Abstract
Human induced pluripotent stem cells (hiPSCs) can be used to mass-produce surrogates of human tissues, enabling new advances in drug screening, disease modeling, and cell therapy. Recent developments in CRISPR/Cas9 genome editing technology use homology directed repair (HDR) to efficiently generate custom hiPSC lines harboring a variety of genomic insertions and deletions. Thus, hiPSCs that encode specific endogenous proteins fused to a fluorescent report protein can be rapidly created by employing CRISPR/Cas9 genome editing, enhancing HDR efficiency, and optimizing homology arm length. These fluorescently-tagged hiPSCs can be used to visualize protein function and dynamics in real time as cells proliferate and differentiate. Since nearly any intracellular protein can be fluorescently tagged, this system serves as a powerful tool to facilitate new discoveries across many biological disciplines. In this unit, we present current protocols for the design, generation, and monoclonal expansion of genetically-customized hiPSCs encoding fluorescently-tagged endogenous proteins.
Keywords: pluripotent stem cells, genome editing, fluorescent reporters, CRISPR, synthetic biology
INTRODUCTION
The discovery of human induced pluripotent stem cells (hiPSCs) during the mid-2000s has revolutionized stem cell biology (Takahashi et al., 2007). Human pluripotent stem cells can now be derived from a small sample of human skin or blood, a process largely devoid of the ethical controversies associated with human embryonic stem cells (hESCs) (Churko, Burridge, & Wu, 2013). These hiPSCs are similar to hESCs in terms of gene expression and epigenetic profile, and cellular differentiation protocols can be used interchangeably between hiPSCs and hESCs (Chin et al., 2009). Additionally, hiPSCs have enabled the large-scale production of non-proliferative human cells such as neurons and cardiomyocytes, although hiPSC-derived cells tend to be “immature” structurally and functionally in comparison to their adult counterparts (X. Yang, Pabon, & Murry, 2014). Nonetheless, hiPSC-derived cells have proved useful in a variety of applications. For example, beating hiPSC-derived cardiomyocytes have recapitulated toxicities clinically associated with certain chemotherapeutic compounds (Sharma et al., 2017). Similarly, patient hiPSC-derived cardiomyocytes have been able to model the cellular phenotypes associated with genetically-acquired, arrhythmogenic disorders such as cardiac long-QT syndrome (Itzhaki et al., 2011). Finally, hiPSC-derived cells are being explored as directed cellular replacement therapies for a variety of neurological disorders (Trounson & DeWitt, 2016). As differentiation protocols improve and methods for in vitro cellular maturation are refined, hiPSC-derived cells should steadily become a mainstream tool in the cell biologist’s toolbox.
Homologous recombination with exogenous DNA has been used to modify eukaryotic and prokaryotic genomes for decades, albeit not always efficiently (Capecchi, 1989). In the 2000s, advances in the field of genome editing focusing on technologies such as zinc finger nucleases (ZFNs) transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, have enabled efficient, site-specific engineering of genetic mutations in a variety of cell types (Gaj, Gersbach, & Barbas, 2013). Perhaps the most easily accessible of these modern genome editing technologies is CRISPR/Cas9, which coopts a naturally-occurring bacterial antiviral defense mechanism to generate a site-specific double-stranded DNA break (DSB) (Ran et al., 2013). The CRISPR/Cas9 genome editing system, usable for both in-vitro and in-vivo applications, is quite simple, employing only a guide RNA (gRNA) to target a protospacer-adjacent motif (PAM) and a Cas9 endonuclease to generate a double-stranded DNA break adjacent to the PAM site (Ran et al., 2013). A vast array of bioinformatic tools have also been developed in parallel to facilitate CRISPR/Cas9 editing, including those that can help design the most effective guide RNAs, identify optimal DNA target sites, and minimize off-target editing (Bae, Park, & Kim, 2014; Naito, Hino, Bono, & Ui-Tei, 2015). Human cell types, including hiPSCs, can be quickly modified using CRISPR/Cas9 (L. Yang, Yang, Byrne, Pan, & Church, 2014). Small genetic insertions or deletions (indels) can be generated via error-prone non-homologous end joining (NHEJ), potentially leading to a genetic reading frame shift and the creation of premature stop codons in a target gene of interest, thereby truncating the translated protein and effectively generating a genetic knockout. Alternatively, when a DNA repair template is introduced in combination with the targeting guide RNA and Cas9 endonuclease, entire regions of the genome can be replaced at the site of the Cas9-induced DNA break with large segments of non-endogenous DNA. This process relies on a coopted version of homology directed repair (HDR), whereby the endogenous DNA repair machinery uses an exogenous DNA repair template in place of a naturally occurring sister chromatid to correct a DNA mutation, caused by the Cas9 endonuclease in this case. The simplicity and efficiency of the CRISPR/Cas9 gene editing system allows for rapid insertion and deletion of genetic sequences using the naturally-occurring mechanisms of NHEJ and HDR.
When used in combination, hiPSCs and improvements in CRISPR/Cas9 gene editing have enabled the creation of patient-specific human induced pluripotent stem cells with highly modified genomes. CRISPR/Cas9-mediated homology directed repair allows for fluorescent tagging of endogenous proteins in a stable hiPSC cell line, whereby a fluorescent protein such as GFP is attached to an endogenous protein of interest. By adding a small fluorescent tag, a variety of applications could be imagined. For instance, endogenous proteins could be tracked spatiotemporally as hiPSCs actively grow, divide, and differentiate. Proteins localized to cellular organelles could be visualized at high resolution in real time. Additionally, transcription factors could be monitored as they undergo translocation from the cytoplasm to the nucleus to activate gene cascades driving stem cell-differentiation. This could prove to be highly useful for studying early embryogenesis and understanding the specification of mesoderm, endoderm, and ectoderm-derived cell lineages. Likewise, functionally-active proteins necessary for cellular locomotion or contraction can be monitored by adding fluorescent tags. A wild-type hiPSC line with a fluorescently-tagged endogenous protein could further serve as a platform on top of which other mutations of interest can be introduced. Subsequently, the effect of genotypic perturbations on a fluorescently-tagged endogenous protein could be visualized in real time in live cells. Ultimately, fluorescent tagging of endogenous proteins presents numerous advantages in studying intracellular protein functions and dynamics. In this unit, we provide current protocols for the design and generation of hiPSC lines with fluorescently-tagged endogenous proteins (Figure 1).
Figure 1.
Workflow for generation of hiPSCs with fluorescently-tagged endogenous proteins. Expect the entire protocol to take up to two months.
STRATEGIC PLANNING
Choosing an endogenous protein to be fluorescently tagged depends on the relevant downstream applications of the proposed hiPSC line. Fluorescent tags could theoretically be fused to the N-terminus, C-terminus, or even in the intra-protein domains of a given protein. However, the advantage to inserting the fluorescent tag at the N- or C-terminus of the selected protein is that there is less of a chance of disrupting a functionally-critical domain within the protein. When deciding between the N- and C-terminus for GFP tagging, the downstream application should be considered. For example, if a GFP tag is fused to the C-terminus of a selected protein, the GFP expression could be lost if new mutations are generated on the same allele as the GFP tag, resulting in premature truncation of the fusion protein such that the GFP tag is never expressed. The expression of the protein to be tagged is also critical, as this will influence the intensity of the fluorescent signal. Expression levels of genes encoding selected proteins can be determined by RNA-sequencing or qPCR. The size of the protein to be tagged is another consideration. For example, GFP is approximately 27 kilodaltons in size, and it is possible that attaching a fluorescent protein to a functionally active endogenous protein could change that protein’s function. Functional domains, such as DNA binding domains or ligand receptors, could be disrupted in this way. Incorporating a flexible linker as an intermediary to connect the fluorescent tag and the protein of interest could potentially alleviate functional disruption of the selected protein.
