Abstract
Cellular growth, development, and differentiation are tightly controlled by a conserved biological mechanism: the cell cycle. This cycle is primarily regulated by cyclin-dependent kinase (CDK)-cyclin complexes, checkpoint kinases, and CDK inhibitors. Deregulation of the cell cycle is a hallmark of the transformation of normal cells into tumor cells. Given its importance in tumorigenesis, several cell cycle inhibitors have emerged as potential therapeutic drugs for the treatment of cancers-both as single-agent therapy and in combination with traditional cytotoxic or molecular targeting agents. In this review, we discuss the mechanisms underlying cell cycle regulation and present small-molecule anticancer drugs that are under development, including both pan-CDK inhibitors and CDK4/6-selective inhibitors. In addition, we provide an outline of some promising CDK inhibitors currently in preclinical and clinical trials that target cell cycle abnormalities in various cancers.
Keywords: Cell cycle regulation, cyclin-dependent kinases (CDK), cyclin, pan-CDK inhibitors, CDK4/6-selective inhibitors
Introduction
Cell division is a highly regulated process that is responsible for the appropriate division of a cell into two daughter cells. The cell cycle combines DNA replication with chromosomal segregation in an oscillatory manner. In this way, it ensures that the duplicated genetic material is distributed equally to each daughter cell. This process is classically described as four sequential phases that progress from quiescence (G0 phase) to proliferation (G1, S, G2, and M phases), and then back to the G0 phase. Cell proliferation is necessary for growth, development, and regeneration of eukaryotic organisms; however, it also causes one of the most devastating diseases of this era—cancer.
Cell cycle progression is primarily controlled by two regulatory processes: phosphorylation of specific proteins by cyclin-dependent kinases (CDKs) and their dephosphorylation by phosphatases; and specific proteolytic degradation by the ubiquitin-proteasome system. These regulatory mechanisms ensure that cells experiencing DNA damage in G1 phase do not enter the S phase, thereby providing a window in which DNA can be repaired before eventual entry into the M phase. In this way, chromosomes are correctly replicated before they segregate to daughter cells.
CDKs are activated by binding to their partner cyclins, which are expressed in a periodic manner. CDKs are also negatively regulated by CDK inhibitors, which are commonly referred to as “CKIs.” Cyclins regulate a series of CDKs, whose phosphorylation of key substrates promotes cell cycle progression. Cell cycle-related genes are usually mutated in tumors, leading to unregulated cell proliferation and tumor growth. For example, components of the CDK pathway are deregulated in most human tumors1. Unregulated expression of cyclins or CDKs is a direct cause of some cancers2, as these events elicit cell proliferation independent of normal extracellular stimuli, or promote the bypass of checkpoints that are designed to prevent the propagation of genomic damage3,4. In line with this, numerous cellular and in vivo models have shown that cyclins and CDKs are bona fide oncogenes.
CDK inhibitors have enormous therapeutic potential in diseases such as diabetes3, renal disorders4, neurodegenerative conditions5, infectious diseases6, and cancer7. However, most of the studies emphasize on the development of anticancer drugs, with a focus on CDKs and the cell cycle. Both academic and industrial drug discovery programs have led to the development of potent small-molecule CDK inhibitors. Many of these compounds can be used as pharmacological tools in fundamental basic research, and some hold promise as novel therapeutic agents against cancers.
This manuscript reviews the cell cycle and its regulation, the relationships among cyclins, CDKs, and cancer, and the latest advances in CDK drug discovery, with a particular emphasis on CDK4- and CDK6-selective inhibitors.
General description of the cell cycle
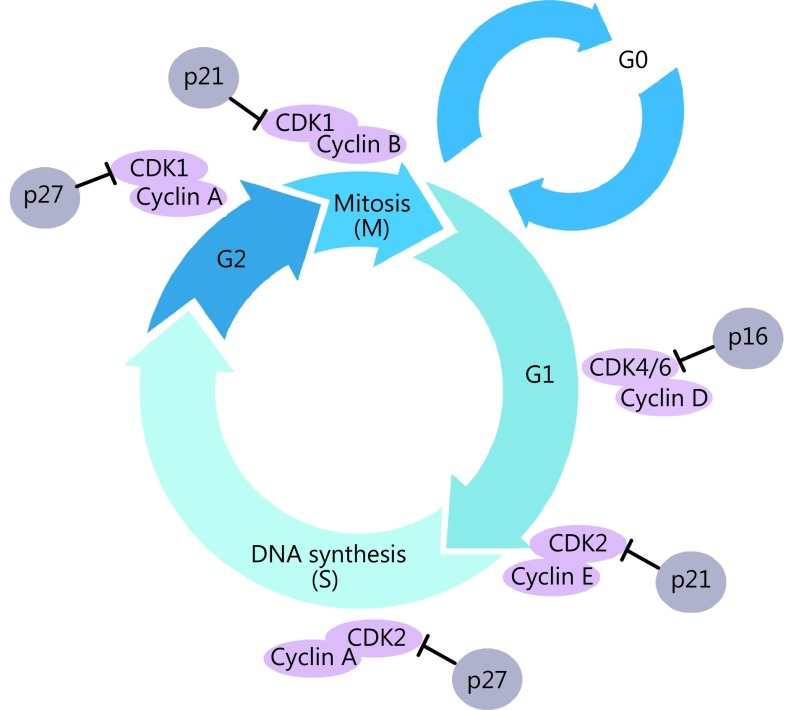
The cell cycle consists of two distinct phases: mitosis (M), in which a cell undergoes cell division, and interphase, which comprises G1 (pre-DNA synthesis), S (DNA synthesis), and G2 (pre-division) phases. Following interphase, the cell returns to the G0 phase (quiescence)8. G0 is typically used to describe cells that are not in the cell cycle but have the potential for division. Cells in G0 account for the majority of non-growing or non-proliferating cells. Cells can enter G1 from the quiescent state G0 if they are proliferating, or are otherwise activated by mitogenic stimuli. The G1 phase is the first step in cell cycle progression. Cells in the S phase synthesize DNA and have DNA content between 2N and 4N. If the chromosomes are correctly duplicated, cells can enter G2 to prepare for the M phase, during which the cell divides into two separate daughter cells (Figure 1).
1.
A schematic view of the cell cycle. Each phase in the cell cycle progression is regulated by cyclin-dependent kinases (CDKs) and their regulatory partner proteins, the cyclins, and CDK inhibitors.
Cyclin/CDK regulation in the cell cycle
Each stage in the cell cycle is tightly regulated by CDKs belonging to a well-conserved family of serine/threonine protein kinases, and their regulatory partners, the cyclins; in turn, the cell cycle is inhibited by CKIs9-11. CDK-cyclin complexes are central regulators of cell cycle progression as they transduce extracellular cues, such as growth factor signals, and the presence of nutrients, to the cell12. Different phases of the cell cycle require different cyclins (Table 1). D-type cyclins (D1, D2, and D3) are associated with CDK4/6, and are essential for entry into G113. Cyclin E is also important in G1. It associates with CDK2 to regulate late G1 phase and the induction of DNA synthesis in early S phase14. The cyclin E/CDK2 complex is of vital importance for G1/S phase transition. As the cell cycle progresses, cyclin A replaces cyclin E as the partner of CDK2, and then controls DNA synthesis and replication in the S phase15. Cyclin A subsequently associates with CDK1 to promote entry into the M phase. CDK1 also cooperates with other kinases, such as polo-like kinases and Aurora, to drive the transition from G2 to M phase, thus contributing to mitotic progression in cell division16,17. In the G2 phase, cyclin B replaces cyclin A, and the cyclin B/CDK1 complex triggers mitosis18.
1.
