ABSTRACT
Eukaryotes possess a vast array of RNA-binding proteins (RBPs) that affect mRNAs in diverse ways to control protein expression. Combinatorial regulation of mRNAs by RBPs is emerging as the rule. No example illustrates this as vividly as the partnership of 3 Drosophila RBPs, Pumilio, Nanos and Brain Tumor, which have overlapping functions in development, stem cell maintenance and differentiation, fertility and neurologic processes. Here we synthesize 30 y of research with new insights into their molecular functions and mechanisms of action. First, we provide an overview of the key properties of each RBP. Next, we present a detailed analysis of their collaborative regulatory mechanism using a classic example of the developmental morphogen, hunchback, which is spatially and temporally regulated by the trio during embryogenesis. New biochemical, structural and functional analyses provide insights into RNA recognition, cooperativity, and regulatory mechanisms. We integrate these data into a model of combinatorial RNA binding and regulation of translation and mRNA decay. We then use this information, transcriptome wide analyses and bioinformatics predictions to assess the global impact of Pumilio, Nanos and Brain Tumor on gene regulation. Together, the results support pervasive, dynamic post-transcriptional control.
KEYWORDS: Brain Tumor (or Brat), combinatorial mRNA regulation, cooperative RNA binding, Nanos, Pumilio
Introduction
Translation of mRNAs is highly regulated to ensure the proper quantity, time and location of protein synthesis. The output of protein from each mRNA is determined in part by its abundance and the status of the translation apparatus. Information within the transcript also controls protein expression, including cis-acting regulatory elements, RNA structure, and codon content. Specific regulatory elements that regulate a transcript's fate are often located in 5´ or 3´ untranslated regions (UTRs). Many regulatory elements are recognized by trans-acting RNA-binding factors that determine whether the transcript is translated or instead silenced, stored, localized, stabilized or destroyed.
In this review, we explore mechanisms of mRNA regulation by RNA-binding proteins (RBPs), focusing on 3 now-classic RBPs, Pumilio (Pum), Nanos (Nos) and Brain Tumor (Brat). To date, >1500 RBPs have been cataloged and the functions of most remain to be discovered.1-4 The sheer number of RBPs signifies the importance of post-transcriptional control. Pum, Nos and Brat were originally identified in Drosophila decades ago and remain relevant because they exemplify key principles of post-transcriptional control and because they regulate crucial biological functions. Recently, important new insights into their molecular mechanisms illuminate our understanding of regulated RNA stability and the spatial and temporal control of protein expression.
Combinatorial control is emerging as a pervasive theme in post-transcriptional regulation, with mRNAs controlled by a dynamic constellation of RNA-binding factors. Pum, Nos and Brat represent an archetypal example where their combined action controls crucial biologic processes including development, stem cell proliferation, fertility and neurological functions. Genetics revealed overlapping functions, and they were shown to physically interact with each other on a target mRNA, leading to a compelling model some 15 y ago.5-7 Yet the mechanism of combinatorial control was not well understood. Recent advances provide the detailed molecular basis of their collaboration. We now understand that Pum, Nos and Brat proteins each define a protein family with unique modes of RNA recognition. Certain transcripts can be targeted by all 3 RBPs, which bind cooperatively to synergistically repress protein expression. Here we introduce the unique features of Pum, Nos and Brat proteins, and integrate new biochemical, structural and functional data into an updated model of their combinatorial regulatory function. We then explore the implications of this model for regulation of mRNAs on a transcriptome-wide scale.
Pumilio
Pum is a founding member of the PUF (Pum and fem-3 binding factor) family of eukaryotic RNA-binding proteins.8 Pum was originally identified as a maternal effect gene necessary for embryonic development.9,10 The name Pumilio is Latin for “dwarf,” referring to the small embryos from the original pum mutant. Subsequently, Pum was shown to regulate diverse biologic processes including germline stem cell proliferation, fertility, neuronal morphology, motor neuron electrophysiology, and memory formation.11-16
Pum is a 1533 amino acid residue protein with a conserved Pum homology domain (Pum-HD) located in the C-terminal third (Fig. 1A). The Pum-HD, which defines the PUF family, is a sequence-specific RNA-binding domain of ∼40 kDa composed of repeated triple α helical units.6,17,18 Pum has 8 repeats that form a crescent shaped molecule, and each repeat presents 3 amino acid residues that recognize a single RNA nucleotide.18,19 Pum thereby binds an 8 nucleotide, single-stranded RNA sequence with the consensus 5´-UGUANAUA (where N = A, G, C or U), herein referred to as the Pum Response Element (PRE) (Fig. 1B).19-23 X-ray crystal structures of the RNA-binding domains of PUF proteins bound to RNA ligands, including the high resolution structure of Pum bound to a PRE, clearly illustrate the modular RNA recognition, and recent reviews provide a comprehensive discussion of the determinants of PUF RNA-binding specificity (Fig. 1C).19,24-26
Figure 1.
