SUMMARY
Background
A total proctocolectomy followed by ileal pouch-anal anastomosis (IPAA) is a potentially curative surgery for ulcerative colitis (UC) or familial adenomatous polyposis (FAP). About 5-35% of UC patients and 0-11% of FAP patients develop subsequent inflammation of the ileal pouch termed pouchitis.
Aim
The aim of this review was to provide a comprehensive analysis of the research studying the possible pathogenesis of pouchitis. The goals were to identify promising areas of investigation, to help focus clinicians, researchers, and patients on how to better understand and then potentially manage ileal pouchitis, and to provide avenues for future research investigations.
Methods
This review examined manuscripts from 1981 through 2015 that discussed and/or proposed hypotheses with supportive evidence for the potential underlying pathogenic mechanism for pouchitis.
Results
The pathogenesis of pouchitis is not definitively understood, but various hypotheses have been proposed, including: 1) recurrence of UC, 2) dysbiosis of the ileal pouch microbiota, 3) deprivation of nutritional short chain fatty acids, 4) mucosal ischemia and oxygen free radical injury, 5) host genetic susceptibility, and 6) immune dysregulation. However, none of these alone are able to fully explain pouchitis pathogenesis.
Conclusions
Pouchitis, similar to IBD, is a complex disorder that is not caused by any one single factor. More likely, pouchitis occurs through a combination of both dysregulated host inflammatory mechanisms and interaction with luminal microbiota.
Keywords: microbiota, immune system, inflammatory bowel disease, genetics, ulcerative colitis, pathogenesis
BACKGROUND
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory condition of the gastrointestinal tract where inflammation is continuous and limited to the colon. In general, there is a gradient of disease severity, with the most severe disease in the rectum which reduces in severity as one proceeds proximally through the colon. The terminal ileum is not inflamed. Patients with UC have frequent episodes of diarrhea (sometimes bloody), abdominal pain, weight loss, and fecal urgency.1 Treatment options for UC vary based on disease severity and may include either a top-down or step-up approach. The top-down approach begins with biological therapy, such as infliximab, to rapidly induce remission while the step-up approach begins with 5-aminosalicylates, followed by immunomodulators (e.g. 6-mercaptopurine), and finally biologic agents before surgical options are exercised.2
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disease characterized by the formation of hundreds to thousands of adenomatous polyps early in life that leads to colon cancer in most patients by the age of 40. FAP is caused by a mutation in the adenomatous polyposis coli (APC) gene. The tumor suppressor, APC, encodes a component of the Wnt signaling pathway where it serves to modulate the levels of the β-catenin transcriptional coactivator. In individuals afflicted with FAP, increased levels of β-catenin accumulate in the nucleus, resulting in upregulation of various proto-oncogenes. Treatment options are limited for these patients. Usually, they undergo colonoscopy screenings every one to two years but ultimately, a prophylactic colectomy is required relatively early in life to reduce or eliminate the risk of colon cancer.3
A potentially curative surgical option for both UC and FAP is a total proctocolectomy preserving the anal sphincters, followed by an ileal pouch-anal anastomosis (IPAA), which recreates a fecal reservoir. First developed by Sir Alan Parks at the St. Mark’s Hospital in London in 1978,4 an ileal pouch is formed from the terminal ileum and connected to the remnant anorectal canal, resulting in a new fecal reservoir. Currently, a J shaped pouch (“J-pouch”) is the most common type of ileal pouch, but S- or W-pouches may also be constructed. The surgery may take place in one-, two-, or three-stages, with the two- and three-stage surgeries including the creation of a temporary upstream ileostomy to allow healing of the ileal pouch reconstruction. Theoretically, once the colon and rectum are removed, so is the disease (FAP or UC); however, about 50% of patients with an IPAA for UC develop at least one episode of subsequent inflammation of the ileal pouch, termed “pouchitis”.5 The symptomology of pouchitis is similar to that of UC and may include fecal urgency, incontinence, straining during defecation, hematochezia, abdominal or pelvic discomfort, fever, and malaise. Unlike UC which is treated with biologics, immunosuppressives, or aminosalicylates, pouchitis is most commonly and very effectively treated with a course of antibiotics. Interestingly, the incidence of pouchitis in FAP patients is considerably less (0-11%),6 suggesting that the underlying inflammatory pathogenesis associated with UC may predispose those individuals to pouchitis, more so than patients with FAP.
Normally, the terminal ileum is the site of vitamin B12 and bile salt absorption, as well as other digestion products not absorbed more proximally in the small bowel. However, the ileal pouch, created during the ileal pouch-anal anastomosis (IPAA) surgery, functions as a fecal reservoir akin to the rectum in healthy individuals. Over time, morphological changes occur which transform the ileal mucosa to a more colonic-type mucosa in response to the new microenvironment associated with fecal stasis. Villus atrophy and crypt hyperplasia (termed “colonic metaplasia”) are two of the most common histological changes that occur in most, if not all, ileal pouches.7–16 In fact, some studies have shown that the degree of colonic metaplasia correlates with pouchitis.17–19
Pouchitis is the most common complication of IPAA but its pathogenesis is still largely unknown. Current hypotheses suggest that the development of pouchitis might be caused by: (1) recurrence of UC in the colon-like ileal reservoir, (2) dysbiosis of ileal pouch microbiota, (3) short chain fatty acid (SCFA) deprivation, (4) mucosal ischemia and oxygen free radical injury, (5) genetic susceptibility, and (6) immune dysregulation. This review will provide an overview of pouchitis and an analysis of the current literature examining its pathogenesis.
METHODS
A PubMed search of the terms “pouchitis pathogenesis”, “pouchitis diet”, “pouchitis ethnicity”, “pouchitis free radical”, “pouchitis ischemia”, “pouchitis fatty acids”, “C. difficile pouchitis”, “CMV pouchitis”, “ileum and ulcerative colitis” and “eosinophilia pouchitis” was performed. Articles from 1981 to September 2015 were included if they reported findings concerning either the normal ileal pouch or pouchitis. Only articles with the full text available in English were included in the review with a focus on the manuscripts describing primary research as opposed to reviews.
RESULTS
Diagnosis and classification of pouchitis
Diagnosis of pouchitis
Pouchitis is defined as acute inflammation of the ileal pouch created in UC or FAP patients following a total proctocolectomy and IPAA. The incidence of pouchitis differs depending on the underlying pathology for which surgery was performed. About 50% of patients with an ileal pouch for UC will develop at least one episode of pouchitis,5 while those with FAP have a much lower incidence of pouchitis (0-11%).6 Many patients with pouchitis may present with clinical symptoms similar to UC, including urgency, hematochezia, and increased frequency. Gross appearance of the inflamed ileal pouch may reveal ulceration, mucous exudates, edema, and friability. These features may correlate with the histological findings of acute inflammatory cell infiltration and ulceration of the epithelial layer (Figure 1).
Figure 1.
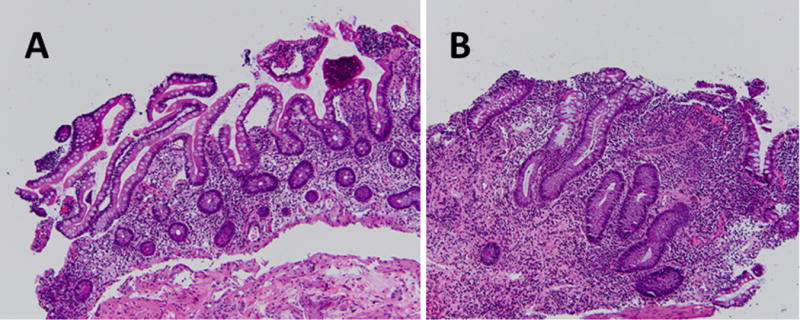
Histological Comparison of Normal Ileal Tissue and Pouchitis.
Histological representations of pouchitis are displayed. (a) 20x; Normal tissue taken during pouchoscopy at 50cm within the terminal ileum above the ileal pouch. No pathogenic alterations or inflammation is seen. (b) 20x; Biopsy obtained during pouchoscopy at 20cm within the ileal pouch showing increased acute inflammatory cell infiltration and ulceration of the epithelium, suggestive of pouchitis. The presence of blunted villi and crypt lengthening indicates significant colonic metaplasia.
The Pouchitis Disease Activity Index (PDAI) is a numerical scale that quantifies the severity of pouchitis by evaluating three separate categories used to describe the severity of pouchitis, including: (1) clinical symptoms, (2) visualized endoscopic inflammation, and (3) microscopic or histological findings. A score ≥7 reflects active pouchitis20 (Table 1). In 2003, a modification of the PDAI (mPDAI) was proposed that omitted the histological criteria, wherein individuals that scored ≥5 were designated as having active pouchitis (Table 1). Both scoring methods were found to be comparable for diagnosing and assessing the severity of pouchitis.21 Since the histological examination of the ileal pouch tissue is time-consuming, invasive, and requires the availability of a trained pathologist, utilizing the mPDAI (gross endoscopic appearance and symptoms) has de facto become the most commonly used method for diagnosing pouchitis. However, multiple other diagnostic methods make comparing pouchitis studies difficult. For example, studies included in this review used the following approaches to define pouchitis: (1) PDAI; (2) mPDAI; (3) clinical, endoscopic, and histological criteria used individually or in some variable combination; (4) patient recall of symptoms; or (5) retrospective medical chart review without overt definition of symptoms or signs justifying the pouchitis diagnosis. Differing stringency of scoring criteria may introduce a source of bias to any study since identifying individuals by only clinical symptoms could result in numerous other pathologies as being included in the pouchitis category (e.g. miscellaneous enteric infections, food poisoning, or transient hypermotility).
Table 1.
Pouchitis disease activity index (PDAI)
| Criteria | Score |
|---|---|
| Clinical | |
| Stool frequency | |
| Usual postoperative normal | 0 |
| 1-2 stool/day > postoperative usual | 1 |
| ≥3 stool/day > postoperative usual | 2 |
| Rectal bleeding | |
| None or rare | 0 |
| Present daily | 1 |
| Fecal urgency or abdominal cramps | |
| None | 0 |
| Occasional | 1 |
| Usual | 2 |
| Fever (temperature >37.8°C | |
| Absent | 0 |
| Present | 1 |
| Maximal total clinical score | 6 |
| Endoscopic inflammation | |
| Edema | 1 |
| Granularity | 1 |
| Friability | 1 |
| Loss of vascular pattern | 1 |
| Mucous exudates | 1 |
| Ulceration | 1 |
| Maximal total endoscopic score | 6 |
| Acute histological inflammation | |
| Polymorphonuclear leukocyte infiltration | |
| Mild | 1 |
| Moderate – crypt abscess | 2 |
| Severe + crypt abscess | 3 |
| Ulceration per low-power field | |
| <25% | 1 |
| 25-50% | 2 |
| >50% | 3 |
| Maximal total histologic score | 6 |
| Maximal total score | 18 |
|
| |
| Pouchitis: ≥7 (PDAI) | |
| Pouchitis: ≥5 (mPDAI) (excluding histologic criteria) | |
Standard medical therapy
Standard medical therapy includes 10-14 days of oral antibiotics most commonly consisting of ciprofloxacin and/or metronidazole. Ciprofloxacin may be preferable since it eradicates potentially pathogenic bacteria while maintaining normal anaerobic bacterial populations. Ciprofloxacin also lowers fecal pH to a level that is more comparable to that seen in uninflamed ileal pouch patients.22
Classification of pouchitis based on therapeutic response
Pouchitis is classified into three categories based on the response to standard antibiotic-based medical therapy: (1) antibiotic-responsive, (2) antibiotic-dependent, and (3) antibiotic-refractory.5 Patients who respond favorably to a 10-14 day course of antibiotics are considered to be antibiotic-responsive. Of those patients with acute pouchitis, it is estimated that about 39% will have only one acute episode that will respond to treatment with antibiotics while 61% will have at least one subsequent episode.23 About 7-19% of pouchitis patients will require long-term continuous use of antibiotics to maintain pouchitis remission and these individuals are considered antibiotic-dependent.24 A small percentage of patients (<5%) may be classified as antibiotic-refractory.25 These patients do not respond to antibiotic therapy and in many cases, require aminosalicylates (e.g. mesalamine), immunosuppressive (e.g. 6-mercaptopurine), or biologics to induce or maintain remission. Secondary causes of pouchitis (e.g. Clostridium difficile, viral, or medication-induced) and the presence of Crohn’s disease (CD), another type of inflammatory bowel disease (IBD), in the ileal pouch should be considered in the differential diagnosis for antibiotic-refractory patients and these potentially confounding etiologies are discussed later in this review.