CDKs/cyclins in cell cycle
| CDKS | Cyclin | Cell cycle |
| CDK, cyclin-dependent kinases; G1, pre-DNA synthesis; S, DNA synthesis; G2, pre-division | ||
| CDK4 | Cyclin D1/2/3 | G1 phase |
| CDK6 | Cyclin D1/2/3 | G1 phase |
| CDK2 | Cyclin E | G1/S phase transition |
| CDK2 | Cyclin A | S phase |
| CDK1 | Cyclin A | G2/M phase transition |
| CDK1 | Cyclin B | Mitosis |
CKI regulation in the cell cycle
The kinase activity of CDKs is not only positively regulated by binding to the cyclins, but is also negatively regulated by the activity of CKIs. CKIs bind either free CDKs or CDK-cyclin complexes to regulate CDK activity. There are two distinct families of CKIs: the INK4 family and the Cip/Kip family19. p16 (INK4a), p15 (INK4b), p18 (INK4c), and p19 (INK4d) specifically inactivate CDK4 and CDK6, which prevents their combination with D-type cyclins20. p21 (Cip1), p27 (Kip1), and p57 (Kip2) form heterotrimeric complexes with cyclin D-, cyclin E-, and cyclin A-dependent kinase complexes21. The p16 protein, a special inhibitor of CDK4/6-cyclin D, prevents phosphorylation of the Rb protein, thereby triggering cell cycle arrest in G1. Cells that do not express p16 proceed unchecked through the G1 phase. The p16 protein is encoded by the ARF-INK4 locus, which also encodes p19 (ARF) through a different reading frame. The p19 transcript variant plays a separate role to that of p16, as it contributes to the stabilization of the p53 tumor suppressor22. Once stabilized, p53 exerts it transcription factor role, leading to the upregulation of the CKI, p2123. The p21 protein can also bind to and inhibit the proliferating cell nuclear antigen (PCNA), which leads to inhibition of DNA synthesis24.
In the G1 phase, CDK4/6-cyclin D promotes cell cycle progression by means of RB phosphorylation and sequestration of p21 and p27; this helps to release CDK2-cyclin E complexes and promote CDK2 kinase activity25,26. Furthermore, CDK2-cyclin E complexes can phosphorylate their own inhibitor, p27, and trigger its degradation by the Skp1/Cul1/F-box protein-ubiquitin-ligase complex27,28. Some antimitogenic signals, such as transforming growth factor-β, block cell cycle progression because they prevent cyclin D synthesis and instead upregulate INK4, which binds to CDK4/6 and results in the redistribution of p27 to CDK2-cyclin E, which prevents RB phosphorylation26. In response to DNA damage or metabolic stress, the p53 protein can transcriptionally activate p21, which in turn inhibits both CDK2 and CDK4 activities, and therefore G1 arrest in mammalian cells29.
Cell cycle in tumorigenicity
CDK4/6-cyclin D complex in tumorigenicity
Although cyclin D is the critical regulator of the cell cycle, pan-cyclin D knockout mice have no obvious proliferation defects during development. However, mice have tissue-specific defects based on the actual D cyclin that is deleted. For example, cyclin D1-deficient animals have a striking reduction in their retinas cell number and pregnancy-related mammary gland development is defective despite normal levels of ovarian steroid hormones30. Cyclin D1 (-/-) mice also exhibited hepatic steatosis and neurological abnormalities31. Aberrant cyclin D1 expression is observed in diverse human cancers, including B-cell malignancies such as mantle cell lymphoma and diffuse large B-cell lymphoma32,33 and many kinds of solid tumors such as breast34, bladder35, lung36, esophageal37, and hepatocellular38 carcinomas. Cyclin D1 is a co-factor of the estrogen receptor (ER), and its amplification is observed in nearly 60% of breast cancers. Moreover, it is reported that cyclin D1 overexpression predicts tamoxifen resistance in breast cancer39.
Knockout studies of cyclin D2 in mice suggest essential roles of this gene in ovarian granulosa and germ cell proliferation40. Although high-level expression of this gene was observed in ovarian and testicular tumors, cyclin D2 is frequently methylated and lost in pancreatic, breast, and prostate cancers, which points to its potential role as a tumor suppressor rather than as an oncogene41-43. Cyclin D3 knockout mice fail to undergo B-lymphocyte maturation, T-lymphocyte development, and neutrophil production, and they cannot regulate erythrocyte size and number. Overexpression of cyclin D3 is implicated in the onset or maintenance of glioblastomas44, renal cell carcinomas45, pancreatic adenocarcinomas46, and several B-cell malignancies, including diffuse large B-cell lymphomas47 and multiple myelomas48.
The activity of CDK4/6 is usually altered through upstream regulators, such as excessive cyclin D1 levels, or p16 inactivation due to gene deletion or epigenetic mechanisms, such as DNA methylation or point mutations49-51. CDK4 is commonly amplified in gliomas52, sarcomas53, and breast54 and cervical carcinomas55, and CDK6 is overexpressed in gliomas and lymphoid tumors56,57.
CDK2-cyclin E/A complexes in tumorigenicity
CDK2 knockout mice are viable; however, both male and female CDK2 (-/-) mice are sterile58. In addition, in p27 knockout mice, ablation of CDK2 does not influence either tumor incidence or latency, although p27 impedes cell division by interrupting the interactions of CDK2 with cyclin E and A59. Moreover, deregulation of CDK2 due to its inappropriate expression, overexpression of its binding partners cyclin E and cyclin A, or loss of its endogenous inhibitors (the Cip/Kip family) may cause different kinds of malignancies, including sarcoma, melanoma, osteosarcoma, and pancreatic, ovarian, breast, lung, and thyroid carcinomas12. Overexpression of cyclin E is related to endometrial intraepithelial carcinoma and uterine serous carcinoma60, ovarian cancer61, osteosarcoma62, non-small cell lung cancer (NSCLC)63, leukemias, and lymphomas64. Cyclin A has been implicated in various human cancers, such as thyroid carcinoma, melanoma, breast cancer, lung, osteosarcoma, ovarian cancer, and endometrial carcinoma65.
CDK1-cyclin B complex and other CDKs in tumorigenicity
CDK1 associates with cyclin A and cyclin B; it is involved in microtubule dynamics and chromosome condensation for cell division. It was reported that a lack of CDK1, but not CDK2, leads to female infertility due to a failure in meiosis in the oocyte. Therefore, reintroduction of CDK1 mRNA into CDK1-null oocytes largely recovers meiosis66,67. Until now, no direct evidence has shown that genetic alteration deregulates CDK1 activity in tumorigenicity. CDK1 amplification has been observed in lymphoma, advanced melanoma, and lung cancer, and targeting CDK1 is a very effective strategy for inhibiting diffuse large B-cell lymphoma proliferation and overcoming drug resistance68. Increased CDK1 activation is often the result of cyclin B overexpression, which is evident in a variety of tumors such as breast69, colon70, prostate71, and thyroid carcinoma72 and NSCLC73. Lack of CDK1 in the cytoplasm predicts poor survival in human lung cancer and confers chemotherapeutic resistance74.
In addition to the cell cycle-related CDKs, CDK5, an unconventional CDK, and its co-factors p35 and p25 are highly expressed in human medullary thyroid carcinoma (MTC). Consistent with this, CDK5 activity promotes MTC proliferation75.
CKIs in tumorigenicity
Abnormal expression of CKIs plays an important role in tumorigenesis. Several mechanisms including deletion, point mutations, and hypermethylation can inactivate p16 gene expression and result in the development of human tumors76. As reported, aberrant methylation of the p16 gene was detected in 62% of esophageal tumor samples77. Moreover, p16 deletions have been reported in approximately 40%–60% of nasopharyngeal cancers, 50% of gliomas and mesotheliomas, and 20%–30% of acute lymphoblastic leukemias78,79. The gene encoding p15 is situated close to p16 and is also often synchronously deleted80. Mutations of p18 and p21 have been found in several kinds of cancers, such as breast and thyroid carcinomas81,82. Low p27 expression caused by increased proteasome-mediated degradation rather than gene deletion has been reported in colorectal tumors83.
The tumorigenicity of cyclins or activated CDKs or CKI was summarized in Table 2.
2.