Additional criteria should be considered when designing the HDR template donor plasmid containing the fluorescent reporter cassette and linker flanked by homology arms. We and others have found that longer homology arms (greater than 500 bp) tend to increase the efficiency of HDR and thus could enhance the integration rate of the fluorescence cassette into the genome (Chu et al., 2015). Previous reports have suggested that having a targeted DSB within 30 bp of the mutation start site can enhance the HDR process, with breaks closer to the mutation site resulting in higher rates of genome editing (Paquet et al., 2016). However, care should be taken to also mutate the target PAM site in the donor HDR plasmid to prevent Cas9 from inducing a double-stranded break in the donor plasmid itself. It may be advantageous to have a guide RNA that targets a non-coding portion of the genome to avoid having to mutate a coding sequence in the HDR template. It is also important to consider that HDR and NHEJ can occur simultaneously during CRISPR transfection, and care should be taken to accurately sequence mutated hiPSC clones after editing to ensure that the donor template integration has been “clean,” without additional off-target DNA indels. Such indels could disrupt the targeted allele, or render the encoded protein nonfunctional if a premature stop codon is introduced in the coding sequence. Bioinformatic tools are available to assist with targeting guide RNA and HDR template design (Hodgkins et al., 2015). Some of these tools incorporate mutation of the PAM site in the design of the HDR template to prevent self-targeting and re-cleavage by Cas9.
To select an appropriate hiPSC line in which to introduce the fluorescent reporter tag, possible single nucleotide polymorphisms (SNPs) in the gene of interest should be identified using whole genome sequencing, as these SNPs could disrupt CRISPR-based targeting. The hiPSC line to be used should be at a relatively low passage number, ideally below passage 30, to minimize the occurrence of karyotypic abnormalities acquired during long-term cell culture. Also, hiPSC lines produced by introduction of the Yamanaka pluripotency factors (Oct4, Sox2, Klf4, and Myc) using a non-integrating sendaivirus would be preferred over those created from a lentiviral vector, which could have the potential for permanent transgene integration and disruption of critical cellular functions. As some lentiviral hiPSC reprogramming vectors express GFP during the hiPSC reprogramming process, hiPSC lines created via lentiviral reprogramming could contain a mistargeted GFP integrated randomly in the hiPSC genome, which could cause problems during PCR-based verification of GFP-fusion HDR template integration. It should be noted that the techniques described in this protocol could readily be applied to hESC lines, if hiPSCs are not available, given that many commonly-used hESC lines have been well-characterized and whole genome-sequenced.
BASIC PROTOCOL 1: DESIGN AND PREPARATION OF GUIDE RNA, Cas9 AND HDR TEMPLATE PLASMIDS
To insert a fluorescent fusion reporter tag into a gene of interest, we first select a target gene and use Benchling, a free, multipurpose genome viewer and integrated lab notebook (www.benchling.com), to design CRISPR guide RNAs targeting a region of interest. Benchling allows for selection of target guide RNAs and ranks them based on previously-published bioinformatic algorithms that predict on-target efficacy, while also simultaneously evaluating guide RNAs for their propensity to induce off-target mutations (Doench et al., 2014; Hsu et al., 2013). HDR repair templates can also be designed in Benchling, with an integrated option to alter the PAM recognition site in the HDR template so that it is not self-targeted by a chosen guide RNA. Both guide RNAs and HDR templates can be rapidly synthesized into double-stranded, sequence-verified genomic blocks (gBlocks) by Integrated DNA Technologies (IDT) and effectively blunt-end cloned into a pCR-Blunt II-TOPO Vector. Additionally, for the double strand break-inducing endonuclease, we prefer the pSpCas9(BB)-2A-Puro (PX459) v2.0 with puromycin selection from the Feng Zhang lab. This Cas9 plasmid can be purchased from Addgene. The basic protocol provided below outlines the steps for generation of the Cas9, guide RNA, and HDR template plasmids for downstream transfection into hiPSCs and is partially adopted from another Current Protocols publication detailing CRISPR/Cas9-mediated editing of hiPSCs (L. Yang et al., 2014).
Materials
pSpCas9(BB)-2A-Puro (PX459) V2.0 (PX459 v2.0; Addgene, cat. No. 62988)
LB agar plates containing 100 µg/mL ampicillin
LB liquid medium containing 100 µg/mL ampicillin
LB agar plates containing 50 µg/mL kanamycin
LB liquid medium containing 50 µg/mL kanamycin
PCR-grade sterile deionized water
Zero Blunt TOPO PCR Cloning Kit (Thermo Fisher Scientific, cat. no. K2800-20)
One Shot Top10 Chemically Competent E. coli cells (Thermo Fisher Scientific, cat. no. C404010)
M13 Forward (5'-GTTTTCCCAGTCACGACG-3') and M13 Reverse (5'-AACAGCTATGACCATG-3') universal sequencing primers (included with Zero Blunt TOPO PCR Cloning Kit)
Plasmid Extraction Mini Kit and Midi Kit (Qiagen)
Sterile pipet tips for picking colonies from agar plates
37°C bacterial incubator-shaker
45°C incubator for heat-shocking bacteria
Nanodrop micro spectrophotometer, or another device for measuring DNA concentration
DNA Sequence analysis software (e.g., NCBI BLAST, UCSC Genome Browser BLAT, DNASTAR LaserGene Suite)
10-mL bacterial culture tubes
Standard 1.5 mL Eppendorf tubes
Access to Sanger sequencing facility
L-shaped bacterial spreaders
Prepare the Cas9 plasmid
-
1
From Addgene, order the selected Cas9 plasmid, which will arrive as a bacterial stock.
-
2
With an L-shaped bacterial spreader, streak the bacterial stock onto an LB agar plate with 100 µg/mL ampicillin. The PX459 v2.0 Cas9 plasmid has an ampicillin resistance cassette. Incubate the plate at 37°C overnight in a designated bacterial incubator.
-
3
The next day, bacterial colonies should have propagated. These bacteria contain the Cas9 plasmid. Pick a single colony from the plate using a sterile pipette tip, and drop the tip into an Erlenmeyer flask containing 200 mL of LB liquid medium with 100 µg/mL ampicillin. Grow this inoculated culture overnight at 37°C in a designated bacterial growth incubator with shaking at 200 rpm.
-
4
After 12–16 hours, extract the Cas9 plasmid using a plasmid midiprep kit. Quantify the Cas9 plasmid DNA concentration with a Nanodrop micro spectrophotometer or another device. The final concentration for the Cas9 plasmid stock should be between 0.5 and 1 µg/mL in water. This is the Cas9 plasmid stock that will be used during the subsequent hiPSC nucleofection process.
Design the guide RNA and HDR template plasmids
-
5
Using a bioinformatics program such as Benchling, identify the genomic region that will be the target of a double-stranded DNA break induced by Cas9. Generate a single-stranded guide RNA for this target region near the selected gene of interest. As a reminder, the DSB is critical to facilitate homology directed repair. The guide RNA target sequence should have the format 5’-N19-NGG-3’, where NGG specifies the protospacer-adjacent motif (PAM) site. The guide RNA target region should be within 30 base pairs of the start codon designating the N-terminus of the selected protein, or the stop codon designating the C-terminus of the selected protein (see Figure 2 for details). DSBs that are closer to the mutation site typically result in higher levels of HDR. The target region for the DSB can be on either strand. We recommend that the guide RNA targets a non-coding region of the selected gene to avoid issues with altering the protein coding sequence of the selected gene. Benchling can provide information as to the on-target and off-target specificity of a selected guide RNA, based on integrated bioinformatic analysis. However, since these algorithms are not perfect predictors, we recommend selecting multiple guide RNAs for genome editing experiments to maximize the chances that one guide RNA will provide efficient genome editing.
-
6
Using the 5’-N19-NGG-3’guide RNA sequence identified in the previous step, generate a 455 bp sequence containing all elements needed for guide RNA expression, such as a U6 promoter, target sequence, guide RNA scaffold, and termination signal. This sequence can be synthesized into a standard Integrated DNA Technologies gBlock without any 5’ modifications. Additional information about the guide RNA gBlock generation process can be found in previous literature (L. Yang et al., 2014).