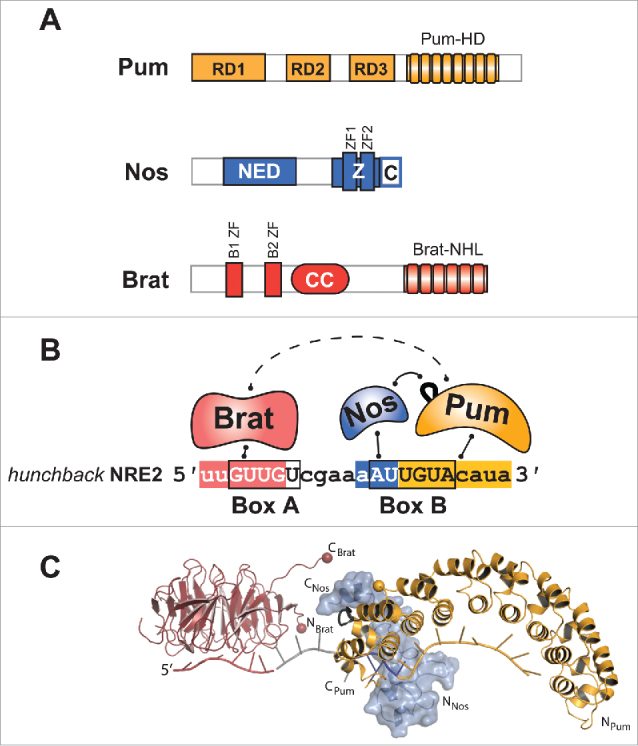
Pum, Nos, and Brat are RNA-binding proteins that bind the hunchback mRNA. (A) Schematic diagrams of Pum, Nos, and Brat proteins with relevant domains labeled: Pum N-terminal Repression Domains (RD1, RD2, and RD3), and Pum Homology Domain (Pum-HD); Nos Effector Domain (NED), Zinc Fingers (Z), and C-terminal extension (C); Brat B-box Zinc Fingers 1 and 2 (B1 and B2), coiled coil (CC), and NCL-1, HT2A, and Lin-41 (NHL) domain. (B) Pum, Nos and Brat bind to the Nanos Response Element 2 (NRE2) RNA from the hunchback 3´UTR with color-coded binding sites for Brat, Nos, and Pum. Box A and B elements of the NRE are outlined by a black box. Direct interactions are indicated by solid lines whereas dashed lines indicate putative interactions. The loop between repeats 7 and 8 of Pum, which mediates protein-protein interaction with Nos, is shown in black. (C) Structural model of Brat (NHL domain), Nos (ZC regions), and Pum (Pum-HD) proteins with NRE RNA. The crystal structures of Brat in complex with a BBS (red, PDB ID 4ZLR) and Nos (blue)/Pum (yellow) in complex with NBS-PRE RNA (PDB ID 5KL1) are shown with the 4 nucleotide spacer RNA (gray) present in the native hunchback NRE2 RNA. Brat and Pum proteins are shown as ribbon diagrams. Nos is shown with a molecular surface superimposed. Residue G1330 of Pum is highlighted by a yellow sphere.
Pum binds and represses specific mRNAs that contain one or more PREs, resulting in reduced protein expression and accelerated mRNA degradation.27-30 We now understand that Pum repression occurs through multiple mechanisms. The Pum-HD represses by targeting the poly(A) tail of target mRNAs. Normally, the poly(A) tail acts to promote translation and stability of an mRNA, mediated by poly(A) binding protein, PABP. We found that the Pum-HD associates with and antagonizes the translational activity of PABP, thereby contributing to repression.28 The Pum-HD also directs repression by promoting removal of poly(A) from target mRNAs by recruiting the Pop2 deadenyase enzyme,28,31,32 which is part of the Ccr4-Not complex (CNOT) that catalyzes deadenylation and causes translational repression.33,34 Notably, poly(A)-dependent repression mechanisms are conserved functions of PUF proteins.28,30-32,35,36
Pum also elicits poly(A)-independent repression. In both cultured cells and embryos, Pum represses reporter mRNAs lacking a poly(A) tail, albeit with reduced efficiency.28,30,37 Structure-function analysis revealed that the N-terminus of Pum, wholly outside of the Pum-HD, conferred poly(A)-independent repression activity.27 The N terminus was largely a mystery as it is not homologous to other proteins or domains. However, genetic evidence indeed supports the importance of the Pum N terminus, as its inclusion in transgenes was necessary to fully rescue developmental defects of a pum mutant.[20] Dissection of the Pum N terminus revealed 3 autonomous repression domains capable of poly(A)-independent repression, potently inhibiting protein expression and stimulating mRNA decay when targeted to a reporter mRNA.27 It remains to be determined how each Pum repression domain operates.
Nanos
Nos is a founding member of the eukaryotic Nos protein family, with orthologs found throughout multicellular eukaryotes. Nos was originally identified as a maternally provided determinant of posterior development.10,38 The name nanos is Greek for “dwarf” and describes the morphology of original mutant embryos, which is identical to the pum phenotype.38 In fact, Nos shares several biological roles with Pum including embryonic development, control of germline stem cell proliferation, neuronal morphology, and long-term memory formation.11,13,15,16,39 These commonalities are indicative of collaborative control by Nos and Pum.
