Abstract
The drug camptothecin has a wide range of antitumor effects in cancers including gastric cancer, rectal and colon cancer, liver cancer, and lung cancer. Camptothecin-based drugs inhibit topoisomerase 1 (Topo 1), leading to destruction of DNA, and are currently being used as important chemotherapeutic agents in clinical antitumor treatment. However, the main obstacle associated with cancer therapy is represented by systemic toxicity of conventional anticancer drugs and their low accumulation at the tumor site. In addition, low bioavailability, poor water solubility, and other shortcomings hinder their anticancer activity. Different from traditional pharmaceutical preparations, nanotechnology-dependent nanopharmaceutical preparations have become one of the main strategies for different countries worldwide to overcome drug development problems. In this review, we summarized the current hotspots and discussed a variety of camptothecin-based nanodrugs for cancer therapy. We hope that through this review, more efficient drug delivery systems could be designed with potential applications in clinical cancer therapy.
Keywords: Camptothecins, nanomedicine, cancer therapy, drug delivery system
Introduction
In 1966, Monroe E. Wall1 first isolated camptothecin (CPT) from the stem of Camptotheca, a plant genus endemic to China. He discovered that CPT had a number of effects on malignant tumors such as gastrointestinal cancer, liver cancer, and leukemia. Later, in 1985, Y. H. Hsiang2 found that CPT could block the synthesis of topoisomerase I (Topo 1), an enzyme closely related to cell division. Blockade of Topo 1 production by CPT prevents cancer cell growth, thus endowing this compound with unique anticancer properties. From this discovery, research on CPT entered a new stage. A large number of CPT derivatives and analogs emerged, among which irinotecan, topotecan, and 10-hydroxycamptothecin (HCPT) were approved for listing. Moreover, various active compounds are currently in the clinical stage. However, similar to most chemotherapeutic agents, application of CPT is limited by its inherent deficiencies such as poor water solubility, low biocompatibility, toxic side effects on healthy tissue3, and a variety of complications4,5.
In recent years, scientists have been trying to overcome these deficiencies. The emergence of nanotechnology provides possibilities to address chemotherapy-associated drawbacks such as toxic side effects of anticancer drugs as well as their low water solubility. Due to the unique features of nanoparticles, including nanoscale size, high specific surface area, and controllable physical and chemical properties, the water solubility and stability of drugs can be improved, resulting in desirable pharmacokinetics and other parameters. In addition, nanopharmaceuticals can accumulate at the tumor site and regulate drug distribution due to their enhanced permeability and retention effect (EPR)6,7. The key advantages of nanomedicines are as follows: (1) improved water solubility and biocompatibility of the drug, (2) prolongation of tolerance time for the anticancer drug in the body by surface-modified nanoparticles, (3) precise accumulation of chemotherapeutics at the tumor site by targeting strategy, (4) stimulus-responsive release of payloads, and (5) reduced toxic side effects against normal cells and tissues7,8. In this regard, an effective combination of conventional chemotherapeutics with nanotechnology-based approaches to achieve efficient tumor treatment with low toxic side effects has become an important research area in cancer treatment and clinical application8-11.
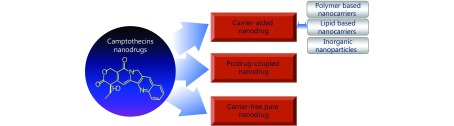
Onivyde, a nanoliposomal dosage form of irinotecan, was approved by the FDA for pancreatic cancer treatment in 2015, and is currently being tested clinically for other malignancies12,13. In this manner, carrier-assisted CPT nanodrug delivery systems have been studied extensively. In addition, prodrug-coupled nanodrug delivery system is more suitable for effective transport and tracking of CPT-based drugs. Carrier-free, self-assembled pure nanodrug delivery systems provide more efficient drug doses for better therapeutic effects. In this review, we present all three nanodrug delivery systems based on CPTs (Figure 1).
1.
Schematic illustration of established camptothecins-based nanodrug platforms.