-
7
To facilitate the generation of the HDR template containing the fluorescent reporter gene of interest, we recommend using Benchling or another similar program. Sequences for common fluorescent tags such as eGFP can be found online or freely on Benchling. To minimize the risk of sterically hindering protein function, we recommend the addition of a flexible glycine-serine multi-repeat linker to connect the fluorescent tag to the N-terminus or C-terminus of the designated protein (see Figure 2 for details) (Chen, Zaro, & Shen, 2013). Linker sequences can be found freely online, and we have had success with a triplet repeat Glycine-Glycine-Glycine-Glycine-Serine linker. Design homology arms that are as large as possible on the 5’ and 3’ side around the fluorescent tag and linker, with homology arms greater than 500 bp recommended to increase the rate of HDR. These homology arms will be recognized by the endogenous DNA repair machinery to facilitate the HDR process. The maximum size of a standard gBlock is typically 2000 bp, so this should be considered when designing homology arms. Ideally, the fluorescent reporter sequence with linker should be under 1000 bp to maximize the size of the homology arms. For example, the eGFP sequence has a length of approximately 700 bp. Within the HDR template sequence, we also highly recommend modifying the PAM sequence that determines the Cas9 target directed by the guide RNA designed in the previous step, to prevent the Cas9 from cleaving the HDR template. Programs such as Benchling can provide HDR templates with modified PAM sites to prevent Cas9 from cutting the HDR template.
-
8
Using the HDR template sequence designed in the previous step, generate a standard gBlock up to 2000 bp without any additional modifications from Integrated DNA Technologies.
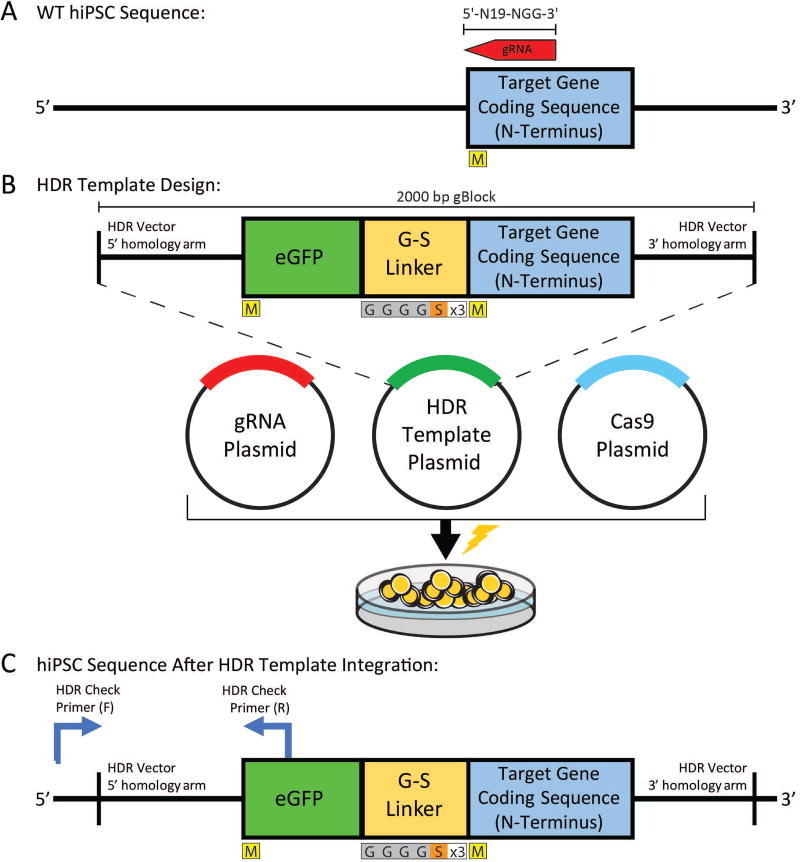
Figure 2.
Example schematic for fluorescent reporter HDR template design and integration at a target gene. Here, the fluorescent tag will be placed at the N-terminus of the encoded target protein. A) Representation of the wild type hiPSC sequence. Guide RNA target sequence (shown in red) should target within 30 bp of the mutation start site. M indicates start codon coding for methionine. B) HDR template design schematic. In a 2000 bp gBlock fragment, design the vector as shown. We recommend a glycine-serine linker with amino acid sequence GGGGSGGGGSGGGGS. C) hiPSC sequence after HDR template integration. Primers at the indicated regions can confirm successful eGFP template vector integration.
Prepare the guide RNA and HDR template plasmids
-
9
Dilute the guide RNA and HDR template gBlocks to 10 ng/µL with nuclease free water.
-
10
Clone the gBlocks into separate pCRII-Blunt-TOPO vectors. For each reaction, mix 4 µL gBlock DNA (10ng/µL) + 1 µL salt solution + 1 µL TOPO plasmid backbone (provided in the Zero Blunt TOPO PCR Cloning Kit) in separate Eppendorf tubes, with one tube per reaction. Leave reactions to incubate at room temperature for 30 minutes.
-
11
For transformation, add each 6 µL reaction to an aliquot of OneShot Top10 Chemically Competent E. coli cells, which have been pre-thawed on ice. Leave this mixture on ice for 30 minutes. Heat-shock the reaction mixture containing the E. coli at 45°C for exactly 1 minute and then return to ice for 1 minute. Return the cells to ice for 1 minute, then add 250 µL of room temperature SOC medium, which is provided in the Zero Blunt TOPO PCR Cloning Kit. Incubate at 37°C for 1 hour with shaking in a designated bacterial culture incubator.
-
12
Using an L-shaped bacterial spreader, plate out 50 µL of transformed bacteria into premade LB agar plates containing 50 µg/mL kanamycin. The pCRII-Blunt-TOPO vector has an integrated kanamycin resistance cassette. Plate the bacteria containing the guide RNA and HDR template vectors on separate agar plates. Spread the solution around the plate evenly with the spreader tool. Incubate the plates at 37°C overnight with the lid upside-down in a designated bacterial incubator. The leftover transformation mixture can be frozen as bacterial stock at −80°C in 25% glycerol in water.
-
13
The next day, pick individual colonies with separate sterile pipet tips, and drop the tips into designated 10 mL bacterial culture tubes containing 3 mL of LB broth with 50 µg/mL kanamycin. Incubate these starter cultures at 37°C in a bacterial incubator with shaking for 12–16 hours, typically overnight.
-
14
The next day, extract the plasmids using a standard plasmid miniprep kit. The final plasmid concentration should be between 0.5–1 µg/µL in water.
-
15
Submit the guide RNA and HDR vector plasmids for Sanger sequencing using the M13 forward and M13 reverse sequencing primers. The sequences returned should match the gBlock sequences for the guide RNA and HDR template gBlocks. Note that because the HDR template gBlock is typically larger than the 455 bp guide RNA gBlock and can be up to 2000 bp, the M13F and M13R sequencing primers may not be able to fully sequence the HDR template. If this is the case, design a new primer that can completely sequence the HDR template. We have also found that there is no need to linearize the HDR template plasmid to facilitate the HDR process.
BASIC PROTOCOL 2: TRANSFECTION OF hiPSCs WITH Cas9, GUIDE RNA, AND HDR TEMPLATE PLASMIDS
This protocol will demonstrate how to transfect the previously-constructed Cas9, guide RNA, and HDR template plasmids into hiPSCs to generate hiPSC lines with fluorescent reporters integrated at genes of interest. We recommend an electroporation-based method of hiPSC transfection, also known as nucleofection. Cells in the logarithmic phase of growth typically demonstrate the highest transfection efficiency and survival post-nucleofection. Since this procedure tends to be stressful for hiPSCs, cells should be allowed to recover for multiple days after nucleofection (See Figure 3 for detail). All cell culture should be conducted in a standard 37°C, 5% CO2 incubator using sterile technique.