Nos protein is 401 amino acid residues in length with 2 unique C-terminal CCHC-type Zinc Finger (ZF) domains that define the Nanos family (Fig. 1A). The ZF domains were reported to mediate non-specific binding to RNA.40,41 We recently found that Nos ZFs bind specifically to a Nanos Binding Site (NBS) in RNA, but only when that RNA includes a downstream PRE sequence that is bound by Pum (discussed further below) (Fig. 1B).19 Crystal structures of the ZFs of Nos bound to RNA in conjunction with Pum provide evidence of specific nucleotide binding pockets formed by the tandem ZF domains (Fig. 1C).19
Like Pum, Nos is a repressor that reduces protein expression and stimulates decay of target mRNAs.42,43 Recent research has revealed that Nos binds and recruits the CNOT complex to repress translation and elicit decay by promoting deadenylation and decapping of the 5´ 7-methyl guanosine cap structure.44-46
Brain Tumor
Brat is an RBP with important roles in oogenesis, embryogenesis and the nervous system.5,47-49 Brat was originally identified as a growth suppressor in the larval brain, its name derived from the mutant phenotype wherein neural cells aberrantly proliferate.48,49 Brat has many documented functions including regulation of neuromuscular junction formation, neuronal differentiation, axon maintenance in mushroom bodies and control of motor neuron electrophysiology.39,50-52 Brat has overlapping functions with Pum and Nos in the germline and embryo, as discussed below, and also has functions independent of Pum and Nos.5,52,53
Brat is a 1037 amino acid residue protein that belongs to the TRIM-NHL class of proteins, which are defined by an N-terminal TRIM (Tripartite Motif) and C-terminal NHL (NCL-1, HT2A, and Lin-41) domain (Fig. 1A).54 The C-terminal NHL domain forms a 6-bladed β propeller structure that is crucial for function.7,48,55 Initially, Brat was thought to function as an adaptor protein that mediated protein-protein interactions5,7; however, the NHL domain of Brat was recently shown to be an RNA-binding domain, specifically recognizing the Brat binding site (BBS) with consensus 5´-WYGUUD (where W = A or U; Y = U or C; D = G, A, or U) (Fig. 1B and Fig. 1C).29,55,56 The TRIM region of Brat contains 2 B-box type ZFs, which are broadly found in DNA- and RNA-binding proteins, but it is unknown whether these domains contact RNA.
Like Pum and Nos, Brat represses translation from target mRNAs and accelerates their decay5,29,53; however, less is known about its mechanism. Evidence from embryos demonstrates that Brat causes turnover of numerous transcripts during the maternal-to-zygotic transition (MZT), a developmental stage in which maternally provided transcripts are degraded and zygotic genome transcription is initiated,57,58 and this effect can be recapitulated in cultured cells with reporter mRNAs.29,55,56 Brat appears to work in conjunction with the translational repressor protein, 4EHP (eIF4E homologous protein); the NHL domain was reported to interact with 4EHP, and 4EHP mutations reduced Brat-mediated repression.53 Brat is also reported to associate with the CNOT deadenylase complex,59 suggesting that it may promote deadenylation of mRNAs, although this supposition remains unproven.
Combinatorial control by Pumilio, Nanos and Brain Tumor: The hunchback mRNA paradigm
No case better exemplifies combinatorial control by Pum, Nos and Brat than collaborative regulation of hunchback mRNA, their first identified target in the early embryo.60-63 Justified by its biological significance, intense focus on the mechanisms of hunchback regulation helped to establish key parameters of the Pum-Nos-Brat partnership.
During early embryogenesis in Drosophila, the zygotic genome is transcriptionally silent and development is directed by maternally supplied gene products.57,58 Maternal mRNAs, including hunchback, must be precisely regulated for development to proceed. Hunchback is a transcription factor that controls body pattern formation, and its expression must be limited to the anterior portion of the syncytial embryo before the MZT.64 Because hunchback mRNA is distributed throughout the embryo, its mRNA is translated only in the anterior while being repressed in the posterior to achieve proper spatial distribution of Hunchback protein.61,63,65 Repression of hunchback is achieved by Pum, Nos and Brat, and mutations that inactivate them result in improper expression of the Hunchback protein in the posterior, subsequent loss of abdominal segments, and developmental failure.5,9,62,66-68
The spatial distribution of Hunchback protein is determined by an opposing concentration gradient of Nos protein.38,61,62,68 nos mRNA is localized to the embryo's posterior where its localized translation, coupled to simultaneous repression of unlocalized nos mRNA in the bulk cytoplasm,69-72 establishes a Nos protein gradient that is highest in the posterior, quickly diminishing toward the anterior. Pum and Brat proteins are distributed throughout the embryo, and although crucial for hunchback mRNA regulation, they do not provide the spatial cue.5,66,73 In addition to controlling hunchback translation during early embryogenesis, the combinatorial action of Pum and Brat (and likely Nos) mediates degradation of maternally provided hunchback mRNA during the MZT.29,65
Early work mapped the features of hunchback mRNA necessary for Nos-mediated repression. Two separate Nanos Response Elements (NREs) were identified within the hunchback mRNA 3´UTR that are necessary and sufficient to confer Nos-mediated repression in embryos.40,42,74 Each NRE contains 2 distinct, conserved elements termed Box A and Box B, which are required for complete regulation (Fig. 1B). These NREs are the nexus for combinatorial regulation of hunchback mRNA by Nos, Pum and Brat.
The Nanos Response Element is directly bound by Pumilio, Nanos and Brain Tumor
A synthesis of early and recent discoveries firmly establishes direct binding and combinatorial regulation of hunchback mRNA by Nos, Pum and Brat. Pum was first shown to bind each NRE element of hunchback.22,74 The Pum-NRE interaction was interrogated through mutational analysis and binding assays, defining a high affinity PRE within each NRE bound by a single Pum.21,22 Structural, high throughput selection and sequencing, and transcriptome-wide analyses corroborated and defined the specificity of the Pum-PRE interaction.18,19,23 We now understand that each hunchback NRE contains a single high affinity 8 nucleotide PRE, the 5´ half of which overlaps with each conserved Box B element (Fig. 1B).