Carrier-aided nanodrug delivery system
Normally, drugs undergo rapid metabolism in the body and lose their pharmacological activity. Therefore, it is important to improve their effective accumulation in the lesion. Based on the remarkable research achievements made in material science and pharmacy, nanomaterials with different sizes, structures, and surface properties have been developed including liposomes, polymeric nanoparticles, and inorganic nanomaterials such as iron oxide, carbon nanotubes, and silica14. These carriers can be widely used for delivering CPT drugs in vivo. The therapeutic effect of the drug is improved by the systemic (intravenous) or in situ route of administration. In addition, existing surface modification techniques can also affect the pharmacokinetic behavior and biodistribution of nanoparticles. For example, PEGylation protects drug-loaded nanoparticles from adsorption in the bloodstream, thereby achieving a long circulation cycle in the body and resulting in enhanced delivery at the tumor site through the EPR effect. Furthermore, the nanoparticle surface can be modified with active ligands to target specific cells. At present, many nanocarriers of CPT drugs have been used in clinical trials (Table 1).
1.
Carrier-aided nanodrug delivery system for camptothecins nanomedicine in clinical trials. Alternative names for the products are given in brackets
| Product [company] | Platform | Drug | Indication | Status | Ref. |
| CPX-1 [Celator] | Liposome | Irinotecan HCI/floxuridine | Colorectal cancer | Phase 2 | [15] |
| IHL-305 [Yakult Honsha] | Liposome | Irinotecan (CPT-11) | Solid tumors | Phase 1 | [16] |
| INX-0076 (Brakiva)
[Tekmira] |
Liposome | Topotecan | Solid tumors | Phase 1 | [17] |
| L9NC
[University of Mexico] |
Liposome
(aerosol) |
9-Nitro-20
(S)-Camptothecin |
Non-small-cell lung cancer | Phase 2 | [18] |
| LE-SN38
[NeoPharm/Insys] |
Liposome | SN38 (active metabolite
of irinotecan) |
Colorectal cancer | Phase 2 | [19] |
| MM-398 (PEP02)
[Merrimack] |
Liposome | CPT-11 (irinotecan) | Pancreatic cancer,
gastric cancer, glioma |
Phase 3, Phase 2 Phase 1 | [20] |
| CRLX-101 (IT-101)
[Cerulean Pharma] |
Cyclodextrin
nanoparticle |
Camptothecin | Solid tumors, renal cell | Phase 1/2 | [21] |
| Lipotecan (TLC388)
[Taiwan Liposome] |
Polymeric micelle | TLC388
(camptothecin derivate) |
Carcinoma, rectal cancer, non-small-cell lung cancer, liver cancer, renal cancer | Phase 1/2
(orphan drug status) |
[22] |
| NK-012
[Nippon Kayaku] |
PEG-PGA
polymeric micelle |
SN-38 (active metabolite
of irinotecan) |
Solid tumors, small cell lung cancer, breast cancer | Phase 2 | [23] |
Polymer-based nanocarriers for delivering CPTs
Delivery systems for transporting CPT drugs based on polymers as building blocks can be divided into two groups: natural polymers (such as proteins, and polysaccharides), and synthetic polymers (such as PLGA-PEG and PCL-PEG). Due to its natural presence in humans as well as its unique shape and excellent biocompatibility, the transferrin nanocarrier has attracted substantial interest. Chen et al24. prepared surface-modified transferrin nanoparticles of irinotecan, containing the specific targeting polypeptide PROM1, to achieve targeted delivery in colorectal cancer. Min et al.25 loaded CPT into glycol-modified chitosan to prepare a nanoscale drug delivery system that shows prolonged blood circulation time and tumor targeting ability, for use in the treatment of human breast cancer. In addition, human serum albumin (HSA) is a multi-gene family protein that exists in the circulatory system with an average molecular weight of 66 kDa and a blood concentration of 50 mg/mL. It has low toxicity, high biocompatibility, and suitable biodegradation rate, and has therefore been widely used in drug delivery systems. Wang et al.26 prepared HSA-modified HCPT-containing nanoparticles, FA-HSA-nHCPT-NPs, in which the drug loading was 7.8%.
