and sent to an accredited laboratory for analysis by ICP-MS. A subset of the samples from the ‘Potholes’ and ‘Riparian Floodplains’ was analyzed for Cd after sieving though both sieve sizes, and the Cd concentrations for both fractions were found not to be different, see Supplementary File A). In total, we obtained concentrations for more than 50 elements from 350 wetlands (mostly 5 replicates, totaling 1733 samples). Loss-on-ignition (LOI, as a measure of organic matter content) was determined on all samples, while soil pH was determined only in the Pothole and Riparian Floodplain studies.
2.1. Statistics
All element concentrations are reported in μmol g−1 dry weight (soil or sediment) and LOI in g g−1. Metal ratios are also reported on a molar basis unless otherwise stated. Concentration data, including LOI, but not pH, were log-transformed prior to statistical analysis. Analysis of Variance (ANOVA) and Analysis of Covariance (ANCOVA) were performed using General Linear Model, followed by a Tukey’s pairwise comparison (p < 0.05) where appropriate. Pearson product-moment correlation analysis was used for the correlations. Because of the large number of observations many elements correlated significantly with each other, but significant correlations with low coefficients of correlation typically do not have much meaning. We chose to consider only significant correlations with r > 0.5, therefore explaining 25% of the total variation. Data analysis was carried out using Minitab 16.2.2 software. Development of maps and GIS analysis was done using ArcGIS Desktop: Release 10, Redlands, CA: Environmental Systems Research Institute. North Dakota surface sediments were determined from North Dakota GIS Hub Data Portal (2012).
3. Results
3.1. The entire dataset
The full set of data pertaining to this paper with coordinates of sampling sites is available online (Supplementary File B). The ranges in concentrations of Cd and of the elements with which Cd showed strong correlations across the entire dataset are listed in Table 1.
Table 1.
Averages standard deviation and range across all sampling sites of concentrations of elements (μmol g−1) that showed correlation with Cd (Pearson product-moment, r > 0.5), n = number of observations (either 1478 or 1733, because the elements detected by two accredited laboratories varied somewhat, datasets did not include the same elements for all samples).
| Element | Average ± standard deviation (μmol g−1) | Range (μmol g−1) | Correlation with Cd
|
|
|---|---|---|---|---|
| r | n | |||
| Cd | 0.0034 ± 0.0015 | 0.000089–0.013 | – | – |
| Al | 410 ± 212 | 3.7–1190 | 0.540 | 1733 |
| Be | 0.06 ± 0.024 | 0.011–0.15 | 0.504 | 1478 |
| Bi | 0.00065 ± 0.00023 | 0.00009–0.0015 | 0.641 | 1478 |
| Cu | 0.23 ± 0.09 | 0.00016–0.54 | 0.696 | 1733 |
| K | 58 ± 31 | 2.6–184 | 0.539 | 1733 |
| Pb | 0.064 ± 0.046 | 0.00048–1.27 | 0.613 | 1733 |
| Rb | 0.20 ± 0.09 | 0.0012–0.53 | 0.607 | 1733 |
| Se | 0.010 ± 0.008 | 0.0013–0.12 | 0.503 | 1478 |
| Tl | 0.00086 ± 0.00029 | 0.00015–0.0020 | 0.577 | 1478 |
| Zn | 0.98 ± 0.37 | 0.0015–3.00 | 0.702 | 1733 |
Cadmium concentrations correlated strongest with Zn concentrations (Fig. 2), followed by, in order of decreasing correlation coefficient, Cu, Bi, Pb, Rb, Tl, Al, K, Be, and Se. None of the other elements analyzed in the studies, including Ag (r = 0.408), As (r = 0.144), P (r = 0.104), and S (r = 0.437), showed strong correlations with Cd, nor did LOI (r = 0.410, average LOI = 8.2 ± 9.6%, range 0–73%). Soil pH (for Potholes and Riparian Floodplains) also did not correlate strongly with Cd (r = −0.219, average pH 7.0 ± 0.9, n = 995, range 3.2–9.8).
Fig. 2.
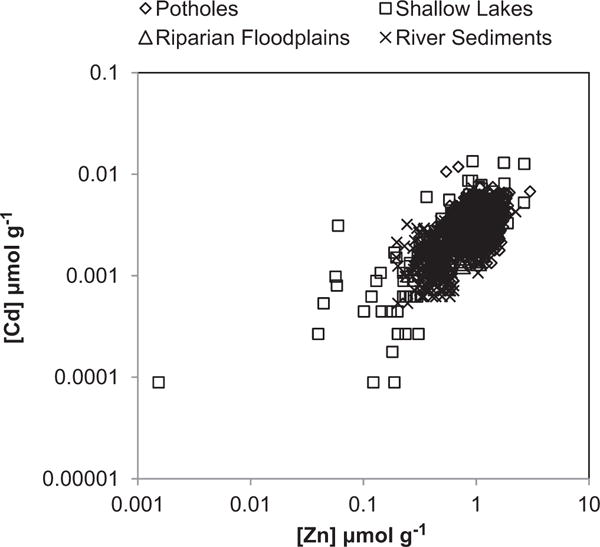
Correlation between Cd and Zn combined for Potholes, Shallow Lakes, Riparian Floodplains, and River Sediments.
The correlations between Cd and Zn further show a much wider range in concentrations for both elements for the Shallow Lakes compared to the Potholes, Riparian Floodplains, and River Sediments (Fig. 2). Very similar patterns apply to Cu and Pb, both of which correlated more strongly with Zn than Cd (Cu–Zn: r = 0.885, and Pb-Zn: r = 0.758). The average molar Cd/Zn ratio across the entire dataset was 0.0037 ± 0.0024.
Within subsets of the dataset, important patterns were observed, as follows.