Risk factors for pouchitis
Disease-associated risk factors
Defining the risk factors associated with pouchitis may help to potentially gain insight into pathogenesis, and to possibly identify those individuals which may be difficult to treat and require long-term maintenance therapy. The presence of extraintestinal manifestations, including arthritis, primary sclerosing cholangitis, reflux ileitis, and osteoporosis are positively associated with the subsequent onset of pouchitis.23, 26–32 Pouchitis can also be influenced by pre-surgical, surgical, and post-surgical factors (Table 2). Patients with a concomitant autoimmune disorder have a higher risk for developing antibiotic-refractory pouchitis (OR: 2.29 [95% CI: 1.52-3.46]).33 Factors that are not associated with pouchitis include anal transition zone inflammatory features,34 straight pull-through vs. J-pouch,35 fecal alpha 1-antitrypsin levels,36 and patients with portal vein thrombi.37 Pancolitis and extensive disease are significant risk factors for pouchitis,30, 38–40 although this association was not seen in all studies.41 Some of these risk factors may suggest differing pathologies, including fecal stasis, technical failures, ischemia, systemic immune dysregulation or other metabolic factors (e.g. anemia).
Table 2.
Disease-associated predictive factors for development of pouchitis
| Pre-surgical | Surgical | Post-surgical |
|---|---|---|
| Pre-operative steroid use27, 42 | Hand sewn anastomosis43 | Positive fecal lactoferrin36 |
| Primary sclerosing cholangitis40, 44 | Anastomosis placed <0.5cm from pectinate line43 | Non-steroidal anti-inflammatory drug (NSAID) use32, 45 |
| Pulmonary comorbidity29 | S-pouch construction29 | Iron deficiency anemia46 |
| Extraintestinal manifestations23, 26–32 | Thrombocytosis27 | |
| Backwash ileitis40, 47 | Longer duration of follow-up/time since pouch construction27, 38, 40 | |
| Pancolitis/extensive disease29, 30, 38, 39 | Ulcerative gastroduodenal lesions48 | |
| UC Disease severity49 | Non-daily proton pump inhibitor (PPI)/H2 antagonist use50 | |
| Steroid dependency49 | ||
| First-degree relative with IBD31 | ||
| Chronic active inflammation of the appendix39 | ||
| Presence of a concomitant autoimmune disorder33 |
Dietary factors and smoking
Diet is potentially one of the most influential factors that determines the composition of the gut microbiota and it therefore potentially represents a significant risk factor for the development of pouchitis.51 A small retrospective pilot study examined whether consuming the FODMAP (fermentable oligo-, di-, and mono-saccharides and polyols) diet influenced pouchitis in seven patients with either ileal pouch or ileorectal anastomosis. This diet avoids foods that are poorly absorbed and thus increase fecal output, such as high lactose dairy, gluten, wheat, rye, barley, beans, some fruits and vegetables, and high fructose corn syrup. Five (71%) patients adhered to the diet and displayed noticeable improvement of pouchitis symptoms, while two (29%) patients who deviated from the diet manifested chronic pouchitis.52 Furthermore, this study also included a small prospective analysis of an additional eight patients. However, the results were inconclusive due to the high withdrawal rate and poor adherence to the diet.52 In a second study reported by an Israeli group, pouchitis-free patients consumed twice as many servings of fruit, more liposoluble antioxidants (e.g. cryptoxanthin commonly found in orange/yellow fruits and vegetables and lycopene found in tomatoes and other red fruits), and more vitamins A and C compared to those who had a history of pouchitis.53 A third study reported that supplementing the diet of ileal pouch patients without clinical signs of pouchitis with 24 g/day of the dietary fiber inulin found a 62% increase in the luminal content of the short chain fatty acid (SCFA) butyrate. Additionally, Bacteroides fragilis abundance was decreased and PDAI endoscopic and histologic scores were reduced compared to individuals consuming a placebo.54 SCFAs are important to maintaining colonic health and in particular, butyrate is the primary fuel source for the colon-like ileal pouch.55 The relationship between butyrate and its potential role in pouchitis will be discussed in more detail later in this review.
Smoking is a recognized protective factor for the development of UC56, but its role in pouchitis is not as well-defined. In a study reported in 2007, acute pouchitis was defined as episodes that were antibiotic-responsive occurring at least four months apart while chronic pouchitis were those patients who were antibiotic-dependent and antibiotic-refractory. Multivariate analysis found that smoking increased the risk of developing acute pouchitis (OR: 2.3 [95% CI: 1.1-5.3]) but reduced the risk for chronic pouchitis (OR: 0.2 [95% CI: 0.05-0.74]).27 The risk of pouchitis for never smokers is similarly conflicting. Some studies have found that individuals that have never smoked had a higher risk for developing pouchitis;32, 57 however, this finding was not confirmed in subsequent studies.30, 31, 58
Pouchitis pathogenesis
Pouchitis suggests itself to be a heterogeneous disease. Clinical, endoscopic, and histological presentation is often dissimilar from patient to patient, presumably reflecting variation in host susceptibility and possibly varying exposure to other factors affecting the disease. Pouchitis is probably a widely variable disease process and the term “pouchitis” might be viewed better as an umbrella term used to describe multiple differing pathologies that end commonly with inflammation of the ileal pouch. This review will discuss six of the main proposed pathogenic mechanisms of pouchitis: (1) recurrence of UC in the colon-like ileal reservoir, (2) dysbiosis of the ileal pouch microbiota, (3) SCFA deprivation, (4) mucosal ischemia and oxygen free radical injury, (5) genetic predisposition, and (6) immune dysregulation (Figure 2).
Figure 2.
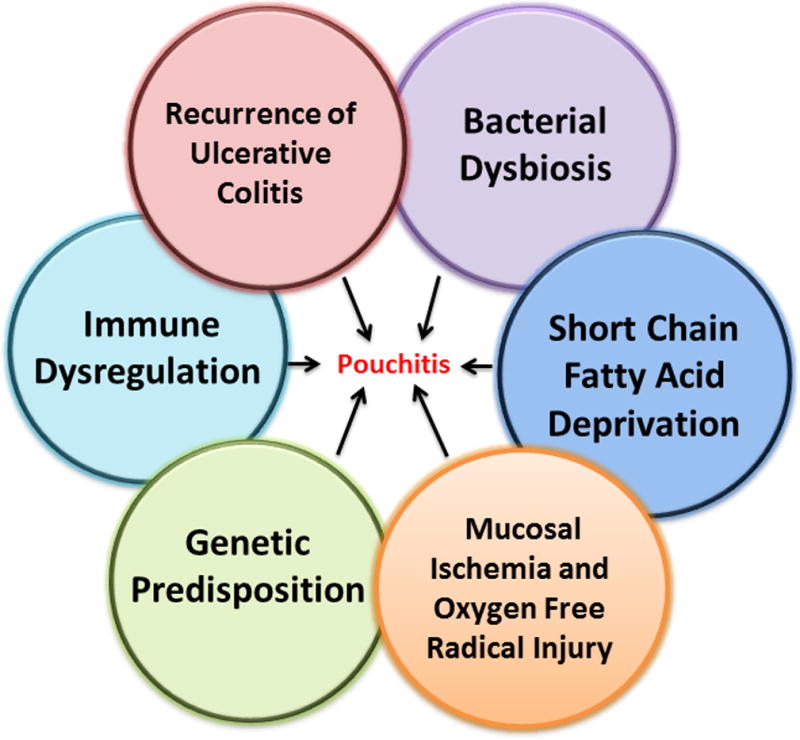
Proposed Etiologies Associated with Pouchitis Pathogenesis.
The pathogenesis of pouchitis is not well defined although multiple etiologies have been suggested, including: bacterial dysbiosis, SCFA deprivation, immune dysregulation, reoccurrence of ulcerative colitis, mucosal ischemia and oxygen free radical injury, and genetic susceptibility. Development of pouchitis probably results from an interplay of several of these factors. Importantly, the relative contribution of these multiple processes may differ from individual to individual.
Pouchitis is a recurrence of ulcerative colitis
One line of thinking suggests that pouchitis is a recurrence of the underlying UC disease within the neorectum. Although the pouch is constructed only from the ileum, over time, villi are lost or blunted and crypts develop to form a more colon-like morphology (Figure 1). This villus blunting and crypt hyperplasia is also known as colonic metaplasia. A study that characterized changes in the ileal pouch mucosa over the first year after ileal pouch construction found significantly reduced villus height and increased crypt depth,14, 59 correlating with increased crypt cell proliferation.14 Additionally, total mucosal thickness was also reduced over this time frame.14 Associated with colonic metaplasia is the presence of a chronic inflammatory cell infiltrate (e.g. lymphocytes, plasma cells, eosinophils, and histiocytes) within the lamina propria.59, 60 Histologically, this is similar in appearance to patients with quiescent or chronic UC. Pouchitis is defined by acute inflammatory changes (e.g. polymorphonuclear infiltration) superimposed on the chronic changes with or without the presence of ulceration and crypt abscesses and this is analogous to an acute UC flare.59, 60
A line of research suggests that UC represents a defect in colonic barrier function and this is further paralleled by investigations into the protective epithelial mucus layer. Mucin, a protein that helps to protect the intestinal epithelium from luminal microbiota, is secreted by goblet cells in the gastrointestinal tract to form a mucus barrier.61 Similar to the colon of healthy individuals, Mucin 2 (MUC2) is the primary secreted mucin in the colon of UC patients.62 Mucins are O-glycosylated to form the “bottle-brush” appearance of the mucus barrier. The O-glycans may be further modified by sialation and sulfation which results in the addition of additional residues, including sialic acid and sulfate.63 An end product of this modification is the formation of sialomucin found primarily in the small bowel and sulfomucin found primarily in the colon and rectum.64 A switch from predominating sialomucin (in the native ileum) to a mixed or predominating sulphomucin phenotype (in the ileal pouch) has been described and this correlates with the development of colonic metaplasia.17 These changes are not commonly described in patients with an ileostomy, suggesting that the stasis associated with the fecal stream and subsequent bacterial overgrowth and mucosal inflammation, is necessary for these mucin changes to occur.10, 14, 15, 59 Studies have shown that patients with a history of pouchitis have a higher degree of colonic metaplasia compared to those without pouchitis,18, 65 confirming the previously described hypothesis that colonic metaplasia is associated with inflammation. These data provide evidence to support the hypothesis that pouchitis may actually be a recurrence of UC, likely due to the development of a colonic-like morphology, and possibly a recurring compromised mucus barrier.65
Specific changes have been identified within the uninflamed terminal ileum of UC patients prior to colectomy which may influence the risk for development of pouchitis. The mucosal barrier is integral to protecting the underlying epithelium from invasion by luminal microbiota and disruption of this protective layer increases the risk for chronic inflammation. Although the small bowel is not inflamed in UC patients, increased epithelial gaps, cell shedding, and leakage have been described in the ileum and duodenum,66, 67 which increases the risk of luminal microbes and antigens crossing the epithelial barrier. Tight junctions, comprised of claudins, zonula occludens, and other proteins, function to maintain appropriate permeability of the intestinal epithelium. Reduced epithelial barrier function, accompanied by reduced claudin-1 and increased claudin-2 proteins, was found in the inflamed ileal pouch tissue during pouchitis.68 Aquaporins (AQP) are membrane proteins that mediate cellular water homeostasis. The AQP8 aquaporin transports hydrogen peroxide in addition to water. In the terminal ileum of UC patients, reduced expression of AQP8 correlated with reduced levels of oxidative stress.69, 70
In order to maintain homeostasis within the intestinal milieu, pathogen recognition receptors (PRRs) are expressed by the innate immune cells to recognize luminal antigens for presentation to the immune system. Children with pancolitis or left-sided colitis had increased expression of the PRR nucleotide-binding oligomerization domain 2 (NOD2) and the pro-inflammatory cytokines IL-1β and TNFα in the uninflamed terminal ileum.71 Another PRR, toll-like receptor 4 (TLR4) was also up-regulated in the terminal ileum of UC patients.72 These data suggest that the terminal ileum, and consequently ileal pouch, have reduced immune tolerance in UC patients which may result in the increased risk for developing pouchitis compared to those with FAP.