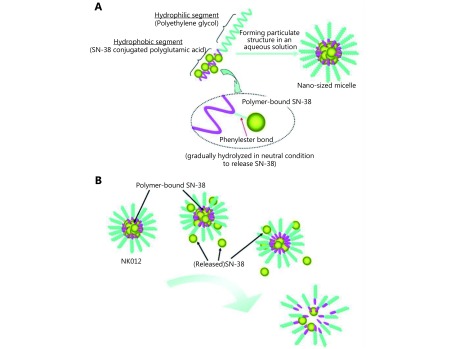
Owing to the diversity of chemical properties, synthetic polymer nanocarriers are a promising tool for nanotechnology-based therapy. Svenson et al.27 prepared CPT nanoparticles CRLX101, based on cyclodextrin as a carrier. Preclinical data indicated that CRLX101 showed a complete tumor response of 55.6% at day 125 after treatment at a dose of 10 mg/kg, whereas no complete tumor responses were observed in irinotecan-treated mice. Because of its antitumor properties such as inhibition of tumor cell proliferation and angiogenesis, CRLX101 has entered the clinical stage21. Hamaguchi et al. 23have developed NK012 into clinical phase II. SN-38 was first connected to polyglutamate through an ester bond, and then assembled with polyethylene glycol as a shell to form nanopolymeric micelles (Figure 2). The size of the resulting nanoparticles was about 20 nm, even in different patients who have shown a stable effect23. The copolymer was also considered a promising carrier. For example, Lee et al.28 loaded SN-38 onto poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic F-108) and poly(PEG-b-PCL) to form nanoparticles. The drug loading capacity was (20.73±0.66)% and good biodistribution was observed, which improved the drug’s ability to kill tumor cells. Apart from these, PDMA-block-poly(ε-caprolactone) (PDMA-b-PCL)28, poly-lactide-co-glycolide-poly-Ethylene glycol-folate (PLGA-PEG-FOL) conjugate29 and other polymers have the potential to improve cell penetration and enhance the antitumor effect of CPT drugs.
2.
Schematic structure of NK012 and release of SN-38 from NK012. Reprinted with permission from Ref [23]; Copyright © 2010, American Association for Cancer Research.
Lipid-based nanocarriers for delivering CPTs
Liposomes are vesicles composed of a phospholipid bilayer with an aqueous phase as the core. This structure allows loading of therapeutic drugs either into the hydrophobic region of the liposome (the partition of the lipid layer) or in the hydrophilic part (the aqueous phase). This feature makes liposomes very promising nanocarriers30. A new technique was first demonstrated for stabilizing the embedded drugs using liposomes by encapsulating irinotecan (CPT-11) into long-term circulating liposomal nanoparticles in 1999. After that, the most critical achievement is that irinotecan nanoliposomes (Onivyde) was approved by the FDA for the treatment of metastatic pancreatic cancer in 201531. In addition, the presence of polyethylene glycol (PEG) on the nanocarrier surface can prevent absorption of liposomes by the vascular endothelial system (RES). Atyabi et al.32 found that the blood concentration of PEGylated nanoliposomes carrying SN-38 is nearly 50%, higher than that of non-PEGylated liposomes, and their accumulation in the liver, spleen, lungs, kidneys, and other organs is lower than that of non-PEGylated nanomicelles. Recently, Zhang et al.33 obtained (HCPT-Ch-LDH)@LS nanostructures by liposome surface modification through a co-assembling strategy, thus providing better water solubility and sustained release of HCPT compared to those of unmodified-liposome nanoparticles.
Inorganic nanoparticles for delivering CPTs
Inorganic nanoparticles are widely used in tumor imaging, radiotherapy, and drug delivery34-39. Single-walled carbon nanotubes have low immunocompetence and show effective cell endocytosis, and were used by Lee et al.40 as carriers of SN-38 for targeted delivery and controlled drug release. Liu et al.41 developed a lipid carrier coated with mesoporous silica (LB-MSNP), which shows better biocompatibility and therapeutic effects than those shown by liposomes and free drugs, and is expected to be used as the first-line medication in the treatment of pancreatic duct cancer (PDAC). This group also attached the iRGD polypeptide to the liposome surface in order to enhance the efficacy of irinotecan and reduce tumor metastasis42. In addition, Song et al.43 utilized PEG-modified hollow tantalum oxide with the payload SN-38 (H-TaOx-PEG@SN-38) for magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) multimodal imaging, as well as to achieve the combined effect of radiotherapy and chemotherapy. Muniesa et al.44 also designed a nanomaterial with CPT and mesoporous silica, thereby achieving a response in the glutathione environment.