3.2. Potholes
Cd concentrations in the Pothole group did not correlate significantly with LOI or soil pH.
One way to categorize pothole wetlands across the region is by one of four Level III Ecoregions (Wiken et al., 2011), see Fig. 3. These Ecoregions show ecological regions of similar biotic, abiotic, or ecosystem quality and integrity. Tested by one-way ANOVA, Cd concentrations between the Ecoregions were found to differ significantly (p < 0.000). Neither Cd nor Zn correlated with P across the Potholes, but Cd correlated strongly with Zn concentrations, and so differences in Cd concentrations between Ecoregions were tested again with Zn as a covariate. This relationship was highly significant (p < 0.000), as well as the differences between Ecoregions (p < 0.000). The Cd/Zn ratios between the Ecoregions were also highly significant (p < 0.000), with the Lake Agassiz Plain region (range 0.0026–0.0196) showing the highest average ratio and the Northwestern Great Plains the lowest (range 0.0011–0.0033) (Table 2).
Fig. 3.
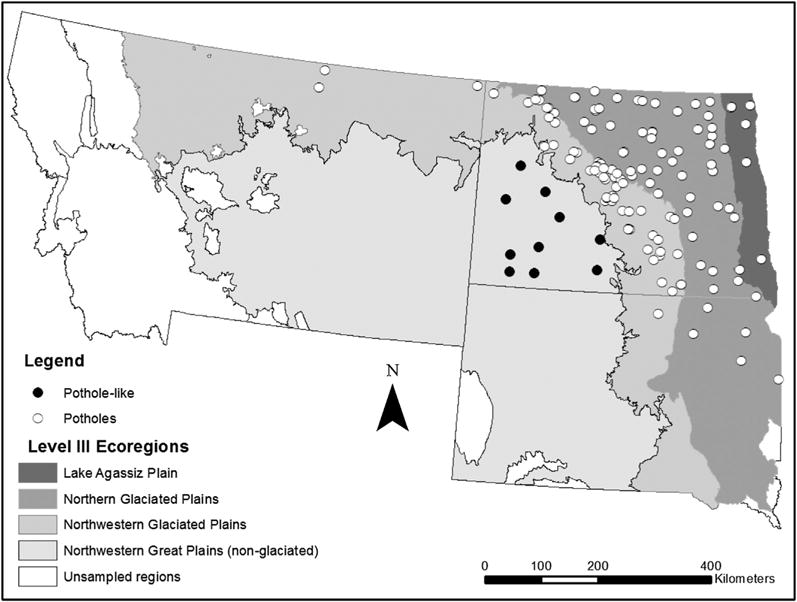
Distribution of potholes and pothole-like depressional wetlands across the study area (Montana, North Dakota, and South Dakota, USA) according to Level III Ecoregions (Wiken et al., 2011).
Table 2.
Average standard deviation of Cd and Zn concentrations and their ratios for potholes in North Dakota based on Level III Ecoregion (Wiken et al., 2011) and surface sediments type. Different letters indicate significant differences in Cd/Zn ratio by sediment after one-way ANOVA and Tukey’s pairwise comparisons of means.
| Level III Ecoregion | No. of wetlands sampled | Cd (μmol g−1) |
Zn (μmol g−1) |
Cd/Zn (mol mol−1) |
|---|---|---|---|---|
| Lake Agassiz Plain | 8 | 0.0048 ± 0.0014 | 1.24 ± 0.32 | 0.0043 ± 0.0027c |
| Northwestern Glaciated Plains | 78 | 0.0036 ± 0.0009 | 1.15 ± 0.25 | 0.0032 ± 0.0010 b |
| Northern Glaciated Plains | 85 | 0.0037 ± 0.0012 | 1.13 ± 0.30 | 0.0034 ± 0.0013 b |
| Northwestern Great Plains | 10 | 0.0030 ± 0.0012 | 1.44 ± 0.24 | 0.0021 ± 0.0006 a |
| Surface sediment type | ||||
| Clay | 2 | 0.0036 ± 0.0027 | 1.03 ± 0.41 | 0.0050 ± 0.0056 ab |
| Cross-Bedded Sand | 11 | 0.0040 ± 0.0009 | 1.15 ± 0.22 | 0.0036 ± 0.0011 b |
| Sand | 9 | 0.0035 ± 0.0016 | 1.15 ± 0.45 | 0.0031 ± 0.0008 ab |
| Silt | 10 | 0.0037 ± 0.0013 | 1.36 ± 0.25 | 0.0027 ± 0.0010 a |
| Till | 140 | 0.0037 ± 0.0011 | 1.15 ± 0.27 | 0.0033 ± 0.0012 b |
Potholes can also be categorized based on the lithology of the surface sediments (Table 2). Differences in Cd concentrations between surface sediments were also highly significant (p = 0.002), as were covariate Zn concentrations as (p = 0.000). It is unclear why samples composed of cross-bedded sand have the highest Cd concentrations. However, as might be expected, samples composed of clay exhibit the highest Cd/Zn ratios. Significant differences were also observed for Cd/Zn ratios between surface sediments (one-way ANOVA), with potholes in silt showing the lowest ratios.
3.3. Differences between shallow lakes of MN
The shallow lakes of MN were sampled in four distinct clusters from north to south: “Red Lake”, all from the Red Lake Indian Reservation; “Itasca”, from around the headwaters of the Mississippi River; “Grant”, from the west central County of Grant; and “Windom”, from southern MN near the town of Windom. Parent materials include glacial till or outwash (Soil Survey Staff, 2012). When tested separately by one-way ANOVA, concentrations of Cd, Zn, and P varied significantly between regions, as did LOI (all p < 0.000), see Table 3.
Table 3.