Summary and comment
As the ileal pouch matures, the architecture switches from an ileal phenotype to one that is more colon-like, termed colonic metaplasia. The degree of colonic metaplasia correlates with the presence of pouchitis, suggesting inflammation drives these changes. Prior to surgery, the terminal ileum of UC patients demonstrates reduced barrier function in spite of this tissue not being overtly inflamed. Thus, insight into the physiology of the terminal ileum may help guide research of the ileal pouch to examine how possible ileal deficits may result in pouchitis development. Such investigation may also identify how the ileal pouch has adapted to its new reservoir function.
Dysbiosis of pouch microbiota
Alterations in microbial communities influence risk for pouchitis
In most cases, pouchitis is easily treated with antibiotics, such as ciprofloxacin, metronidazole, or rifaximin. Based upon this empiric observation, pouchitis may be the result of some form of bacterial overgrowth or imbalance. Although constructed from the ileum, the pouch bacterial community changes to become more similar to that of the colon, likely in response to fecal stasis. In patients with multiple stage IPAA surgery, biopsies obtained from the upstream ileostomy site at the time of stoma closure has predominately facultative anaerobes (such as lactobacilli, enterococci, and coliforms), a paucity of sulfate-reducing bacteria (SRB), and low levels of Clostridium perfringens.73 After ileostomy closure, the bacterial population changes with decreased facultative anaerobes and increased obligate anaerobes, resulting in a bacterial population that is more similar to that seen in the colon.73–76
A comparison of the bacterial populations in the ileal pouches of UC and FAP patients may provide important information about beneficial as well as potentially causative or pathogenic microbial families. By comparing bacterial flora from UC patients which have a high incidence of pouchitis, and FAP patients, which have a low incidence of pouchitis, one might gain insight into how the microbiota might influence risk of pouchitis. One of the major differences between pouches in these two diagnoses is the greater presence of SRB in UC pouches compared to FAP pouches (Table 3).73, 77 These bacteria utilize sulfur for anaerobic respiration rather than oxygen, producing hydrogen sulfide. Hydrogen sulfide inhibits butyrate oxidation which reduces concentrations available as nutrition to the intestinal epithelial cells that may then result in injury to the ileal pouch mucosa.78 SRB and increased concentrations of hydrogen sulfide have been associated with active pouchitis.79 The presence of SRB is hypothesized to be a consequence of colonic metaplasia which results in the higher concentration of sulfomucin. Commensal bacterial species, such as Bacteroides fragilis, degrade mucin (in particular, sulfomucin), releasing free sulfate which promotes colonization of SRB.80 Furthermore, Enterobacteriaceae were also increased in uninflamed UC pouches compared to FAP pouches81 and have also been previously implicated in pouchitis.22, 81, 82 Ruminococcus gnavus, found in higher abundance in uninflamed UC pouches, produces ruminococcin A, a lantibiotic that is active against various pathogenic clostridial species and other Gram-positive organisms, which may help to maintain bacterial homeostasis in the ileal pouch (Table 3).83 The butyrogenic Bacteroidetes and Faecalibacterium prausnitzii were increased in uninflamed FAP pouches (Table 3).81 F. prausnitzii also has an anti-inflammatory effect84 through the production of a microbial anti-inflammatory molecule (MAM) that inhibits the NF-κB pathway.85 A study that transfected the gene encoding the MAM into Lactobacillus lactis confirmed its protective effect in the 2,4-dinitrobenzenesulfonic acid (DNBS)-induced colitis mouse model. Animals fed the modified L. lactis had smaller weight loss and lower abundance of cytokines IL-17A and IFN-γ compared to those fed with L. lactis without the MAM modification.85 However, not all studies have identified differences in the microbiota composition between FAP and UC pouches.79, 82, 86 These data suggest that the microenvironment of the ileal pouch in UC patients predisposes individuals to colonization by specific bacterial populations, such as SRB, which can then increase the likelihood for developing pouchitis.87
Table 3.
Comparison of the microbial communities between the uninflamed ileal pouch and pouchitis
| FAP ileal pouches compared to UC ileal pouches
| |
|---|---|
| Taxa with increased abundance in UC ileal pouches compared to FAP ileal pouches | Taxa with increased abundance in FAP ileal pouches compared to UC ileal pouches |
| Alcaligenaceae81 | Bacteroidaceae81, 86, 95 |
| Akkermansia spp. 95 | Bacteroides vulgatus81 |
| Clostridium spp. 95 | Lactobacillus spp.95 |
| Comamonadaceae81 | Prevotellaceae81 |
| Eubacterium spp.95 | Ruminococcaceae81 |
| Lachnospiraceae95 | Faecalibacterium spp.95 |
| Moraxellaceae81 | Faecalibacterium prausnitzii81 |
| Prevotella spp. 95 | Streptococcus spp.95 |
| Roseburia spp.95 | |
| Sulfate-reducing bacteria73, 77 | |
| Veillonella spp. 95 | |
|
| |
| Changes in the bacterial composition during pouchitis | |
|
| |
| Taxa with increased abundance during pouchitis | Taxa with reduced abundance during pouchitis |
|
| |
| Clostridium perfringens22, 90 | Alcaligenaceae81 |
| Enterobacteriaceae81, 82 | Bacteroides spp.82, 86 |
| Escherichia coli22, 81 | Bifidobacterium spp.90 |
| Fusobacterium spp.96 | Enterococcaceae97 |
| Ruminococcus obeum96 | Faecalibacterium spp.82, 96 |
| Sulfate-reducing bacteria79 | Lachnospiraceae82 |
| Total number of aerobic bacteria22, 90 | Blautia spp.86 |
| Lactobacillus spp.90 | |
| Parabacteroides spp.86 | |
| Ruminococcaceae82 | |
| Ruminococcus gnavus96 | |
| Streptococcus spp.81, 96 | |
| Total number of anaerobic bacteria22 | |
During a pouchitis episode, reduced bacterial diversity of the ileal pouch has been described,81, 82, 86 that includes a reduction of the anaerobic to aerobic ratio, seen in both mucosal biopsies81, 88, 89 and fecal samples.13, 82, 90 In particular, an increase in the abundance of Enterobacteriaceae81, 82 and reduced abundance of Bacteroides82, 86 and Faecalibacterium prausnitzii82 have been described. However, a study by Johnson et al. identified a trend towards increased bacterial diversity and richness in UC pouchitis patients, although, this may have been due to the increased aerobe population associated with pouchitis.91 Identification of seventeen non-dominant operational taxonomic units (OTUs) exclusive to pouchitis, including Leptospira, Pseudoaltermonas, Desulfosporosinus, Microcystis, Methylobacter, and twelve unknown OTUs, have been described92. Many of these are environmental bacteria found in soil or water and are difficult to culture in the laboratory. Changes of the bacterial composition during pouchitis can be seen in Table 3. There is lack of conclusive evidence to suggest that the presence of a common pathologic bacterium, Clostridium difficile, is associated with the development of conventional pouchitis.93, 94 However, C. difficile pouchitis has been described, but is effectively treated in a similar fashion to C. difficile colitis with metronidazole or vancomycin.
Probiotic therapy
Alteration of the commensal gut microbiota has been implicated in pouchitis pathogenesis, including the reduction of anaerobic bacteria. Therefore, the use of probiotics may be beneficial to restore the natural anaerobic nature of the ileal pouch and to promote an anti-inflammatory immune response.98, 99 VSL #3 is a medical grade probiotic that contains 3×1011 colony forming units (CFU)/gram of the following lyophilized bacterial species: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophilus. Another probiotic Ecologic 825 contains 2.5×109 CFU/g of Bifidobacterium bifidum, 2 species of Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus salivarius, and Lactococcus lactis. Gionchetti et al. examined whether probiotic therapy can prevent the onset of pouchitis in the year following ileostomy closure and found that 90% of patients (N = 18) on VSL#3 did not develop pouchitis compared to 60% (N = 12) on placebo.100 Additionally, using four capsules per day of 0.5-1×1010 CFU/capsule Lactobacillus rhamnosus GG for 3 months during a pouchitis episode (PDAI range 5-13) to induce remission increased abundance of lactobacilli but did not improve PDAI score.101 A few studies have examined if probiotics can help maintain remission when it is first induced by antibiotics. Ecologic 825 maintained remission in pouchitis and uninflamed IPAA pouches after 8 weeks of probiotic therapy.102 Similarly, VSL#3 maintained remission in 85-100% of pouchitis patients compared to placebo in three small trials.103–105
Summary and comment
The initial treatment regimen for pouchitis is antibiotics, suggesting that the disease is at least partially mediated by a bacterial component, at least for those who respond to antibiotics. Increases in the bacterial populations that are commonly considered pathogenic are seen in the uninflamed UC pouch compared to FAP pouches. Increased abundance of beneficial bacteria, such as Bacteroidetes and Faecalibacterium prausnitzii are seen in FAP pouches and may be a potential reason why FAP patients rarely develop pouchitis. Probiotics do not seem to benefit induction of remission but some data is available to indicate that it may be helpful to maintain remission after standard antibiotic therapy in some patients. Longitudinal studies in the same patients would be of interest since multiple factors can influence the gut microbiota and many studies do not account for differences in diet, medication usage, and miscellaneous environmental or host genetic factors in different patients.