Tumorigenicity of CDK-cyclin complexes
| CDK/cyclin | Deficiency in some kind of cancers | Over-expression in some kind of cancers |
| CDK, cyclin-dependent kinases. NSCLC, non-small cell lung cancer. | ||
| CyclinD1 | B-cell malignancies and many kinds of solid tumors, including breast, bladder, lung, esophageal, and hepatocellular carcinomas | |
| CyclinD2 | Pancreatic, breast, and prostate cancers | Ovarian and testicular tumors |
| CyclinD3 | Glioblastomas, renal cell carcinomas, pancreatic adenocarcinomas, and several B-cell malignancies | |
| CDK4 | Gliomas, sarcomas, and breast and cervical carcinomas | |
| CDK6 | Gliomas and lymphoid tumors | |
| Cyclin E | Uterine, pancreatic, and ovarian cancers, NSCLC, leukemias, lymphomas, and osteosarcoma | |
| Cyclin A | Thyroid carcinoma, melanoma, breast cancer, lung, osteosarcoma, and ovarian and endometrial carcinomas | |
| CDK2 | Sarcomas, melanoma, osteosarcoma, and pancreatic, ovarian, breast, lung, and thyroid carcinomas | |
| Cyclin B | Breast, colon, prostate, and thyroid carcinomas and NSCLC | |
| CDK1 | Lymphoma, advanced melanoma, and lung cancer | |
CDK inhibitors: from discovery to therapy
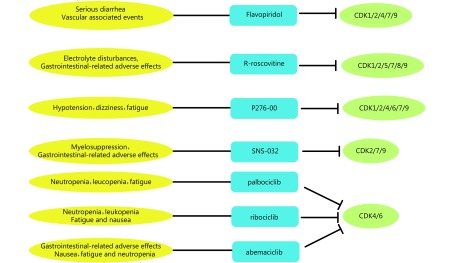
The clinical benefits of CDK inhibition in cancer has received growing attention during the last two decades. On the basis of differences in binding sites, the inhibitors are divided into ATP-competitive CDK inhibitors and non-ATP-competitive inhibitors. Despite different chemical structures, all ATP-competitive CDK inhibitors bind the ATP-binding pocket of CDK proteins, mimicking ATP structure84. Because of the high conservation of amino acid chains in the ATP-binding pocket, many first generation CDK compounds are pan-CDK inhibitors. Promising progress has been made in developing non-ATP competitive CDK inhibitors, which inhibit the cyclin binding groove or CDK-cyclin association, or which simulate the inhibitory CDK substrates. As the binding interactions and docking sites usually differ among different protein regulators, non-ATP competitive CDK inhibitors are more selective than ATP-competitive compounds. Currently, both pan-CDK inhibitors and next-generation relatively selective CDK inhibitors (especially CDK4/6-selective drugs) are being developed as promising agents for cancer therapies (Figure 2). The following section of this review provides a brief overview of the use of CDK inhibitors in cancer treatment.
2.
The side effects and targets of cyclin-dependent kinase (CDK) inhibitors as anti-cancer drugs. Pan and CDK4/6-selective CDK inhibitors have different side effects and targets.
Pan-CDK inhibitors
Among the first-generation pan-CDK inhibitors, flavopiridol, R-roscovitine, P276-00, and SNS-032 are the most tested in clinical trials. Unfortunately, although these CDK inhibitors initially offered great hope, none of them has received final approval as a therapeutic option. The main issue is related to the low specificity toward the target kinase, which also causes many side effects. Flavopiridol and R-roscovitine are representative pan-CDK inhibitors and are discussed in detail below.
Flavopiridol
Flavopiridol (Alvocidib) is a semi-synthetic flavonoid derived from rohitukine, an alkaloid isolated from Dysoxylum binectariferum, a plant that is native to India. Flavopiridol is primarily used for the treatment of rheumatoid arthritis85. As an ATP-competitive CDK inhibitor, flavopiridol can induce cell cycle arrest at the G1 or G2/M phase by association with CDKs 1, 2, 4, 6, 7, and 9 at nanomolar concentrations86. Flavopiridol can induce apoptosis and inhibits angiogenesis by targeting vascular endothelial growth factor at micromolar concentrations87.
Among the pan-CDK inhibitors, flavopiridol was the first to reach clinical trials, and has been the most comprehensively studied. In preclinical studies, it was reported to induce cell cycle arrest and tumor growth inhibition in a majority of solid tumor cell lines especially those of B-cell origin and xenografts88. phase II clinical trials were completed with flavopiridol in chronic lymphocytic leukemia89, endometrial adenocarcinoma90, multiple myeloma91, and melanoma92. The drug shows dose-limiting toxicities (DLTs) such as serious diarrhea and vascular-associated events, including deep vein thrombosis, pulmonary embolism, and myocardial infarction. Although these events limit the agent’s use in clinical practice, combining it with other antineoplastic agents to reduce its side effects is a promising therapeutic strategy. In several preclinical studies, the synergistic effects of flavopiridol with other chemotherapeutic agents, including paclitaxel, gemcitabine, docetaxel, cisplatin, vorinostat, TNF-α, and HA14-1, have proven beneficial93. In phase II clinical trials, flavopiridol combined with cytarabine and mitoxantrone drugs achieved a complete response in 75% of patients with acute myelogenous leukemia94. Additionally, a clinical trial of flavopiridol in advanced sarcoma showed that its sequential combination with doxorubicin was more effective than administration of doxorubicin alone95.
R-roscovitine
R-roscovitine (Seliciclib, CYC202), the second CDK inhibitor to enter clinical trials in humans, is an oral purine analog that inhibits CDKs 1, 2, 5, 7, 8 and 9. Its inhibition of CDK2 (mediated by ATP-competitive binding) is stronger than its inhibition of CDK196. In preclinical studies, R-roscovitine, which inhibits RNA processing and RNA polymerase II-dependent transcription, led to growth inhibition and apoptosis in vitro by activating p53. The drug also reduced the expression of anti-apoptotic proteins, such as MCL-1 and X-linked inhibitor of apoptosis97,98, but not BCL-2, most likely due to the longer half-life of the latter protein.
R-roscovitine is orally administered and distributes rapidly to body tissues. It then decomposes rapidly to a carboxylate derivative and is excreted through the kidneys. The major side effects of R-roscovitine, such as electrolyte disturbances (hypokalemia, hyponatremia) and gastrointestinal adverse effects (nausea, emesis, anorexia), are DLTs. Benson and colleagues99 conducted a phase I clinical trial including patients suffering from intractable solid tumors; R-roscovitine was administered orally twice daily for 7 days of a 21-day cycle. A maximum tolerated dose (MTD) of 800 mg was reached with DLTs (fatigue, rash, hyponatremia, and hypercalcemia). Although no tumor reductions were recorded in the 21 patients, stable disease (SD) was observed in eight patients. Another phase I trial was conducted in patients with advanced cancer. The MTD was 1250 mg orally twice daily for 5 consecutive days every 3 weeks. Partial response (PR) was observed in a patient with hepatocellular carcinoma and the other patients had SD100. R-roscovitine was also evaluated in a phase II trial with 187 NSCLC patients. The drug led to longer median survival, but the trial failed to meet the primary endpoint of improving progression-free survival (PFS)101. Combinations of R-roscovitine and other drugs are undergoing clinical trials, including the nucleoside analog sapacitabine and the EGFR inhibitor erlotinib, in patients with advanced solid tumors and in patients with rheumatoid arthritis who are not sensitive to current conventional therapies. More information about all these trials can be found at the ClinicalTrials.gov website (http://clinicaltrials.gov/).
Because of the high homology of the ATP-binding sites among most cellular kinases, many of the early CDK inhibitors were relatively unselective for CDKs; this likely explains their undesired toxicities and side effects. To obtain more specific CDK inhibitors, researchers have increasingly focused on different approaches to creating a new generation of compounds. Considering that cyclin D plays a vital role in tumorigenesis and tumor progression, selective CDK4/CDK6 inhibitors have been the focus of drug discovery efforts to develop novel anticancer drugs.