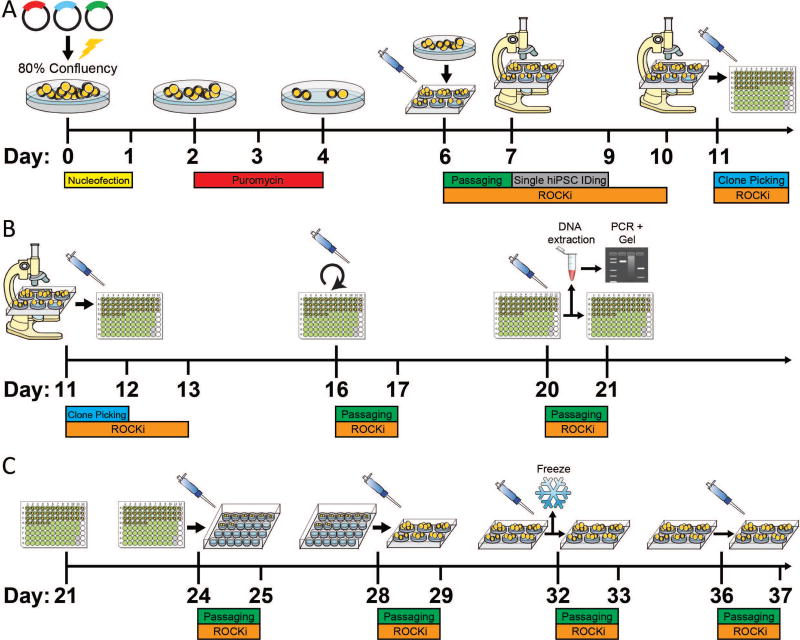
Figure 3.
Timeline for nucleofection, subcloning, and expansion of fluorescently-tagged hiPSCs. Assume a daily mTeSR1 media change for all hiPSC steps. Rho kinase inhibitor is recommended for passaging and steps where hiPSCs are plated sparsely or as single cells. A) Steps from nucleofection until initial clone picking. Begin by nucleofecting 80% confluent, low passage hiPSCs and conduct puromycin selection. After selection, allow clones to grow before replating cells in a serial dilution at very low density. Then, identify individual cells under a microscope and allow single, monoclonal colonies to grow to 100 cells in rho kinase inhibitor before picking into a 96-well plate. B) Steps from clone picking to positive HDR clone identification. After repicking monoclonal hiPSC colonies into a new 96-well plate, allow the colonies to grow in rho kinase inhibitor before redistributing them within the same well. Then, allow colonies to into 95% confluent monolayers before passaging half into a new plate while simultaneously harvesting half for HDR verification with DNA extraction and PCR/gel electrophoresis. Once positive clones are identified, proceed to C. If no positive clones are identified, re-nucleofect with the same guide RNA or order new guide RNAs. C) Once positive clones are identified, grow the clones in 96-well plates and passage positive clones into 24 and 6-well plates, consecutively. After clones are grown to 6-well plate format, freeze a subset of clones before passaging and expanding for long-term culture.
Materials
Wild type, low passage hiPSCs adapted for feeder cell-free growth on Matrigel
Matrigel (hESC-qualified; BD Sciences, cat. no. 354277)
DMEM/F12 medium (Thermo Fisher Scientific, cat. no. 11320033)
mTeSR1 medium (StemCell Technologies, cat. no. 05850)
Rho kinase inhibitor (ROCKi; Tocris cat. no. 1254)
Human Stem Cell Nucleofector Kit 2 (Lonza, cat. no. VPH-5022)
GFP plasmid as nucleofection positive control (pMax GFP vector supplied with Human Stem Cell Nucleofector Kit 2)
Cas9 plasmid DNA (from Basic Protocol 1)
gRNA expression vector (from Basic Protocol 1)
HDR template vector (from Basic Protocol 1)
Phosphate-buffered saline (PBS; Life Technologies, cat. no. 20012-050)
0.5M EDTA (Thermo Fisher Scientific, cat. no. 15575020)
6-well tissue culture–treated plates
15- and 50-mL conical centrifuge tubes (e.g., BD Falcon)
Hemocytometer for cell counting
Tabletop centrifuge with plate adapter
Amaxa Nucleofector II Device
Transfect hiPSCs with CRISPR plasmids
Prepare 6-well plates coated with Matrigel extracellular matrix, on which hiPSCs will grow in a feeder cell-free format. To coat 6-well plates with Matrigel, thaw Matrigel on ice and add 125 µL Matrigel to 50 mL of cold DMEM/F12 at a 1:400 dilution. Make sure pipette tips are cooled to 4°C before pipetting Matrigel. Two mL of Matrigel solution should be added per well of a 6-well dish. Matrigel should be allowed to coat the dish for at least 1 hour before use. Stocks of Matrigel-coated plates can typically be kept at 37°C for 1 month.
When ready for cell culture, remove Matrigel mixture in a designated plate and add wild type hiPSCs (ideally below passage 30) resuspended in mTeSR1 pluripotent stem cell growth medium containing 10 µM rho kinase inhibitor. The rho kinase inhibitor has been shown to dramatically improve survival of pluripotent stem cells during passaging and at low confluency (Watanabe et al., 2007). These hiPSCs can be freshly cultured (hiPSC passaging covered in steps 8–10 below) or obtained from a frozen stock.
At 24 hours after plating hiPSCs, replace media with fresh mTeSR1 without rho kinase inhibitor.
Cells should be allowed to grow, with daily replacement of mTeSR1, until they have reached approximately 80% confluency and are in the logarithmic phase of growth. These hiPSCs are ready for nucleofection. Note that one million cells are required for each nucleofection reaction.
At approximately 2 hours before nucleofection, warm fresh mTeSR1 with 10 µM rho kinase inhibitor to 37°C.
At 1 hour before nucleofection, prepare a pre-coated Matrigel plate by replacing the DMEM/F12 containing 1:400 diluted Matrigel with 2 mL of prewarmed mTeSR1 with 10 µM rho kinase inhibitor. Return this new plate to 37°C, as it will be the destination for nucleofected cells.
-
Prepare the nucleofection reaction solution in a sterile cell culture hood. From the Human Stem Cell Nucleofector Kit 2, add 82 µl of Nucleofector® Solution plus 18 µL of supplement to make 100 µL supplemented nucleofection solution per reaction. To this 100 µL, add 1.5 µg Cas9, 1.5 µg HDR vector, and 1.5 µg targeting guide RNA plasmids. Ideally, the total reaction mixture per reaction should be under 110 µL.
We also recommend having a separate transfection reaction with 100 µL supplemented nucleofection solution plus 2 µg GFP plasmid as a positive nucleofection control. A GFP plasmid is supplied as part of the Human Stem Cell Nucleofector Kit 2.
Start the hiPSC dissociation and passaging process by removing mTesR1 and adding 1 mL 0.5 mM EDTA diluted in PBS to wells of approximately 80% confluent wild type hiPSCs. Incubate the plate at 37°C for 5 minutes.
-
Gently aspirate off the 0.5 mM EDTA.
Cells should not be completely dissociated, but if examined under the microscope, the hiPSC colonies will be seen breaking apart into individual cells and lightly adhered to the cell culture plate.
Using a 1000 µL manual pipetman, spray off the loosened hiPSCs with 1 mL fresh, prewarmed mTesR1 with 10 µM rho kinase inhibitor. Collect the 1 mL cell suspension in a new 15 mL tube. Collect hiPSCs from as many wells needed for nucleofection, remembering that 1 million cells will be required per reaction.
Count the cells with a cell counter and determine the volume needed for 1 million cells per nucleofection reaction.
Separate the cells needed for different nucleofection reactions into separate 15 mL conical tubes. Centrifuge the cells at 200 × g for 5 minutes.
Aspirate off the supernatant and resuspend the hiPSC cell pellet of 1 million cells into the designated, prepared nucleofection reaction solution containing the Cas9 plasmid, guide RNA plasmid, and HDR template plasmid.
Transfer the cell suspension into the center chamber of a Nucleocuvette (provided with Human Stem Cell Nucleofector Kit 2). Insert the Nucleocuvette into the Nucleofector II device and nucleofect with the appropriate nucleofection program. We recommend program B-020 for the Nucleofector II device.
Immediately transfer the nucleofected cells into the destination 6-well plate containing prewarmed mTeSR1 with 10 µM rho kinase inhibitor. Add the cells from 1 reaction into 1 well and distribute the cells evenly around the well. Return the cells to a 37°C cell culture incubator.