Short chain fatty acid deprivation
Bacterial dysbiosis may influence the abundance of short chain fatty acids
The SCFAs butyrate, propionate, and acetate are principal metabolic products of fermentation of dietary carbohydrates and fiber by obligate anaerobic bacteria. Of particular interest are the butyrogenic bacteria. These bacteria are Gram positive Firmicutes belonging to Clostridium clusters XIVa and IV with notable bacterial species including F. prausnitzii, Roseburia spp., and Eubacterium rectale.106 Normally, enterocytes utilize glutamine and butyrate as cellular fuel; however, after construction of the ileal pouch, the ileal pouch mucosa shows a decreased rate of glutamine oxidation while butyrate oxidation remains constant.55 This data suggests that the ileal pouch mucosa is able to adapt to loss of glutamine oxidation by relying primarily on butyrate oxidation. This adaptation benefits the ileal pouch by not only providing a higher concentration of available butyrate due to the changes in bacterial composition to reflect that of the colon but also to appropriately fuel the developing colonic metaplasia. In addition, butyrate promotes colonic health by reducing oxidative stress, acting as an anti-inflammatory factor, and maintaining the intestinal mucosal barrier.107 Conversely, reduced metabolism of butyrate may result in damage to the intestinal epithelial cell barrier.108
It has been shown that UC patients have impaired butyrate metabolism within the terminal ileum prior to IPAA construction. The rate of butyrate oxidation in the ileum is significantly reduced in UC patients compared to non-UC controls,109 suggesting a potential predisposition to reduced butyrate metabolism within the subsequently constructed ileal pouch. The mechanism for this impaired butyrate utilization has not been established. One potential cause however may be due to the bacterial composition of the ileal pouch. In vitro analysis has shown that hydrogen sulfide can inhibit butyrate oxidation108 and as already discussed, the uninflamed UC ileal pouch demonstrates increased abundance of hydrogen sulfide-producing SRB. During pouchitis, reduced levels of SCFAs have been described compared to the uninflamed ileal pouch.110–112 Treatment with antibiotics to resolve pouchitis restored SCFA concentrations to normal.110
Although SCFAs may display a beneficial role in treating pouchitis, very few studies have examined SCFA enemas in humans. Anecdotal reports using SCFA enemas have been done with contradictory results. Both referenced studies used an SCFA enema preparation consisting 60mM sodium acetate, 30mM sodium propionate, and 40 mM sodium n-butyrate.113, 114 Treating a patient with 100 ml of an SCFA enema twice a day for four weeks resolved pouchitis and maintained remission for two years.113 However, in another study, treatment of two other pouchitis patients with an SCFA enema (60mL) resulted in early termination of the study (14 and 28 days) due to endoscopic deterioration of the pouch.114
Summary and comment
SCFAs are the primary metabolites produced by bacterial fermentation of indigestible dietary carbohydrates and fiber and have been shown to have beneficial effects for colonic health, particularly butyrate. Reduced butyrate metabolic capacity has been demonstrated in the ileum of UC patients and reduced butyrate has been reported during pouchitis, correlating with reduced abundance of butyrogenic bacteria, such as F. prausnitzii. However, an altered SCFA profile is not likely the primary cause of pouchitis since SCFA levels are comparable between the ileal pouch and the normal terminal ileum. During pouchitis, increased fecal output may result in decreased total SCFA content in the ileal pouch however and could explain the reduced SCFA content seen during pouchitis.
Mucosal ischemia and oxygen free radical injury
Ischemia is unlikely to be the primary cause of pouchitis
Ischemia is defined as an inadequate blood flow to an organ. Following construction of the ileal pouch, blood flow to the ileal reservoir is reduced compared to the normal colon.115 One method to measure ischemia is by monitoring the intramucosal pH since mucosal hypoperfusion results in acidosis. And, in fact, reduced intramucosal pH has been associated with the development of pouchitis.116 Ischemia is likely to cause increased production of reactive oxygen species which may then lead to injury of the mucosa. Patients with uninflamed ileal pouches have reduced plasma levels of the lipophilic antioxidants α-carotene, β-carotene, and lycopene compared to controls.117 In addition, increased levels of the lipid peroxidation marker malondialydehyde suggest basal levels of oxidative stress are present in uninflamed ileal pouches.117 However, it is unlikely that ischemia is the primary cause of pouchitis since patients with FAP undergo the same surgical procedure as UC patients and patients with FAP rarely develop pouchitis. These findings have been confirmed in rat IPAA surgical models in which an ileal pouch was constructed and attached to the remnant rectum. Increased oxidative stress markers, mucosal myeloperoxidase activity and urinary 8-isoprostane, were seen in rats with a U-pouch. Administration of the antioxidants vitamin E and allopurinol alone, or in combination, reduced oxidative stress as seen by reduced myeloperoxidase activity and urinary 8-isoprostane levels.118
Pharmacologic therapy to reduce oxidative stress has been suggested for pouchitis patients; however, the results are not conclusive. A study of 184 UC IPAA patients found no difference in pouchitis incidence over 24 months following prophylactic administration of the antioxidant allopurinol immediately after IPAA surgery compared to placebo.119 A smaller study saw a successful response to allopurinol in 50% of acute (4/8) and chronic (7/14) pouchitis patients who had a history of recurrent pouchitis symptoms after failing stand treatment.120 These data suggest that allopurinol may be beneficial to treat acute pouchitis symptoms rather than prevent onset.
Summary and comment
Since both FAP and UC patients undergo the same surgical IPAA procedure, but only UC patients commonly develop pouchitis, ischemia as a result of the surgical procedure is unlikely to be the primary cause for most causes of pouchitis. Treatment with the antioxidant allopurinol has demonstrated some efficacy in patients with pouchitis symptoms but is not effective as a prophylaxis. Although data is limited regarding the mechanism of this theory, evaluating the addition of antioxidants to standard pouchitis treatment would be of interest particularly for those with chronic antibiotic-refractory pouchitis.
Genetic predisposition
Ethnicity
Ethnicity and by implication, genetic background, could be a factor in the incidence of pouchitis. In a Canadian study of Caucasian individuals (N = 399), the Ashkenazi Jewish ethnicity was significantly associated with the development of chronic pouchitis (OR: 2.7, P = 0.008).121 Additionally, a study of South Asian Caucasians living in the United Kingdom found that pouchitis incidence was significantly greater in these individuals (77%) compared to non-South Asian Caucasians (46%).122 A study of African Americans and Caucasians found no differences in chronic pouchitis incidence.123 However, the number of studies examining the relationship between ethnicity and pouchitis are limited and the genetic predisposition to this disease is largely unresolved.
Genetic polymorphisms potentially associated with pouchitis
A meta-analysis of genome wide association studies (GWAS) has identified 47 susceptibility loci that accounted for approximately 16% of UC predisposition.124 However, the heritability estimate varies based on the study. For example, a pooled twin study found a heritability of 0.67 for UC.125 These data further support the role of genetic predisposition in UC and may provide further evidence to potentially explain why FAP patients rarely develop pouchitis. Unfortunately most, if not all, of the studies that have examined the effect of genetics in pouchitis are underpowered and lack multiple testing correction. Nonetheless, of these studies, the most compelling evidence supports the potential association of a NOD2 variant (rs2066847, NOD2insC, 1007fsCins) with pouchitis in the Caucasian population. NOD2 recognizes muramyl dipeptide (MDP) of Gram positive and Gram negative bacteria that then stimulates the enteric immune system. Reduction or loss of function of the NOD2 protein may result in an altered immune response to bacterial pathogens.126–128 In a multicenter study of ileal pouch patients, a significant association of the NOD2insC variant was found with chronic pouchitis (OR: 3.21 [95% CI: 1.38-7.47]) after Bonferroni correction (Table 4).129 This association is interesting because unlike the strong association of NOD2 variants with CD, these variants are not commonly associated with UC,130, 131 but such an association is intuitively appealing since an IPAA is contraindicated in CD patients due to disease recurrence. Thus, these CD-associated alleles may suggest more of a Crohn’s-like phenotype in UC pouchitis patients akin to IBD occurrence in the neo-rectum. Although these associations need to be confirmed in larger studies, polymorphisms found in other genes of the innate immune system (besides NOD2) may also have an influence on pouchitis risk. For example, tumor necrosis factor superfamily 15 (TNSFS15) variants were associated with severe pouchitis in addition to NOD2 variants.132
Table 4.
Genetic association studies increasing risk for pouchitis
| Genes | SNP (rs#) | Population | N | Reference |
|---|---|---|---|---|
| IL-1RN*2 | N/A | Northern European | Controls N = 82 Pouchitis N = 29 |
135 |
|
IL-1RN*1 TNF*1 |
N/A N/A |
USA | Controls N = 52 ≤2 Pouchitis Episodes/Year N = 11 >2 Pouchitis Episodes/Year N = 39 |
136 |
|
ATG16L1 U6 JAK2 |
rs2241880 rs6927210 rs7849191 |
USA | Controls N = 63 Mild Pouchitis N = 41 |
132 |
|
TNFSF15 NOD2 S100Z |
rs7869487 rs2066844 rs7712957 |
USA | Controls N = 63 Severe Pouchitis N = 12 |
132 |
|
TLR9 CD14 |
rs574383, rs2569190 | Italian | Controls N = 224 Pouchitis N = 78 |
137 |
| NOD2 | rs2066844 |
USA | Controls N = 37 Mild Pouchitis N = 33 |
138 |
|
NOD2 |
rs2066847 | USA | Controls N = 37 Severe Pouchitis N = 9 |
138 |
| NOD2 | rs2066847 | USA and Canada | Controls N = 487 Chronic Pouchitis N = 118 |
129 |
Gene expression
Studies that identify differences in either mRNA transcript levels or protein concentrations may help guide researchers to develop more clinically relevant laboratory tests and to identify new candidate genes for genetic association studies. Matrix metalloproteinase (MMP)-1 and MMP-2 are involved in degrading the extracellular matrix. As expected, with increased ulceration and tissue destruction associated with inflammatory bowel diseases, mRNA levels of MMP-1 and MMP-2 were up-regulated in the inflamed ileal pouch tissue of pouchitis patients.133 These changes may be more prevalent in the final stages of tissue injury due to the function of the MMPs in tissue remodeling.133 Additionally, increased epithelial apoptosis was seen in patients that were symptom-free but with a history of pouchitis, as identified by an increase in TUNEL and M30 staining. Increased Fas-L mRNA which encodes a transmembrane protein involved in inducing the extrinsic apoptotic pathway, was seen in the intestinal epithelium of patients with a history of pouchitis.134 This observation indicate that increased apoptotic rates in these patients could contribute to increased damage and epithelial turnover.
Summary and comment
Many of the genetic association studies for pouchitis that have been performed are small and underpowered. Clinical utilization of genetic variants still requires larger, more powerful studies that examine various phenotypically uniform cohorts to confirm or exclude associations. The NOD2 frameshift variant (rs2066847) is currently the strongest genetic factor for the inherited risk of pouchitis. This variant is of particular interest since NOD2 is not commonly associated with UC. Gene expression and protein abundance studies of various candidate pathways identified up-regulation of genes involved in tissue injury and apoptosis in patients with a history of pouchitis. One limitation of these genetic studies is the lack of a uniform definition of pouchitis, which makes comparing studies challenging. Additionally, identifying a control group is difficult since the risk of pouchitis increases over time. Comparing UC IPAA patients with and without pouchitis may not be the most appropriate control since the “control” patients may eventually develop pouchitis.
Immune dysregulation
The underlying inflammatory disease associated with UC predisposes individuals to pouchitis
The inflammatory response in pouchitis is interesting since both UC and FAP patients undergo the same surgery but only UC patients commonly develop pouchitis, suggesting that the underlying inflammatory disease in UC patients may be a contributing factor to pouchitis pathogenesis. Genetic predisposition may also influence the underlying inflammatory disease as shown by the genetic polymorphisms associated with the immune system described in the previous section. Unfortunately, research comparing the immunologic differences between uninflamed UC and FAP ileal pouches is limited. In one study that examined the transcriptomic profile of UC versus FAP, a gene ontology analysis found that the innate immune response showed the most significant difference, with inflammatory response and the acquired immune system among the subsequent top hits.139 In addition, analysis into the differentially expressed genes between uninflamed UC and FAP pouches found transcripts encoding for Charcot-Leyden crystal protein (CLC) (fold change 1.54, P = 0.032), inositol 1,4,5-trisphosphate receptor interacting protein-like 2 (ITPRIPL2) (fold change 1.97, P = 0.039), and component of oligomeric golgi complex 5 (COG5) (fold change 1.72, P = 0.045) as most different between the two groups.139 CLC are microscopic crystals commonly found in individuals with allergic diseases. These crystals are found within eosinophils and basophils and have a lysophospholipase activity which converts lysophosphatidylcholine to glycerophosphocholic and a free fatty acid. The level of CLC has been associated with the inflammation seen in celiac disease.140 ITPRIPL2 is a paralog of ITPRIP which increases the sensitivity of the inositol 1,4,5-trisphosphate receptor to calcium signaling and COG5 is one of five subunits comprising the golgi-localized complex. Neither of these genes has been previously studied in colitis.