CDK4/6-selective CDK inhibitors
Currently, there are at least three CDK4/6 inhibitors in various stages of clinical trials: palbociclib (PD-0332991; Pfizer), ribociclib (LEE011; Novartis), and abemaciclib (LY2835219; Lilly) (Table 3). They have been studied most extensively in pRB-positive tumor types. All three compounds inhibit CDK4 and CDK6 with half maximal inhibitory concentration (IC50) values less than 40 nM; other CDK family members are far less sensitive to inhibition.
3.
CDK4/6-selective Small-molecule Inhibitors Currently used in Clinical Trials*
| Clinical trials identifier | Patients/diseases included | Trial stage | Trial status | Drugs |
| *Data retrieved from www.clinicaltrials.gov and current as of March 2017. | ||||
| Palbociclib
(PD-0332991) |
||||
| NCT00141297 | Advanced cancer | 1 | Completed | Palbociclib |
| NCT01037790 | Advanced and metastatic colorectal cancer | 2 | Recruiting participants | Palbociclib |
| NCT00721409 | ER-positive/HER2-negative advanced breast cancer in postmenopausal women | 1/2 | Ongoing, but not recruiting participants | Palbociclib; letrozole |
| NCT01740427 | Postmenopausal women with
ER-positive/HER2-negative breast cancer who have not received any prior systemic anti-cancer treatment for advanced disease |
3 | Ongoing, but not recruiting participants | Palbociclib; letrozole |
| NCT02513394 | Patients with HR-positive/HER2-negative early-stage breast cancer | 3 | Recruiting participants | Palbociclib; standard adjuvant endocrine therapy |
| NCT01864746 | HR-positive/HER2-normal patients with residual disease after neoadjuvant chemotherapy and surgery | 3 | Recruiting participants | Palbociclib |
| NCT01942135 | HR-positive/HER2-negative metastatic breast cancer after endocrine treatment failure | 3 | Ongoing, but not recruiting participants | Palbociclib; fulvestrant |
| Ribociclib (LEE011) | ||||
| NCT01237236 | Advanced solid tumors and lymphoma | 1 | Completed | LEE011 |
| NCT01958021 | Postmenopausal women with HR-positive/HER2-negative advanced breast cancer who received no prior therapy for advanced disease | 3 | Ongoing, but not recruiting participants | LEE011; Letrozole;
LEE011 + placebo |
| NCT02278120 | Premenopausal women with HR-positive/HER2-negative
advanced breast cancer |
3 | Ongoing, but not recruiting participants | LEE011; tamoxifen; Letrozole; anastrozole; goserelin; LEE011 + placebo |
| NCT02422615 | Men and postmenopausal women with HR-positive/HER2-negative advanced breast cancer who have received no or only 1 line of prior endocrine treatment | 3 | Ongoing, but not recruiting participants | LEE011; fulvestrant; LEE011 + placebo |
| NCT01872260 | Advanced ER-positive breast cancer | 1 | Recruiting participants | LEE011; letrozole; BYL719 |
| NCT01857193 | HR-positive/HER2-negative advanced breast cancer | 1 | Recruiting participants | LEE011; exemestane; everolimus |
| Abemaciclib (LY2835219) | ||||
| NCT01394016 | Advanced cancer | 1 | Ongoing, but not recruiting participants | Abemaciclib; fulvestrant |
| NCT02107703 | Women with HR-positive/HER2-negative locally advanced or metastatic breast cancer | 3 | Ongoing, but not recruiting participants | Abemaciclib; fulvestrant; placebo |
Palbociclib
Palbociclib (PD0332991) is an oral, reversible, selective, low nanomolar (IC50, 0.011 micromol/L for CDK4 and 0.016 micromol/L for CDK6) small-molecule inhibitor of CDK4/6 developed by Pfizer. It acts primarily by inactivating early G1 kinases. It does not exhibit obvious activity against other kinases, such as CDK2, which is driven by cyclin E/A, and CDK1, which is activated by cyclin B/A. In February 2015, this drug, in combination with letrozole, received accelerated approval by the United States Food and Drug Administration (FDA). Palbociclib became the first-line treatment for postmenopausal patients with ER-positive/HER2-negative locally advanced or metastatic breast cancer102.
In preclinical studies, palbociclib showed antiproliferative effects on RB-positive cells and selectively led to G1 arrest in a series of cancer cell lines. The drug is most potent in cell lines with highly activated CDK4/6 pathways induced by increased expression of cyclin D1 or loss of p16. However, loss of RB will result in drug resistance. Generally, RB positivity is an important biomarker of palbociclib efficacy103. Inhibition of CDK4 and CDK6 caused by palbociclib leads to complete suppression of RB phosphorylation at Ser780/Ser795. Oral administration of palbociclib suppresses the growth of Colo-205 human colon carcinoma xenografts. In tumor tissue, palbociclib can lead to the elimination of phospho-RB and Ki-67, as observed in immunohistochemical analysis and downregulation of E2F target genes by reverse transcription-polymerase chain reaction (RT-PCR)104.
As described in the previous section, flavopiridol, a pan-CDK inhibitor, can enhance the effects of cytotoxic drugs. In contrast, palbociclib has a more complicated effect when combined with cytotoxic chemotherapy in vitro. Cytotoxic chemotherapies require cell cycle progression, but palbociclib causes cytostatic G1 arrest. In tumor-bearing mice that harbor a functional RB pathway, co-administration of palbociclib and carboplatin decreased antitumor activity compared to carboplatin alone105. Palbociclib also antagonizes the cytotoxic response to anthracycline therapy in triple-negative breast cancer cell lines106. However, when palbociclib was used 24 h in advance to synchronize the cell cycle, this antagonistic effect was significantly impaired105.
Moreover, the combination of radiation therapy and palbociclib remarkably improved the potential benefit when compared with either therapy alone107. Furthermore, palbociclib shows promising synergistic effects with molecularly targeted therapies, such as trastuzumab in HER2-amplified cell lines, tamoxifen in ER-positive cell lines, and a SMAD inhibitor in pancreatic adenocarcinoma cell lines103.
In a phase I clinical trial (NCT00141297), palbociclib was administered to patients with advanced cancers expressing RB. The aim of the study was to establish tolerated dosing levels and determine the optimal oral administration periods. Two schedules of oral dosing were evaluated: in the 2/1 schedule, doses ranged from 100 to 225 mg daily for 2 weeks, followed by 1 week off treatment; in the 3/1 schedule, doses were 75, 125, or 150 mg daily for 3 weeks with a 1-week rest. In the 2/1 schedule, six out of a total of 33 patients had DLTs (neutropenia and thrombocytopenia). Non-hematological treatment-related adverse events (fatigue, diarrhea, anemia, and nausea) occurred in 29 (88%) patients during cycle 1 and 27 (82%) patients thereafter. Of 31 evaluable patients, one patient with testicular cancer achieved a PR and nine patients had SD (≥ 10 cycles in three cases). The MTD of the 2/1 schedule was 200 mg daily108. A total of 41 patients were enrolled in the 3/1 schedule. DLTs were observed in five (12%) patients, and neutropenia was the only dose-limiting effect. Non-dose-limiting, non-hematologic adverse effects reported after cycle 1 were grade 3 neutropenia (12%), anemia (7%), and leukopenia (2%). Of 37 patients evaluable for tumor response, 10 (27%) had SD for at least four cycles; six of these achieved prolonged benefit (≥ 10 cycles). The MTD and recommended phase II dose of palbociclib on this schedule was 125 mg daily109.
In most phase II and III trials, palbociclib was administered orally at a dosage of 125 mg in the 3/1 schedule, unless indicated otherwise. This drug has been studied in various disease states, including liposarcoma, breast cancer, mantle-cell lymphoma, and germ-cell tumors.
