Abstract
Overcoming sensor induced tissue reactions is an essential element of achieving successful continuous glucose monitoring (CGM) in the management of diabetes, particularly when used in closed loop technology. Recently, we demonstrated that basement membrane (BM) based glucose sensor coatings significantly reduced tissue reactions at sites of device implantation. However, the biocompatible BM based bio-hydrogel sensor coating rapidly degraded over a less than a three-week period, which effectively eliminated the protective sensor coating. In an effort to increase the stability and effectiveness of the BM coating, we evaluated the impact of cross-linking BM utilizing glutaraldehyde as a cross-linking agent, designated as X-Cultrex. Sensor performance (non-recalibrated) was evaluated for the impact of these X-Cultrex coatings in vitro and in vivo. Sensor performance was assessed over a 28-day time period in a murine CGM model and expressed as mean absolute relative difference (MARD) values. Tissue reactivity of Cultrex coated, X-Cultrex coated, and uncoated glucose sensors was evaluated over a 28-day time period in vivo using standard histological techniques. These studies demonstrated that X-Cultrex based sensor coatings had no effect on glucose sensor function in vitro. In vivo, glucose sensor performance was significantly enhanced following X-Cultrex coating throughout the 28-day study. Histological evaluations of X-Cultrex treated sensors demonstrated significantly less tissue reactivity when compared to uncoated sensors.
Keywords: extracellular matrices, basement membrane, Cultrex, glutaraldehyde, biocompatibility, glucose sensor coatings, Trevigen, implantable glucose sensor, continuous glucose monitoring
INTRODUCTION
Implantable glucose sensors frequently demonstrate insufficient reliability with respect to performance (accuracy and response time) and often require re-calibration of the sensor device to compensate for sensor output drift when considering long-term functionality in vivo. Generally, the lack of sensor biocompatibility is thought to be the reason for this poor sensor function and short lifespan of sensors in vivo.
Previous efforts to overcome implant induced biofouling have generally focused on the uses of synthetic polymer coatings with limited success (1–5). However synthetic sensor coatings often manifest poor biocompatibility, thus making them less resistant to foreign body tissue reactions (inflammation, fibrosis and loss of vasculature). Therefore, materials with greater degrees of biocompatibility are required. Our approach has been one of employing protein-based tissue engineering. We hypothesize that extracellular matrices (ECM) are particularly well suited as sensor coatings given that they are biologic hydrogels that permit rapid diffusion of glucose to the sensor. Thus, autologous BM preparations are highly biocompatible resulting in fewer tissue reactions such as inflammation or fibrosis. For this reason, we hypothesized that these ECM’s would be highly effective coatings on glucose sensors. The importance of the usage of autologous ECM, i.e. BM, is underscored by previous ECM-based sensor coating studies that employed xenogenic collagen. However, these previous studies were limited in both number and scope and demonstrated marginal effects on sensor function in vivo (6). It is likely that tissue reactions were induced by the cross-linked collagens (6). To our knowledge, basement membrane (BM) preparations (e.g. Cultrex or Matrigel) have not been used as glucose sensor coatings or for any other implantable devices. Previously, we investigated two different sources of ECM (Cultrex and Matrigel) and we found that Cultrex induced far fewer tissue reactions compared to Matrigel, such that Cultrex was used for all studies.
Basement Membranes and sensor function
Cultrex Basement Membrane Extract components comprise the soluble ECM proteins of ECM including laminin (60%), type IV collagen (30%), as well as entactin, heparin sulfate, metalloproteinases, and a variety of growth factors such as PDGF, EGF, and TGFB, which are purified from the murine Engelbreth-Holm-Swarm tumor. In vivo, these ECM proteins create continuous sheets of specialized matrices. In addition to supporting cells and providing a supporting interface between cell layers and their adjacent stroma, they serve a critical role in wound healing and tissue regeneration. We believe that the use of ECM coatings (e.g. Cultrex BM), around glucose sensor implantation sites may attenuate the unwanted induction of tissue reactions, such as inflammation, which results in biofouling and reduced glucose sensor function. Our previous studies demonstrated that Cultrex-based BM coatings of glucose sensors accomplished the dual goals of decreased tissue reactivity of the glucose sensors in vivo as well as extending the life span and function of glucose sensors in our murine model of continuous glucose monitoring (CGM).
Specifically our data demonstrated better performance of the BM (designated as Cultrex) coated sensors versus non-coated sensors in the first three weeks post sensor implantation. Specifically, these studies demonstrated that Cultrex based BM-coatings of glucose sensors accomplished the dual goals of 1) decreasing tissue reactivity of glucose sensors in vivo (i.e. inflammation and fibrosis) as well as 2) improving the performance of glucose sensors in a murine model of CGM until the third week after sensor implantation. However, there was a subsequent decline in sensor performance beyond week 3, which was associated with the natural degradation processes related to the extracellular turnover including the impact of inflammation on the BM degradation. Periodic histological evaluation of the BM coated sensors revealed that the BM coating was inconsistently aligned or not completely covering the sensor’s surface. We speculated that the BM coating might misalign during implantation, in part, due to lack of sensor-coating stability. We hypothesize that misalignment of the coating and degradation of the BM coating surrounding the sensor exposes the original sensor surface, which triggers the commonly observed implant-induced foreign body tissue reaction. Thus, better coating alignment and extending the lifespan of the BM based bio-hydrogel coatings would delay tissue reaction at the sensor implantation site and thereby extend sensor performance. To overcome these obstacles, we hypothesized that cross-linking the Cultrex BM would increase sensor-coating stability. This would mitigate degradation and misalignment and thus increase sensor function and lifespan in vivo.
Cross-linked Basement Membranes and sensor function
Previous studies using cross-linked collagen coatings demonstrated marginal effects on sensor function in vivo (6). This lack of enhanced sensor function was likely due to tissue reactions that were induced by the cross-linked collagens, i.e. inflammation and fibrosis (6). These tissue reactions were likely the result of excessive crosslinking of the collagen by gluteraldehyde (14). Because gluteraldehyde prompts tissue reactions, our strategy was to utilize low concentrations of the linking agent (0.2–0.3 %). When cross-linked Cultrex (X-Cultrex) coated sensors were tested in vivo in our murine CGM model (7) we demonstrated that the X-Cultrex coated sensors considerably enhanced glucose sensor function and lifespan in vivo, while inducing virtually no tissue reactions in a 28 day time period. This was evident in comparisons to non-cross-linked Cultrex coatings and sensors without any coatings. It is also important to note that X-Cultrex coatings contributed significantly to a substantial sensor functional increase immediately following device implantation. Suboptimal sensor performance is often experienced in the first 24-hours post implantation. To our knowledge, these are the first studies that demonstrate the effectiveness of cross-linked BM-based coatings in reducing sensor-induced tissue reactions as well as enhancing and extending glucose sensor function in vivo.