Inflammatory cells
Due to the ongoing inflammatory condition of pouchitis, it is expected that particular inflammatory cells will be present while the disease is active. Individuals with pouchitis have been described to have an increase in CD4+ T cells within the inflamed pouch mucosa, as indicated by the CD4:CD8 ratio.141 Additionally, relative abundance of CD4+/CD25+ regulatory T cells and CD8+/HLA-DR+ T cells are increased during pouchitis.141 CD40+ and CD40L+ leukocytes were described in the mucosa of active pouchitis compared to uninflamed pouches.142 In the lamina propria of inflamed pouch mucosa, CD19+ and CD138+ plasma cells were significantly increased, suggesting an abnormality of the B-cell lineage in pouchitis pathogenesis.143
Presence of antibodies associated with pouchitis
The presences of antibodies, either to bacterial antigens or autoantibodies have been studied as potential biomarkers for identifying individuals at high-risk for pouchitis. Anti-neutrophilic cytoplasmic antibodies (ANCA) are autoantibodies against antigens within the cytoplasm of neutrophil granulocytes and monocytes previously implicated in various autoimmune diseases. Antigens for ANCAs may include myeloperoxidase, proteinase 3 and bacterial permeability increasing factor, and staining can either be perinuclear (pANCA) or cytoplasmic (cANCA). The mechanisms causing their development are not well understood although one hypothesis suggests that they develop based on both genetic predisposition and environmental factors.144 cANCA positivity was associated with development of CD-like pouchitis.145 The presence of antibodies specific for bacterial pathogens have also been implicated in pouchitis. Anti-CBir1 antibodies against bacterial flagellin are associated with increased complications after IPAA surgery,146 CD of the pouch,121, 146 and pouchitis development in patients who are also pANCA positive.147 Anti-OmpC is an antibody against the outer membrane protein of Escherichia coli. Anti-OmpC has been studied in conjunction with the presence of anti-ANCA and no associations were described with pouch complications146 or pouchitis.145, 148 Antibodies against fungal proteins have also been described in the serum of pouchitis patients. Anti-Saccharomyces cerevisiae antibody (ASCA) is an antibody against mannan present in the cell wall of the yeast S. cerevisiae and its serum levels are positively associated with increased complications after surgery,146 CD of the pouch,121, 146, 149 and chronic pouchitis.150 On the contrary, one study found reduced fecal and serum ASCA antibodies in pouchitis patients compared to CD of the pouch and irritable pouch syndrome/normal pouch.149 Immunoglobulin G4 (IgG4) antibodies are commonly associated with the development of IgG4-related diseases, including various autoimmune disorders. IgG4 production is mediated by T helper 2 cytokines that facilitate an allergic reaction and the production of IgE. However, it’s pathogenicity in diseases is not well-understood.151 Similar to the trend seen in patients with IgG4-related diseases, patients with high serum IgG4 were more likely to develop chronic pouchitis.152
Peyer’s patches
The ileum also contains Peyer’s patches, comprised of gut-associated lymphoid tissue (GALT). GALT is derived from isolated and aggregated lymphoid follicles enclosed by an epithelial layer (follicle-associated epithelium) that contain microfold (M) cells. M cells transport antigens and luminal bacteria to the underlying immune cells which stimulate or suppress an immune response by discriminating between pathogens and commensal organisms. Peyer’s patches are susceptible to bacterial invasion due to increased permeability of the epithelium required for its normal function.153 They are exclusive to the small bowel and how their function might change in the context of an ileal pouch would be of interest. Currently, very little data describes the function of the Peyer’s patches within the ileal pouch and what adaptations might occur.
Summary and comment
Since pouchitis is commonly considered to be a lower grade recurrence of UC, understanding how the uninflamed UC ileal pouch differs immunologically from uninflamed FAP ileal pouches is an important area for new research. Studies have demonstrated altered adaptive immune response, as seen by abnormality of the B-cell lineage, as well as an altered innate immune response, identified by gene ontology analysis of transcriptomic data. The presence of ASCA and anti-CBir1 antibodies seems to be most suitable for distinguishing patients with CD of the pouch; however, clinical use of these antibodies still require a larger, more comprehensive analysis. Understanding how the normal population of immune cells differs between UC and FAP patients prior to IPAA surgery and within the ileal pouch may help ascertain why UC patients are relatively predisposed to pouchitis.
Secondary pouchitis
Secondary pouchitis is defined as an identifiable cause of pouchitis, distinct from the conventional or idiopathic pouchitis that has been previously discussed. In general, about 20-30% of patients with chronic pouchitis that do not respond to antibiotics can be diagnosed with some form of secondary pouchitis.154 This may include various etiologies or triggers that cause onset of disease, including strip pouchitis, C. difficile infection, cytomegalovirus (CMV) infection, non-steroidal anti-inflammatory drugs (NSAIDs), or eosinophilia.
Strip pouchitis
Another variant “strip pouchitis” is actually a misnomer in that this represents inflammation of the retained rectum below the pouch anastomosis. The pouch may not be inflamed at all but the urgency, bleeding, and frequent stooling comes from an exacerbation of the underlying UC of the remnant rectal tissue. Treatment is usually transanal medications such as steroid suppositories or in cases with inappropriately long segments, re-operation.
Clostridium difficile-associated pouchitis
The presence of C. difficile in the stool is commonly found with antibiotic-associated pseudomembranous colitis in patients with recent hospitalization and antibiotic therapy. Antibiotic-associated dysbiosis of the normal gut microbiota allows overgrowth of the opportunistic pathogen C. difficile. As described in Navaneethan et al., C. difficile infection (CDI) has been reported in ileal pouch patients and should be a consideration for patients who either do not respond to standard antibiotic therapy (metronidazole and/or ciprofloxacin) or those known to have chronic antibiotic-refractory pouchitis.154, 155 Risk factors for CDI-associated pouchitis may include recent hospitalization,156 weight loss,156 increased stool frequency,156 male gender,157 pre-operative comorbidities,157 pre-operative antibiotic use,157 and low serum IgG1.158 Pre-operative CDI was not found to be associated with post-operative CDI-associated pouchitis.157 Identification of C. difficile toxin in the stool defines a diagnosis of CDI-associated pouchitis and administration of appropriate medical therapy (e.g. vancomycin). Fecal microbiota transplant (FMT) has been shown to be efficacious in treating patients with CDI who are unresponsive to antibiotics. The use of FMT to treat CDI-associated pouchitis has not be widely studied; however, a case study presented evidence that FMT was capable of treating a patient with CDI-associated pouchitis.159
Cytomegalovirus pouchitis
CMV is another possible infective cause of pouchitis, although very rare. CMV is a β-herpes virus that usually remains dormant in infected individuals; however, infants and immunosuppressed individuals are at risk for developing reactivated CMV infection. If suspected, the virus may be identified by CMV DNA PCR or the detection of viral inclusion bodies on hematoxylin and eosin stained histopathology slides. A retrospective study of 2,559 ileal pouch patients identified seven that had positive CMV infection in the ileal pouch. Of the seven patients, four were on immunosuppressive agents following liver transplant for primary sclerosing cholangitis, one was on azathioprine and steroids for refractory pouchitis, and two had no immunosuppressive therapy. Logistic regression analysis identified that individuals of the female gender or on immunosuppressives were at highest risk for CMV pouchitis.160 Whether CMV is the cause of pouchitis or simply a bystander is unknown. Nonetheless, if CMV pouchitis is encountered, treatment with ganciclovir was shown to reduce viral load and improve symptoms.160
NSAID-associated pouchitis
Regular usage of NSAIDs has been shown to promote development of pouchitis. NSAIDs inhibit cyclooxygenase-1 (COX-1) and COX-2 which are normally increased during inflammation to promote mucosal healing. As described in Table 2, NSAID use is a known risk factor for pouchitis as well as IBD. Symptoms of NSAID-associated pouchitis are similar to that of UC, including bloody stools and abdominal pain. If the patient is believed to have NSAID-associated pouchitis, the most appropriate treatment is to withdraw NSAID use and prescribe antibiotics.161
Eosinophilic pouchitis
Eosinophils are inflammatory cells whose levels are normally increased during allergic reaction and parasitic infection. Mucosal eosinophils have been found in intestinal tissue of patients with inflammatory bowel disease (IBD); but, the exact contribution of these cells to IBD has yet to be established. However, eosinophils are known to affect inflammation and the normal gastrointestinal microenvironment.162 Eosinophilia of the ileal pouch was associated with left-sided colitis compared to those with pancolitis (OR: 3.3 [95% CI: 1.3-8.1]). Individuals with no history of NSAID use were at higher risk for eosinophilia of the afferent pouch limb (OR: 3.5 [95% CI: 1.2-10.4]).163 It is not known if ileal pouch eosinophilia is associated with allergy or specific microbiota. If associated with allergy, antihistamines would be a more appropriate treatment than antibiotic therapy.
CONCLUSION
The pathogenesis of pouchitis remains elusive; however, researchers and clinicians are gaining a better understanding of factors that may be involved in development. Nonetheless, of the hypotheses discussed in this review, none are able to stand alone as a conclusive factor in the pathogenesis of pouchitis. Both FAP and UC patients may undergo IPAA for two different diseases; however, one group (UC) is more likely to develop pouchitis. Once it is understood why pouchitis favors UC patients, the pathogenesis may be more easily elucidated.
As discussed in this review, the exact mechanism of pouchitis has yet to be fully established but multiple hypotheses have arisen which may predispose certain individuals to disease. Changes in the ileal pouch resulting in a reduction of villi and increased presence of crypts, developing a colon-like phenotype may allow a recurrence of UC to occur. Additionally, alterations within the terminal ileum of UC patients may reduce the strength of the epithelial barrier. Based on the effectiveness of standard antibiotic therapy, bacterial dysbiosis seems to play a role in the development of pouchitis. Alterations in the abundance of anaerobic and aerobic bacteria can result in many changes within the ileal pouch ecosystem. Overgrowth of bacterial populations can lead to an increased immune response and resultant inflammation. Alterations in the anaerobic population may deprive the ileal pouch mucosa of SCFAs, potentially resulting in mucosal injury or influencing overgrowth of aerobic bacteria. In addition, the symptomology of pouchitis, including increased fecal excretion, may decrease SCFA concentration in the ileal pouch, resulting in mucosal nutritional deficiency and injury. Genetic susceptibility may be an additional contributing factor of pouchitis pathogenesis. A better understanding of the genetic variations between FAP patients with normal pouches and UC patients with pouchitis may help identify candidate genes and genetic biomarkers for identifying individuals at high-risk for developing pouchitis and pathways involved in pathogenesis. Finally, UC patients have an altered immune response, leading to their primary disease. It is thought that this immune dysregulation recrudesces in some patients in the form of pouchitis. This is further supported by the chronic low level inflammatory response seen in UC ileal pouch patients, without the acute pouchitis effect. Therefore, pouchitis is most likely caused by a dysregulated immune response (potentially due to genetic predisposition) to commensal bacteria, resulting in increased mucosal injury and altered barrier function. Other secondary types of pouchitis, most usually with infection, should be considered when working up the difficult to treat patient to ensure an appropriate diagnosis and treatment.