Insight into the role of Nos in hunchback regulation emerged from structure-function analysis using Nos transgenes, which identified the ZFs and C terminus as being critical for hunchback regulation.40 Purified, recombinant Nos was also reported to bind the NRE without apparent specificity, though we now understand that the NRE mutations tested were in fact outside the Nos binding site.19,40 Key insights into the Nos-NRE interaction came from yeast 3-hybrid assays, which showed that Nos binds to the NRE in a Pum-dependent manner, and the resulting ternary complex could also be detected by in vitro pulldown assays.75 Mutations that disrupt Nos, Pum or NRE function prevented formation of the ternary complex. Nos did not bind the NRE in the absence of Pum; however, when Pum was included, Nos could be crosslinked to the RNA. Nos did not stably bind Pum in the absence of NRE RNA. These results indicated that Pum provides sequence-specific RNA-binding, whereas Nos recognizes a combination of Pum and RNA. Importantly, nucleotides upstream of the PRE were shown to be important for incorporation of Nos into this ternary complex.75
We recently reported biochemical, structural and cell-based studies that show how Nos and Pum cooperatively bind NRE RNA.19 Using RNA electrophoretic mobility shift assays (EMSA), we found that Nos tightly binds the Pum-NRE complex and increases the affinity of Pum for the NRE, correlating with its ability to enhance translational repression in cells in a dosage dependent manner. A critical revelation is that Pum does not merely recruit Nos for repression activity; Nos enhances the binding of Pum to hunchback RNA, bringing the combined repressive activities of Nos and Pum to bear on hunchback mRNA exclusively in the embryonic posterior where Nos concentration is highest.19
Our crystal structure of the Nos-Pum-NRE ternary complex illuminated the mechanism of Nos-Pum cooperativity.19 Pum recognizes the PRE sequence in the recognizably modular fashion, while Nos embraces both Pum and RNA, effectively clamping them together (Fig. 1C). The Nos C terminus interacts with a loop region between the 7th and 8th repeats of the Pum-HD. Conformational changes in the loop of Pum are induced by the Nos interaction, which enables an α helix at the C terminus of the Pum-HD to unfold and contact the NRE. Mutational analysis affirmed the importance of observed interactions for complex formation in vitro and repression activity in cells.19 The observed contacts also illustrate how mutants of Pum (mutations in the loop between repeat 7 and 8), Nos (mutations in the ZFs or C terminus) and Box B of the NRE result in loss of hunchback mRNA regulation in embryos.20,38,42,68
Our Nos-Pum-NRE structure revealed that the tandem ZFs of Nos bind 3 nucleotides immediately upstream of the PRE, defining the NBS (Fig. 1B).19 By performing Nos-Pum selection of a randomized RNA library and high throughput sequencing (SEQRS), we showed that Nos confers specificity for A/U rich NBS sequences in the presence of Pum.19,76 Nos-NBS specificity is verified in cells and embryos, where mutations of the NBS prevent Nos-mediated repression.19,20,42,74 Together, these data support a model wherein Nos acts as a clamp that promotes the binding of Pum to the NRE, and together they recognize an extended NBS+PRE sequence encompassing the Box B region of the NRE (Fig. 1C). In turn, the ternary complex elicits robust repression of hunchback mRNA.
Brat was identified as a third protein recruited by the Nos-Pum-NRE ternary complex.5 Using a yeast 4-hybrid strategy, the Brat NHL domain was found to bind the Pum-Nos-hb NRE complex. Yeast interaction and in vitro pulldown assays indicated that Nos and Pum were both needed to recruit Brat to the NRE. Yet no direct interaction of Brat with NRE, Nos or Pum individually could be detected by these means. A model was put forth wherein Pum and Nos bind the NRE and then recruit Brat through simultaneous protein-protein interactions with Nos and Pum to form a quaternary complex.5-7 Genetic analysis showed that brat mutants disrupted hunchback mRNA regulation and abdominal segmentation in embryos, mirroring the effects of Nos, Pum or NRE mutants.5,53
New data on Brat's RNA-binding properties and its interaction with the NRE warrant a re-evaluation of the quaternary complex model. Three studies have now shown that Brat is an RNA-binding protein that directly contacts the Box A sequence in each hunchback NRE (Fig. 1B).29,55,56 A crystal structure of the Brat NHL domain bound to RNA revealed an electropositive surface of the NHL domain that recognizes the 6 nucleotide, single-stranded RNA element, and mutation of observed Brat-RNA contacts (including R875A, F916A, and N933A) disrupted its RNA-binding and cellular repression activities.55,56 Based on these data, it is now apparent that Brat does not require Pum and Nos to bind the NRE. Although Brat and Pum are able to bind to the NRE cooperatively,55 it is unclear whether protein-protein interactions underlie this cooperative binding, since a Brat-Pum interaction could not be detected in the absence of NRE RNA.55 One proposal is that cooperative Brat-NRE-Pum binding is mediated by changes in RNA secondary structure induced by protein-RNA contact.56 More importantly, it remains uncertain whether the observed cooperative binding even impacts repression activity, since synergism between Brat and Pum has not yet been demonstrated.55
New insights from structure-function analyses also prompt reassessment of the effects of specific mutations on the quaternary complex, as summarized in Table 1. For example, Pum mutants C1365R, T1366D, or N1368S were reported to disrupt Brat recruitment,6 but the Nos-Pum-NRE structure shows that these residues are at the Nos-Pum interface, and thus are unlikely to interact with Brat in a quaternary complex.19 Pum mutant G1330D, which is located in a loop between repeats 6 and 7 of the Pum-HD (Fig. 1C), does not affect RNA binding or cellular repression activities.5,27 Moreover, G1330D does not contact Nos in the ternary complex structure, nor does it compromise Nos-Pum synergy (Fig. 1C).19,27 Instead, G1330D was proposed to mediate Brat-Pum interaction,5-7 but no assay has detected this putative protein-protein interaction and its effect on cooperative RNA binding by Pum and Brat has not been evaluated.