Average standard deviation of Cd, Zn, and P concentrations, and LOI of sediments from shallow lakes in MN by region. Different letters within each column indicate significant differences determined by Tukey’s pairwise comparison of means. n = number of replications.
| Region | Cd (μmol g−1) | Zn (μmol g−1) | P (μmol g−1) | LOI (g g−1) | n |
|---|---|---|---|---|---|
| Red Lake | 0.0033 ± 0.0022 b | 0.76 ± 0.41 b | 18.6 ± 6.3 b | 0.041 ± 0.015 c | 70 |
| Itasca | 0.0022 ± 0.0017 a | 0.51 ± 0.50 a | 14.1 ± 6.3 a | 0.016 ± 0.011 b | 36 |
| Grant | 0.0035 ± 0.0011 b | 0.87 ± 0.31 bc | 24.5 ± 5.7 c | 0.013 ± 0.008 ab | 34 |
| Windom | 0.0044 ± 0.0018 b | 1.02 ± 0.32 c | 24.0 ± 5.6 c | 0.009 ± 0.004 a | 114 |
Cd concentrations within this dataset did not correlate with LOI, but correlated strongly with Zn (r = 0.828, n = 251) and with P (r = 0.659). Zn concentrations also strongly correlated with P (r = 0.729) (Fig. 4). Subsequent ANCOVA testing Cd differences between regions with Zn and P concentrations as covariates showed covariation with both Zn and P to be highly significant (p < 0.000), but differences between regions were not (p = 0.335). The Cd/Zn ratios were not significantly different between regions (average over all lakes 0.0048 mol mol−1).
Fig. 4.
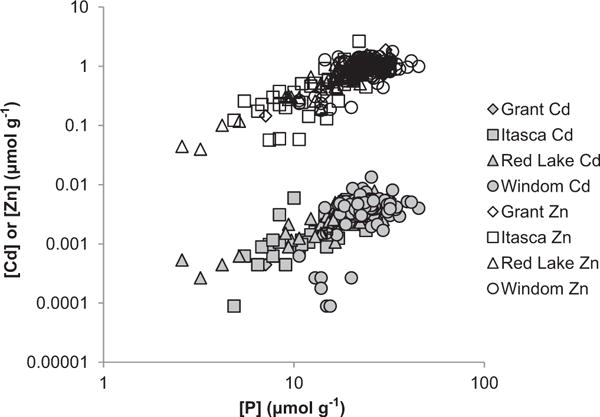
Relationships between concentrations of Cd, Zn, and P for each of the four Shallow Lakes regions.
3.4. Differences between rivers – Riparian Floodplains
Concentrations of Cd (μmol g−1, n = 20) in sediments of the riparian floodplains of the rivers in our study, the Red (average 0.0035 ± 0.0006, range 0.0026–0.0049), Sheyenne (0.0030 ± 0.0008, 0.0016–0.0045), James (0.0025 ± 0.0004, 0.0019–0.0034), Missouri (0.0018 ± 0.0003, 0.0012–0.0024), and the Little Missouri (0.0023 ± 0.0005, 0.0014–0.0035), varied significantly between rivers (one-way ANOVA, p < 0.000), as did Zn (p < 0.000) and P concentrations (p < 0.000). No differences were found in LOI (average 2.9 ± 2.2, n=100) or pH (average 7.5 ± 0.3, n = 100) between the rivers. When tested by ANCOVA, Cd concentrations strongly co-varied with Zn concentrations (p < 0.000), significantly, but less strongly, with P (p = 0.024), and also varied significantly between rivers (p < 0.000). The highest concentrations for both metals were recorded along the Red River (Cd 0.005, Zn 1.6 μmol g−1, different samples) in eastern ND (Fig. 5). The Cd/Zn ratio (mol mol−1) of the Red River soils (0.0030 ± 0.0002) was significantly higher (p < 0.000) than the other rivers, while the Missouri River had a significantly lower ratio (0.0020 ± 0.0003). The Cd/Zn ratios of the Sheyenne (0.0025 ± 0.007), James (0.0024 ± 0.0006), and Little Missouri (0.0023 ± 0.0002) rivers were intermediate values and did not differ from each other.
Fig. 5.
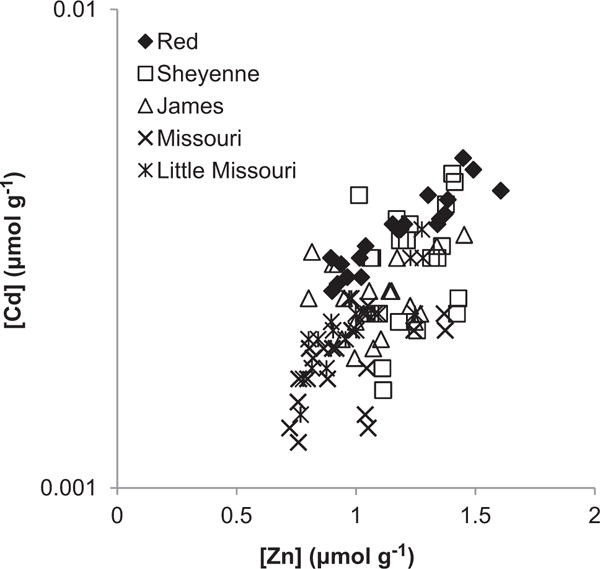
Correlations between Cd and Zn concentrations (μmol g−1) in riparian floodplain soils of the Red, Sheyenne, James, Missouri, and Little Missouri rivers in North Dakota.