Acknowledgments
Declaration of funding interests: Kathleen Schieffer was supported by Grant UL1 TR000127 and TL1 TR000125 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
AUTHORSHIP
Guarantor of the article: Walter A. Koltun
Author contributions:
Study concept and design: Schieffer, Williams, Koltun
Acquisition and analysis of data: Schieffer
Interpretation of data: Schieffer, Yochum, Koltun
Drafting of the manuscript: Schieffer
Critical revision of the manuscript for important intellectual content: all authors
Study supervision: Koltun
Declarations of personal interests: None
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ. Step-up versus top-down therapy in the treatment of ulcerative colitis. Gastroenterol Hepatol (N Y) 2007;3:16–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Hagen CE, Setia N, Lauwers GY. Familial adenomatous polyposis: a review of gastrointestinal manifestations. Diagn Histopathol. 2015;21:152–160. [Google Scholar]
- 4.Parks A, Nicholls R. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85–8. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen B, Lashner BA. Diagnosis and treatment of pouchitis. Gastroenterol Hepatol (N Y) 2008;4:355–61. [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin SD, Clark SK, Tekkis PP, Nicholls RJ, Ciclitira PJ. The bacterial pathogenesis and treatment of pouchitis. Therap Adv Gastroenterol. 2010;3:335–48. doi: 10.1177/1756283X10370611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arashiro RT, Teixeira MG, Rawet V, et al. Histopathological evaluation and risk factors related to the development of pouchitis in patients with ileal pouches for ulcerative colitis. Clinics (Sao Paulo, Brazil) 2012;67:705–10. doi: 10.6061/clinics/2012(07)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell AP, Merrett MN, Kettlewell M, Mortensen NJ, Jewell DP. Expression of colonic antigens by goblet and columnar epithelial cells in ileal pouch mucosa: their association with inflammatory change and faecal stasis. J Clin Pathol. 1994;47:834–8. doi: 10.1136/jcp.47.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell PR, Rankin DR, Weiland LH, Kelly KA. Enteric bacteriology, absorption, morphology and emptying after ileal pouch-anal anastomosis. Br J Surg. 1986;73:909–14. doi: 10.1002/bjs.1800731121. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls RJ, Belliveau P, Neill M, Wilks M, Tabaqchali S. Restorative proctocolectomy with ileal reservoir: a pathophysiological assessment. Gut. 1981;22:462–8. doi: 10.1136/gut.22.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerch MM, Braun J, Harder M, Hofstădter F, Schumpelick V, Matern S. Postoperative adaptation of the small intestine after total colectomy and J-pouch-anal anastomosis. Dis Colon Rectum. 1989;32:600–8. doi: 10.1007/BF02554181. [DOI] [PubMed] [Google Scholar]
- 12.Santavirta J, Mattila J, Kokki M, Matikainen M. Mucosal morphology and faecal bacteriology after ileoanal anastomosis. Int J Colorectal Dis. 1991;6:38–41. doi: 10.1007/BF00703959. [DOI] [PubMed] [Google Scholar]
- 13.de Silva HJ, Gatter KC, Millard PR, Kettlewell M, Mortensen NJ, Jewell DP. Crypt cell proliferation and HLA-DR expression in pelvic ileal pouches. J Clin Pathol. 1990;43:824–8. doi: 10.1136/jcp.43.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Silva HJ, Millard PR, Soper N, Kettlewell M, Mortensen N, Jewell DP. Effects of the faecal stream and stasis on the ileal pouch mucosa. Gut. 1991;32:1166–9. doi: 10.1136/gut.32.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein NS, Sanford WW, Bodzin JH. Crohn’s-like complications in patients with ulcerative colitis after total proctocolectomy and ileal pouch-anal anastomosis. Am J Surg Pathol. 1997;21:1343–53. doi: 10.1097/00000478-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt CM, Lazenby AJ, Hendrickson RJ, Sitzmann JV. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann Surg. 1998;227:654–5. doi: 10.1097/00000658-199805000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Silva HJ, Millard PR, Kettlewell M, Mortensen NJ, Prince C, Jewell DP. Mucosal characteristics of pelvic ileal pouches. Gut. 1991;32:61–5. doi: 10.1136/gut.32.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruin AB, El-Zammer O, Stucchi AF, O’Brien M, Becker JM. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg. 2003;7:246–53. doi: 10.1016/s1091-255x(02)00191-9. discussion 253-4. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Armengol J, Hinojosa J, Lledo S, Roig JV, Garcia-Granero E, Martinez B. Prospective study of morphologic and functional changes with time in the mucosa of the ileoanal pouch: functional appraisal using transmucosal potential differences. Dis Colon Rectum. 1998;41:846–53. doi: 10.1007/BF02235364. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409–15. doi: 10.1016/s0025-6196(12)61634-6. [DOI] [PubMed] [Google Scholar]
- 21.Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003;46:748–53. doi: 10.1007/s10350-004-6652-8. [DOI] [PubMed] [Google Scholar]
- 22.Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden J. Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum. 2004;47:1519–25. doi: 10.1007/s10350-004-0623-y. [DOI] [PubMed] [Google Scholar]
- 23.Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Mowschenson PM, Critchlow JF, Peppercorn MA. Ileoanal pouch operation: long-term outcome with or without diverting ileostomy. Arch Surg. 2000;135:463–5. doi: 10.1001/archsurg.135.4.463. discussion 465-6. [DOI] [PubMed] [Google Scholar]
- 25.Madiba TE, Bartolo DC. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. J R Coll Surg Edinb. 2001;46:334–7. [PubMed] [Google Scholar]
- 26.Teixeira WG, da Silva JH, Teixeira MG, Almeida M, Calache JE, Habr-Gama A. Pouchitis: extracolonic manifestation of ulcerative colitis? Rev Hosp Clin Fac Med Sao Paulo. 1999;54:155–8. doi: 10.1590/s0041-87811999000500005. [DOI] [PubMed] [Google Scholar]
- 27.Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5:952–8. doi: 10.1016/j.cgh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Lian L, Fazio VW, Lavery IC, Hammel J, Remzi FH, Shen B. Evaluation of association between precolectomy thrombocytosis and the occurrence of inflammatory pouch disorders. Dis Colon Rectum. 2009;52:1912–8. doi: 10.1007/DCR.0b013e3181b300f4. [DOI] [PubMed] [Google Scholar]
- 29.Lipman JM, Kiran RP, Shen B, Remzi F, Fazio VW. Perioperative factors during ileal pouch-anal anastomosis predict pouchitis. Dis Colon Rectum. 2011;54:311–7. doi: 10.1007/DCR.0b013e3181fded4d. [DOI] [PubMed] [Google Scholar]
- 30.Achkar JP, Al-Haddad M, Lashner B, et al. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3:60–6. doi: 10.1016/s1542-3565(04)00604-4. [DOI] [PubMed] [Google Scholar]
- 31.Stahlberg D, Gullberg K, Liljeqvist L, Hellers G, Lofberg R. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum. 1996;39:1012–8. doi: 10.1007/BF02054692. [DOI] [PubMed] [Google Scholar]
- 32.Shen B, Fazio VW, Remzi FH, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:81–9. doi: 10.1016/j.cgh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Shen B, Remzi FH, Nutter B, et al. Association between immune-associated disorders and adverse outcomes of ileal pouch-anal anastomosis. Am J Gastroenterol. 2009;104:655–64. doi: 10.1038/ajg.2008.76. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman M, Hyman N, Iyer A, Wilcox R, Osler T. The natural history of anal transition zone inflammation and possible relationship to pouchitis: a long-term longitudinal study. Colorectal Dis. 2013;15:1493–8. doi: 10.1111/codi.12322. [DOI] [PubMed] [Google Scholar]
- 35.Rintala RJ, Lindahl H. Restorative proctocolectomy for ulcerative colitis in children–is the J-pouch better than straight pull-through? J Pediatr Surg. 1996;31:530–3. doi: 10.1016/s0022-3468(96)90489-3. [DOI] [PubMed] [Google Scholar]
- 36.Parsi MA, Shen B, Achkar JP, et al. Fecal lactoferrin for diagnosis of symptomatic patients with ileal pouch-anal anastomosis. Gastroenterology. 2004;126:1280–6. doi: 10.1053/j.gastro.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Ball CG, MacLean AR, Buie WD, Smith DF, Raber EL. Portal vein thrombi after ileal pouch-anal anastomosis: its incidence and association with pouchitis. Surg Today. 2007;37:552–7. doi: 10.1007/s00595-006-3470-8. [DOI] [PubMed] [Google Scholar]
- 38.Hashavia E, Dotan I, Rabau M, Klausner JM, Halpern Z, Tulchinsky H. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14:1365–71. doi: 10.1111/j.1463-1318.2012.02993.x. [DOI] [PubMed] [Google Scholar]
- 39.Yantiss RK, Sapp HL, Farraye FA, et al. Histologic predictors of pouchitis in patients with chronic ulcerative colitis. Am J Surg Pathol. 2004;28:999–1006. doi: 10.1097/01.pas.0000126758.35603.8d. [DOI] [PubMed] [Google Scholar]
- 40.Abdelrazeq AS, K N, BI D, et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2008;10:805–13. doi: 10.1111/j.1463-1318.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 41.Samarasekera DN, Stebbing JF, Kettlewell MG, Jewell DP, Mortensen NJ. Outcome of restorative proctocolectomy with ileal reservoir for ulcerative colitis: comparison of distal colitis with more proximal disease. Gut. 1996;38:574–7. doi: 10.1136/gut.38.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okita Y, Araki T, Tanaka K, et al. Characteristics of extremely early-onset pouchitis after proctocolectomy with ileal pouch-anal anastomosis. J Gastrointest Surg. 2013;17:533–9. doi: 10.1007/s11605-012-2120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gozzetti G, Poggioli G, Marchetti F, et al. Functional outcome in handsewn versus stapled ileal pouch-anal anastomosis. Am J Surg. 1994;168:325–9. doi: 10.1016/s0002-9610(05)80158-8. [DOI] [PubMed] [Google Scholar]
- 44.Wasmuth H, Tranø G, Endreseth B, Wibe A, Rydning A, Myrvold H. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch-anal anastomosis. J Gastrointest Surg. 2010;14:1099–104. doi: 10.1007/s11605-010-1223-x. [DOI] [PubMed] [Google Scholar]
- 45.Shen B, Fazio VW, Remzi FH, et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am J Gastroenterol. 2005;100:93–101. doi: 10.1111/j.1572-0241.2005.40778.x. [DOI] [PubMed] [Google Scholar]
- 46.Pastrana RJ, Torres EA, Arroyo JM, Rivera CE, Sanchez CJ, Morales L. Iron-deficiency anemia as presentation of pouchitis. J Clin Gastroenterol. 2007;41:41–4. doi: 10.1097/01.mcg.0000212641.90477.d0. [DOI] [PubMed] [Google Scholar]
- 47.Kroesen AJ, Dullat S, Schulzke JD, Fromm M, Buhr HJ. Permanently increased mucosal permeability in patients with backwash ileitis after ileoanal pouch for ulcerative colitis. Scand J Gastroenterol. 2008;43:704–11. doi: 10.1080/00365520701873206. [DOI] [PubMed] [Google Scholar]
- 48.Hisabe T, Matsui T, Miyaoka M, et al. Diagnosis and clinical course of ulcerative gastroduodenal lesion associated with ulcerative colitis: possible relationship with pouchitis. Dig Endosc. 2010;22:268–74. doi: 10.1111/j.1443-1661.2010.01006.x. [DOI] [PubMed] [Google Scholar]
- 49.Kalkan IH, Dağli Ü, Önder F, et al. Evaluation of preoperative predictors of development of pouchitis after ileal-pouch-anastomosis in ulcerative colitis. Clin Res Hepatol Gastroenterol. 2012;36:622–7. doi: 10.1016/j.clinre.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Poritz LS, Sehgal R, Berg AS, Laufenberg L, Choi C, Williams ED. Chronic use of PPI and H2 antagonists decreases the risk of pouchitis after IPAA for ulcerative colitis. J Gastrointest Surg. 2013;17:1027–31. doi: 10.1007/s11605-013-2172-y. [DOI] [PubMed] [Google Scholar]