Prodrug-coupled nanodrug delivery systems
At present, prodrug-coupled nanodrugs are one of the successful drug delivery systems in clinical treatment. A prodrug is a compound that is metabolized to a pharmacologically active drug after administration. Nanotherapeutics are designed by covalently linking prodrugs with nanosized carriers composed of antibody molecules, lipids, proteins, polysaccharides, polypeptides, or polymers, which can improve bioavailability when a drug itself is poorly absorbed, and thus reduce the severe unintended and undesirable side effects of the drug45.
Only a few nanoparticles conjugated with CPT, such as N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers46,47, polyglutamic acid (PGA), polyethylene glycol (PEG)48-50, and carboxymethyldextran polyalcohol polymer51,52 have entered clinical practice so far. Prodrug-coupled nanodrug delivery systems have also been used in clinical trials (Table 2). These block copolymers are assembled to form micelles. Drug coupling to the polymer carrier can improve the water solubility of hydrophobic drugs, thus making it easier to inject them in patients. Although there are many polymers that can be used for drug delivery, only few of them can be used in the clinic due to their toxicity and immunogenicity, resulting in less effective drug delivery45.
2.
Prodrug-coupled nanodrug delivery system for camptothecins nanomedicine in clinical trials. Alternative names for the products are given in brackets
| Product [company] | Platform | Drug | Indication | Status | Ref. |
| CT-2106
[CTI Biopharma] |
Polyglutamic acid drug conjugate | Camptothecin | Colon cancer, ovarian cancer | Phase 1/2 | [57] |
| DE-310
[Daiichi Pharmaceutical] |
Carboxymethyldextran polyalcohol drug conjugate | DX-8951
(camptothecin derivate) |
Solid tumors | Phase 1 | [52] |
| Delimotecan
(Men 4901/T-0128) |
Carboxymethyldextran
drug conjugate |
T-2513
(camptothecin analogue) |
Solid tumors | Phase 1 | [51] |
| MAG-CPT (PNU166148/
Mureletecan) [Pfizer] |
HPMA drug conjugate | Camptothecin | Solid tumors | Phase 1 | [47] |
| NKTR-102 (Etirinotecan
pegol) [Nektar] |
PEG drug conjugate | Irinotecan | Breast cancer, ovarian
cancer, colorectal cancer |
Phase 3 | [48] |
| Pegamotecan
(EZ-246) [Enzon] |
PEG drug conjugate | Camptothecin | Gastric cancer | Phase 2 | [50] |
| XMT-1001 [Mersana] | Fleximer drug conjugate | Camptothecin | Gastric cancer, lung cancer | Phase 1 | [58] |
In addition, most nanoparticles conjugated with CPT using polymers show tumor targeting dependent on tumor vascular permeability (EPR effect53). Han et al.54 connected herceptin (an antibody to HER2) to the surface of MAP-CPT nanoparticles via a boronic acid bond to achieve targeted combination therapy for HER2-overexpressing breast cancer. Peng et al.55 conjugated the anticancer drug CPT with a polypeptide that was self-assembled to form nanofibers that could release CPT by hydrolysis. Zhang et al.56 prepared a combination of irinotecan and fatty acids to achieve a high drug load with 77.2% irinotecan content, high drug availability, and enrichment at the tumor site.
Carrier-free pure nanodrug delivery systems
While drug-loaded nanocarriers have many advantages, there are still some concerns regarding potential issues8,59,60 in the fields of environment, health, and safety61,62. Furthermore, almost all carriers have no therapeutic efficacy by themselves63,64. It is complicated to establish a proper manufacturing process65 for drug-loaded nanocarriers, and most of them demonstrate low drug-loading capacity66. Even worse, many carriers may cause high toxicity and serious inflammation in the kidneys and other organs61,63,64. Therefore, development of alternative targeted delivery strategies with minimum use of inert materials is highly desirable67. There is no doubt that carrier-free pure nanodrugs will thus become candidates in the next generation of drugs63,68-71.