3.5. Differences between rivers – the Souris and Turtle River sediments
The Souris and Turtle Rivers were sampled as part of a study on the use of multi-element fingerprinting in rivers in ND (Wijeyaratne, 2011). The Souris River watershed is much larger (63 714 km2) than the Turtle River (1645 km2), but both contribute to the Red River. Because the Red River flows from the US into Canada, water quality of the two tributaries is of concern due to cross-border issues with contaminant transport. Cd concentrations across both rivers correlated strongly with Zn (r = 0.707, n = 475), Pb (r = 0.846), and Cu (r = 0.898). There was a clear difference between the sediments of the Turtle and Souris rivers (Fig. 6) in relation to Cd (Turtle River average 0.0038 ± 0.0015, range 0.0010–0.0072, n = 192, Souris average 0.0021 ± 0.0011, range 0.0005–0.0054, n = 283). ANCOVA showed Cd significantly (p < 0.000) co-varied with Zn, and Cd/Zn ratios were significantly higher (p = 0.000) for the Turtle River (0.0057 ± 0.0012 mol mol−1) compared to the Souris River (0.0030 ± 0.0009 mol mol−1). Cd concentrations also correlated with P in both the Souris (r = 0.532, n = 283, average 22 ± 6 μmol g−1) and Turtle River (r = 0.654, n= 196, average 2.7 ± 0.3 μmol g−1). In contrast, the Pb/Zn ratios (Souris 0.055 ± 0.015, Turtle 0.054 ± 0.013) and Cu/Zn (Souris 0.24 ± 0.04, Turtle 0.25 ± 0.10 mol mol−1) did not vary between rivers.
Fig. 6.
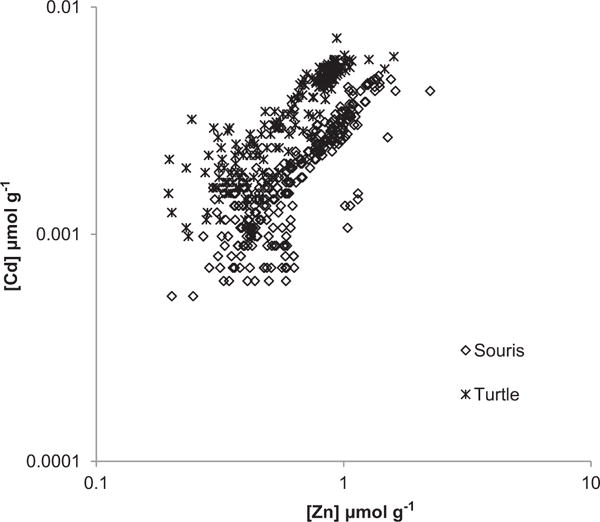
Correlations between Cd and Zn (μmol g−1) in sediments of the Souris and Turtle rivers, North Dakota.
4. Discussion
The five samples with the highest Cd concentrations are listed in Table 4. These five wetlands had concentrations averaging well above the mean of 0.0034 μmol g−1 (0.38 mg kg−1) for the entire dataset. The average values are within the range of Cd concentrations of 0.003–0.008 μmol g−1 (0.17 to 0.87 mg kg−1) reported by Martin and Hartman (1984) for wetlands in the Northern Plains, but different methods were used in their study and ours, thus making direct comparisons difficult. The high concentrations of well over 2 mg kg−1 (0.018 μmol g−1) reported by Garrett (1994), Hopkins et al. (1999), and Jyoti (2010) for drylands were not observed in our study. This is most likely due to the fact that the studies listed above reported concentrations in soils originating from Cd-rich shales, whereas the wetlands in our study were situated over a wide variety of soil types, and it also hints at a possible buffering effect for Cd concentrations in wetlands. For North Dakota dryland soils, Holmgren et al. (1993) reports soil Cd concentrations of 0.316 mg kg−1, and for the Northern Great Plains as 0.369 mg kg−1 (both geometric means), whereas our equivalent geometric mean concentrations were similar at 0.414 mg kg−1 for North Dakota potholes and 0.394 mg kg−1 for the entire data set. Other reports on background Cd soil concentrations include up to average 0.4 mg kg−1 worldwide (United Nations, 2010) and in the US (US EPA, 2005); and median 0.3 mg kg−1 in the Canadian Great Plains and adjoining US (Garrett, 1994). In Europe (Forum of European Geological Surveys website) stream sediments were median 0.29 mg kg−1 and floodplain sediments were median 0.3 mg kg−1 Cd. The average cadmium concentrations we observed in the potholes (0.0037 μmol g−1, 0.41 mg kg−1), the shallow lakes (0.0037 μmol g−1, 0.41 mg kg−1), the riparian floodplains (0.0026 μmol g−1, 0.30 mg kg−1), and the river sediments (0.0028 μmol g−1, 0.31 mg kg−1) were comparable to the concentrations reported above.
Table 4.
The five samples with highest cadmium concentration out of the entire dataset (1733 samples). Three were from the Shallow Lakes group and two were from the Potholes group, n = number of replicates.