Table 1.
List of documented Pum, Nos, and Brat mutants which disrupt protein-protein and protein-RNA interactions, comparing the interpretations from the “Original Model” relative to the “New Model” based on recent studies, as cited in the table.
| Protein | Mutation | Original Model | New Model | Reference |
|---|---|---|---|---|
| Pum | G1130D | Binding to Nos-Pum-NRE | Unknown | 5 |
| Pum | C1365R | Blocked binding to Nos-Pum-NRE | Located at the interface of Nos-Pum, predicted to disrupt binding to Nos | 6,19 |
| Pum | T1366D | Blocked binding to Nos-Pum-NRE | Located at the interface of Nos-Pum, predicted to disrupt binding to Nos | 6,19 |
| Pum | N1368S | Blocked binding to Nos-Pum-NRE | Located at the interface of Nos-Pum, predicted to disrupt binding to Nos | 6,19 |
| Nos | M378A or K | Blocked binding of Brat to Nos-Pum-NRE | Necessary for Nos-Pum binding to NRE | 5,19,27 |
| Bratfs1 | G774D | Blocked binding to Nos-Pum-NRE | Effect on NRE binding unknown. Reported to disrupt Brat binding to Mira. | 5,50,56 |
| Bratfs3 | H802L | Blocked binding to Nos-Pum-NRE | Reduced affinity for NRE, but does not directly contact RNA | 5,55,56 |
| Brat | Y829A | Blocked binding to Nos-Pum-NRE | Reduced binding to NRE | 7,55,56 |
| Brat | R847A | Blocked binding to Nos-Pum-NRE | Reduced binding to NRE | 7,55,56 |
| Brat | R875A | Blocked binding to Nos-Pum-NRE | Blocked binding to NRE | 7,55,56 |
| Brat | G860D | Disrupt 4EHP interaction | 53 | |
| Brat | K809A/E810A | Disrupt 4EHP interaction | 53 | |
| Brat | K882E | Disrupt 4EHP interaction | 53 |
Nos was previously thought to be necessary for Brat recruitment to the NRE,5 but direct binding of Brat to Box A obviates that conclusion. Moreover, the Nos mutant M378K (referred to as M379K in the original study) prevented Brat recruitment, but the Nos-Pum-NRE ternary complex revealed that this residue is at the interface of the Nos-Pum interaction and is necessary for Nos-Pum synergism.19,27 The potential influence of Nos on Brat-NRE interaction remains to be re-evaluated considering this new information. What effect might Nos have on the Brat-NRE-Pum interaction? Since Nos enhances Pum affinity for the NRE, and Pum and Brat cooperatively bind the NRE, we speculate that Nos may enhance the Brat-NRE interaction acting through Pum. If true, this potential mutual cooperativity would be expected to contribute to spatiotemporal control of hunchback mRNA.
Several Brat mutations were originally attributed to disrupt protein-protein interactions with the ternary complex.5 Instead, new information shows that these Brat mutations negatively affect its ability to bind RNA, including H802L (bratFS3 allele)5 and 3 residues located on the “top” electropositive interface (Y829A, R847A, R875A)(Table 1).7,55,56 With the exception of H802, the crystal structure of a Brat-RNA complex shows that these mutated residues line the RNA-binding interface.55,56
Using current information, we illustrate a model of the quaternary complex on the hunchback NRE RNA (Fig. 1C). The model depicts interaction of the Brat NHL domain with its binding site overlapping Box A, the contacts of the Pum-HD bound to the PRE and the Nos-NBS interactions.19,56 From a structural standpoint, it is not possible to dock the proteins consistent with the proposed Brat-Pum contacts. First, the Nos-Pum interface occludes the previously proposed interaction site for Brat on Pum.7 As mentioned above, the previously modeled Brat-Pum interface is now known to be the RNA-binding interface. Moreover, the intervening 4 nucleotides between Box A and the NBS-PRE do not provide enough distance to permit interaction between the Brat NHL domain and Pum G1330 (Fig. 1C). Multiple features are missing from this model including the N termini of Brat, Nos and Pum, for which structural information is currently not available. Future biochemical and structural analyses are necessary to provide a more complete understanding of the quaternary complex architecture.