Palbociclib displays remarkable clinical activity in breast cancer. A phase II study (NCT01037790; UPCC03909) enrolled 37 patients with RB-positive metastatic breast cancer: 31 (84%) patients were hormone receptor (HR)-positive/HER2-negative, two (5%) patients were HR-positive/HER2-positive, and four (11%) patients were HR-negative/HER2-negative. All patients had received a median of two previous cytotoxic therapies. Palbociclib administered as a single agent was associated with a median PFS of 3.7 months, and its clinical benefit rate was 19% among all patients enrolled, 21% among HR-positive patients, and 29% among HR-positive/HER2-negative patients who had advanced through at least two previous lines of endocrine therapy110. This result shows that palbociclib monotherapy is effective in patients with hormone-resistant, HR-positive, RB-positive breast cancer.
Another randomized, open-label phase II trial (PALOMA-1/TRIO-18; NCT00721409) compared letrozole with palbociclib plus letrozole in patients with stage IV, ER-positive/HER2-negative breast cancer. It was the first study to determine the efficacy of palbociclib/letrozole combined therapy111. Patients were enrolled into two separate cohorts: in the first group, 66 patients were characterized by ER-positive/HER2-negative disease alone; and in the second group 99 patients had amplification of cyclin D1, loss of p16, or both. PFS was the primary endpoint. Response rates were similar between the two populations. Among the entire population of 165 patients, the median PFS was 10.2 months for the letrozole group and 20.2 months for the combined group. This indicates improved PFS when palbociclib is combined with letrozole. However, grade 3 or 4 neutropenia was reported in 54% of patients in the combined group but only 1% in the letrozole group. Leukopenia (19% vs. 1%) and fatigue (4% vs. 1%) were also reported more frequently in the combined group than in the letrozole group.
On the basis of these data, phase III trials combining palbociclib with other endocrine therapies in the metastatic and post-neoadjuvant settings (NCT01740427, NCT02513394, NCT01864746) are ongoing and awaiting further analysis. Early results of a phase III trial (NCT01942135) comparing fulvestrant/placebo or fulvestrant/palbociclib were recently published112. Consistent with the results of the study with letrozole, the addition of palbociclib to fulvestrant improved PFS compared with fulvestrant alone in patients with advanced breast cancer (9.2 months vs. 3.8 months). Grade 3 or 4 neutropenia was the most common adverse event, accounting for 62.0% of all events in the combined group and only 0.6% in the fulvestrant/placebo group. Other side effects that occurred more frequently in the combined group included leukopenia (25.2% vs. 0.6%), anemia (2.6% vs. 1.7%), thrombocytopenia (2.3% vs. 0%), and fatigue (2.0% vs. 1.2%).
These results show an obvious improvement in PFS of advanced breast cancer patients when palbociclib is combined with letrozole or fulvestrant. However, these molecularly targeted therapies require long-term use, and some adverse effects may become unacceptable or lead to a reduced quality of life following combination therapy strategies. Therefore, in order to optimize clinical combinations, reduce side effects, and ultimately improve prognosis, further studies are urgently required.
Ribociclib
Ribociclib (LEE011) is the second highly selective CDK4/6-targeting agent which was approved in both the US and EU in 2017 as the first-line treatment of postmenopausal women with HR-positive/HER2-negative advanced or metastatic breast cancer.
The exploration of this drug is quite advanced. Ribociclib promotes dephosphorylation of RB, which results in G1 arrest and senescence in various cancer cells. In preclinical in vivo tumor models, ribociclib inhibits the growth of established xenograft models of RB-positive tumors as monotherapy or in combination with other drugs. The initial phase I clinical trial (NCT01237236) of single-agent ribociclib enrolled 132 patients harboring RB-positive advanced solid tumors or lymphomas113. The MTD and recommended doses for expansion were 900 and 600 mg/day, respectively, on a 3/1 schedule. Among paired skin and tumor biopsies from 40 patients, reductions in Ki67 and phospho-pRB were observed in 55% and 42% of the samples, respectively. Among 110 evaluable patients, three patients had confirmed PR, 43 patients achieved a best response of SD, and eight patients were progression free for more than six months. Common treatment-related adverse events were neutropenia, leukopenia, fatigue, and nausea. The majority of adverse events were grade 1 or 2 and reversible. Grade 3/4 adverse events included neutropenia, leukopenia, and lymphopenia.
Ribociclib is now moving ahead into more advanced studies in ER-positive breast cancer. At present, it is being evaluated in a phase Ib/II open-label study in combination with the phosphoinositide 3-kinase inhibitor alpelisib (BYL719) and letrozole (NCT01872260)114 and in a phase Ib/II study with the mammalian target of rapamycin inhibitor (mTOR) everolimus and the steroidal aromatase inhibitor exemestane (NCT01857193)115. Preliminary data from the NCT01857193 study showed that the triplet combination seems efficacious. Of course, these data are limited and more robust data are required. There are many ongoing clinical trials investigating the effects of ribociclib in postmenopausal advanced breast cancer patients with HR-positive/ HER2-negative disease. A phase III trial (NCT01958021) studied the effect of combination therapy (ribociclib plus letrozole) and found the duration of progression-free survival was significantly longer in the ribociclib group than in the placebo group. This clinical trial established foundation of ribociclib as first-line therapy for HR-positive, advanced breast cancer. Other similar study (NCT02278120) is currently enrolling patients to investigate ribociclib plus a non-steroidal aromatase inhibitor (letrozole or anastrozole) or tamoxifen in combination with the gonadotropin-releasing hormone agonist goserelin. The phase III trial (NCT02422615) is recruiting patients to study the combination of ribociclib plus fulvestrant in first- and second-line settings.
Ribociclib is also undergoing clinical evaluation, both alone and in combination with chemotherapeutic agents, in other tumors, including advanced NRAS-mutant melanoma, locally advanced or metastatic BRAF-mutant melanoma, advanced melanoma, advanced solid tumors, lymphoma, and neuroblastoma. More information about all these trials can be found at the ClinicalTrials.gov website (http://clinicaltrials.gov/).
Abemaciclib
Abemaciclib (LY2835219) is another orally bioavailable CDK4/6 inhibitor in clinical development; it achieves CDK4/6 inhibition at nanomolar concentrations. Preclinical data indicate that it causes G1 arrest in RB-proficient cells and results in antitumor activity in human tumor xenograft models as both monotherapy and in combination with other chemotherapies116. It also inhibits the growth of intracranial tumors, as the drug appears to be able to cross the blood–brain barrier117. Abemaciclib improves the effect of cytotoxic drugs, underscoring the fact that it can be used in combined therapeutic regimens118.
Abemaciclib is currently being tested in various clinical trials against breast cancer and a variety of other tumors. The first phase I study (NCT01394016) involving abemaciclib included 75 patients with advanced solid tumors; the primary results of the study were presented in Invest New Drugs in 2014116. The MTD was defined at 200 mg twice daily. The most common treatment-related toxicities were mild to moderate and included diarrhea, nausea, fatigue, vomiting, and neutropenia. This phase I study showed promising activity in a metastatic breast cancer expansion cohort in which patients were administered abemaciclib after systemic therapies (median of seven cycles)119. Among 44 evaluable patients, nine (20%) patients achieved PR, 24 (55%) patients had SD, and 11 (25%) patients showed disease progression. Patnaik and colleagues119 reported that the PR rate was 25% and the SD rate was 56% in the HR-positive subgroup. They reported PFS in HR-positive patients was 9.1 months compared to 5.8 months in all patients. The trial also included a small cohort to investigate tolerance to the combination of abemaciclib plus fulvestrant; the assessment concluded that the combination was safe and the toxicities were acceptable. These two drugs are currently undergoing evaluation in another randomized, double-blind, placebo-controlled phase III study (NCT02107703). A total of 550 women with HR-positive/HER2-negative locally advanced or metastatic breast cancer were enrolled. The results have not yet been published.