| Wetland description | Cd concentration in sample, μmol g−1 | Average for the same wetland, μmol g−1 |
|---|---|---|
| Shallow lake in Windom region, MN | 0.013 (1.46 mg kg−1) | 0.006 (0.67 mg kg−1), n = 5 |
| Shallow lake in Red Lake region, MN | 0.013 (1.46 mg kg−1) | 0.006 (0.67 mg kg−1), n = 5 |
| Shallow lake in Red Lake region, MN | 0.013 (1.46 mg kg−1) | 0.005 (0.56 mg kg−1), n = 8 |
| Pothole in central ND | 0.012 (1.35 mg kg−1) | 0.005 (0.56 mg kg−1), n = 5 |
| Pothole in northeast ND below the Pembina escarpment | 0.011(1.24 mg kg−1) | 0.005 (0.56 mg kg−1), n = 5 |
Across the entire dataset, the average Cd/Zn ratio was 0.0037 (mol mol−1), but higher values were observed for potholes in clay sediments (0.0050), the Red Lake (0.0047) and Itasca (0.0064) shallow lakes, and the Turtle River (0.0057). The higher Cd concentrations and enrichment relative to Zn may be explained by both natural processes and anthropogenic activities. One of the five wetlands with the highest Cd concentrations, the pothole site in the Pembina area, had a very high Cd/Zn ratio (0.0196 mol mol−1 in one sample, 0.0086 average for the wetland). This wetland is in the Lake Agassiz Plain Ecoregion (associated with clay soils), and is very close to the Pembina Escarpment, where high Cd concentrations are associated with outcrops of the Pierre Formation (Hopkins et al., 1999; Jyoti, 2010). The same shale also hosts the Pierre aquifer, which partly feeds the Turtle River (Kelly and Paulson, 1970), and this may explain the relatively higher Cd/Zn ratios in the sediments of the Turtle River compared to those in the Souris and other rivers. The US EPA classifies the Turtle River as “impaired” due to Cd concentrations in the water, likely originating from the shales in the Pembina area, which eventually flows into the Red River (US EPA, 2008). Holmgren et al. (1993) report Cd/Zn ratios (geometric mean) for North Dakota dryland soils in the range of 0.0050–0.0067 and Minnesota down to 0.0040 g g−1. In our study, the proportion of Cd to Zn was comparatively lower in the Riparian floodplains, but higher in the Turtle River sediments and for the Minnesota shallow lakes (Table 5).
Table 5.
Proportions of Cd and Zn in sediments of wetland groups converted from molar concentrations (stochiometrically relevant) to weight-based units (grams).
| Wetland group | Arithmetic average Cd/Zn ratio
|
Geometric average Cd/Zn ratio
|
|||
|---|---|---|---|---|---|
| mol mol−1 | Range | g g−1 | Range | g g−1 | |
| Potholes | 0.0033 | 0.0010–0.0196 | 0.0057 | 0.0017–0.0336 | 0.0053 |
| Shallow lakes | 0.0048 | 0.0005–0.0582 | 0.0083 | 0.0008–0.0999 | 0.0071 |
| Riparian floodplains | 0.0024 | 0.0013–0.0040 | 0.0041 | 0.0022–0.0069 | 0.0040 |
| River sediments (all) | 0.0041 | 0.0010–0.0132 | 0.0070 | 0.0018–0.0226 | 0.0064 |
| Souris River | 0.0030 | 0.0010–0.0062 | 0.0051 | 0.0018–0.0106 | 0.0048 |
| Turtle River | 0.0057 | 0.0023–0.0132 | 0.0098 | 0.0040–0.0226 | 0.0095 |
This link with the underlying geology and hydrology is also emphasized by the correlations between Cd and other metals across the entire dataset. It is well known that Cd is a member of the chalcophile group which also includes Ag, Cu, Ga, Hg, In, Tl, joined by As, Bi, Pb, S, Sb, Se, and Te (Faure, 1998). In many cases these elements are strongly correlated because of their very similar biogeochemistry (Salomons and Förstner, 1984; Dudka and Adriano, 1997). The association with Se is likely due to its chemistry being similar to S, even though the correlation between Cd and S in this study was not strong. Schultz et al. (1980) reported that Cd in Pierre Shale, the bedrock source material in much of the region, is associated with both sulfide minerals and with organic matter. It is likely that some Se substituted for S in these materials. Under oxidizing conditions, sulfide is transformed to sulfate and can be transported by aqueous solutions. This type of process can decouple metals from S or Se. Any selenates formed by such oxidation are more easily (as compared to sulfate reduction) redeposited as selenide when in a reducing environment, such as a wetland soil (Misra, 2000). Al, Be, Bi, K, and Rb are associated with clay minerals and are typically strongly correlated. The Red River showed a high Cd concentration and Cd/Zn ratio, reflecting the relationship between the metal and the high proportion of clay particles in this river (Stoner et al., 1998). Thallium(I) has biogeochemical properties similar to K+ and is strongly correlated with Al, Be, Bi, K, and Rb. The correlation between Cd and these elements is likely related to the origin of Cd in these soils and sediments, but Cd may also have become associated with minerals containing these elements at a later stage, perhaps even as a result of anthropogenic activity (Dolor et al., 2012).
Another important source of Cd is the use of mineral phosphate fertilizer, which typically contains high Zn and Cd concentrations in addition to other elements (Mortvedt, 1996; Lambert et al., 2007). Phosphate fertilizer application can directly increase both the P and Cd concentrations in the soil solution (Lambert et al., 2007) and agricultural land (Mortvedt, 1996). Across the region of this study, the predominant land use grades from cattle ranching in the west to crop production in the east. The shallow lakes in the Grant and Windom areas are surrounded by cropland more than any other region in this study, while the lakes in the Red lake and Itasca regions have more organic-rich soils (bogs and forest). The strong correlations between Cd, Zn, and P observed for the shallow lakes in our study, in addition to the high Cd/Zn ratio for this group, particularly when compared with the lower ratios predicted by Holmgren et al. (1993), support the hypothesis of Cd association with land use.
In contrast to wetlands, much research has been done on the cadmium in dryland soils (e.g. Holmgren et al., 1993; Garrett, 1994; Smith et al., 2005, 2009; Klassen, 2009; Woodruff et al., 2009; Chaney, 2010). Wetland soils show different biogeochemical behavior compared with dryland soils, and the lack of data for Cd concentrations in wet ecosystems is a major gap in our scientific knowledge. Under stable waterlogged, reducing conditions Cd is immobilized, but can become mobile when these conditions are disrupted and soils become oxidized and pH decreases (Gambrell, 1994). Cd in agricultural soils is soluble and mobile (Narwal et al., 1999), and leaching from oxidized, previously reduced sediments can occur under natural conditions (Gambrell, 1994). It is not known how periodic drying of pothole wetlands affects metal concentrations, but it is possible that fluctuating oxidation and reduction processes induce alternating high and low Cd mobility in the environment. Similarly, the fluctuating water levels in the riparian floodplains may affect the Cd mobility in the sediments. The repercussions of these effects on ecosystems when human activity is the direct cause of wetland oxidation, e.g. through widespread land drainage programs and wetlands being cultivated during dry years, as is common in the region in our study, is not known.