Multiple mechanisms of synergistic repression by Pumilio, Nanos and Brain tumor
Why are 3 repressors necessary to regulate hunchback mRNA? Redundancy is a possibility, but the fact that Pum, Nos and Brat are all required in vivo argues against that explanation. Instead, 4 principles have emerged. First, cooperative RNA binding contributes to the observed synergistic regulation through increased RNA affinity and specificity, as documented for Nos and Pum.19,27 Whether cooperative RNA binding by Brat and Pum also contributes to synergism remains unknown. Second, each regulator is independently capable of repression, and therefore their collaborative regulation provides multiple repression mechanisms that inhibit translation and accelerate mRNA decay. Pum can repress PRE-containing mRNAs independent of Nos or Brat.27 Brat can repress mRNAs bearing BBS motifs independent of Nos and Pum.55,56 Nos also possesses its own repression activity, as demonstrated by artificial tethering Nos directly to mRNA31,44; however, in the natural context, it requires Pum.19,27 Acting together, the combined activities of Brat, Nos and Pum offers increased magnitude of repression, as shown by synergistic repression by Nos and Pum,19,27 though synergism between Brat and Pum has not been demonstrated.55 The third principle is that the repressors each contact specific subunits of the same effector complex, as described for the CNOT complex below, resulting in enhanced recruitment to the target mRNA. A fourth principle is that collaboration imparts versatility in the means of controlling protein expression. For instance, repression of Hunchback protein synthesis is caused by translational inhibition and deadenylation early in embryogenesis followed by hunchback mRNA degradation during the MZT.29,37,43,65
Collaborative repression by Pum, Nos and Brat is mediated through multiple mechanisms, as shown in Fig. 2. Repression of translation by Pum, Nos and Brat is caused by inhibition of both 5´ cap and poly(A)-mediated translation (Fig. 2A). First, Pum antagonizes the translational activity of PABP; PABP interference has been demonstrated in cellular repression assays,28 but the use of this poly(A)-mediated mechanism in the embryo must be verified. Second, Brat recruits 4EHP, which inhibits translation by displacing eIF4E from the 5´ cap structure.53 Supporting this mechanism, the cap binding activity of 4EHP is required in vivo. However, it is important to note that mutation of 4EHP reduces, but does not eliminate, repression of Hunchback expression in vivo,53 consistent with multiple repression mechanisms. Brat mutants (G860D, K809A/E810A, R837D, K882E) that prevent 4EHP recruitment were identified, and Brat R837D or K882E did not repress hunchback mRNA in embryos.53 In cellular repression assays, however, the Brat R837D mutation had no effect, providing conflicting information about the importance of 4EHP recruitment for Brat-mediated repression in all contexts.55 Third, Nos causes translational repression in cell-based assays via a Nos Effector Domain (NED) in the protein's N terminus.44 The mechanism of this Nos-mediated translational repression is currently unknown, but might involve the action of CNOT complex and associated translational repressors 4E-T and Me31B (homolog of mammalian DDX6).77-79
Figure 2.
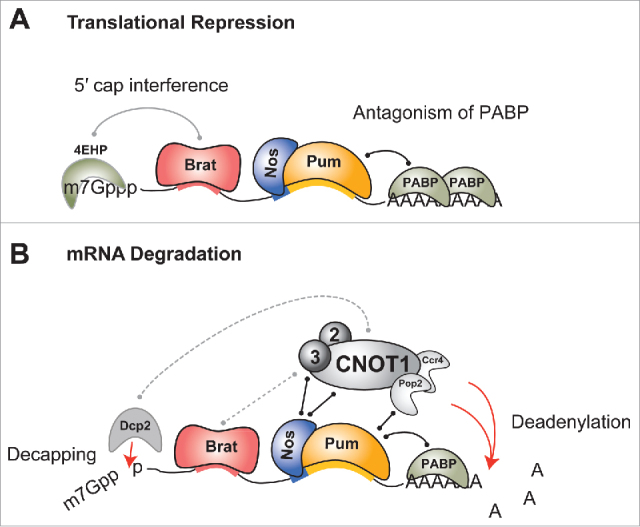
Multiple mechanisms of repression by Pum, Nos, and Brat. (A) Translational repression of target mRNAs can be mediated through recruitment of alternative cap-binding protein 4EHP by Brat, which is proposed to prevent binding of eIF4F translation initiation complex to the 5´ cap structure (7-methyl guanosine 5´-5´ triphosphate, m7Gppp). In addition, Pum antagonizes the translation activity of Poly(A) binding protein (PABP). (B) mRNA decay can be initiated through recruitment of the Ccr4-NOT (CNOT) complex, which catalyzes deadenylation and promotes decapping of the target mRNA. Pum recruits the Pop2 deadenylase to stimulate deadenylation, and Nos directly recruits Not1 and Not3 of the CNOT complex to stimulate deadenylation and decapping. Solid lines indicate documented interactions whereas dashed lines indicate putative interactions.