The other expansion cohorts from NCT01394016 recruited 49 NSCLC patients (19 KRAS wild-type, 26 KRAS mutant, and four unknown KRAS status)120. The enrolled patients had advanced NSCLC and were treated with single-agent abemaciclib following a median of four previous systemic therapies. The disease control rate was 51% [37% for KRAS wild-type (n=19) and 54% for KRAS mutants (n=26)] with one confirmed patient achieving PR. In this clinical study, abemaciclib was associated with acceptable safety and tolerability profiles, with diarrhea (2%), nausea (4%), fatigue (2%), vomiting (2%), and anemia (2%) being the most common adverse effects. There were no grade 4 side effects.
There are other ongoing evaluations of abemaciclib in clinical trials in other disease states, such as squamous cell carcinoma in NSCLC, NSCLC with KRAS mutant, and mantle cell lymphoma. Information about all these trials can be found at the ClinicalTrials.gov website (http://clinicaltrials.gov/). Results from the trials that are currently in progress will improve understanding of the activity of abemaciclib and help distinguish and differentiate it from other CDK4/6 inhibitors.
There are some similarities among the three CDK4/6-selective inhibitors in functional and toxicity profiles. As they are RB-dependent, loss of RB will result in drug resistance. However, these three inhibitors have various treatment-related adverse effects, especially when used in combination with other drugs; these effects may limit the uses of the inhibitors as cancer therapies. Several phase III trials involving these three selective CDK4/6 inhibitors are currently under way and may uncover differences among them in terms of niche activity and tolerability. According to the information obtained from those clinical trials, clinical combinations can hopefully be optimized, treatment-related side effects can be reduced, and, ultimately, the prognoses of various cancers can be improved.
Conclusions
In all organisms, from yeast to humans, it is well documented that the cell cycle is controlled by the sequential activation of a group of serine-threonine kinases called CDKs, which associate with the cyclin proteins. Since the discovery of the key role of CDK1 in driving the cell cycle, significant progress has been made in understanding the controlled processes of the cell cycle. Deregulation of the cell cycle is a hallmark of the transformation of normal cells into tumor cells. Tumor-associated alterations in CDKs or their activators and inhibitors (cyclins and CKIs, respectively) contribute to sustained proliferation that is independent of the external signals, which is a hallmark of cancer.
In the cell cycle, CDKs regulate critical checkpoints, and are thus important antiproliferative drug targets for several diseases, including cancer. Indeed, therapeutic approaches based on CDK inhibition represent a unique opportunity for drug discovery and a promising strategy for cancer treatment.
In the past two decades, the major research focus has been on ATP-competitive small molecules that target several CDK proteins. However, none of these compounds has yet received approval following clinical trials, primarily due to the lack of specificity in targeting CDKs, as well as their accompanying toxicities and side effects. CDK4 and CDK6 play pivotal roles in regulating cell cycle progression at the G1 restriction point, and are therefore important anticancer drug targets. Indeed, the development of CDK4/6-selective inhibitors has made promising progress: palbociclib, abemaciclib, and ribociclib have been approved or are currently being evaluated in various cancers in ongoing clinical trials. Other ongoing evaluations of CDK4/6-selective inhibitors are in clinical trials. Although CDK4/6 inhibitors are showing great promise in many cancers, there are some questions and concerns that have emerged from the preclinical and clinical data. First, some cancers such as melanomas, colorectal cancer, triple-negative breast cancer are resistant to CDK4 and CDK6 inhibition. Second, there remains a strong need to identify predictive markers that can help define the patient subgroup(s) most likely to benefit from CDK4/6 inhibition. Another question about the future direction of anti-cell cycle therapy is how to optimize treatment protocols: by combining CDK4/6 inhibitors with other targeted therapies or cytotoxic chemotherapy. The preclinical trials are expected to identify the most promising and most well-tolerated combination therapeutic strategies and improve our understanding of novel functioning and resistance mechanisms. In the near future, more efficient and specific CDK selective inhibitors should be available. New strategies based on CDK inhibition will thus accelerate the development of precision cancer therapy.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–64. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Danesh FR, Sadeghi MM, Amro N, Philips C, Zeng L, Lin S, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci USA. 2002;99:8301–5. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankland SJ, Wolf G. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am J Physiol Renal Physiol. 2000;278:F515–29. doi: 10.1152/ajprenal.2000.278.4.F515. [DOI] [PubMed] [Google Scholar]
- 5.Park DS, Obeidat A, Giovanni A, Greene LA. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiol Aging. 2000;21:771–81. doi: 10.1016/s0197-4580(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 6.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nature Med. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 7.Mayer EL. Targeting breast cancer with CDK inhibitors. Curr Oncol Rep. 2015;17:443. doi: 10.1007/s11912-015-0443-3. [DOI] [PubMed] [Google Scholar]
- 8.Norbury C, Nurse P. Animal cell cycles and their control. Ann Rev Biochem. 1992;61:441–68. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 9.Malumbres M. Physiological relevance of cell cycle kinases. Physiol Rev. 2011;91:973–1007. doi: 10.1152/physrev.00025.2010. [DOI] [PubMed] [Google Scholar]
- 10.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–41. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–69. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–66. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–24. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson RL, Maller JL. Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis. Curr Biol. 2010;20:856–60. doi: 10.1016/j.cub.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–75. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 17.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J cell Sci. 2007;120:2987–96. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 18.Ito M. Factors controlling cyclin B expression. Plant Mol Biol. 2000;43:677–90. doi: 10.1023/a:1006336005587. [DOI] [PubMed] [Google Scholar]
- 19.Chim CS, Fung TK, Wong KF, Lau JS, Law M, Liang R. Methylation of INK4 and CIP/KIP families of cyclin-dependent kinase inhibitor in chronic lymphocytic leukaemia in Chinese patients. J Clin Pathol. 2006;59:921–6. doi: 10.1136/jcp.2005.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cánepa ET, Scassa ME, Ceruti JM, Marazita MC, Carcagno AL, Sirkin PF, et al. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life. 2007;59:419–26. doi: 10.1080/15216540701488358. [DOI] [PubMed] [Google Scholar]
- 21.Ullah Z, Lee CY, DePamphilis ML. Cip/Kip cyclin-dependent protein kinase inhibitors and the road to polyploidy. Cell Div. 2009;4:10. doi: 10.1186/1747-1028-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YP, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways . Cell. 1998;92:725–34. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 23.Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21WAF1/Cip1 gene by the p53 tumor suppressor protein . J Biolog Chem. 2001;276:29116–25. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- 24.Law M, Forrester E, Chytil A, Corsino P, Green G, Davis B, et al. Rapamycin disrupts cyclin/cyclin-dependent kinase/p21/proliferating cell nuclear antigen complexes and cyclin D1 reverses rapamycin action by stabilizing these complexes. Cancer Res. 2006;66:1070–80. doi: 10.1158/0008-5472.CAN-05-1672. [DOI] [PubMed] [Google Scholar]
- 25.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression . Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 26.Blain SW. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle. 2008;7:892–8. doi: 10.4161/cc.7.7.5637. [DOI] [PubMed] [Google Scholar]