Hitoshi Kasai68 proposed the use of CPT (SN-38) to form a dimer and first assembled it to form nanoparticles successfully. These nanoparticles showed reduced side effects due to the absence of other components, and the drug-loading capacity was nearly 100%. Since then, pure drugs with the ability to self-assemble into nanodelivery systems have opened a new chapter in drug delivery. Subsequently, disulfide-linked CPT and irinotecan nanoparticles achieved a response to glutathione and drug release in a specified area72. Improvement of the water solubility of CPT and multiple attacks against tumor cells could be achieved through co-assembly of CPT and other drug components. In a previous work, Chen et al.73 provided a new strategy for the combination of drugs by co-loading HCPT and doxorubicin (DOX)73,74, which resulted in a synergistic effect in overcoming tumor drug resistance. Wen et al.75 combined HCPT with dihydroporphyrin (Ce6). These two components co-formed a nanorod structure by π-π conjugation and hydrophobic interaction. This hybrid nanodrug not only circumvents the extreme hydrophobicity of HCPT (with a solubility at least 100-fold higher than that of free HCPT in water), but also integrates two tumor treatment modalities into one system. It provided a simple and green solution to develop pure carrier-free nanodrugs that combine two treatment modalities, chemotherapy and photodynamic therapy, into a single platform to circumvent the drawbacks of traditional small molecules and to achieve highly potent antitumor capacity, which could be easily expanded to other drugs and modalities. The rationale of this facile and green strategy for carrier-free pure nanodrugs might provide new opportunities for the development of combinatorial therapeutics for tumors75.
Conclusions
Due to their unique physical and chemical properties, CPT drugs have received widespread attention in the field of pharmaceutical preparations. However, there are many obstacles for nanodrugs in their journey from the laboratory to the clinical stage. Although CPT drug delivery systems have been extensively studied, most nanodrug-dependent nanopharmaceuticals are limited by the potentially toxic side effects of nanomaterials and in vivo metabolism and controllable problems. Thus, nanodrugs that can be used without nanocarriers, relying on self-assembled drug molecules, are thought to be the new generation of pharmaceutical preparations of clinical value, but they still need to be tested. As a general rule, the simpler and easier the development of a system is, the better are its chances of reaching the clinic.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Xiaoli Liu, Email: liuxiaoli@nanoctr.cn.
Xingjie Liang, Email: liangxj@nanoctr.cn.
References
- 1.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata . J Am Chem Soc. 1966;88:3888–90. [Google Scholar]
- 2.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–8. [PubMed] [Google Scholar]
- 3.Wall ME. Camptothecin and taxol: discovery to clinic. Med Res Rev. 1998;18:299–314. doi: 10.1002/(sici)1098-1128(199809)18:5<299::aid-med2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34:1500–8. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 5.Potmesil M. Camptothecins: from bench research to hospital wards. Cancer Res. 1994;54:1431–9. [PubMed] [Google Scholar]
- 6.Maeda H, Greish K, Fang J. The EPR effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century. Adv Polym Sci. 2006;193:103–21. [Google Scholar]
- 7.Xin Y, Yin MM, Zhao LY, Meng FL, Luo L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol Med. 2017;14:228–41. doi: 10.20892/j.issn.2095-3941.2017.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–87. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 10.D'Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12:523–9. doi: 10.1038/nnano.2017.67. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Zhang XN, Li XD, Chang J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol Med. 2016;13:339–48. doi: 10.20892/j.issn.2095-3941.2016.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HJ. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001–7. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passero FC Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev Anticancer Ther. 2016;16:697–703. doi: 10.1080/14737140.2016.1192471. [DOI] [PubMed] [Google Scholar]