BASIC PROTOCOL 3: MONOCLONAL EXPANSION AND GENETIC VALIDATION OF hiPSCs WITH FLUORESCENTLY-TAGGED ENDOGENOUS PROTEINS
Human induced pluripotent stem cells represent a powerful, multipurpose cell type since they can be differentiated into most somatic human tissues and can be derived from a small sample of skin or blood. Care must be taken to maintain hiPSCs in an optimal, highly pluripotent state for downstream differentiation by regularly passaging hiPSCs and preventing cells from becoming overconfluent. Following the electroporation process described previously, hiPSCs that successfully received the Cas9-Puro PX459 v2.0 plasmid are selected using puromycin treatment and subsequently replated for monoclonal expansion and genetic validation. This monoclonal expansion process ensures that hiPSC colonies are homogenously composed of genotypically identical cells (See Figure 3 for details). All cell culture should be conducted in a standard 37°C, 5% CO2 incubator using sterile technique. Portions of this protocol are adapted from previous literature (L. Yang et al., 2014).
Materials
HiPSCs transfected with guide RNA, Cas9, and HDR template plasmids (from Basic Protocol 2)
Matrigel (hESC-qualified; BD Sciences, cat. no. 354277)
DMEM/F12 medium (Thermo Fisher Scientific, cat. no. 11320033)
mTeSR1 medium (StemCell Technologies, cat. no. 05850)
Rho kinase inhibitor (ROCKi; Tocris cat. no. 1254)
Phosphate-buffered saline (PBS; Life Technologies, cat. no. 20012-050)
0.5M EDTA (Thermo Fisher Scientific, cat. no. 15575020)
6-, 24-, and 96-well tissue culture–treated plates
15- and 50-mL conical centrifuge tubes (e.g., BD Falcon)
Tabletop centrifuge with plate adapter
Standard cell culture laminar flow hood
Puromycin dihydrochloride (Thermo Fisher Scientific, cat. no. A1113803)
Tissue culture hood microscope
Standard thermocycler and gel apparatus for PCR/gel electrophoresis
96-well thermocycler compatible plate
prepGEM Tissue Kit for DNA extraction (VWR cat. no. 95044-034)
KOD Xtreme™ Hot Start DNA Polymerase for PCR (Millipore, cat. no. 71975-3)
Bambanker Cell Freezing Medium (Fisher Scientific, cat. no. NC9582225)
CoolCell LX (Sigma-Aldrich, cat. no. BCS-405)
Nunc™ Biobanking and Cell Culture Cryogenic Tubes (Fisher Scientific, cat. no. 375418)
Perform puromycin selection and monoclonal expansion of HDR-edited hiPSCs
-
1
Twenty-four hours after nucleofecting hiPSCs with guide RNA, Cas9, and HDR template plasmids, replace the media on the nucleofected cells by changing from mTesR1 with 10 µM rho kinase inhibitor to 2 mL of regular mTesR1.
It is normal to observe above-average cell death at this stage. If a positive-control GFP plasmid was used, GFP expression should begin.
-
2Forty-eight hours after nucleofection, begin puromycin selection of nucleofected hiPSCs. Cells that are successfully transfected with pSpCas9(BB)-2A-Puro (PX459) v2.0 will be resistant to selection with puromycin, whereas cells not receiving the Cas9 plasmid should die.
- A range between 0.5–1 µg/mL puromycin diluted in mTeSR1 should be added to the transfected cells. If there is low cell survival post-nucleofection, use a puromycin concentration closer to 0.5 µg/mL.
-
3
Twenty-four hours after initiating puromycin selection, replace media with fresh mTeSR1 with the same concentration of 0.5–1 µg/mL puromycin.
-
4
After 48 hours of puromycin selection, significant cell death should have occurred. Replace media with fresh mTeSR1 without puromycin and leave for 24 hours.
-
5
Twenty-four hours after ending puromycin selection, replace old mTeSR1 with 2 mL fresh mTeSR1.
-
6
After 24 hours, begin replating transfected, puromycin-selected hiPSCs for monoclonal expansion. Replate the selected cells into a new Matrigel pre-coated 6-well dish, using the same technique as used previously for hiPSC passaging with 0.5 mM EDTA in PBS and mTeSR1 with 10 µM rho kinase inhibitor. Perform a serial dilution of cells in mTeSR1 with 10 µM rho kinase inhibitor, so that each well of the new 6-well dish has half as many cells as the preceding well.
For example, we recommend replating into a Matrigel pre-coated 6-well dish with new wells receiving 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 dilutions of cells in mTeSR1 with 10 µM rho kinase inhibitor. The goal here is to obtain a cell distribution where individual hiPSCs can survive at a very sparse density and eventually grow into colonies in a monoclonal, homogenous format that can be easily picked for expansion and downstream analysis.
-
7
Twenty-four hours after replating cells in a serial dilution, use a fine-tipped marker to circle individual hiPSCs under a microscope. Pending survival, these cells should grow out into monoclonal, genetically homogenous hiPSC colonies. Replace the media with fresh mTeSR1 with 10 µM rho kinase inhibitor.
-
8
Continue replacing mTeSR1 with 10 µM rho kinase inhibitor on the transfected cells daily for 3–4 days. Monoclonal cells should slowly grow out into 100–200 cell colonies, at which point mTeSR1 with 10 µM rho kinase inhibitor should be swapped for regular mTeSR1 without rho kinase inhibitor.
While in 10 µM rho kinase inhibitor, it is normal for the cells to have a spread-out morphology. They should revert to the standard, tightly packed hiPSC colony morphology when the rho kinase inhibitor is removed.
-
9
Pre-coat 96-well plates with 1:400 dilution Matrigel in DMEM/F12, as demonstrated previously with 6-wells. Twenty-hours after monoclonal, 100–200 cell large colonies have been returned to mTeSR1 without rho kinase inhibitor, pick the colonies under a tissue culture hood microscope and re-plate them into 96-well plates with mTeSR1 containing 10 µM rho kinase inhibitor.
We recommend picking up to 96 colonies for examining HDR efficiency, which tends to be lower than NHEJ.
-
10
Twenty-four hours after clone picking, replace media with fresh mTeSR1 containing 10 µM rho kinase inhibitor.
-
11
Forty-eight hours after clone picking, replace media with fresh mTeSR1 without 10 µM rho kinase inhibitor. Continue changing mTeSR1 for 3–4 days until colonies have reached approximately 1000 cells, or approximately 1 mm diameter, at which point they will become visible to the naked eye in the 96-well plate.
-
12
When the picked colonies in the 96-well plate become visible to the naked eye, passage the colonies at a 1:1 dilution in mTeSR1 with 10 µM rho kinase inhibitor into the same wells of the 96-well plate.
When the monoclonal, 1000-cell large colonies are re-passaged, they will be broken apart and individual cells will be evenly redistributed in the same well. This will allow for even growth of the hiPSC colonies into a monolayer distribution within the same well.
-
13
Twenty-four hours after redistributing the colonies in the 96-well plate, change media back to mTeSR1 without rho kinase inhibitor. Continue changing mTeSR1 daily until the cells in the 96 well plate have achieved approximately 95% confluency.
Harvest transfected, puromycin-selected clones for PCR and DNA sequencing validation
-
14
Design a primer set to validate proper integration of the fluorescent tag at the site of interest. Design the forward primer located beyond the 5’ homology arm and the reverse primer within the DNA sequence of the fluorescent tag (See Figure 2 for details). Ideally, the primer set should generate a DNA amplicon between 500–1000 bp.
-
15
When cells replated into the 96-well plate have reached 90% confluency, perform a 1:2 cell passage in mTeSR1 with 10 µM rho kinase inhibitor into a new, Matrigel pre-coated 96-well plate. Return the newly passaged cells to the 37°C cell culture incubator. Transfer the remaining 1:2 dilution of cells resuspended in mTeSR1 with 10 µM rho kinase inhibitor into a 96-well thermocycler compatible plate.
-
16
Spin the 96-well thermocycler compatible plate in a centrifuge with a plate adaptor for 5 minutes at 200 × g.