Pum, Nos and Brat also accelerate mRNA decay through multiple mechanisms, with collaborative recruitment of the CNOT complex emerging as a central theme.27-30,42,43 Both Nos and Pum promote deadenylation by recruiting the CNOT complex,28,31,44-46 The Pum-HD binds the Pop2 deadenylase subunit,28,30-32 whereas the Nos NED contacts the CNOT1 and CNOT3 subunits.44 Brat also associates with the CNOT complex,59 but the contacts and its effect on deadenylation remain to be determined. When combined on NRE-containing mRNA, Brat, Nos and Pum may synergistically enhance deadenylase recruitment, resulting in accelerated deadenylation and subsequent mRNA decay.33 To test this prediction, the contributions of the individual RBP-CNOT contacts on decay of hunchback mRNA should be evaluated in embryos. Nos also accelerates 5´ decapping (Fig. 2), and inactivation of the decapping enzyme Dcp2 blocked Nos-mediated mRNA decay, as did depletion of CNOT3.44 It remains unclear whether Nos directly contacts the decapping enzyme. Alternatively, Dcp2 may be linked to Nos through the CNOT complex, which associates with decapping factors.78,79
Additional repression mechanisms appear to contribute to the repression of hunchback mRNA. For instance, we identified 3 repression domains (RDs) in the N terminus of Pum, each of which potently represses translation and promotes mRNA decay in cell-based assays.27 Although the cofactors necessary for the activity of the Pum RDs must be identified, PABP and poly(A) are not essential for their activity.28 Future research will focus on the mechanism of Pum RD repression and how they contribute to synergistic regulation to ensure proper spatial and temporal control of Hunchback protein expression.
Global impact of Pumilio, Nanos, and Brain Tumor on gene expression
With our new understanding of combinatorial control by Brat, Nos and Pum, it is now possible to survey their potential impact on the transcriptome (and thus proteome), both individually and collaboratively. Here we integrate experimental and transcriptome-wide predictions, revealing broad potential impact on gene expression. Target mRNAs fall into several categories (Fig. 3) including those individually targeted by Brat or Pum, jointly targeted by Brat and Pum, jointly targeted by Nos and Pum, and combinatorially controlled by all 3 RBPs.
Figure 3.
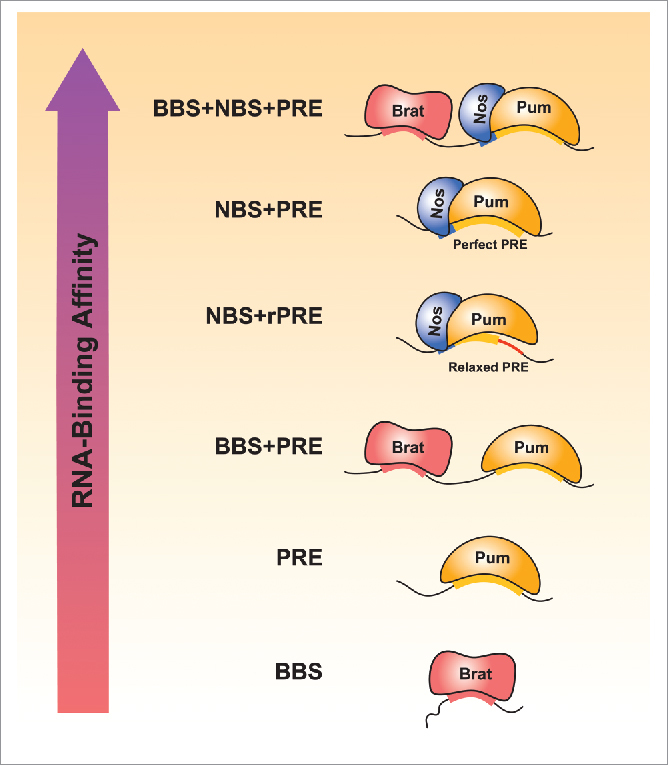
Classification of Pum, Nos, and Brat target mRNAs. Classification of target mRNAs regulated by Pum, Nos, and/or Brat, based on experimental evidence and bioinformatics analysis of Drosophila 3´UTRs using RNA-binding affinity, specificity, and cooperativity. Brat Binding Site (BBS), Pum Response Element (PRE), relaxed PRE (rPRE), and Nos Binding Site (NBS), described in the text, are indicated for each of 6 categories.
To begin, we integrated experimental evidence from several transcriptome-wide studies that used RNA-protein coimmunoprecipitation with microarray (i.e. RIP-Chip) to identify mRNAs bound by Pum and Brat. Unfortunately, no such data set exists for Nos. Gerber et al. identified mRNAs enriched by epitope-tagged Pum-HD purified from embryos or adult ovaries,23 and Laver et al. performed RIP-Chip of endogenous Pum from early embryos.29 Together, 1163 Pum-associated mRNAs were reported; of these, 679 have a consensus PRE. Laver et al. also identified mRNAs that copurified with endogenous Brat from early embryos, and Loedige et al. identified mRNAs enriched by epitope-tagged Brat purified from late-stage embryos.29,56 When combined, 3601 mRNAs were associated with Brat, with 605 shared between data sets, and 3117 mRNAs contain a BBS. Together, this evidence indicates widespread targeting of mRNAs by Pum or Brat, a conclusion that is bolstered by the fact that Pum and Brat repress translation and promote decay of many mRNAs during embryogenesis.29
Comparison of the Pum- and Brat-bound mRNAs reveals an overlap of 484 mRNAs, indicating the potential coregulation of many mRNAs. Functional assays lend support for dual regulation of several mRNAs by Brat and Pum.29,55,56 For example, the dMyc mRNA, which contains 6 PREs and 46 BBS motifs, is repressed by Pum and Brat in the differentiating cystoblast and in cellular repression assays, where Nos is absent.56,80 However, since the majority of targets identified for each regulator did not overlap, Loedige et al and Laver et al (2015) concluded that Brat and Pum individually regulate most of their respective target mRNAs. It is noteworthy that these analyses focused on the early embryo and adult female germline and likely miss targets in different tissues and life stages, such as the nervous system, where Pum and Brat have documented roles.13,15,39,48,50-52,81-86
To survey the genome-wide regulatory potential of each RBP, we searched the 3´UTRs of all Drosophila mRNAs for potential binding sites. The results are summarized in Table S1, including number and location of each predicted binding site in annotated 3´UTRs isoforms (the numbers of sites cited in the text pertain to the longest isoform of unique genes). The PRE consensus 5´-UGUANAUA was derived from multiple approaches, including SEQRS, RNAcompete, and RIP-Chip, and validated by EMSA and structural analyses.3,19,23,25,29,87 In total, 2477 mRNAs possess one or more PREs in their 3´UTR, including many potential targets with interesting biological implications. The mRNA encoding the antiproliferative protein, Tob, contains the highest number of PREs, with 12 found in its 3´UTR. The second and third highest number of PREs were in the cpx mRNA (11 PREs), encoding a protein involved in synaptic transmission, and eag mRNA (9 PREs), encoding a voltage gated potassium ion channel that controls neuronal excitability.