- 14.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 15.Batist G, Gelmon KA, Chi KN, Miller WH, Chia SKL, Mayer LD, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 16.Infante JR, Keedy VL, Jones SF, Zamboni WC, Chan E, Bendell JC, et al. Phase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;70:699–705. doi: 10.1007/s00280-012-1960-5. [DOI] [PubMed] [Google Scholar]
- 17.Tardi P, Choice E, Masin D, Redelmeier T, Bally M, Madden TD. Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res. 2000;60:3389–93. [PubMed] [Google Scholar]
- 18.Verschraegen CF, Gilbert BE, Huaringa AJ, Newman R, Harris N, Leyva FJ, et al. Feasibility, phase I, and pharmacological study of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann N Y Acad Sci. 2000;922:352–4. doi: 10.1111/j.1749-6632.2000.tb07063.x. [DOI] [PubMed] [Google Scholar]
- 19.Pal A, Khan S, Wang YF, Kamath N, Sarkar AK, Ahmad A, et al. Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res. 2005;25:331–42. [PubMed] [Google Scholar]
- 20.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer. 2013;109:920–5. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur S, Wang YF, Kretzner L, Chen LL, Yen T, Wu XW, et al. Pharmacodynamic and pharmacogenomic study of the nanoparticle conjugate of camptothecin CRLX101 for the treatment of cancer. Nanomedicine. 2014;10:1477–86. doi: 10.1016/j.nano.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Ghamande S, Lin CC, Cho DC, Shapiro GI, Kwak EL, Silverman MH, et al. A phase 1 open-label, sequential dose-escalation study investigating the safety, tolerability, and pharmacokinetics of intravenous TLC388 administered to patients with advanced solid tumors. Invest New Drugs. 2014;32:445–51. doi: 10.1007/s10637-013-0044-7. [DOI] [PubMed] [Google Scholar]
- 23.Hamaguchi T, DoiT , Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, et al. Phase I study of NK012, a novel SN-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res. 2010;16:5058–66. doi: 10.1158/1078-0432.CCR-10-0387. [DOI] [PubMed] [Google Scholar]
- 24.Chen JLY, Tsai YC, Tsai MH, Lee SY, Wei MF, Kuo SH, et al. Prominin-1-specific binding peptide-modified apoferritin nanoparticle carrying irinotecan as a novel radiosensitizer for colorectal cancer stem-like cells. Part Part Syst Charact. 2017; 34: 1600424.
- 25.Min KH, Park K, Kim Y-S, Bae SM, Lee S, Jo HG, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127:208–18. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang WC, Liang H, Sun BH, Xu JL, Zeng Z, Zhao XJ, et al. Pharmacokinetics and tissue distribution of folate-decorated human serum albumin loaded with nano-hydroxycamptothecin for tumor targeting. J Pharm Sci. 2016;105:1874–80. doi: 10.1016/j.xphs.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Svenson S, Wolfgang M, Hwang J, Ryan J, Eliasof S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release. 2011;153:49–55. doi: 10.1016/j.jconrel.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Yang CY, Peng CL, Wei MF, Chen KC, Yao CJ, et al. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92–105. doi: 10.1016/j.biomaterials.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 29.Ebrahimnejad P, Dinarvand , R , Sajadi , A. , Jaafari MR, Nomani AR, Azizi E, et al. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomedicine. 2010;6:478–85. doi: 10.1016/j.nano.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse DN, Yapp D, Verreault M, Anantha M, Sutherland B, Bally MB. Lipid-based nanoformulation of irinotecan: dual mechanism of action allows for combination chemo/angiogenic therapy. Nanomedcine. 2011;6:1645–54. doi: 10.2217/nnm.11.140. [DOI] [PubMed] [Google Scholar]
- 31.Ur Rehman SS, Lim K, Wang-Gillam A. Nanoliposomal irinotecan plus fluorouracil and folinic acid: a new treatment option in metastatic pancreatic cancer. Expert Rev Anticancer Ther. 2016;16:485–92. doi: 10.1080/14737140.2016.1174581. [DOI] [PubMed] [Google Scholar]