Many of the wetlands in this study foster plants and animals that contribute to food webs and are eaten by humans. Cd uptake in organisms is strongly affected by the Zn concentrations in the substrate (e.g. Brzóska and Moniuszko-Jakoniuk, 2001; Wang et al., 2011), because both metals compete for the same uptake mechanisms. As a result, higher uptake and translocation in plants are observed when the Cd/Zn ratio is relatively high (Chaney, 2010). The impact and hazard to wild animals from Cd exposure is disputed (Beyer, 2000), but it has been long recognized that plants can be adversely affected and take up Cd into their edible tissues (Chaney, 2010) and most documented cases of plant accumulation concern dryland crops. Regarding wetland plants, Asian rice (Oryza spp.), which had been grown in Cd-contaminated water, caused human disease (Chaney, 2010). Of course Oryza does not grow in the Northern Plains, but another edible wetland plant, wild rice (Zizania palustris) is common in Minnesota shallow lakes, is sold widely, and plays an important cultural role for Native Americans. Cd uptake and translocation into the edible seeds has been documented (e.g. Pip, 1993). Because wild rice grows in wetlands and thus in landscape positions that receive drainage and runoff from larger catchment areas, it is vulnerable to both natural and anthropogenic sources of cadmium in the environment.
Overall, our data show important trends in relation to Cd concentrations in wetland soils and sediments:
On average, the wetlands in this study, prairie potholes, shallow lakes, riparian floodplains and river sediments, contain similar Cd concentrations (average 0.0034 ± 0.0015 μmol g−1, 0.38 mg kg−1) compared to dryland soils/sediments of the region (average 0.0025 μmol g−1; 0.28 mg kg−1, Garrett, 1994).
Within the dataset, the shallow lakes of Minnesota displayed a much larger range in Cd concentrations than the other groups, including the potholes, which span a much larger land area (about 800 km from SD to MT sites), compared to the shallow lakes (about 400 km Windom to Red Lake).
Within the dataset, Cd concentrations in the potholes (MT, SD and ND) increased from the non-glaciated southwestern ecoregion to the shale-derived, clay-rich, glaciated eastern ecoregions.
The higher Cd concentrations in our study appear to be associated with dryland areas of naturally high concentrations or with areas of intense agriculture, indicating that both underlying geology and land use play an important role.
Cd concentrations in the soils/sediments in all wetland types strongly co-vary with Zn concentrations, as well as with P in the case of riparian sediments and shallow lakes.
Supplementary Material
Acknowledgments
This project was part-supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM12345 (ND INBRE). The US Environmental Protection Agency (EPA/ND Department of Health Wetland Program Development Grant, National Center for Research Resources (5P20RR016471-1), the ND Agricultural Experiment Station/NDSU College of Science and Mathematics Small Grant Program, and the Upper Midwest Aerospace Consortium/NASA (prime award no. NNX10AH20G, subaward no. 1177-16116) provided funding for Prairie Pothole and Riparian Floodplains studies; the MN Department of Natural Resources (The Legislative-Citizen Commission on Minnesota Resources), the Red Lake Nation Department of Natural Resources (EPA Wetland Program Development Grant), ND ESPCoR/NSF (grant #EPS-0814442), The Wetland Foundation, and Sigma Xi Grants-in-Aid of Research provided funding the Shallow Lake study; and the ND Water Resources Research Institute and the ND Department of Health jointly funded the Souris and Turtle river studies.
Thanks to Dr. E. Shawn DeKeyser, Mike Ell, Dr. Mark Hanson, Dr. Christina Hargiss, Dr. Michael Hill, Dr. David Hopkins, Dr. Wei Lin, Dr. Donald Schwert, Dr. Changhui Yan, Winston Allen, Stefan Bischof, Shane Bowe, John Charles, Brian Herwig, Joshua Norenberg, Brandon Palesh, Shaminda Samaraweera, Vince Smith, and Josh Suckow. We are particularly indebted to the following undergraduates, several supported by ND INBRE: Joshua Borchardt, Kevin Christensen, Patrick Culhane, Fawad Dawlaty, Ankit Dinghra, Emily Fischbach, Eden Friedrich, Alex Hoehle, Xiao Liang, Brett Lyslo, Bryan Marquardt, Jeremy McLeod, Aude Monthean, Hannah Passolt, Nicholas Peterson, Madhulika Potukuchui, Brandi Roshau, John Schmidt, Travis Strehlow, Ryan Sullivan, Christina Swensen, Justin Tabaka, Tasnia Tarannum, Chen Tian, and Yiqing Xu.
Appendix A. Supplementary material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.envpol.2013.03.005.