-
17
Aspirate the supernatant and resuspend the cell pellets with 100 µL PBS. Re-spin the 96-well thermocycler compatible plate in PBS to wash the pellets for 5 minutes at 200 × g.
-
18Aspirate the supernatant. Prepare a DNA extraction reaction with the following reagents:
- Per 40 µL reaction for each pellet: 35.6 µL water, 4 µL 10x prepGEM gold buffer (Zygem), 0.4 µL prepGEM tissue protease enzyme.
-
19
Resuspend the cell pellets in the DNA extraction reaction shown above.
-
20Incubate the reaction in a thermocycler:
- 75°C for 5 minutes
- 95°C for 5 minutes, then cool down to 4°C
-
21Prepare a 25 µL KOD Hotstart PCR reaction (1 per hiPSC clone) to obtain an amplicon to validate fluorescent reporter integration into the appropriate target location of the selected gene:
- 2.5 µL of template hiPSC DNA from the previous step
- 12.5 µL 2X Xtreme Buffer
- 5 µL dNTPs (2mM)
- 0.75 µL Forward primer (10 uM)
- 0.75 µL Reverse primer (10 uM)
- 0.5 µL KOD Polymerase
- Water to 25 µL
-
22Perform PCR for all hiPSC clone DNA samples with the following parameters:
i. 2 min 94°C (polymerase activation) ii. 10 secs 98°C (denaturation) iii. 30 secs lowest primer Tm°C (annealing) iv. 1 min 68°C (extension) Repeat steps ii–iv for 40 cycles. -
23
Run PCR products on an agarose gel to determine if fluorescent reporter integration was successful.
Based on the primer design, bands will only appear if the fluorescent reporter was successfully integrated into the genome of hiPSCs.
-
24
Submit positive clones for Sanger sequencing to verify proper sequence integration.
Expand positively-identified candidate clones for long-term storage and downstream use
-
25
If hiPSC clones containing a fluorescent reporter insert were positively identified by PCR and Sanger sequencing, begin expanding the candidate clones from the 96-well culture plate in the 37°C cell culture incubator.
-
26
When candidate hiPSC clones reach 90% confluency in the 96 well-plate, perform standard hiPSC passaging procedures, as mentioned previously, at 1:1 dilution in mTeSR1 with 10 µM rho kinase inhibitor into a new, Matrigel pre-coated 24-well plate.
-
27
The day after passaging, return the cells to mTeSR1 without rho kinase inhibitor. Change mTeSR1 daily until cells reach 90% confluency in the 24-well plate.
-
28
When candidate hiPSC clones reach 90% confluency in the 24 well-plate, perform standard hiPSC passaging procedures, as mentioned previously, at 1:1 dilution in mTeSR1 with 10 µM rho kinase inhibitor into a new, Matrigel pre-coated 6-well plate.
-
29
The day after passaging, return the cells to mTeSR1 without rho kinase inhibitor. Change mTeSR1 daily until cells reach 90% confluency in the 6-well plate.
-
30
When candidate hiPSC clones reach 90% confluency in the 6-well plate, perform standard hiPSC passaging procedures, as mentioned previously, at 1:12 dilution in mTeSR1 with 10 µM rho kinase inhibitor into a new, Matrigel pre-coated 6-well plate. These cells can now be regularly maintained with an mTeSR1 change daily, optimally performing a 1:12 passage on 90% confluent hiPSCs every 4 days using mTeSR1 with 10 µM rho kinase inhibitor.
-
31We recommend creating a backup stock of the leftover cells from this 6-well passaging step by freezing hiPSCs in Bambanker cell freezing medium.
- For long term cryo-storage of edited hiPSCs, first centrifuge the leftover cells at 200 × g for 5 minutes.
- Aspirate the supernatant and resuspend the cells directly in Bambanker freezing medium, at approximately 1 million cells per mL.
- Place the Bambanker-resuspended cells into a standard 1.8 mL cryo-tube and place into a CoolCell LX freezing container.
- Place the freezing container at −80°C to allow the cells to slow-freeze (approximately 1°C per minute) to −80°C.
- When the cells have reached −80°C, the CoolCell LX freezing container can be removed, and the cryo-tubes can be kept at −80°C. For long term storage, the cells can be stored in liquid nitrogen.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps, unless otherwise specified. Shelf life for all cell culture media is 1 month at 4°C.
Matrigel-diluted medium
Add Matrigel stock (hESC-qualified; BD Sciences, cat. no. 354277) to DMEM/F12 medium (Thermo Fisher Scientific, cat. no. 11320033) at a dilution of 1:400 to pre-coat plates for hiPSC maintenance. Thaw Matrigel stock on ice at 4°C, and dilute Matrigel in cold DMEM/F12 quickly, with cooled pipette tips, to prevent premature solidification. Once plates are pre-coated with Matrigel dilution, store plates at 37°C until needed. When ready for use, aspirate old Matrigel dilution from plates and add fresh cell culture media specific to hiPSCs, such as mTeSR1. We do not recommend repeatedly freeze-thawing the Matrigel stock.
hiPSC maintenance medium (for daily hiPSC culture)
mTeSR1 feeder-free pluripotent stem cell maintenance medium (StemCell Technologies, cat. no. 05850)
hiPSC passaging and subcloning medium (for passaging hiPSCs and maintaining single hiPSCs and small colonies after passaging)
mTeSR1 feeder-free pluripotent stem cell maintenance medium containing: 10 µM rho kinase inhibitor
COMMENTARY
Background Information
Within the past 10 years, hiPSC technology has dramatically advanced the fields of stem cell biology and regenerative medicine (Takahashi et al., 2007). With only a small sample of a patient’s own skin or blood, cells nearly identical to human embryonic stem cells can be derived, albeit mostly without the associated ethical controversies. The recent revolution in genome editing technologies, particularly in CRISPR/Cas9 editing, has further unlocked the potential to make highly genetically-customized hiPSCs in a matter of weeks (L. Yang et al., 2014). Gene knockouts, frameshift mutations, and large deletions are relatively easy to generate, requiring only the Cas9 endonuclease and the targeting guide RNA(s) to either create small insertions and deletions or to even eliminate kilobase-large segments of DNA (Hsu, Lander, & Zhang, 2014). Nearly any region of the genome in almost any dividing cell type can now be genetically modified by introducing only a Cas9 endonuclease, an HDR donor repair template, and a targeting guide RNA (Hsu et al., 2014). Single point mutations can be generated, or kilobases of DNA can be inserted to generate reporter cell lines and drug selection resistance cassettes, among other applications. Such cellular customization was more technically challenging prior to the development of CRISPR/Cas9 for use in mammalian systems. Finally, these genetic customization possibilities can be easily multiplexed by using multiple targeting guide RNAs and HDR templates (Cong et al., 2013). Although much work remains to be done in further improving Cas9 efficacy and specificity, genome editing technologies can be combined with hiPSCs to make genetically unique stem cells such as these GFP-fusion reporter hiPSCs, which can be further differentiated into a variety of cell types such as contracting cardiomyocytes (Figure 4, Video 1) (CPHG Unit 21.12, Sharma et al 2017, Curr Protoc, “Differentiation and Contractile Analysis of GFP-Sarcomere Fusion Reporter hiPSC-Cardiomyocytes”.)
Figure 4.
Example of cells differentiated from genetically-modified hiPSCs with fluorescently-labeled endogenous proteins. These are hiPSC-derived cardiomyocytes (contractile heart muscle cell) exhibiting an eGFP tag at the N-terminus of titin, a protein specific to the sarcomere. These cardiomyocytes spontaneously contract as demonstrated in Video 1. Cells were imaged using a 100x objective. For more information on the differentiation and analysis of hiPSC-derived cardiomyocytes with fluorescently-labeled sarcomeres, please see UNIT 21.12, Sharma et al 2017, Curr Protoc, “Differentiation and Contractile Analysis of GFP-Sarcomere Fusion Reporter hiPSC- Cardiomyocytes”.