These studies support our hypothesis that naturally occurring matrix coatings (e.g. biohydrogels) hold great potential as highly biocompatible coatings for implantable devices such as glucose sensors. Carefully developed crosslinking protocols aid in the enhancement of biohydrogel stability and biocompatibility in vitro and in vivo without compromising loss of biocompatibility, i.e. X-Cultrex BM coatings. Additionally, the development of effective sensor coatings should lead to their use in other implantable devices that require biocompatibility for short and long-term functionality in vivo.
MATERIALS AND METHODS
Trevigen Cultrex basement membrane preparations
Cultrex Basement Membrane Extract (Type 2) Clearpath, was purchased from Trevigen, Inc. (Gaithersburg, MD). Cultrex Basement Membrane Extract (referred to as Cultrex, Cultrex basement membrane or Cultrex BM) is a soluble form of basement membrane purified from murine Engelbreth-Holm-Swarm tumor. The basement membrane is stored in Dulbecco’s Modified Eagle’s medium without phenol red, with 10-ug/ml gentamicin sulfate, at a storage and working concentration of approximately 15 mg protein/ml (Table 1). Generally the Cultrex preparations are kept frozen at −80 C, thawed in ice, and maintained on ice water for general use.
Table 1. Statistical Comparisons of Average Total MARD Values for Sensors Coated with Cultrex, Cross-linked Cultrex, and Uncoated sensors (Control) in CD-1 Mice.
Table 1 lists in a three by three box the statistical comparisons of all pairs of average total mean absolute relative difference (MARD) values for the three treatment groups: non-cross-linked Cultrex, cross-linked Cultrex, and uncoated control CD-1 mice. Total MARD values represent MARD values for each mouse, which are the averages of all the MARD values over the entire or total experimental time course. P-values within the boxes represent the statistical significance of the comparisons of the average total MARD values of two treatment groups indicated in the axes, calculated by student t-tests, since all three groups of average total MARD values are normally distributed. Error values following the +/− are standard deviations from the average of the individual treatment group’s total MARD values. The n values listed in the axes describe the sample sizes of the specific treatment group. The sensors were not recalibrated, thus their readings represent raw sensor output in nano-Amperes (nA). Thus MARD values used in these studies are derived from raw sensor output.
| Total mean MARD data student t-test | Control (no coating) Average mean MARD= 20.5 +/−6.6% n=39 |
Cultrex Average mean MARD= 16.7+/−5.9% n=34 |
x-Cultrex Average mean MARD= 10.6+/−2.9% n=26 |
|---|---|---|---|
|
Control (no coating) Average mean MARD= 20.5 +/−6.6% n=39 |
_______ | <0.01 | <0.01 |
|
Cultrex Average mean MARD= 16.7+/−5.9% n=34 |
<0.01 | _______ | <0.01 |
Dialysis of Cultrex basement membrane preparations
To eliminate salts, vitamins, amino acids and glucose present in the Cultrex preparations, the Cultrex was dialyzed against sterile deionized water with 3 changes of water using Thermo Scientific Slide-A-Lyzer Mini Dialysis devices. Generally 2 ml of Cultrex is dialyzed against 45 ml of sterile pyrogen free water/exchange, for a total of 3 dialysis exchanges.
Coating of Glucose Sensors with Cross-linked Cultrex (X-Cultrex) Basement Membrane
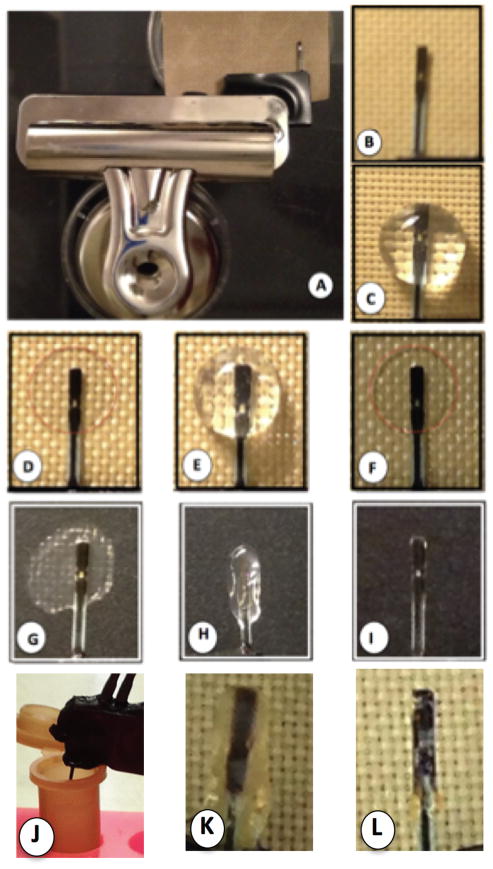
The modified Abbott Navigator glucose sensors used in the in vitro and in vivo studies were obtained from Abbott Diabetes Care [Alameda, CA]. Sensors were sterilized by exposure to UV light overnight prior to applying the sensor coating. Aseptic techniques were utilized during the coating process and before implantation. To coat the glucose sensors with cross-linked basement membrane, the glucose sensors were placed on a sterile polytetrafluoroethylene (PTFE) liner by modification of our previously described methods (8). Specifically, 50 uL of dialyzed Cultrex (15 mg protein /ml) was applied on one side of each sensor and placed in a 37°C incubator for two hours (Figure 1). The glucose sensor was then turned over and an additional 50 uL of dialyzed Cultrex was applied on the opposite side of the sensor before placing it in 37°C incubator for two hours. The resulting coated sensor was briefly dipped into sterile pyrogen-free water to shape the coating around the sensor (Figure 1H) followed by drying at 37°C. Next, the dry coated sensor was place in a 0.2–0.4 % glutaraldehyde solution (Figure 1J) for five minutes followed by at least one hour of dialysis in sterile, pyrogen-free water, followed by drying at 37°C for at least two hours. The resulting cross-linked Cultrex was designed as X-Cultrex. This coating technique allowed for a simple and consistent coating process. The Cultrex or X-Cultrex coated glucose sensors are stored dehydrated in a tissue culture hood until in vitro testing or implantation in mice.