References
- Beyer WN. Hazards to wildlife from soil-borne cadmium reconsidered. Journal of Environmental Quality. 2000;29:1380–1384. [Google Scholar]
- Burger J. Assessment and management of risk to wildlife from cadmium. Science of the Total Environment. 2008;389:37–45. doi: 10.1016/j.scitotenv.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Brzóska MM, Moniuszko-Jakoniuk J. Interactions between cadmium and zinc in the organism. Food Chemistry and Toxicology. 2001:39967–39980. doi: 10.1016/s0278-6915(01)00048-5. [DOI] [PubMed] [Google Scholar]
- Chaney RL. Cadmium and zinc. In: Hooda PS, editor. Trace Elements in Soils. John Wiley and Sons Ltd; West Sussex, UK: 2010. pp. 409–439. [Google Scholar]
- Comis D. Getting cadmium out of sunflower seeds. Agricultural Research Magazine. 1995;43:21. [Google Scholar]
- Communication Department of the European Commission. Chemicals/REACH: EU to ban cadmium in jewelry, brazing sticks and all plastics. Europa Press Release Rapid. 2011 December 6, 2011. http://europa.eu/rapid/press-release_IP-11-620_en.htm#PR_metaPressRelease_bottom.
- Dalrymple J, Dwelle T. North Dakota 2012 Integrated Section 305(b) Water Quality Assessment Report and Section 303(d) List of Waters Needing Total Maximum Daily Loads. North Dakota Department of Health, Division of Water Quality; Bismarck, North Dakota: 2012. p. 241. [Google Scholar]
- Department of Health and Human Services. Public Health Statement: Cadmium. Agency for Toxic Substances and Disease Registry, CDC; 2008. p. 10. [Google Scholar]
- Dolor MK, Helz GR, McDonough WF. Cause of the chalcophile trace element enrichments marking the Holocene to Anthropocene transition in northern Chesapeake Bay sediments. Geochimica et Cosmochimica Acta. 2012;82:79–91. [Google Scholar]
- Dudka S, Adriano DC. Environmental impacts of metal ore mining and processing: a review. Journal of Environmental Quality. 1997;26:590–602. [Google Scholar]
- European Food Safety Authority. Cadmium in food, scientific opinion of the panel on contaminants in the food chain. The EFSA Journal. 2009;980:1–139. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure G. Principles and Applications of Geochemistry. second. Prentice Hall; 1998. p. 600. [Google Scholar]
- Forum of European geological surveys. Salminen R, editor. Geochemical Atlas of Europe. http://weppi.gtk.fi/publ/foregsatlas/ (accessed 07.12.12)
- Franzen DW, Nanna T, Norvell WA. A survey of soil attributes in North Dakota by landscape position. Agronomy Journal. 2006;98:1015–1022. [Google Scholar]
- Gambrell RP. Trace and toxic metals in wetlands – a review. Journal of Environmental Quality. 1994;23:883–891. doi: 10.2134/jeq1994.00472425002300050005x. [DOI] [PubMed] [Google Scholar]
- Garrett RG. The distribution of cadmium in a horizon soils of Canada and adjoining United States. Current Research B: Geological Survey of Canada. 1994:73–82. [Google Scholar]
- Goyer R, Golub M, Choudhury H, Hughes M, Kenyon E, Stifelman M. Issue Paper on the Human Health Effects of Metals. Risk Assessment Forum; Washington DC. ERG, Lexington, MA: 2004. p. 44. (Submitted to the U.S. Environmental Protection Agency). http://www.epa.gov/raf/publications/pdfs/HUMANHEALTHEFFECTS81904.PDF (accessed 07.12.12) [Google Scholar]
- Holmgren GGS, Meyer MW, Chaney RL, Daniels RB. Cadmium, lead, zinc, copper, and nickel in agricultural soils of the United States of America. Journal of Environmental Quality. 1993;22:335–348. [Google Scholar]
- Hopkins DG, Norvell WA, Wu J. Formation and distribution of traceelement-enriched soils near the Pembina Escarpment, Cavalier County, North Dakota. Grand Forks, ND: 1999. (Proc. 91st ND Academy of Science). [Google Scholar]
- Jacob DL, Otte ML. Conflicting processes in the wetland plant rhizosphere: metal retention or mobilization? Water, Air and Soil Pollution, Focus. 2003;3:91–104. [Google Scholar]
- Jyoti V. North Dakota State University MS Thesis. NDSU; 2010. Trace element distribution in soils of the Pembina Escarpment; p. 174. [Google Scholar]
- Kantrud HA, Krapu GL, Swanson GL. Prairie basin wetlands of the Dakotas: a community profile. US Fish and Wildlife Service Biological Report. 1989;85(7.28):111. [Google Scholar]
- Kelly TE, Paulson QF. North Dakota Geological Survey/County Groundwater Studies 13, 58. North Dakota State Water Commission; 1970. Geology and groundwater resources of Grand Forks County. Part III, groundwater resources. Bulletin 53. mapservice.swc.state.nd.us/4dlink9/4dcgi/…/GrandForks_Part_3.pdf. [Google Scholar]
- Klassen RA. Geological controls on soil parent material geochemistry along a northern Manitoba-North Dakota transect. Applied Geochemistry. 2009;24:1382–1393. [Google Scholar]
- Lambert R, Grant C, Sauvé S. Cadmium and zinc in soil solution extracts following the application of phosphate fertilizers. Science of the Total Environment. 2007;378:293–305. doi: 10.1016/j.scitotenv.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Li YM, Chaney RL, Schneiter AA, Miller JF, Elias EM, Hammond JJ. Screening for low grain cadmium phenotypes in sunflower, durum wheat and flax. Euphytica. 1997;94:23–30. [Google Scholar]
- Lively RS, Thorleifson LH. Minnesota Soil, Till, and Ground-water Geochemical Data. Minnesota Geological Survey Open File Report OFR-09-02. 2009:19. 1 poster, 69 atlas pages, 8 digital tables, 1 geodatabase. [Google Scholar]
- Martin DB, Hartman WA. Arsenic, cadmium, lead, mercury, and selenium in sediments of riverine and pothole wetlands of the north central United States. Journal of the Association of Analytical Chemists. 1984;67:1141–1146. [Google Scholar]