- 51.Graf D, Di Cagno R, Fåk F, et al. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164. doi: 10.3402/mehd.v26.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croagh C, Shepherd SJ, Berryman M, Muir JG, Gibson PR. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm Bowel Dis. 2007;13:1522–8. doi: 10.1002/ibd.20249. [DOI] [PubMed] [Google Scholar]
- 53.Ianco O, Tulchinsky H, Lusthaus M, et al. Diet of patients after pouch surgery may affect pouch inflammation. World J Gastroenterol. 2013;19:6458–64. doi: 10.3748/wjg.v19.i38.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welters C, Heineman E, Thunnissen F, van den Bogaard A, Soeters P, Baeten C. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–7. doi: 10.1007/s10350-004-6257-2. [DOI] [PubMed] [Google Scholar]
- 55.Chapman MA, Hutton M, Grahn MF, Williams NS. Metabolic adaptation of terminal ileal mucosa after construction of an ileoanal pouch. Br J Surg. 1997;84:71–3. [PubMed] [Google Scholar]
- 56.Bastida G, Beltran B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol. 2011;17:2740–7. doi: 10.3748/wjg.v17.i22.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38:362–4. doi: 10.1136/gut.38.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joelsson M, Benoni C, Oresland T. Does smoking influence the risk of pouchitis following ileal pouch anal anastomosis for ulcerative colitis? Scand J Gastroenterol. 2006;41:929–33. doi: 10.1080/00365520500527482. [DOI] [PubMed] [Google Scholar]
- 59.Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987;40:601–7. doi: 10.1136/jcp.40.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setti Carraro P, Talbot IC, Nicholls RJ. Longterm appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut. 1994;35:1721–7. doi: 10.1136/gut.35.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–30. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tytgat KM, Opdam FJ, Einerhand AW, Buller HA, Dekker J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut. 1996;38:554–63. doi: 10.1136/gut.38.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergstrom KS, Xia L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology. 2013;23:1026–37. doi: 10.1093/glycob/cwt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One. 2011;6:e24447. doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luukkonen P, Jarvinen H, Tanskanen M, Kahri A. Pouchitis–recurrence of the inflammatory bowel disease? Gut. 1994;35:243–6. doi: 10.1136/gut.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–53. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim LG, Neumann J, Hansen T, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2014;20:892–900. doi: 10.1097/MIB.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 68.Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1149–56. doi: 10.1007/s00384-009-0737-8. [DOI] [PubMed] [Google Scholar]
- 69.Ricanek P, Lunde LK, Frye SA, et al. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin Exp Gastroenterol. 2015;8:49–67. doi: 10.2147/CEG.S70119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zahn A, Moehle C, Langmann T, et al. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J Gastroenterol. 2007;13:1687–95. doi: 10.3748/wjg.v13.i11.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stronati L, Negroni A, Pierdomenico M, et al. Altered expression of innate immunity genes in different intestinal sites of children with ulcerative colitis. Dig Liver Dis. 2010;42:848–53. doi: 10.1016/j.dld.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–74. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith FM, Coffey JC, Kell MR, O’Sullivan M, Redmond HP, Kirwan WO. A characterization of anaerobic colonization and associated mucosal adaptations in the undiseased ileal pouch. Colorectal Dis. 2005;7:563–70. doi: 10.1111/j.1463-1318.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 74.Young VB, Raffals LH, Huse SM, et al. Multiphasic analysis of the temporal development of the distal gut microbiota in patients following ileal pouch anal anastomosis. Microbiome. 2013;1:9. doi: 10.1186/2049-2618-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falk A, Olsson C, Ahrné S, Molin G, Adawi D, Jeppsson B. Ileal pelvic pouch microbiota from two former ulcerative colitis patients, analysed by DNA-based methods, were unstable over time and showed the presence of Clostridium perfringens. Scand J Gastroenterol. 2007;42:973–85. doi: 10.1080/00365520701204238. [DOI] [PubMed] [Google Scholar]
- 76.Kohyama A, Ogawa H, Funayama Y, et al. Bacterial population moves toward a colon-like community in the pouch after total proctocolectomy. Surgery. 2009;145:435–447. doi: 10.1016/j.surg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Duffy M, O’Mahony L, Coffey JC, et al. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45:384–8. doi: 10.1007/s10350-004-6187-z. [DOI] [PubMed] [Google Scholar]
- 78.Gibson GR, Medical Research Council DCNC, Cambridge, UK, Cummings JH, Medical Research Council DCNC, Cambridge, UK, Macfarlane GT, Medical Research Council DCNC, Cambridge, UK. Growth and activities of sulphate‐reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiology Ecology. 2016;9:103–111. [Google Scholar]
- 79.Ohge H, Furne JK, Springfield J, Rothenberger DA, Madoff RD, Levitt MD. Association between fecal hydrogen sulfide production and pouchitis. Dis Colon Rectum. 2005;48:469–75. doi: 10.1007/s10350-004-0820-8. [DOI] [PubMed] [Google Scholar]
- 80.Coffey JC, Rowan F, Burke J, Dochery NG, Kirwan WO, O’Connell PR. Pathogenesis of and unifying hypothesis for idiopathic pouchitis. Am J Gastroenterol. 2009;104:1013–23. doi: 10.1038/ajg.2008.127. [DOI] [PubMed] [Google Scholar]
- 81.McLaughlin SD, Walker AW, Churcher C, et al. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg. 2010;252:90–8. doi: 10.1097/SLA.0b013e3181e3dc8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reshef L, Kovacs A, Ofer A, et al. Pouch Inflammation is Associated with a Decrease in Specific Bacterial Taxa. Gastroenterology. 2015;149:718–727. doi: 10.1053/j.gastro.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 83.Dabard J, Bridonneau C, Phillipe C, et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl Environ Microbiol. 2001;67:4111–8. doi: 10.1128/AEM.67.9.4111-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quevrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2015;65:415–25. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tyler AD, Knox N, Kabakchiev B, et al. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PloS One. 2013;8:e66934. doi: 10.1371/journal.pone.0066934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bambury N, Coffey J, Burke J, Redmond H, Kirwan WO. Sulphomucin expression in ileal pouches: emerging differences between ulcerative colitis and familial adenomatous polyposis pouches. Dis Colon Rectum. 2008;51:561–7. doi: 10.1007/s10350-008-9200-0. [DOI] [PubMed] [Google Scholar]
- 88.Onderdonk AB, Dvorak AM, Cisneros RL, et al. Microbiologic assessment of tissue biopsy samples from ileal pouch patients. J Clin Microbiol. 1992;30:312–7. doi: 10.1128/jcm.30.2.312-317.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgan XC, Kabakchiev B, Waldron L, et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol. 2015;16:67. doi: 10.1186/s13059-015-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruseler-van Embden J, Schouten W, van Lieshout L. Pouchitis: result of microbial imbalance? Gut. 1994;35:658–64. doi: 10.1136/gut.35.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson MW, Rogers GB, Bruce KD, et al. Bacterial community diversity in cultures derived from healthy and inflamed ileal pouches after restorative proctocolectomy. Inflamm Bowel Dis. 2009;15:1803–11. doi: 10.1002/ibd.21022. [DOI] [PubMed] [Google Scholar]
- 92.Lim M, Adams JDW, Wilcox M, Finan P, Sagar P, Burke D. An assessment of bacterial dysbiosis in pouchitis using terminal restriction fragment length polymorphisms of 16S ribosomal DNA from pouch effluent microbiota. Dis Colon Rectum. 2009;52:1492–500. doi: 10.1007/DCR.0b013e3181a7b77a. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin SD, Clark SK, Roberts CH, et al. Extended spectrum beta-lactamase-producing bacteria and Clostridium difficile in patients with pouchitis. Aliment Pharmacol Ther. 2010;32:664–9. doi: 10.1111/j.1365-2036.2010.04401.x. [DOI] [PubMed] [Google Scholar]
- 94.Shen B, Jiang Z-D, Fazio VW, et al. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:782–8. doi: 10.1016/j.cgh.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 95.Zella GC, Hait EJ, Glavan T, et al. Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm Bowel Dis. 2011;17:1092–1100. doi: 10.1002/ibd.21460. [DOI] [PubMed] [Google Scholar]
- 96.Komanduri S, Gillevet PM, Sikaroodi M, Mutlu E, Keshavarzian A. Dysbiosis in pouchitis: evidence of unique microfloral patterns in pouch inflammation. Clin Gastroenterol Hepatol. 2007;5:352–60. doi: 10.1016/j.cgh.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Scarpa M, Grillo A, Faggian D, et al. Relationship between mucosa-associated microbiota and inflammatory parameters in the ileal pouch after restorative proctocolectomy for ulcerative colitis. Surgery. 2011;150:56–67. doi: 10.1016/j.surg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 98.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444S–450. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 99.Corthesy B, Gaskins HR, Mercenier A. Cross-Talk between Probiotic Bacteria and the Host Immune System. J Nutr. 2007;137:781S–790. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- 100.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 101.Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Therap. 2003;17:509–15. doi: 10.1046/j.1365-2036.2003.01465.x. [DOI] [PubMed] [Google Scholar]
- 102.Persborn M, Gerritsen J, Wallon C, Carlsson A, Akkermans LMA, Soderholm JD. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Therap. 2013;38:772–83. doi: 10.1111/apt.12451. [DOI] [PubMed] [Google Scholar]
- 103.Kuhbacher T, Ott SJ, Helwig U, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL # 3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL # 3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 106.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 107.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–28. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–9. doi: 10.1023/a:1018851723920. [DOI] [PubMed] [Google Scholar]
- 109.Chapman MA, Grahn MF, Hutton M, Williams NS. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. Br J Surg. 1995;82:36–8. doi: 10.1002/bjs.1800820115. [DOI] [PubMed] [Google Scholar]
- 110.Sagar PM, Taylor BA, Godwin P, et al. Acute pouchitis and deficiencies of fuel. Dis Colon Rectum. 1995;38:488–93. doi: 10.1007/BF02148848. [DOI] [PubMed] [Google Scholar]
- 111.De Preter V, Bulteel V, Suenaert P, et al. Pouchitis, similar to active ulcerative colitis, is associated with impaired butyrate oxidation by intestinal mucosa. Inflamm Bowel Dis. 2009;15:335–40. doi: 10.1002/ibd.20768. [DOI] [PubMed] [Google Scholar]
- 112.Clausen MR, Tvede M, Mortensen PB. Short-chain fatty acids in pouch contents from patients with and without pouchitis after ileal pouch-anal anastomosis. Gastroenterology. 1992;103:1144–53. doi: 10.1016/0016-5085(92)91497-r. [DOI] [PubMed] [Google Scholar]