Figure 1. Coating of Glucose Sensors in vitro Utilizing Cultrex Basement Membrane Extract (Cultrex) and Crosslinked Cultrex Preparations.
To produce a simple and reproducible coating of glucose sensors with Cultrex for in vitro and in vivo studies, a clamp based system, which utilizes a standard magnetic office clamp (OC), a modified Abbott glucose sensor (GS) and a polytetrafluoroethylene sheet (PTFE) was utilized (A). The Abbott sensor is centered on top of the PTFE sheet (B), and 50ul of dialyzed Cultrex is added on top of the sensor (C) and allowed to dry at 37°C, resulting in a thin protein layer on the sensor and associated PTFE sheet (D, red dotted line). The sensor is then removed from the PTFE sheet, flipped over and an additional 50ul of dialyzed Cultrex is added (E) and allowed to dry (F). This process can be repeated as needed to form a Cultrex coating on the sensor. Finally the sensor with a dry Cultrex coating is removed from the PTFE sheet (g), dipped in sterile water (h) and then allowed to dry at 37°C until dry (i). Cross-linked Cultrex (X-Cultrex) sensors were produced in the same process as the non-cross-linked Cultrex sensors, except with the addition of two steps in which the coated dry Cultrex coated sensors are dipped in 0.2 – 0.4% glutaraldehyde for 5 minutes, and then dipped in sterile pyrogen free water for at least 1 hour. Then the X-Cultrex coated sensors are dried as described above at 37°C. The resulting non-cross-linked (Cultrex) or cross-linked Cultrex (X-Cultrex) sensor is then utilized for in vitro studies, or implanted subcutaneously for in vivo studies.
In vitro glucose sensor testing
In order to determine if X-Cultrex coating negatively impacted sensor performance, sensor sensitivity of uncoated glucose sensors (controls), were evaluated pre and post X-Cultrex coating in vitro using our standard CGM system (9). Sensor sensitivity was characterized in tissue culture medium with an initial glucose concentration of 50 mg/dL at 200 mV. Background current was allowed to stabilize for about 15 minutes before sensors were subjected to increased glucose concentrations in the culture medium. Sensors were then rinsed in sterile water and left in a tissue culture hood to dry. After the sensors completely dried they were coated with X-Cultrex as described above. Sensors were then retested in vitro using the same protocol as described above. Sensor sensitivity for both pre and post X-Cultrex coatings was determined as described below (6, 10).
Glucose sensor Implantation and CGM in a murine model
Once it was established that sensor coating did not negatively impact sensor performance in vitro, we evaluated the performance of the X-Cultrex coated sensors versus uncoated sensors in our CGM mouse model (11). Cultrex and X-Cultrex coated and non-coated sensors were implanted in CD-1 mice (Jackson Laboratory, Bar Harbor, ME) and CGM was undertaken for a period up to 28 days as described previously (9, 11). Blood glucose reference measurements from the tail vein were obtained over the 28-day implantation period using Bayer Contour blood glucose monitors. The sensors were not recalibrated throughout the study period, thus their readings represent raw sensor output in nano-Amperes (nA). As such, MARD values used in these studies are derived from raw sensor output. The Institutional Animal Care and Use Committee of the University of Connecticut Health Center (Farmington, CT) approved the murine studies.
Continuous Glucose Monitoring Data Analysis
Reference blood measurements were used to calculate the mean absolute relative difference (MARD) over a four-week experiment for the two groups of mice with and without basement membrane coated sensors (12, 13). Equations (1), (2) and (3) below describe the MARD calculation in detail. Sensitivity (S; mg/dl/nA) is calculated for each mouse based on the reference blood glucose and the sensor output (I; nA) measurements in an initial reference stage of the experiment, i.e., k in Equation (2) is approximately 5, for the first initial five measurements across two days.
| (1) |
| (2) |
| (3) |
Histopathological Analysis of Tissue Reactions at Glucose Sensor Implantation Sites
In order to evaluate tissue responses to non-Cultrex coated, Cultrex and X-Cultrex coated glucose sensors, sensors containing tissue explants were obtained from mice at various time points post sensor implantation. For these studies, mice were euthanized and the full thickness of the skin and sensors were removed end bloc in approximately 3 × 3 cm2 sections and immediately placed in tissue fixative. Tissues were fixed in formalin for 24 to 48 hours, transferred to 70% ethanol, followed by standard processing, embedded in paraffin, and sectioning. The resulting 5-μm sections on glass slides were then stained using standard protocols for hematoxylin and eosin stain and Masson Trichrome (fibrosis). Histopathological evaluation of tissue reactions at sites of sensor implantation was performed on mouse specimens obtained at 1–28 days post-sensor implantation. The tissue section slides were viewed and assessed by a blinded experienced histopathologist (DLK) using a modified histologic scale (6, 9, 10, 14). Histologic parameters included inflammatory response, foreign body reaction, fibrotic response, collagen organization, and neovascularization. After an initial review of all slides to gain a baseline measure of histologic parameters, each sample was re-evaluated and scored against one another in order to obtain a semi-quantitative measure of the tissue responses to the implanted sensors. For the inflammatory response, the degree of infiltration of chronic inflammatory cells, principally lymphocytes and macrophages, surrounding the sensor were noted. Foreign body reaction was determined by the relative quantity of foreign body giant cells (FBGC) surrounding each sensor or adjoining tissue of sensor. Fibrotic change was a function of relative abundance of new collagen deposition at sites of sensor implantation, while collagen organization was determined by factors such as connective tissue density (loose versus dense) and arrangement of collagen bundles (parallel versus haphazard pattern). Neovascularization was a reflection of the number of new blood vessels per high power field (14).
Immunohistchemical staining of macrophages in sensor implanted tissue using anti-mouse F4/80 antibodies
To confirm the observations of the presence of macrophages in tissue sections, we utilized a mouse macrophage specific antibody designated anti-mouse F4/80. Anti-mouse F4/80 (@F4/80) (Invitrogen Catalog # A14800) was validated using mouse spleen tissue and standard immunohistochemical (IHC) techniques (9).
Statistical Analysis
The mean MARD values for each group, together or separated by week, were evaluated statistically, including tests to determine if the group MARD values were normally distributed. In cases where the mean MARD values were non-normal in distribution, non-parametric tests [e.g. Mann Whitney U test] were used to assess for statistical significance between the two groups of average mean MARD values. When three groups were compared, the Bonferroni correction method yielded a stricter statistical significance threshold of p<0.017. Microsoft Excel for Mac 2011 (version 14.1.4) and IBM SPSS Statistics 20 (release 20.0.0) were the software packages used for the calculations/graphing and statistical analyses, respectively.