Perhaps one of the greatest strengths of CRISPR/Cas9-mediated gene editing is the ease with which large segments of DNA can be introduced into a genome both in vitro and in vivo using homology directed repair. This enables rapid generation of the fluorescent reporter lines shown here, but many other applications can be envisioned. Although the HDR template that we describe here is contained with a single 2 kilobase-large gBlock, multiple gBlocks can be assembled contiguously using the Gibson Assembly method (Gibson et al., 2009). This procedure allows for efficient conjoining of multiple DNA fragments such as gBlocks in a single reaction, enabling the creation of HDR templates that could be many kilobases in size. Using Gibson assembly, highly complex protein tags can be assembled into a single HDR vector, or alternatively, the length of homology arms used to facilitate the HDR process can be dramatically increased. This possibility, combined with the ability to easily multiplex Cas9 to target several regions of the genome, unlocks the ability to simultaneously modify megabases of genomic DNA. Large-scale CRISPR-mediated genome editing could be extremely useful in the burgeoning field of synthetic biology, which explores rewriting large portions of the genome to create custom gene circuits or potentially artificial organisms (Lienert, Lohmueller, Garg, & Silver, 2014).
Critical Parameters and Troubleshooting
Design and generation of fluorescent reporter hiPSCs can be accomplished within two months. We have provided details that may further promote the generation of hiPSCs with fluorescence-tagged endogenous proteins and provide tips that may serve to troubleshoot the process if difficulties are experienced.
Plasmid design and generation
Rigorous planning is needed to properly design guide RNAs and HDR templates that will insert a fluorescent reporter into a gene of interest in the correct orientation and frame. When selecting a gene to target, the number of guide RNA target sites within the gene start codon (N-terminus GFP reporter) or stop codon (C-terminus GFP reporter) may be a limiting factor. We highly recommend picking a guide RNA that has a high on-target editing frequency, low-off target editing frequency, and is within 30 base pairs of the mutational start site. A variety of bioinformatic tools are available online to assist with guide RNA design, and we recommend the free-to-use Benchling software (www.benchling.com). Once a guide RNA is chosen, the sequence can be readily inserted into an approximately 450 bp Integrated DNA Technologies gBlock with all components needed for guide RNA expression, as previously demonstrated (L. Yang et al., 2014).
Likewise, when generating an HDR template vector containing the fluorescent reporter of interest, we highly recommend using a template design program such as Benchling. Using this software, a custom HDR template optimized for the targeting guide RNA can be created. Benchling will automatically modify the PAM site in the HDR template so that the selected guide RNA does not self-target the generated HDR template. Benchling also allows for length customization for the 5’ and 3’ homology arms surrounding the fluorescent gene and linker of choice. We recommend that homology arms be at least 500 bp on each side, with a total maximum HDR template size of 2000 bp, as this is the upper size limit for IDT gBlocks. On rare occasions, IDT has been unable to generate a gBlock for the HDR template of interest because of significant DNA repeat sequences within the homology arms. If this occurs, the homology arms may have to be shortened or lengthened to optimize gBlock generation. We have generally preferred to use the PX459 v2.0 Cas9 vector, as in our hands it enables efficient generation of DSBs. A variety of DSB-inducing endonucleases have been generated by the Feng Zhang lab and are available through Addgene. These include the enhanced-specificity Cas9 (espCas9), as well as similar endonucleases such as Cpf1 (Slaymaker et al., 2016; Zetsche et al., 2015).
We recommend using the Zero Blunt TOPO PCR Cloning Kit to perform straightforward blunt-end cloning of the gBlocks into a PCR-Blunt II-Topo vector. If no E. coli colonies are obtained after transformation and bacterial selection, make sure that the antibiotic resistance of choice is no older than 1 month, or try re-transforming the cells. Competent E. coli cells for bacterial transformation should also be handled carefully and allowed to slowly thaw on ice before transformation. If there is low plasmid yield after plasmid transformation and bacterial expansion, we recommend retrying with fresh reagents for optimal bacterial transformation and plasmid growth. Always sequence plasmids after generation to verify that the plasmids have the correct guide RNA and HDR template sequences. A single point mutation anywhere in the gBlock or expression vector can alter the efficacy of the genome editing process, or worse, completely alter the protein structure of the targeted protein of interest. Save time and effort downstream by double checking that all plasmids are of optimal quality and contain the correct DNA sequences. For long-term storage of plasmids, we recommend TE buffer instead of water.
Nucleofection of CRISPR plasmids into hiPSCs
Once the CRISPR plasmids have been correctly generated, the nucleofection process can commence. The best hiPSCs to use for nucleofection are of low passage, highly-proliferative, and not spontaneously differentiating. It may also be worthwhile to karyotype hiPSCs before use, to ensure that hiPSCs do not contain any irregular chromosomal abnormalities that may arise during long-term cell culture. We do not recommend using feeder cells for hiPSC culture. Maintain hiPSCs carefully by changing mTeSR1 every day and not allowing cells to become overconfluent during maintenance. If cells begin to behave irregularly, divide slowly, or spontaneously differentiate, use a new batch of hiPSCs. Ensure that the nucleofection process is performed quickly to avoid cell death. A major hurdle for generating custom hiPSCs is the nucleofection process and low cell survival post-nucleofection. This could be caused by many factors, including but not limited to:
Transfecting overconfluent hiPSCs not in the logarithmic phase of growth
Waiting too long after nucleofection to return the cells to the 37°C incubator
Using old or expired nucleofection reagents or cell culture media
Replating onto Matrigel plates that are older than 1 month
Not using rho kinase inhibitor after nucleofection
Using a DNA volume that is greater than 10% of the total reaction volume
Not pre-warming the mTeSR1 that the cells are replated in post-nucleofection
Even if these issues are all avoided, there may still be some cell death occurring post-nucleofection, which is not surprising as electroporation is a highly-stressful process for hiPSCs.
Monoclonal expansion and genetic validation of edited hiPSCs
After nucleofection, allow cells to recover for two days, while changing mTeSR1 daily, before proceeding to puromycin selection of cells that have taken up the Cas9 plasmid. Puromycin selection is yet another critical step where significant cell death can occur. To determine the optimal concentration of puromycin to use on genome-edited cells, we highly recommend optimizing the concentration first on wild type hiPSCs at different confluencies. Anywhere between 0–1 µg/mL puromycin could be effective for selection. Ideally after the selection process, dozens of small colonies of 5–10 cells will be present, scattered throughout the plate of transfected hiPSCs. At this point, we recommend waiting for the cells to grow out to 100 colonies large before replating them, in mTeSR1 with rho kinase inhibitor, in a serial dilution so that individual cells can be identified. Notably, this contrasts with other protocols, which recommend directly picking individual colonies into new 96-well plates once the colonies have become 100 cells in size after puromycin selection (L. Yang et al., 2014). The reason we recommend conducting a serial dilution is to increase the chances of monoclonal colony formation. After the puromycin selection process is complete, the remaining colonies may not be monoclonal and may consist of wild-type cells that possibly survived the puromycin selection process and migrated to join the edited cells. Alternatively, colonies picked immediately after puromycin selection could consist of genome-edited cells of multiple genotypes. Downstream, genotypically mixed colonies can be a nuisance as this will require additional laborious rounds of monoclonal subcloning. By performing a serial dilution after puromycin selection and immediately identifying individual, isolated hiPSCs under a microscope the next day, you can increase the probability that the colonies picked subsequently are monoclonal and coming from a single ancestral cell. However, extremely important to the serial dilution process and ensuring that individual hiPSCs survive during replating is the addition of rho kinase inhibitor. This may in fact be the most critical aspect of the cell culture portion of this protocol, as rho kinase inhibitor dramatically improves hiPSC survival, especially when these cells are plated at low density (Watanabe et al., 2007). We find that multiday treatment with rho kinase inhibitor can exponentially increase the number of surviving hiPSCs during this protocol.