- Misra K. Understanding Mineral Deposits. Kluwer Academic Publishers; Boston, USA: 2000. p. 864. [Google Scholar]
- Mortvedt JJ. Heavy metal contaminants in inorganic and organic fertilizers. Fertilizer Research. 1996;43:55–61. [Google Scholar]
- Narwal RP, Singh BR, Salbu B. Association of cadmium, zinc, copper, and nickel with components in naturally heavy metal-rich soils studied by parallel and sequential extractions. Communications in Soil Science and Plant Analysis. 1999;30:1209–1230. [Google Scholar]
- North Dakota GIS Hub Data Portal. Boundaries and geology. 2012 http://web.apps.state.nd.us/hubdataportal/srv/en/main.home (accessed 21.11.12)
- Pillatzki AE, Neiger RD, Chipps SR, Higgins KF, Thiex N, Afton AD. Hepatic element concentrations of Lesser Scaup (Aythyaaffinis) during spring migration in the Upper Midwest. Archives of Environmental Contamination and Toxicology. 2011;61:144–150. doi: 10.1007/s00244-010-9587-1. [DOI] [PubMed] [Google Scholar]
- Pip E. Cadmium, copper and lead in wild rice from central Canada. Archives of Environmental Contamination and Toxicology. 1993;24:179–181. doi: 10.1007/BF01141345. [DOI] [PubMed] [Google Scholar]
- Rojas-Cifuentes GA, Johnson BL, Berti MT, Norvell WA. Zinc fertilization effects on seed cadmium accumulation in oilseed and grain crops grown on North Dakota soils. Chilean Journal of Agricultural Research. 2012;72:117–124. [Google Scholar]
- Salomons W, Förstner U. Metals in the Hydrocycle. Springer-Verlag; Berlin: 1984. p. 349. [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure and health outcomes. Environmental Health Perspectives. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LG, Tourtelot HA, Gill JR, Boerngen JG. Composition and properties of the Pierre Shale and equivalent rocks, Northern Great Plains Region US Geological Survey Professional Paper 1064-B. United States Government Printing Office; Washington D.C: 1980. p. 114. [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, Garrett RG, Klassen R, Kilburn JE, Horton JD, King HD, Goldhaber MB, Morrison JM. Major- and trace element concentrations in soils from two continental-scale transects of the United States and Canada. US Geological Survey. Open-File Rep #1253. 2005 < http://pubs.usgs.gov/of/2005/1253/>.
- Smith DB, Woodruff LG, O’Leary RM, Cannon WF, Garrett RG, Kilburn JE, Goldhaber MB. Pilot studies for the North American Soil Geochemical Landscapes Project – site selection, sampling protocols, analytical methods, and quality control protocols. Applied Geochemistry. 2009;24:1357–1368. [Google Scholar]
- Soil Survey Staff. Official Soil Series Descriptions. Natural Resources Conservation Service, United States Department of Agriculture. 2012 http://soils.usda.gov/technical/classification/osd/index.html (accessed 07.12.12)
- Spurgeon DJ, Rowland P, Ainsworth G, Rothery P, Lond S, Black HIJ. Geographical and pedological drivers of distribution and risks to soil fauna of seven metals (Cd, Cu, Cr, Ni, Pb, V and Zn) in British soils. Environmental Pollution. 2008;153:273–283. doi: 10.1016/j.envpol.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Stoner JD, Lorenz DL, Goldstein RM, Brigham ME, Cowdery TK. Water Quality in the Red River of the North Basin, Minnesota, North Dakota, and South Dakota, 1992–95. US Geological Survey Circular 1169 1998 [Google Scholar]
- United Nations. United Nations Environment Programme Chemicals Branch DTIE, Final review of scientific information on cadmium, Version of December 2010. 2010. [Google Scholar]
- US EPA. Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs), Review of Background Concentrations for Metals. Office of Solid Waste and Emergency Agency 2003 Nov; [Google Scholar]
- US EPA. Ecological Soil Screening Levels for Cadmium. Office of Solid Waste and Emergency Agency. Interim Final 2005 Mar; [Google Scholar]
- US EPA. North Dakota impaired waters, cause of impairment: cadmium, reporting year 2008. Watershed assessment, tracking and environmental results. 2008 http://ofmpub.epa.gov/tmdl_waters10/attains_impaired_waters.control?p_cause_name=CADMIUM&p_state=ND&p_cycle=2008&p_report_type (last updated 06.12.12)
- US Geological Survey. Geochemistry of soils in the United States from the PLUTO database. US Geological Survey; Reston, VA: 2001. http://mrdata.usgs.gov/pluto/soil/ (accessed 07.12.12) [Google Scholar]
- US Geological Survey. Geochemical Survey. http://tin.er.usgs.gov/geochem/doc/home.htm (accessed 07.12.12)
- Waalkes MP. Cadmium carcinogenesis in review. Journal of Inorganic Biochemistry. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Wiken E, Jiménez Nava F, Griffith G. North American Terrestrial Ecoregionsd—Level III. Commission for Environmental Cooperation; Montreal, Canada: 2011. p. 149. [Google Scholar]
- Wijeyaratne DN. Thesis (Ph D) North Dakota State University; 2011. Multi-element fingerprinting of river sediments to identify diffuse pollution sources; p. 202. [Google Scholar]
- Wang G, Ottman MJ, Chaney RL, Sanchez CA, Spiller M. 2011 AGRPC Final Report. Arizona Grain Research and Promotion Council, Arizona Department of Agriculture; 2011. [Google Scholar]
- Woodruff LG, Cannon WF, Eberl DD, Smith DB, Kilburn JE, Horton JD, Garrett RG, Klassen R. Continental-scale patterns in soil geochemistry and mineralogy: results from two transects across the United States and Canada. Applied Geochemistry. 2009;24:1369–1381. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