The difference in MARD values among all three groups was evaluated statistically by ANOVA testing and its non-parametric equivalent, Kruskal-Wallis (K-W) tests. The differences in MARD values between any two groups were assessed with a Student t-test for normally distributed data whereas the non-parametric The Mann-Whitney U-test was employed when the data were not normally distributed. For multiple comparisons, the Bonferroni correction method was used in order to adjust the level of statistical significance required to reject the null hypothesis.
RESULTS
Impact of Cultrex coatings on glucose sensor function in vitro and in vivo
We previously demonstrated that glucose sensors coated with non-cross-linked Cultrex did not affect sensor function in vitro (8). We also demonstrated that sensors coated with non-crosslinked Cultrex showed significantly less tissue reaction at the sites of sensor implantation (8). These Cultrex coated sensors also demonstrated an increased performance as compared to uncoated sensors. However, the Cultrex sensor coating began to degrade by day 21 post sensor implantation such that sensor-induced tissue reactions were observed (8). We believe that the loss of the Cultrex coatings eliminated the protective sensor coating resulting in the exposure of the underlying sensor surface. In an effort to increase the longevity of the Cultrex based sensor coating, we utilized glutaraldehyde cross-linked Cultrex for the coating of a glucose sensor. As a first step in validating these X-Cultrex sensor coatings, we assessed whether or not the cross-linked Cultrex coating compromised sensor function in vitro by evaluating the impact of varying coatings of Cultrex on glucose sensor performance in vitro. Sensor sensitivity remained unchanged before and after the Cultrex coating within the range of 0 to 2 mg Cultrex/sensor, and was determined to be 45.9 ± 4.8 mg/(dL*nA) and 48.6 ± 5.1 mg/(dL*nA), respectively. For the cross-linked Cultrex (X-Cultrex), sensor sensitivity was similar before and after the X-Cultrex coating within the range of 0 to 3 mg X-Cultrex/sensor. These values were 37.9 ± 3.2 mg/(dL*nA) and 41.3 ± 2.2 mg/(dL*nA), respectively.
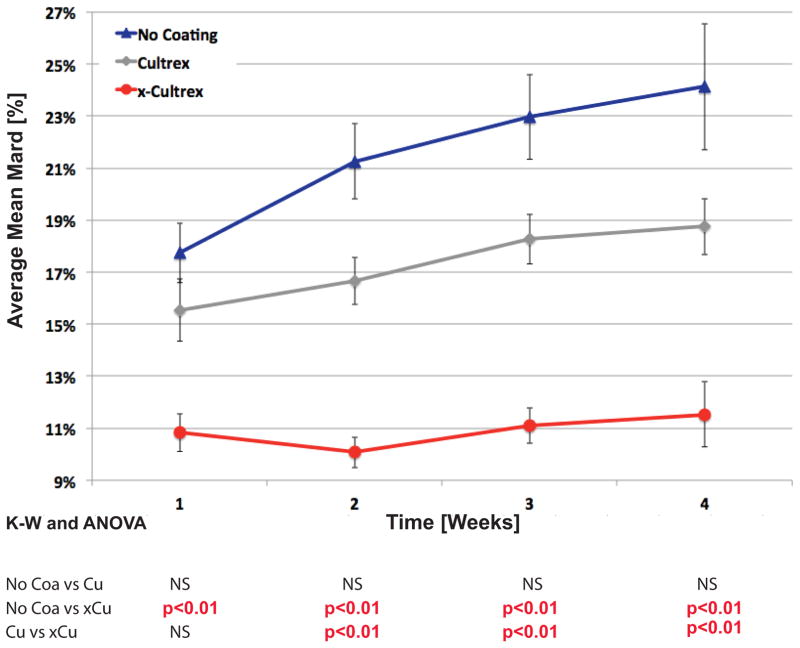
Once we demonstrated that the cross-linking of Cultrex coatings did not negatively affect sensor performance in vitro, the effect of X-Cultrex coatings on sensor performance in vivo for a period of up to 28 days post sensor implantation was assessed. These in vivo studies were performed utilizing our established murine model of CGM (9, 11). For these studies, we utilized mean absolute relative difference (MARD) values of non-cross-linked Cultrex and X-Cultrex coated sensors as well as uncoated, control sensors in CD-1 mice as a measure of sensor performance and error of the CGM sensors over time; the lower the MARD values, the lower the error, the better the performance. It is important to note that no sensor recalibration occurred for these studies. Thus MARD values used in these studies are derived from raw sensor output. As illustrated in Figure 2, the cross-linking of the Cultrex on the sensor resulted in a significant improvement of sensor functionality when compared to uncoated or non-cross-linked Cultrex coated sensors for the entire 28 day in vivo evaluation save for the first week in which no statistically significant difference was detected between the non-cross-linked Cultrex and the X-Cultrex groups (Figure 2). Statistical comparisons of the sensor performance during the entire four week, 28-day post-implantation experimental time course, showed that the X-Cultrex had significantly better MARD values as compared to non-coated control and Cultrex coated sensors except for the previously mentioned non-significant difference (Figure 2). By the fourth week, the total average MARD value for the CD-1 mice with X-Cultrex coated sensors was 10.6% in a group of 26 mice. The average MARD value in Cultrex coated sensors was 16.7% in a group of 34 mice, as compared to the CD-1 control mice with an average MARD value of 20.5% in a group of 39 mice (Table 1). These sample sizes are relatively large for such investigations. A calculation of standard deviation of the MARD values for these groups of mice indicates that the CD-1 controls present a greater standard deviation of 6.6%, while the Cultrex coated CD-1 MARD values present a smaller standard deviation of 5.9%, whereas the X-Cultrex coated sensors had the lowest standard deviation of 2.9% (Table 1). An analysis of the total mean MARD revealed that both Cultrex and X-Cultrex demonstrated a statistically significant difference from the control group.
Figure 2. Trend Analysis of Sensor MARD between Sensors Coated with Cultrex, Cross-linked Cultrex or Uncoated (Control) Sensors in CD-1 Mice.