When performing DNA extraction on picked hiPSC colonies to evaluate editing efficiency, make sure that the cells in the 96-well plate are at 95–100% confluency before passaging for extraction. This will maximize the amount of DNA that can be PCR amplified. It may be worthwhile to have multiple primer sets available for checking fluorescent reporter integration. In this protocol, we recommend a primer set where one primer sits beyond the 5’ homology arm and one primer sits in the fluorescent sequence itself. This will ensure that only cells with the reporter integrated into the genome will generate an amplicon with this primer set. If the primer set appears to have non-specific banding or other irregularities, another primer set can be designed with one primer in the fluorescent reporter sequence and one primer beyond the 3’ homology arm. Final editing efficiency can vary dramatically based on the efficiency of the nucleofection, integration of the HDR template, rate of Cas9 cutting, and luck in picking positive monoclonal colonies. Proper HDR template integration should be expected in under 10% of the total picked clones. If no edited colonies are detected via PCR and gel electrophoresis, consider repeating the nucleofection process with a new targeting guide RNA. If positive clones are identified, expand them carefully from 96- to 24- to 6-well plates before freezing a subset of them as a backup. Mistakes and contamination can happen in cell culture, so be sure to freeze a backup of candidate clones as soon as possible.
Interpreting Results
Plasmid design and generation
We highly encourage designing targeting guide RNAs as close as possible to the intended mutation site, as closer guides tend to result in higher HDR frequency. For generating the HDR template vector containing a fluorescent reporter construct, as well as the guide RNA vector, gBlocks (available from IDT) can be quickly designed, given that they do not contain significant repeat sequences. We recommend using the Zero Blunt TOPO PCR blunt end cloning system because it allows for straightforward, one-step cloning of blunt-end gBlocks directly into the PCR-Blunt II-TOPO vector. Directionality of gBlock integration into the vector does not matter because the gBlock designed in previous literature contains all the necessary components needed for expression (L. Yang et al., 2014). Plasmids can be quickly transformed into OneShot TOP10 chemically-competent E. coli and plated onto kanamycin resistant plates, after which dozens of clones should be visible. We recommend picking two transformed, kanamycin-resistant colonies for each plasmid for expansion, plasmid extraction, and Sanger sequencing. Expect to be unable to sequence the center of the HDR vector with the M13F and M13R primers if the corresponding gBlock is close to 2000 bp. In this scenario, design a new primer for the center of the gBlock.
Plasmid Nucleofection and Monoclonal Expansion of Edited Cells
We recommend using hiPSCs in the logarithmic phase of growth and under passage 30 for nucleofection of plasmids. Expect to see significant cell death following nucleofection and during the puromycin selection process. Following the puromycin selection process, small colonies should remain, and these colonies should have taken up the PX459 v2.0 Cas9 plasmid, conferring puromycin resistance. After allowing these colonies to grow, there should be approximately 50–100 small colonies in a single well of a 6-well dish. However, these colonies will likely still be mixed with some residual wild type cells or composed of multiple edited genotypes. Thus, after serial dilution and replating at low density, you should be able to identify single, isolated surviving hiPSCs. These cells, in the presence of multiday rho kinase inhibitor treatment, should survive and grow out into monoclonal colonies for clone picking into a 96-well plate. After allowing picked clones to grow in a 96-well plate, DNA extraction and subsequent PCR with gel electrophoresis should give a positive, HDR templated-integrated clone percentage of 10% or so for the total number of clones picked. However, this percentage can be highly variable based on the efficiency of the initial nucleofection and subsequent steps.
Time Considerations
Generation of hiPSCs with fluorescently-tagged endogenous proteins will require a considerable amount of time and effort in cell culture. Work involving hiPSCs requires daily maintenance and replenishment of pluripotency-sustaining media such as mTeSR1. Subcloning of genome-edited hiPSCs is often required to obtain truly homogenous, monoclonal populations of the cell line of interest, and can be a tedious process of routine clone picking, passaging, and waiting for growth. Fluorescence-activated cell sorting of individual edited hiPSCs may speed up the subcloning process as well, and could be considered as an alternative to puromycin-based selection and manual monoclonal hiPSC expansion. We have observed that regular use of rho kinase inhibitor during culture of small hiPSC colonies and individual hiPSCs for monoclonal expansion can dramatically improve cell survival, thus increasing the number of colonies that may have the mutation of interest. Finally, we highly recommend freezing hiPSCs at multiple stages after genome editing to save time and effort in the case of potential cell culture contamination or other unforeseen circumstances. See figures for more information about time constraints in plasmid design and genome editing.
Supplementary Material
Video of cardiomyocytes differentiated from hiPSCs exhibiting a N-terminus GFP fusion tag in the sarcomeric protein titin. These cardiomyocytes spontaneously contract and express GFP beginning at day 7–10 post-differentiation from hiPSCs. Cells were recorded using a 100x objective. For more information on the differentiation and analysis of hiPSC-derived cardiomyocytes with fluorescently-labeled sarcomeres, please see CPHG UNIT 21.12, Sharma et al 2017, Curr Protoc, “Differentiation and Contractile Analysis of GFP-Sarcomere Fusion Reporter hiPSC- Cardiomyocytes”.
SIGNIFICANCE STATEMENT.
Cas9/Crispr engineering of human induced pluripotent stem cells (hiPSCs) provides an important tool for assessing human ‘tissues’ such as neurons and heart cells. HiPSCs have unlocked new possibilities in human disease modeling, drug discovery, and cell therapy. Improvements in CRISPR/Cas9-mediated genome editing allow rapid and precise introduction of reporter genes. Exemplifying this union of two emerging technologies, we describe a method for rapid generation of hiPSCs with fluorescently-tagged proteins. These fluorescently-labeled hiPSCs can be used to non-invasively examine how intracellular proteins behave as stem cells grow, divide, and differentiate. Since pluripotent stem cells can differentiate into nearly any cell type, hiPSCs with fluorescently-labeled endogenous proteins are useful to researchers in a broad range of fields.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) Grants 1R01HL080494 and 1R01HL084553 (to C.E.S.), the Foundation Leducq (C.E.S.), the Howard Hughes Medical Institute (C.E.S.), the NIH NHLBI Cardiovascular Development Consortium (CVDC) Postdoctoral Research Fellowship (A.S.), and Wellcome Trust Grant 206466/Z/17/Z (C.N.T.).
LITERATURE CITED
- Bae S, Park J, Kim JS. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30(10):1473–1475. doi: 10.1093/bioinformatics/btu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–1369. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kuhn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol. 2015;33(5):543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- Churko JM, Burridge PW, Wu JC. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods in molecular biology. 2013;1036:81–88. doi: 10.1007/978-1-62703-511-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I, Root DE. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol. 2014;32(12):1262–1267. doi: 10.1038/nbt.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Hodgkins A, Farne A, Perera S, Grego T, Parry-Smith DJ, Skarnes WC, Iyer V. WGE: a CRISPR database for genome engineering. Bioinformatics. 2015;31(18):3078–3080. doi: 10.1093/bioinformatics/btv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Lienert F, Lohmueller JJ, Garg A, Silver PA. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat Rev Mol Cell Biol. 2014;15(2):95–107. doi: 10.1038/nrm3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Hino K, Bono H, Ui-Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics. 2015;31(7):1120–1123. doi: 10.1093/bioinformatics/btu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533(7601):125–129. doi: 10.1038/nature17664. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Wu JC. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science translational medicine. 2017;9(377) doi: 10.1126/scitranslmed.aaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351(6268):84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang JL, Byrne S, Pan J, Church GM. CRISPR/Cas9-Directed Genome Editing of Cultured Cells. Curr Protoc Mol Biol. 2014;107:31, 31, 31–17. doi: 10.1002/0471142727.mb3101s107. [DOI] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation research. 2014;114(3):511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of cardiomyocytes differentiated from hiPSCs exhibiting a N-terminus GFP fusion tag in the sarcomeric protein titin. These cardiomyocytes spontaneously contract and express GFP beginning at day 7–10 post-differentiation from hiPSCs. Cells were recorded using a 100x objective. For more information on the differentiation and analysis of hiPSC-derived cardiomyocytes with fluorescently-labeled sarcomeres, please see CPHG UNIT 21.12, Sharma et al 2017, Curr Protoc, “Differentiation and Contractile Analysis of GFP-Sarcomere Fusion Reporter hiPSC- Cardiomyocytes”.