- 32.Atyabi F, Farkhondehfai A, Esmaeili F, Dinarvand R. Preparation of pegylated nano-liposomal formulation containing SN-38: In vitro characterization and in vivo biodistribution in mice . Acta Pharm. 2009;59:133–44. doi: 10.2478/v10007-009-0020-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YF, Wu WX, Mi YM, Li HP, Hou WG. Engineering of (10-hydroxycamptothecin intercalated layered double hydroxide)@liposome nanocomposites with excellent water dispersity. J Phys Chem Solids. 2017;108:125–32. [Google Scholar]
- 34.Huang H-C, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155:344–57. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Rivera Gil P, Hühn D, del Mercato LL, Sasse D, Parak WJ. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010;62:115–25. doi: 10.1016/j.phrs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Ross RW, Zietman AL, Xie WL, Coen JJ, Dahl DM, Shipley WU, et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009;33:301–5. doi: 10.1016/j.clinimag.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Na HB, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009;21:2133–48. [Google Scholar]
- 38.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61:1027–40. [Google Scholar]
- 40.Lee PC, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials. 2013;34:8756–65. doi: 10.1016/j.biomaterials.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 41.Liu XS, Situ A, Kang YN, Villabroza KR, Liao YP, Chang CH, et al. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano. 2016;10:2702–15. doi: 10.1021/acsnano.5b07781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XS, Lin P, Perrett I, Lin J, Liao YP, Chang CH, et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J Clin Invest. 2017;127:2007–18. doi: 10.1172/JCI92284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song GS, Chao Y, Chen YY, Liang C, Yi X, Yang GB, et al. All-in-One Theranostic Nanoplatform Based on Hollow TaOx for Chelator-Free Labeling Imaging, Drug Delivery, and Synergistically Enhanced Radiotherapy. AdvFunct Mat. 2016;26:8243–54. [Google Scholar]
- 44.Muniesa C, Vicente V, Quesada M, Sáez-Atiénzar S, Blesa JR, Abasolo I, et al. Glutathione-sensitive nanoplatform for monitored intracellular delivery and controlled release of Camptothecin. RSC Adv. 2013;3:15121–31. [Google Scholar]
- 45.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 46.Williams CC, Thang SH, Hantke T, Vogel U, Seeberger PH, Tsanaktsidis J, et al. RAFT-derived polymer-drug conjugates: poly(hydroxypropyl methacrylamide) (HPMA)-7-ethyl-10-hydroxycamptothecin (SN-38) conjugates. ChemMedChem. 2012;7:281–91. doi: 10.1002/cmdc.201100456. [DOI] [PubMed] [Google Scholar]
- 47.Bissett D, Cassidy J, de Bono JS, Muirhead F, Main M, Robson L, et al. Phase I and pharmacokinetic (PK) study of MAG-CPT (PNU 166148): a polymeric derivative of camptothecin (CPT) Br J Cancer. 2004;91:50–5. doi: 10.1038/sj.bjc.6601922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awada A, Garcia AA, Chan S, Jerusalem GH, Coleman RE, Huizing MT, et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol. 2013;14:1216–25. doi: 10.1016/S1470-2045(13)70429-7. [DOI] [PubMed] [Google Scholar]
- 49.Patnaik A, Papadopoulos KP, Tolcher AW, Beeram M, Urien S, Schaaf LJ, et al. Phase I dose-escalation study of EZN-2208 (PEG-SN38), a novel conjugate of poly(ethylene) glycol and SN38, administered weekly in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;71:1499–506. doi: 10.1007/s00280-013-2149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott LC, Yao JC, Benson AB III, Thomas AL, Falk S, Mena RR, et al. A phase II study of pegylated-camptothecin (pegamotecan) in the treatment of locally advanced and metastatic gastric and gastro-oesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2009;63:363–70. doi: 10.1007/s00280-008-0746-2. [DOI] [PubMed] [Google Scholar]
- 51.Veltkamp SA, Witteveen EO, Capriati A, Crea A, Animati F, Voogel-Fuchs M, et al. Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors. Clin Cancer Res. 2008;14:7535–44. doi: 10.1158/1078-0432.CCR-08-0438. [DOI] [PubMed] [Google Scholar]
- 52.Soepenberg O, de Jonge MJ, Sparreboom A, de Bruin P, Eskens FA, de Heus G, et al. Phase I and pharmacokinetic study of DE-310 in patients with advanced solid tumors. Clin Cancer Res. 2005;11:703–11. [PubMed] [Google Scholar]