Figure 2 represents the impact of Cultrex and X-Cultrex coating on in vivo trends of continuous glucose monitoring (CGM), by average mean absolute relative difference (MARD) from weeks 1 to 4. Cultrex, X-Cultrex coated sensors were compared with uncoated sensors implanted subcutaneously in CD-1 genetic background mice over a 4-week time period. The p-values at the bottom represent the significance of the difference among the three treatment groups in average mean MARD value for each individual week, by the Analysis of Variance (ANOVA) and Kruskal-Wallis tests (as the non-parametric equivalent to ANOVA). The three treatment groups were assessed against one another for a statistically significant difference at weekly intervals for a total of four weeks. The weekly MARD values, particularly in weeks 2 to 4, are cumulative MARD values, meaning the average MARD values used in these calculations in weeks 2 to 4 represent the average of MARD values for all weeks prior to that point, i.e. a cumulative average of all MARD values up to that week, not simply the MARD values just in that specific week. The error bars around each data point represent the standard error (S.E.) of the mean MARD for the particular time point and treatment group. It is important to note that no sensor recalibration occurred for these studies. The sensors were not recalibrated, thus their readings represent raw sensor output in nano-Amperes (nA). Thus MARD values used in these studies are derived from raw sensor output.
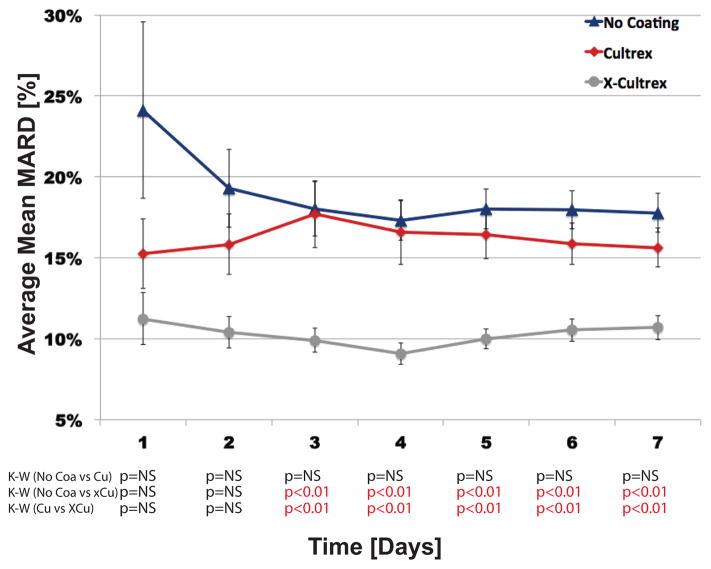
It is noteworthy that the overall performance of the X-Cultrex coated sensors already surpassed sensor performance of Cultrex and uncoated sensors within the first 7 days of the study (Figure 3). This high accuracy underscores the enhancing effect of X-Cultrex even within the first seven days post sensor implantation. Specifically we observed that the X-Cultrex coating enhanced sensor function significantly post implantation (Figure 3).
Figure 3. Trend Analysis of First Week Sensor MARDs, Between Sensors Coated with Non-Cross-linked Cultrex, Cross-linked Cultrex or Uncoated (Control) Sensors in CD-1 Mice.
Figure 3 represents the impact of Cultrex and X-Cultrex coating on in vivo trends of continuous glucose monitoring (CGM), by average mean absolute relative difference (MARD) in the first week, separated by day. Cultrex, X-Cultrex coated sensors were compared with uncoated sensors implanted subcutaneously in CD-1 genetic background mice over a four (4) week time period, but the analysis and calculations for this figure were limited to the first week, separated by day. The p-values at the bottom represents the significance of the difference among the three treatment groups in average mean MARD value for each individual week, by Kruskal-Wallis (K-W) tests, as the non-parametric equivalent to ANOVA (Analysis of Variance). The daily MARD values, particularly in days 2 to 7, are cumulative MARD values, meaning the calculated average MARD values for days 2 to 7 represents the average of MARD values for all days prior to, and including, that point, i.e. a cumulative average of all MARD values up to that day, not simply the MARD values in that specific day. The error bars around each data point represents the standard error (S.E.) of the mean MARD for the particular time point and treatment group. Thus MARD values used in these studies are derived from raw sensor output.
As illustrated in Figure 2, a trend analysis of the MARD values of the Cultrex and X-Cultrex coated sensors in CD-1 mice and their CD-1 control mice, over the course of the four (4) week experiment, demonstrates a significant improvement in sensor performance, [i.e. lower MARD values, for the X-Cultrex coated sensors throughout the entire experiment].
Impact of Cultrex coatings on sensor induced tissue reactions at sites of glucose sensor implantations
Based on the in vivo functional sensor data previously described, we hypothesize that the increase in the sensor performance of the X-Cultrex coated sensor was a direct result of the diminished sensor-induced tissue reactions in the X-Cultrex coated sensors, [i.e. increased biocompatibility and stability of the X-Cultrex coatings]. We previously reported that non-cross-linked Cultrex BM preparations, in the form of a gel, may be implanted in mouse subcutaneous tissue for extended periods of time without inducing significant tissue reactions (8). Unfortunately, the non-cross-linked Cultrex began degrading around 14–21 days post sensor implantation resulting in increased tissue reactions at the sensor implantation site (8). We hypothesize that the delayed tissue reactions seen in the non-cross-linked Cultrex coated sensors were triggered by the exposure of the sensor surfaces as the non-cross-linked Cultrex degraded. As such, the biocompatibility of the non-cross-linked Cultrex in vivo suggested that it could be a strong candidate for biocompatibility coating for implanted devices, such as glucose sensors, if it was more stable and remained highly biocompatible.