- 27.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–78. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 28.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 29.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression . Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 30.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–30. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 31.Mullany LK, White P, Hanse EA, Nelsen CJ, Goggin MM, Mullany JE, et al. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle. 2008;7:2215–24. doi: 10.4161/cc.7.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shakir R, Ngo N, Naresh KN. Correlation of cyclin D1 transcript levels, transcript type and protein expression with proliferation and histology among mantle cell lymphoma. J Clin Pathol. 2008;61:920–7. doi: 10.1136/jcp.2008.057455. [DOI] [PubMed] [Google Scholar]
- 33.Zlamalikova L, Moulis M, Salek D, Jarkovsky J, Smarda J, Smardova J. Expression of D-type cyclins in mantle cell and diffuse large B-cell lymphomas. Oncol Rep. 2016;35:2673–80. doi: 10.3892/or.2016.4658. [DOI] [PubMed] [Google Scholar]
- 34.Burandt E, Grünert M, Lebeau A, Choschzick M, Quaas A, Jänicke F, et al. Cyclin D1 gene amplification is highly homogeneous in breast cancer. Breast Cancer. 2016;23:111–9. doi: 10.1007/s12282-014-0538-y. [DOI] [PubMed] [Google Scholar]
- 35.Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, et al. Expression of cyclin d1 and its association with disease characteristics in bladder cancer. Anticancer Res. 2013;33:5235–42. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Jin DH, Lee BB, Cho EY, Han J, Shim YM, et al. Overexpression of β-catenin and cyclin d1 is associated with poor overall survival in patients with stage IA-IIA squamous cell lung cancer irrespective of adjuvant chemotherapy. J Thorac Oncol. 2016;11:2193–201. doi: 10.1016/j.jtho.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Jiang W, Kahn SM, Tomita N, Zhang YJ, Lu SH, Weinstein IB. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992;52:2980–3. [PubMed] [Google Scholar]
- 38.Ao R, Zhang D-R, Du Y-Q, Wang Y. Expression and significance of Pin1, β-catenin and cyclin D1 in hepatocellular carcinoma. Mol Med Rep. 2014;10:1893–8. doi: 10.3892/mmr.2014.2456. [DOI] [PubMed] [Google Scholar]
- 39.Stendahl M, Kronblad Å, Rydén L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004;90:1942–8. doi: 10.1038/sj.bjc.6601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hefnawy T, Zeleznik AJ. Synergism between FSH and activin in the regulation of proliferating cell nuclear antigen (PCNA) and cyclin D2 expression in rat granulosa cells. Endocrinology. 2001;142:4357–62. doi: 10.1210/endo.142.10.8438. [DOI] [PubMed] [Google Scholar]
- 41.Matsubayashi H, Sato N, Fukushima N, Yeo CJ, Walter KM, Brune K, et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin Cancer Res. 2003;9:1446–52. [PubMed] [Google Scholar]
- 42.Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, Niranjan B, et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 2001;61:2782–7. [PubMed] [Google Scholar]
- 43.Padar A, Sathyanarayana UG, Suzuki M, Maruyama R, Hsieh J-T, Frenkel EP, et al. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation . Clin Cancer Res. 2003;9:4730–4. [PubMed] [Google Scholar]
- 44.Büschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G. Amplification and expression of cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas . Brain Pathol. 1999;9:435–42. doi: 10.1111/j.1750-3639.1999.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedberg Y, Roos G, Ljungberg B, Landberg G. Cyclin D3 protein content in human renal cell carcinoma in relation to cyclin D1 and clinico-pathological parameters. Acta Oncolog. 2002;41:175–81. doi: 10.1080/028418602753669562. [DOI] [PubMed] [Google Scholar]
- 46.Ito Y, Takeda T, Wakasa K, Tsujimoto M, Matsuura N. Expression and possible role of cyclin D3 in human pancreatic adenocarcinoma. Anticancer Res. 2001;21:1043–8. [PubMed] [Google Scholar]
- 47.Filipits M, Jaeger U, Pohl G, Stranzl T, Simonitsch I, Kaider A, et al. Cyclin D3 is a predictive and prognostic factor in diffuse large B-cell lymphoma. Clin Cancer Res. 2002;8:729–33. [PubMed] [Google Scholar]
- 48.Shaughnessy J Jr, Gabrea A, Qi Y, Brents L, Zhan F, Tian E, et al. Cyclin D3 at 6p21 is dysregulated by recurrent chromosomal translocations to immunoglobulin loci in multiple myeloma. Blood. 2001;98:217–23. doi: 10.1182/blood.v98.1.217. [DOI] [PubMed] [Google Scholar]
- 49.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 50.Cui CH, Gan Y, Gu LK, Wilson J, Liu ZJ, Zhang BZ, et al. P16-specific DNA methylation by engineered zinc finger methyltransferase inactivates gene transcription and promotes cancer metastasis . Genome Biol. 2015;16:252. doi: 10.1186/s13059-015-0819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quesnel B, Fenaux P, Philippe N, Fournier J, Bonneterre J, Preudhomme C, et al. Analysis of p16 gene deletion and point mutation in breast carcinoma. Br J Cancer. 1995;72:351–3. doi: 10.1038/bjc.1995.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciznadija D, Liu Y, Pyonteck SM, Holland EC, Koff A. Cyclin D1 and cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 2011;71:6174–83. doi: 10.1158/0008-5472.CAN-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez M, Muñoz-Galván S, Jiménez-García MP, Marín JJ, Carnero A. Efficacy of CDK4 inhibition against sarcomas depends on their levels of CDK4 and p16ink4 mRNA. Oncotarget. 2015;6:40557–74. doi: 10.18632/oncotarget.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013;15:R5. doi: 10.1186/bcr3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol oncol. 1999;73:223–8. doi: 10.1006/gyno.1999.5346. [DOI] [PubMed] [Google Scholar]
- 56.Costello JF, Plass C, Arap W, Chapman VM, Held WA, Berger MS, et al. Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res. 1997;57:1250–4. [PubMed] [Google Scholar]
- 57.Kollmann K, Sexl V. CDK6 and p16INK4A in lymphoid malignancies . Oncotarget. 2013;4:1858–9. doi: 10.18632/oncotarget.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–85. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–5. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Karst AM, Jones PM, Vena N, Ligon AH, Liu JF, Hirsch MS, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74:1141–52. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhn E, Bahadirli-Talbott A, Shih I-M. Frequent CCNE1 amplification in endometrial intraepithelial carcinoma and uterine serous carcinoma . Mod Pathol. 2014;27:1014–9. doi: 10.1038/modpathol.2013.209. [DOI] [PubMed] [Google Scholar]
- 62.Lockwood WW, Stack D, Morris T, Grehan D, O'Keane C, Stewart GL, et al. Cyclin E1 is amplified and overexpressed in osteosarcoma. J Mol Diagn. 2011;13:289–96. doi: 10.1016/j.jmoldx.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koutsami MK, Tsantoulis PK, Kouloukoussa M, Apostolopoulou K, Pateras IS, Spartinou Z, et al. Centrosome abnormalities are frequently observed in non-small-cell lung cancer and are associated with aneuploidy and cyclin E overexpression. J Pathol. 2006;209:512–21. doi: 10.1002/path.2005. [DOI] [PubMed] [Google Scholar]
- 64.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G. Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas-prognostic implications. Blood. 1998;92:770–7. [PubMed] [Google Scholar]
- 65.Santo L, Siu KT, Raje N. Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Semin Oncol. 2015;42:788–800. doi: 10.1053/j.seminoncol.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Adhikari D, Zheng WJ, Shen Y, Gorre N, Ning Y, Halet G, et al. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Human Mol Genet. 2012;21:2476–84. doi: 10.1093/hmg/dds061. [DOI] [PubMed] [Google Scholar]
- 67.Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 68.Zhao MY, Auerbach A, D'Costa AM, Rapoport AP, Burger AM, Sausville EA, et al. Phospho-p70S6K/p85S6K and cdc2/cdk1 are novel targets for diffuse large B-cell lymphoma combination therapy. Clin Cancer Res. 2009;15:1708–20. doi: 10.1158/1078-0432.CCR-08-1543. [DOI] [PubMed] [Google Scholar]
- 69.Aaltonen K, Amini RM, Heikkila P, Aittomäki K, Tamminen A, Nevanlinna H, et al. High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer. 2009;100:1055–60. doi: 10.1038/sj.bjc.6604874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bondi J, Husdal A, Bukholm G, Nesland JM, Bakka A, Bukholm IRK. Expression and gene amplification of primary (A, B1, D1, D3, and E) and secondary (C and H) cyclins in colon adenocarcinomas and correlation with patient outcome. J Clin Pathol. 2005;58:509–14. doi: 10.1136/jcp.2004.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27:4733–44. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 72.Nar A, Ozen O, Tutuncu NB, Demirhan B. Cyclin A and cyclin B1 overexpression in differentiated thyroid carcinoma. Med Oncol. 2012;29:294–300. doi: 10.1007/s12032-010-9800-0. [DOI] [PubMed] [Google Scholar]