- 113.den Hoed PT, van Goch JJ, Veen HF, Ouwendijk RJ. Severe pouchitis successfully treated with short-chain fatty acids. Can J Surg. 1996;39:168–9. [PMC free article] [PubMed] [Google Scholar]
- 114.de Silva HJ, Ireland A, Kettlewell M, Mortensen N, Jewell DP. Short-chain fatty acid irrigation in severe pouchitis. N Engl J Med. 1989;321:1416–7. doi: 10.1056/nejm198911163212019. [DOI] [PubMed] [Google Scholar]
- 115.Perbeck L, Lindquist K, Liljeqvist L. The mucosal blood flow in pelvic pouches in man. A methodologic study of fluorescein flowmetry. Dis Colon Rectum. 1985;28:931–6. doi: 10.1007/BF02554310. [DOI] [PubMed] [Google Scholar]
- 116.Kienle P, Weitz J, Reinshagen S, et al. Association of decreased perfusion of the ileoanal pouch mucosa with early postoperative pouchitis and local septic complications. Arch Surg. 2001;136:1124–30. doi: 10.1001/archsurg.136.10.1124. [DOI] [PubMed] [Google Scholar]
- 117.El Muhtaseb MS, Talwar D, Duncan A, et al. Free radical activity and lipid soluble anti-oxidant vitamin status in patients with long-term ileal pouch-anal anastomosis. Colorectal Dis. 2009;11:67–72. doi: 10.1111/j.1463-1318.2008.01517.x. [DOI] [PubMed] [Google Scholar]
- 118.Shebani KO, Stucchi AF, McClung JP, Beer ER, LaMorte WW, Becker JM. Role of stasis and oxidative stress in ileal pouch inflammation. J Surg Res. 2000;90:67–75. doi: 10.1006/jsre.2000.5842. [DOI] [PubMed] [Google Scholar]
- 119.Joelsson M, Andersson M, Bark T, et al. Allopurinol as prophylaxis against pouchitis following ileal pouch-anal anastomosis for ulcerative colitis. A randomized placebo-controlled double-blind study. Scand J Gastroenterol. 2001;36:1179–84. doi: 10.1080/00365520152584815. [DOI] [PubMed] [Google Scholar]
- 120.Levin KE, Pemberton JH, Phillips SF, Zinsmeister AR, Pezim ME. Role of oxygen free radicals in the etiology of pouchitis. Dis Colon Rectum. 1992;35:452–6. doi: 10.1007/BF02049401. [DOI] [PubMed] [Google Scholar]
- 121.Tyler AD, Milgrom R, Xu W, et al. Antimicrobial antibodies are associated with a Crohn’s disease-like phenotype after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2012;10:507–12.e1. doi: 10.1016/j.cgh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 122.Norwood MG, Mann CD, West K, Miller AS, Hemingway D. Restorative proctocolectomy. Does ethnicity affect outcome? Colorectal Dis. 2009;11:972–5. doi: 10.1111/j.1463-1318.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 123.Moore L, Tang L, Lopez R, Shen B. Clinical features of ileal pouch-anal anastomosis in African American patients with underlying ulcerative colitis. Aliment Pharmacol Ther. 2009;30:385–91. doi: 10.1111/j.1365-2036.2009.04054.x. [DOI] [PubMed] [Google Scholar]
- 124.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–52. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gordon H, Trier Moller F, Andersen V, Harbord M. Heritability in inflammatory bowel disease: from the first twin study to genome-wide association studies. Inflamm Bowel Dis. 2015;21:1428–34. doi: 10.1097/MIB.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lecine P, Esmiol S, Metais JY, et al. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 127.Kabi A, McDonald C. FRMBP2 directs NOD2 to the membrane. Proc Natl Acad Sci U S A. 2012;109:21188–9. doi: 10.1073/pnas.1219395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barnich N, JE A, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-kappaB activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–6. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tyler AD, Milgrom R, Stempak JM, et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut. 2013;62:1433–9. doi: 10.1136/gutjnl-2011-301957. [DOI] [PubMed] [Google Scholar]
- 130.Lesage S, Zouali H, Czard J-P, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–57. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McGovern DPB, Gardet A, Trkvist L, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–7. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sehgal R, Berg A, Polinski JI, et al. Genetic risk profiling and gene signature modeling to predict risk of complications after IPAA. Dis Colon Rectum. 2012;55:239–48. doi: 10.1097/DCR.0b013e31823e2d18. [DOI] [PubMed] [Google Scholar]
- 133.Stallmach A, Chan CC, Ecker KW, et al. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415–22. doi: 10.1136/gut.47.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coffey JC, Bennett MW, Wang JH, et al. Upregulation of Fas-Fas-L (CD95/CD95L)-mediated epithelial apoptosis–a putative role in pouchitis? J Surg Res. 2001;98:27–32. doi: 10.1006/jsre.2001.6129. [DOI] [PubMed] [Google Scholar]
- 135.Carter MJ, Di Giovine F, Cox A, et al. The interleukin 1 receptor antagonist gene allele 2 as a predictor of pouchitis following colectomy and IPAA in ulcerative colitis. Gastroenterology. 2001;121:805–11. doi: 10.1053/gast.2001.28017. [DOI] [PubMed] [Google Scholar]
- 136.Aisenberg J, Legnani PE, Nilubol N, et al. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am J Gastroenterol. 2004;99:432–41. doi: 10.1111/j.1572-0241.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- 137.Lammers KM, Ouburg S, Morré S, et al. Combined carriership of TLR9-1237C and CD14-260T alleles enhances the risk of developing chronic relapsing pouchitis. World J Gastroenterol. 2005;11:7323–9. doi: 10.3748/wjg.v11.i46.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sehgal R, Berg A, Hegarty JP, et al. NOD2/CARD15 mutations correlate with severe pouchitis after ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:1487–94. doi: 10.1007/DCR.0b013e3181f22635. [DOI] [PubMed] [Google Scholar]
- 139.Paziewska A, Horbacka K, Goryca K, et al. Transcriptional changes between uninflamed ulcerative colitis and familial adenomatous polyposis pouch mucosa can be attributed to an altered immune response. Acta Biochim Pol. 2015;62:69–75. doi: 10.18388/abp.2014_778. [DOI] [PubMed] [Google Scholar]
- 140.De Re V, Simula MP, Caggiari L, et al. Protein expression profile of celiac disease patient with aberrant T cell by two-dimensional difference gel electrophoresis. Ann N Y Acad Sci. 2007;1109:429–40. doi: 10.1196/annals.1398.049. [DOI] [PubMed] [Google Scholar]
- 141.Stallmach A, Schäfer F, Hoffmann S, et al. Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchitis. Gut. 1998;43:499–505. doi: 10.1136/gut.43.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Polese L, Angriman I, Giuseppe D, et al. Persistence of high CD40 and CD40L expression after restorative proctocolectomy for ulcerative colitis. World J Gastroenterol. 2005;11:5303–8. doi: 10.3748/wjg.v11.i34.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hirata N, Oshitani N, Kamata N, et al. Proliferation of immature plasma cells in pouchitis mucosa in patients with ulcerative colitis. Inflamm Bowel Dis. 2008;14:1084–90. doi: 10.1002/ibd.20447. [DOI] [PubMed] [Google Scholar]
- 144.Reumaux D, Duthilleul P, Roos D. Pathogenesis of diseases associated with antineutrophil cytoplasm autoantibodies. Hum Immunol. 2004;65:1–12. doi: 10.1016/j.humimm.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 145.Kaditis AG, Perrault J, Sandborn WJ, Landers CJ, Zinsmeister AR, Targan SR. Antineutrophil cytoplasmic antibody subtypes in children and adolescents after ileal pouch-anal anastomosis for ulcerative colitis. J Pediatr Gastroenterol Nutr. 1998;26:386–92. doi: 10.1097/00005176-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 146.Coukos JA, Howard LA, Weinberg JM, Becker JM, Stucchi AF, Farraye FA. ASCA IgG and CBir antibodies are associated with the development of Crohn’s disease and fistulae following ileal pouch-anal anastomosis. Dig Dis Sci. 2012;57:1544–53. doi: 10.1007/s10620-012-2050-6. [DOI] [PubMed] [Google Scholar]
- 147.Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:561–8. doi: 10.1016/j.cgh.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Esteve M, Mallolas J, Klaassen J, et al. Antineutrophil cytoplasmic antibodies in sera from colectomised ulcerative colitis patients and its relation to the presence of pouchitis. Gut. 1996;38:894–8. doi: 10.1136/gut.38.6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tang LY, Cai H, Navaneethan U, et al. Utility of fecal and serum anti-Saccharomyces cerevisiae antibodies in the diagnosis of Crohn’s disease-like condition of the pouch. Int J Colorectal Dis. 2012;27:1455–63. doi: 10.1007/s00384-012-1444-4. [DOI] [PubMed] [Google Scholar]
- 150.White E, Melmed GY, Vasiliauskas EA, et al. A prospective analysis of clinical variables, serologic factors, and outcome of ileal pouch-anal anastomosis in patients with backwash ileitis. Dis Colon Rectum. 2010;53:987–94. doi: 10.1007/DCR.0b013e3181dcb3f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119–24. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 152.Navaneethan U, Venkatesh PG, Kapoor S, Kiran RP, Remzi FH, Shen B. Elevated serum IgG4 is associated with chronic antibiotic-refractory pouchitis. J Gastrointest Surg. 2011;5:1556–61. doi: 10.1007/s11605-011-1587-6. [DOI] [PubMed] [Google Scholar]
- 153.Jung C, Hugot JP, Barreau F. Peyer’s Patches: The Immune Sensors of the Intestine. Intl J Inflamm. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Navaneethan U, Shen B. Secondary pouchitis: those with identifiable etiopathogenetic or triggering factors. Am J Gastroenterol. 2010;105:51–64. doi: 10.1038/ajg.2009.530. [DOI] [PubMed] [Google Scholar]
- 155.Suzuki H, Ogawa H, Shibata C, et al. The long-term clinical course of pouchitis after total proctocolectomy and IPAA for ulcerative colitis. Dis Colon Rectum. 2012;55:330–6. doi: 10.1097/DCR.0b013e3182417358. [DOI] [PubMed] [Google Scholar]
- 156.Li Y, Qian J, Queener E, Shen B. Risk factors and outcome of PCR-detected Clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2013;19:397–403. doi: 10.1097/MIB.0b013e318280fcb9. [DOI] [PubMed] [Google Scholar]
- 157.Sun C, Du P, Wu XR, Queener E, Shen B. Preoperative Clostridium difficile infection is not associated with an increased risk for the infection in ileal pouch patients. Dig Dis Sci. 2014;59:1262–8. doi: 10.1007/s10620-014-3047-0. [DOI] [PubMed] [Google Scholar]
- 158.Seril DN, Ashburn JH, Lian L, Shen B. Risk factors and management of refractory or recurrent clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2014;20:2226–33. doi: 10.1097/MIB.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 159.Patel LN, Schairer J, Shen B. Fecal transplantation therapy for Clostridium difficile-associated pouchitis. Int J Colorectal Dis. 2014;29:263–4. doi: 10.1007/s00384-013-1771-0. [DOI] [PubMed] [Google Scholar]
- 160.McCurdy JD, Loftus EV, Jr, Tremaine WJ, et al. Cytomegalovirus infection of the ileoanal pouch: clinical characteristics and outcomes. Inflamm Bowel Dis. 2013;19:2394–9. doi: 10.1097/MIB.0b013e3182a52553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tonolini M. Acute nonsteroidal anti-inflammatory drug-induced colitis. J Emerg Trauma Shock. 2013;6:301–3. doi: 10.4103/0974-2700.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650–61. doi: 10.1097/MPG.0b013e3182128512. [DOI] [PubMed] [Google Scholar]
- 163.Shen B, Plesec T, Remzi FH, et al. Evaluation of tissue eosinophilia in the pouch and afferent limb in patients with restorative proctocolectomy. Inflamm Bowel Dis. 2008;14:744–9. doi: 10.1002/ibd.20401. [DOI] [PubMed] [Google Scholar]