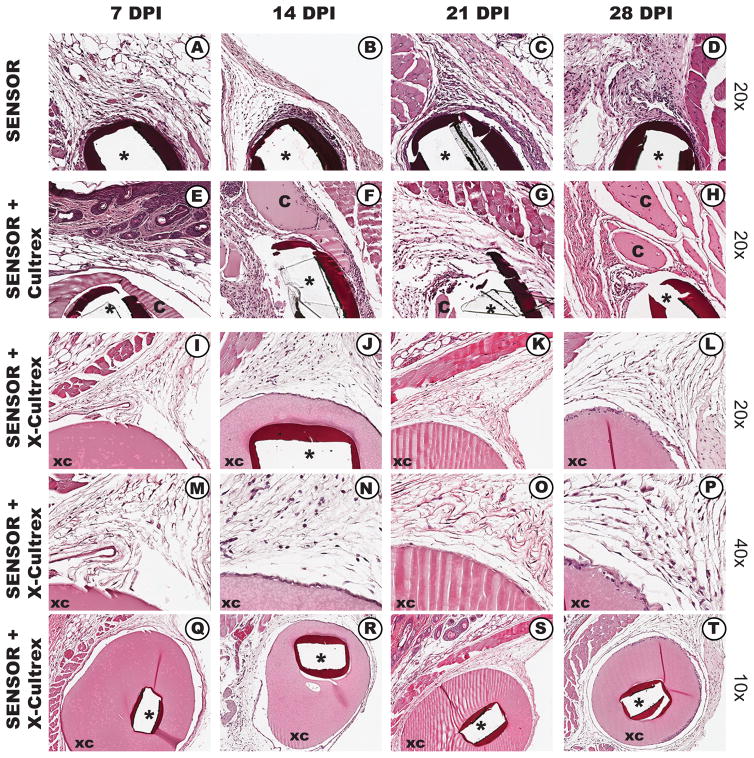
To investigate whether cross-linked Cultrex enhances coating stability, while retaining biocompatibility, we evaluated the tissue reactions associated with non-coated, non-cross linked Cultrex and X-Cultrex coated sensors implanted in mouse subcutaneous tissue over a 28 day time period (Figure 4). As expected from our previous studies, during the first 14 days post sensor implantation, the non-cross-linked Cultrex appeared intact on the sensor surface and demonstrated significantly less tissue reaction at the implantation site (Figure 4, E and F). However, by 21 and 28 days, there was significant loss of the Cultrex coating on the sensor surface and an early indication of tissue reactions being induced at the sensor implantation site (Figure 4, G and H). When tissue reactions induced by X-Cultrex coated sensors, X-Cultrex demonstrated that it was very stable and biocompatible for the tested time period up to 28 days post sensor implantation (see Figure 4, I–T v) when compared to the non-cross linked Cultrex (Figure 4, e–h) as well as to the uncoated sensors (Figure 4, a–d). To extend these observations, we also assessed for the presence of macrophages at the sensor implantation sites. Macrophages were selected because they have been implicated in the biofouling of glucose sensors in vivo (9, 11). For these studies, immunohistochemical staining of tissue section using mouse macrophage specific anti-F4/80 antibodies was employed (9, 11). As noted in Figure 5, non-Cultrex coated sensors triggered significant macrophage accumulation at the uncoated sensor implantation site (Figure 5 c and d). Alternatively coating the sensors with Cultrex (Figure 5 e and f) or X-Cultrex (Figure 5 g and h) resulted in a significant reduction in macrophage accumulation at the coated sensor implantation sites. In conclusion, these histologic studies are consistent with the sensor function study presented in Figures 2 and 3, which support our hypotheses regarding the uses of X-Cultrex as a sensor coatings in vivo.
Figure 4. Histological Analysis of the Tissue Reactions Induced in CD-1 Murine Models of CGM by Cultrex coated, X-Cultrex coated, or uncoated Glucose Sensors CD-1 Mice.
X-Cultrex, Cultrex, and non-coated sensors were obtained from the implantation sites at 7, 14, 21 and 28 days post implantation and processed for standard histopathology using paraffin embedding and H&E staining. Tissue reactions to X-Cultrex and Cultrex only implantations demonstrated that X-Cultrex and Cultrex (smooth pink staining and indicated with the letter “XC” or “C”, respectively) is very biocompatible, and does not induce any significant tissue reactions over the 28-day test period (E–T). As expected sensor only (black bands are remnants of sensor) implantation sites displayed significant tissue reaction characterized by inflammation (A–D). Tissue reactions to Cultrex coated sensors (e–h) demonstrated minimized tissue reactions at site of glucose sensors, particularly in the first three weeks (E–G). However starting as early as two weeks post sensor implantation degradation of the Cultrex coating is observed, leading to the exposure of the sensor implant with subsequent increased tissue reaction at the sensor site (H). The lack of Tissue reactions to X-Cultrex coated sensors (I–T) demonstrated that X-Cultrex coating significantly minimized tissue reactions at site of glucose sensors. The absence of tissue reaction likely contributed to the enhanced sensor functionality seen in the X-Cultrex coated sensors when compared to non-cross-linked Cultrex on non-coated sensors.
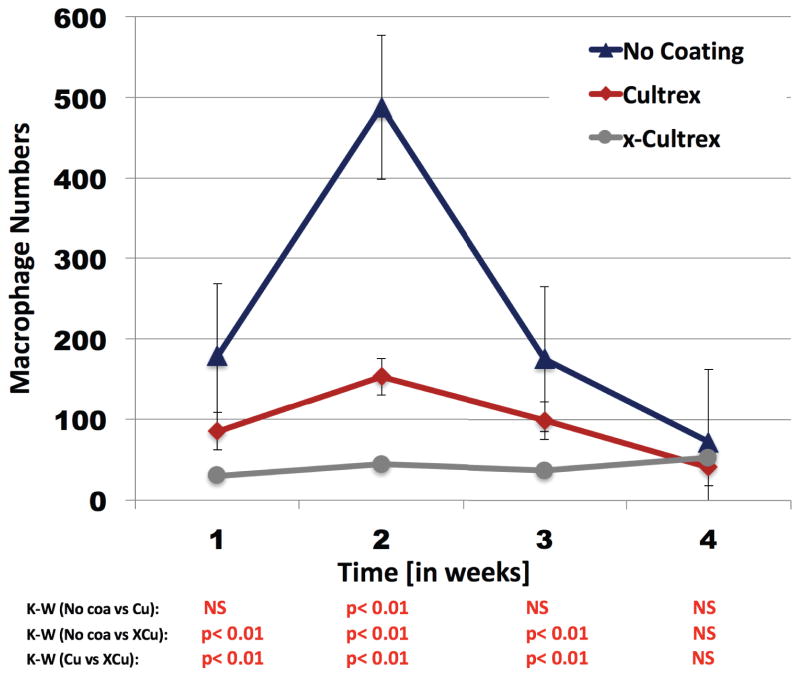
Figure 5. Characterization of the presence and distribution of F4/80 positive macrophages at Cultrex coated, X-Cultrex coated, or uncoated Glucose Sensor Implantation Sites in CD-1 Mice.
To evaluate the presence and distribution of macrophages at sensor implantation sites, anti mouse F4/80 antibodies were using with standard immunohistochemistry technology.