- 73.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4. [PubMed] [Google Scholar]
- 74.Zhang CY, Elkahloun AG, Robertson M, Gills JJ, Tsurutani J, Shih JH, et al. Loss of cytoplasmic CDK1 predicts poor survival in human lung cancer and confers chemotherapeutic resistance. PLoS ONE. 2011;6:e23849. doi: 10.1371/journal.pone.0023849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pozo K, Castro-Rivera E, Tan CF, Plattner F, Schwach G, Siegl V, et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell. 2013;24:499–511. doi: 10.1016/j.ccr.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romagosa C, Simonetti S, López-Vicente L, Mazo A, Lleonart ME, Castellvi J, et al. p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors . Oncogene. 2011;30:2087–97. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 77.Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, Moaven O, et al. p16INK4a hypermethylation and p53, p16 and MDM2 protein expression in esophageal squamous cell carcinoma . BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okamoto A, Demetrick DJ, Spillare EA, Hagiwara K, Hussain SP, Bennett WP, et al. p16INK4 mutations and altered expression in human tumors and cell lines . Cold Spring Harb Symp Quant Biol. 1994;59:49–57. doi: 10.1101/sqb.1994.059.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 80.Jen J, Harper JW, Bigner SH, Bigner DD, Papadopoulos N, Markowitz S, et al. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994;54:6353–8. [PubMed] [Google Scholar]
- 81.Lapointe J, Lachance Y, Labrie Y, Labrie C. A p18 mutant defective in CDK6 binding in human breast cancer cells. Cancer Res. 1996;56:4586–9. [PubMed] [Google Scholar]
- 82.Shi Y, Zou M, Farid NR, al-Sedairy ST. Evidence of gene deletion of p21 (WAF1/CIP1), a cyclin-dependent protein kinase inhibitor, in thyroid carcinomas. Br J Cancer. 1996;74:1336–41. doi: 10.1038/bjc.1996.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 84.Davies TG, Pratt DJ, Endicott JA, Johnson LN, Noble ME. Structure-based design of cyclin-dependent kinase inhibitors. Pharmacol Ther. 2002;93:125–33. doi: 10.1016/s0163-7258(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 85.Mohanakumara P, Sreejayan N, Priti V, Ramesha BT, Ravikanth G, Ganeshaiah KN, et al. Dysoxylum binectariferum Hook.f (Meliaceae), a rich source of rohitukine . Fitoterapia. 2010;81:145–8. doi: 10.1016/j.fitote.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Shapiro GI. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res. 2004;10:4270s–5s. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- 87.Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–70. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 88.Blachly JS, Byrd JC. Emerging drug profile: cyclin-dependent kinase inhibitors. Leuk Lymphoma. 2013;54:2133–43. doi: 10.3109/10428194.2013.783911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lanasa MC, Andritsos L, Brown JR, Gabrilove J, Caligaris-Cappio F, Ghia P, et al. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res. 2015;39:495–500. doi: 10.1016/j.leukres.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grendys EC Jr, Blessing JA, Burger R, Hoffman J. A phase II evaluation of flavopiridol as second-line chemotherapy of endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;98:249–53. doi: 10.1016/j.ygyno.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 91.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Fitch TR, Fenton RG, et al. Flavopiridol in patients with relapsed or refractory multiple myeloma: a phase 2 trial with clinical and pharmacodynamic end-points. Haematologica. 2006;91:390–3. [PubMed] [Google Scholar]
- 92.Burdette-Radoux S, Tozer RG, Lohmann RC, Quirt I, Ernst DS, Walsh W, et al. phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs. 2004;22:315–22. doi: 10.1023/B:DRUG.0000026258.02846.1c. [DOI] [PubMed] [Google Scholar]
- 93.Wang L-M, Ren D-M. Flavopiridol, the first cyclin-dependent kinase inhibitor: recent advances in combination chemotherapy. Mini Rev Med Chem. 2010;10:1058–70. doi: 10.2174/1389557511009011058. [DOI] [PubMed] [Google Scholar]
- 94.Karp JE, Smith BD, Levis MJ, Gore SD, Greer J, Hattenburg C, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:4467–73. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 95.Luke JJ, D'Adamo DR, Dickson MA, Keohan ML, Carvajal RD, Maki RG, et al. The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: preclinical investigations and results of a phase I dose-escalation clinical trial. Clin Cancer Res. 2012;18:2638–47. doi: 10.1158/1078-0432.CCR-11-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wesięrska-Gądek J, Maurer M, Zulehner N, Komina O. Whether to target single or multiple CDKs for therapy? That is the question. J Cell Physiol. 2011;226:341–9. doi: 10.1002/jcp.22426. [DOI] [PubMed] [Google Scholar]
- 97.Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, et al. Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma . Blood. 2005;106:1042–7. doi: 10.1182/blood-2005-01-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim EH, Kim SU, Shin DY, Choi KS. Roscovitine sensitizes glioma cells to TRAIL-mediated apoptosis by downregulation of survivin and XIAP. Oncogene. 2004;23:446–56. doi: 10.1038/sj.onc.1207025. [DOI] [PubMed] [Google Scholar]
- 99.Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le Tourneau C, Faivre S, Laurence V, Delbaldo C, Vera K, Girre V, et al. phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies . Eur J Cancer. 2010;46:3243–50. doi: 10.1016/j.ejca.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Cyclacel. Cyclacel Press Release 21/12/10. Cyclacel Website. [updated 2010 Dec 21]. Available from: http://investor.cyclacel.com/releasedetail.cfm?ReleaseID=614395.
- 102.Dhillon S. Palbociclib: first global approval. Drugs. 2015;75:543–51. doi: 10.1007/s40265-015-0379-9. [DOI] [PubMed] [Google Scholar]
- 103.Clark AS, Karasic TB, DeMichele A, Vaughn DJ, O'Hara M, Perini R, et al. Palbociclib (PD0332991)-a selective and potent cyclin-dependent kinase inhibitor: a review of pharmacodynamics and clinical development. JAMA Oncol. 2016;2:253–60. doi: 10.1001/jamaoncol.2015.4701. [DOI] [PubMed] [Google Scholar]
- 104.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 105.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J Biol Chem. 2012;287:29075–87. doi: 10.1074/jbc.M112.365494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michaud K, Solomon DA, Oermann E, Kim J-S, Zhong W-Z, Prados MD, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–38. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, et al. phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) Br J Cancer. 2011;104:1862–8. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flaherty KT, Lorusso PM, DeMichele A, Abramson VG, Courtney R, Randolph SS, et al. phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568–76. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 110.DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment . Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 111.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 112.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. New Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 113.Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2016;22:5696–705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munster PN, Hamilton EP, Estevez LG, De Boer RH, Mayer IA, Campone M, et al. Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. J Clin Oncol. 2014;32:143. [Google Scholar]
- 115.Bardia A, Modi S, Gregor MC-M, Kittaneh M, Marino AJ, Matano A, et al. phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2-metastatic breast cancer. ASCO Meeting Abstracts. 2014;32:535. [Google Scholar]
- 116.Gelbert LM, Cai SF, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig New Drugs. 2014;32:825–37. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanchez-Martinez C, Gelbert LM, Shannon H, De Dios A, Staton BA, Ajamie RT, et al. Abstract B234: LY2835219, a potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts. Mol Cancer Ther. 2011; 10: B234.
- 118.Gelbert LM, Cai SF, Lin X, Sanchez-Martinez C, del Prado M, Lallena MJ, et al. Abstract B233: Identification and characterization of LY2835219: A potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) with broad in vivo antitumor activity. Mol Cancer Ther. 2011; 10: B233.
- 119.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with metastatic breast cancer. Cancer Res. 2014; 74: CT232.
- 120.Goldman JW, Gandhi L, Patnaik A, Rosen LS, Hilton JF, Papadopoulos KP, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with non-small cell lung cancer. J Clin Oncol. 2014; 32: 8026.