- 53.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Han H, Davis ME. Single-antibody, targeted nanoparticle delivery of camptothecin. Mol Pharm. 2013;10:2558–67. doi: 10.1021/mp300702x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng MY, Qin SY, Jia HZ, Zheng DW, Rong L, Zhang XZ. Self-delivery of a peptide-based prodrug for tumor-targeting therapy. Nano Res. 2015;9:663–73. [Google Scholar]
- 56.Zhang CQ, Jin SB, Xue XD, Zhang TB, Jiang YG, Wang PC, et al. Tunable self-assembly of Irinotecan-fatty acid prodrugs with increased cytotoxicity to cancer cells. J Mater Chem B. 2016;4:3286–91. doi: 10.1039/c6tb00612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Homsi J, Simon GR, Garrett CR, Springett G, De Conti R, Chiappori AA, et al. Phase I trial of poly-L-glutamate camptothecin (CT-2106) administered weekly in patients with advanced solid malignancies . Clin Cancer Res. 2007;13:5855–61. doi: 10.1158/1078-0432.CCR-06-2821. [DOI] [PubMed] [Google Scholar]
- 58.Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Deliv Rev. 2009;61:1193–202. doi: 10.1016/j.addr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine. 2005;1:101–9. doi: 10.1016/j.nano.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–62. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vega-Villa KR, Takemoto JK, Yáñez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60:929–38. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 63.Huang P, Wang DL, Su Y, Huang W, Zhou YF, Cui DX, et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc. 2014;136:11748–56. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 64.Yu DS, Peng P, Dharap SS, Wang Y, Mehlig M, Chandna P, et al. Antitumor activity of poly(ethylene glycol)-camptothecin conjugate: the inhibition of tumor growth in vivo . J Control Release. 2005;110:90–102. doi: 10.1016/j.jconrel.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 65.Tan WB, Zhang Y. Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications. J Biomed Mater Res A. 2005;75:56–62. doi: 10.1002/jbm.a.30410. [DOI] [PubMed] [Google Scholar]
- 66.Cao ZQ, Yu QM, Xue H, Cheng G, Jiang SY. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew Chem Int Ed Engl. 2010;49:3771–6. doi: 10.1002/anie.200907079. [DOI] [PubMed] [Google Scholar]
- 67.Li YN, Yang YL, An FF, Liu Z, Zhang XJ, Zhang XH. Carrier-free, functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform. Nanotechnology. 2013;24:015103. doi: 10.1088/0957-4484/24/1/015103. [DOI] [PubMed] [Google Scholar]
- 68.Kasai H, Murakami T, Ikuta Y, Koseki Y, Baba K, Oikawa H, et al. Creation of pure nanodrugs and their anticancer properties. Angew Chem Int Ed. 2012;51:10315–8. doi: 10.1002/anie.201204596. [DOI] [PubMed] [Google Scholar]
- 69.Li W, Yang YL, Wang C, Liu Z, Zhang XJ, An FF, et al. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem Commun. 2012;48:8120–2. doi: 10.1039/c2cc33214k. [DOI] [PubMed] [Google Scholar]
- 70.Cheetham AG, Zhang PC, Lin YA, Lock LL, Cui HG. Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc. 2013;135:2907–10. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Zhang XJ, Zhou MJ, Tian BS, Yu CT, Jie JS, et al. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv Healthc Mater. 2014;3:1475–85. doi: 10.1002/adhm.201300577. [DOI] [PubMed] [Google Scholar]
- 72.He DX, Zhang W, Deng HZ, Huo SD, Wang Y-F, Gong NQ, et al. Self-assembling nanowires of an amphiphilic camptothecin prodrug derived from homologous derivative conjugation. Chem Commun. 2016;52:14145–8. doi: 10.1039/c6cc07595a. [DOI] [PubMed] [Google Scholar]
- 73.Chen F, Zhao YY, Pan YM, Xue XD, Zhang X, Kumar A, et al. Synergistically enhanced therapeutic effect of a carrier-free HCPT/DOX nanodrug on breast cancer cells through improved cellular drug accumulation. Mol Pharm. 2015;12:2237–44. doi: 10.1021/mp500744m. [DOI] [PubMed] [Google Scholar]
- 74.Zhao YY, Chen F, Pan YM, Li ZP, Xue XD, Okeke CI, et al. Nanodrug formed by coassembly of dual anticancer drugs to inhibit cancer cell drug resistance. ACS Appl Mater Interfaces. 2015;7:19295–305. doi: 10.1021/acsami.5b05347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen Y, Zhang W, Gong NQ, Wang YF, Guo HB, Guo WS, et al. Carrier-free, self-assembled pure drug nanorods composed of 10-hydroxycamptothecin and chlorin e6 for combinatorial chemo-photodynamic antitumor therapy in vivo . Nanoscale. 2017;9:14347–56. doi: 10.1039/c7nr03129g. [DOI] [PMC free article] [PubMed] [Google Scholar]