As can be seen in Figure 5 both glucose sensors coatings of either Cultrex basement membrane or X-Cultrex basement membrane showed statistically better performance when compared to sensors with no coatings. Additionally the X-Cultrex basement membrane significantly outperformed the non-crosslinked Cultrex basement membrane. Statistical analysis was done using the Kruskal-Wallis test (KW)
Analysis of Macrophage influx in to tissue sites implanted with BM-coated and non-coated glucose sensors
Quantitative analysis of the macrophage influx into tissue sites implanted with BM-coated and non-coated glucose sensors, indicated that non-coated sensors showed significantly more MQ, when compared to either Cultrex BM coated or X-Cultrex BM coated (Figure 5). The decrease in MQ numbers at 3–4 weeks post implantation in the non-coating sensor was the result of MQ disappearance associated with fibrosis occurring as part of wound healing. Additionally it is also important to note that the X-Cultrex coated sensors had the lowest number of MQ accumulation at the implantation site when compared non-cross-linked Cultrex (Figure 5). The increased in MQ accumulation seen in the non-crosslinked Cultrex coating likely is the result of the degradation of the non-cross-linked Cultrex by 14 days and beyond. This membrane degradation would expose the underlying sensor and thereby trigger a more intense inflammatory reaction (Figure 4). Alternatively X-Cultrex did not experience degradation over the study period and as such the underlying sensor was not exposed to the tissue. We argue that this prevented any delayed inflammation at the sensor implantation site including prevention of recruitment of MQ to the X-Cultrex-sensor implantation site (Figure 4).
DISCUSSION
Implantable glucose sensor monitoring of blood glucose levels in diabetic patients has been available for over 40 years (15). However, despite sensor functionality improvements recalibration of the sensor device is often a necessity in order to compensate for unreliable sensor performance. Generally, poor sensor performance has been attributed to the triad of inflammation, fibrosis, and vessel regression. Efforts to overcome these tissue reactions include localized release of steroids (5, 16–19) and/or growth factors (18, 20, 21).
Our laboratory’s recent efforts demonstrated that basement membrane (BM) based bio-hydrogels as coatings (i.e. Cultrex) for glucose sensors enhance their biocompatibility and function in vivo (8). Specifically, our studies demonstrated that Cultrex based BM sensor coatings accomplished the dual goals of decreased tissue reactivity at the site of glucose sensor implantation in vivo as well as better device performance in a CGM murine model. Nonetheless, these effects were transient given the degradation of the bio-hydrogel when implanted into tissue. Onset of the bio-hydrogel degradation related to extracellular turnover resulted in a subsequent decline in sensor performance that was observed approximately three weeks after sensor implantation (1). We hypothesized that this degradation process exposes the original sensor surface and induces the commonly observed foreign body tissue reaction, ( i.e. inflammation and fibrosis). As such, the bio-hydrogel was only able to delay the tissue reaction at the sensor implantation site. This delay appears to be dependent on the degradation rate of the outer sensor Cultrex coating.
In response to these observations, we undertook the present studies in order to investigate whether chemical crosslinking of the Cultrex would increase the stability and biocompatibility of Cultrex based biohydrogels in vivo designed to increasethe function and lifespan of sensors and CGM. Previous studies using cross-linked collagen coatings demonstrated marginal effects on sensor function in vivo (6). This lack of enhanced sensor function likely occurred as a result of tissue reactions that were induced by the cross-linked collagens,( i.e. inflammation and fibrosis) (6). These tissue reactions were likely the result of excessive crosslinking of the collagen by gluteraldhyde (14). Due to issues of gluteraldehyde induced tissue reactions, our strategy was to utilize low concentrations of gluteraldehyde (0.2–0.3 %) and only expose BM (Culturex) briefly to the gluteraldehyde fixative, (e.g. five minutes). Upon fixation, the sensor/BM gluteraldehyde coating was exposed to pyrogen-free water in order to minimize any fixative induced tissue reactions. Cultrex BM was then cross-linked upon the completion of post sensor Cultrex BM coating and drying. In vivo murine CGM studies demonstrated that X-Cultrex coated sensors demonstrated superior performance as compared to CD-1 non-coated controls and Cultrex coating only. This performance was observed beyond the study’s third week. Overall, Cultrex coated sensor performance demonstrated better MARD values when compared to control (e.g. no Cultrex coating). Nevertheless, statistical significance between the two groups was not established with a p<0.01 (Figure 2). We hypothesized that the increased MARD values, especially those observed in weeks three and four post sensor implantations of the Cultrex coated CD-1 mice, was likely the result of the degradation of the Cultrex coating. This is a result of the normal tissue remodeling that hardly ever occurred in the X-Cultrex coated sensors (Figure 2). This hypothesis was further supported as noted in histologic studies (Figure 4). This degradation ultimately exposes the underlying glucose sensor, which induces a cascade of reactions including inflammation and tissue remodeling leading to further degradation of the Cultrex sensory coating degradation. The use of glutaraldehyde to cross-link Cultrex appears to prevent appreciable degradation thus minimizing tissue remodeling and inflammatory processes. X-Cultrex outperformed both Cultrex coated and control sensors during the first week post sensor implantation (Figure 3). We hypothesize that this results from the X-Cultrex coating’s ability to remain intact during the implantation process. We also hypothesize that Cultrex coating sensors experience a higher rate of variability during the implantation process from Cultrex coating dislocation. Nonetheless, these studies demonstrate that a biocompatible hydrogel such as Cultrex can result in an increased long-term sensor performance. We intend to repeat these studies in diabetic mouse and porcine models of CGM in order to confirm the effectiveness of these X-Cultrex coatings.
Natural bio-hydrogels such as basement membranes also provide numerous binding sites for specific proteins such as growth factors and cytokines within their molecular structures (22–31). These binding sites serve a critical role in the regulation of cell and tissue responses to injury including inflammation, repair and regeneration. Future studies could utilize these binding sites and incorporate additional proteins and/or drugs in an effort to prolong an implantable device’s functionality. Furthermore, addition of bioactive factor to X-Cultrex coatings may result in fewer implant associated limitations and enhance glucose sensor function. Future human studies will require an assessment of the BM subcomponents that are responsible for its biocompatibility, which would allow the use of recombinant proteins. Alternatively, if intact BM based coatings are required, we would anticipate using BM obtained from placentas for use in human trials. In conclusion, our data indicate that cross-linked BM based bio-hydrogels represent novel biocompatible coatings for implantable devices such as glucose sensors that attenuate the foreign body reaction.
Acknowledgments
The Leona M. and Harry B. Helmsley Charitable Trust and the National Institute of Health provided funding for this study. We would like to thank Mr. Omar Antar for his assistance in the statistical analysis. We would also like to thank Abbott Diabetes Care (ADC), Alameda, CA, for providing us with the glucose sensors.
Abbreviations
- BM
basement membrane
- BME
basement membrane extract
- MARD
mean absolute relative difference
- ECM
extracellular matrices
- CGM
continuous glucose monitoring
- X-Cultrex
glutaraldehyde cross-linked Cultrex extract
Footnotes
Declaration of Conflicting Interests The author(s) declared the following potential appearance of conflicts of interest with respect to the research, authorship, and/or publication of this article since UK and DK are founders of Cell and Molecular Tissue Engineering, LLC.
References
- 1.Hu Y, Liang B, Fang L, Ma G, Yang G, Zhu Q, et al. Antifouling Zwitterionic Coating via Electrochemically Mediated Atom Transfer Radical Polymerization on Enzyme-Based Glucose Sensors for Long-Time Stability in 37 degrees C Serum. Langmuir. 2016;32(45):11763–70. doi: 10.1021/acs.langmuir.6b03016. [DOI] [PubMed] [Google Scholar]
- 2.Vallejo-Heligon SG, Brown NL, Reichert WM, Klitzman B. Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 2016;30:106–15. doi: 10.1016/j.actbio.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N, Burugapalli K, Song W, Halls J, Moussy F, Ray A, et al. Electrospun fibro-porous polyurethane coatings for implantable glucose biosensors. Biomaterials. 2013;34(4):888–901. doi: 10.1016/j.biomaterials.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Burugapalli K, Wijesuriya S, Far MY, Song W, Moussy F, et al. Electrospun polyurethane-core and gelatin-shell coaxial fibre coatings for miniature implantable biosensors. Biofabrication. 2014;6(1):015002. doi: 10.1088/1758-5082/6/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J Control Release. 2013;169(3):341–7. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Ju YM, Yu B, West L, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. II. Long-term in vitro/in vivo sensitivity characteristics of sensors with NDGA- or GA-crosslinked collagen scaffolds. Journal of biomedical materials research Part A. 2010;92(2):650–8. doi: 10.1002/jbm.a.32400. [DOI] [PubMed] [Google Scholar]
- 7.Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, et al. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):402–12. doi: 10.1089/dia.2006.8.402. [DOI] [PubMed] [Google Scholar]
- 8.Klueh U, Qiao Y, Czajkowski C, Ludzinska I, Antar O, Kreutzer DL. Basement Membrane-Based Glucose Sensor Coatings Enhance Continuous Glucose Monitoring in Vivo. J Diabetes Sci Technol. 2015 doi: 10.1177/1932296815598776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35(10):3145–53. doi: 10.1016/j.biomaterials.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. Journal of biomedical materials research Part A. 2008;87(1):136–46. doi: 10.1002/jbm.a.31756. [DOI] [PubMed] [Google Scholar]
- 11.Klueh U, Qiao Y, Frailey JT, Kreutzer DL. Impact of macrophage deficiency and depletion on continuous glucose monitoring in vivo. Biomaterials. 2014;35(6):1789–96. doi: 10.1016/j.biomaterials.2013.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obermaier K, Schmelzeisen-Redeker G, Schoemaker M, Klotzer HM, Kirchsteiger H, Eikmeier H, et al. Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment. J Diabetes Sci Technol. 2013;7(4):824–32. doi: 10.1177/193229681300700404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zschornack E, Schmid C, Pleus S, Link M, Klotzer HM, Obermaier K, et al. Evaluation of the performance of a novel system for continuous glucose monitoring. J Diabetes Sci Technol. 2013;7(4):815–23. doi: 10.1177/193229681300700403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW. Comparative Analysis of Histopathologic Effects of Synthetic Meshes Based on Material, Weight, and Pore Size in Mice. The Journal of surgical research. doi: 10.1016/j.jss.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Gifford R. Continuous glucose monitoring: 40 years, what we’ve learned and what’s next. Chemphyschem. 2013;14(10):2032–44. doi: 10.1002/cphc.201300172. [DOI] [PubMed] [Google Scholar]
- 16.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–7. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 17.Klueh U, Kaur M, Montrose DC, Kreutzer DL. Inflammation and glucose sensors: use of dexamethasone to extend glucose sensor function and life span in vivo. J Diabetes Sci Technol. 2007;1(4):496–504. doi: 10.1177/193229680700100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. Journal of biomedical materials research Part A. 2007;81(4):858–69. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Vaddiraju S, Qiang L, Xu X, Papadimitrakopoulos F, Burgess DJ. Effect of dexamethasone-loaded poly(lactic-co-glycolic acid) microsphere/poly(vinyl alcohol) hydrogel composite coatings on the basic characteristics of implantable glucose sensors. J Diabetes Sci Technol. 2012;6(6):1445–53. doi: 10.1177/193229681200600626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J Control Release. 2015;214:103–11. doi: 10.1016/j.jconrel.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Klueh U, Dorsky DI, Kreutzer DL. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials. 2005;26(10):1155–63. doi: 10.1016/j.biomaterials.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Hall H, Baechi T, Hubbell JA. Molecular properties of fibrin-based matrices for promotion of angiogenesis in vitro. Microvasc Res. 2001;62(3):315–26. doi: 10.1006/mvre.2001.2348. [DOI] [PubMed] [Google Scholar]
- 23.Wagner WR, Hubbell JA. Local thrombin synthesis and fibrin formation in an in vitro thrombosis model result in platelet recruitment and thrombus stabilization on collagen in heparinized blood. J Lab Clin Med. 1990;116(5):636–50. [PubMed] [Google Scholar]
- 24.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13(15):2214–24. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 25.Ye Q, Zund G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17(5):587–91. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 26.Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18(4):415–9. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 27.Herbert CB, Nagaswami C, Bittner GD, Hubbell JA, Weisel JW. Effects of fibrin micromorphology on neurite growth from dorsal root ganglia cultured in three-dimensional fibrin gels. J Biomed Mater Res. 1998;40(4):551–9. doi: 10.1002/(sici)1097-4636(19980615)40:4<551::aid-jbm6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Herbert CB, Bittner GD, Hubbell JA. Effects of fibinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. J Comp Neurol. 1996;365(3):380–91. doi: 10.1002/(SICI)1096-9861(19960212)365:3<380::AID-CNE4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69(1):149–58. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 30.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 31.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001;72(1–3):101–13. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]