We used the consensus BBS, 5´-WYGUUD, derived from RIP-Chip and RNAcompete analyses and supported by EMSA and structural analyses,29,55,56 and found that 9018 mRNAs contain at least one BBS. Note that we opted to include a C at nucleotide position 2 of the BBS search motif to be inclusive of the functional BBS of hunchback NRE1, which contributes to regulation55 in vivo, though it is reported to be lower affinity relative to the BBS in NRE2.55 Of all mRNAs containing BBS motifs, the mei-P26 mRNA, which encodes another TRIM-NHL tumor suppressor involved in germline differentiation, contains the most at 76 potential sites. Brat mRNA contains the second highest number with 70 BBS motifs, supporting the potential for autoregulation, as suggested by Laver et al, 2015. Interestingly, the smooth mRNA, encoding a regulator of axon guidance, has 66 BBS sites.
A consensus binding site has not been found for Nos alone, despite our attempts.19 Instead, Nos requires Pum for specific binding to RNA, and we identified a consensus NBS, encompassing 4 nucleotides upstream of the PRE, using SEQRS and corroborated by EMSA, Nos-Pum-RNA structures, and functional assays.19 Nos binding to the NBS enhances binding of Pum to “perfect” consensus PREs, 5’-DDWWUGUANAUA (NBS+PRE), including those in the hunchback NREs. Some 1077 mRNAs have a 3´UTR with a NBS+PRE, with the cpx (8 motifs), tob (7 motifs), and kruppel (5 motifs) mRNAs containing the highest number of these motifs (Table S1).
Additionally, Nos enables Pum binding to “relaxed” PRE sites (rPRE), wherein nucleotides in position 5-8 of the PRE do not match the consensus (NBS+rPRE: 5’-DDWWUGUA).19 This category includes Nos-Pum targets bicoid and Cyclin B and was validated by SEQRS, EMSA, a Nos-Pum-Cyclin B RNA crystal structure and cellular repression assays.16,19,31,42,88 NBS+rPREs are present in 6225 mRNAs (Table S1). The mei-P26 mRNA contains 47 such motifs in its 3´UTR, consistent with its CNOT-dependent repression by Nos and Pum in germline stem cells.46 The smooth mRNA has the second most NBS+rPREs (41 motifs), suggesting a possible relationship of Pum and Nos to axon guidance. The longest brat mRNA isoform has 36 NBS+rPRE motifs (and no perfect PREs), which likely underlie the ability of Nos and Pum to repress its translation in germline stem cells.80 We also analyzed the Pum-bound target mRNAs for the presence of these motifs and found significant enrichment: 56% have a PRE, 79% have NBS+rPRE and 28% have NBS+PRE (p values < 0.002) relative to 19%, 52% and 8%, respectively, for all 3´UTRs. Based on these data, we predict that Nos expands the regulatory potential of Pum. In summary, our global target predictions suggest that regulation by Pum, Nos and Brat is pervasive.
We next asked how many transcripts may be combinatorially regulated by Brat and Pum and found that 2124 mRNAs possess both BBS and PRE motifs. In only 182 of these mRNAs, a BBS is located 1-13 nucleotides upstream of a PRE (BBSCPRE) (Table S1), the range of separation found in verified targets that are jointly regulated by Brat and Pum. We also assessed how many mRNAs possess binding sites for Brat, Nos and Pum and found that 1858 mRNAs possess at least one binding site for each protein within the 3´UTR (Table S1). The paralytic (para) mRNA, a known target of Brat, Nos and Pum belongs to this category.39,89 Para encodes a sodium ion channel that functions in the larval motor neurons and its longest 3´UTR has one PRE, 26 NBS+rPRE sites and 19 BBS motifs. In fact, many of the predicted targets have the potential to be combinatorially regulated: nearly 81% of 3´UTRs with PRE or NBS+rPRE/PRE sites also possess BBS motifs, and reciprocally, 66% of 3´UTRs with BBS motifs also contain a PRE or NBS+rPRE/PRE. However, if we restrict the distance between the BBS and PRE motifs to <13 nucleotides, as is the case in the hunchback NREs, only 24% of 3´UTRs with PRE or NBS+rPRE/PRE sites also possess an upstream BBS motif and only 19% of 3´UTRs with BBS motifs also contain a downstream PRE or NBS+rPRE/PRE. Together, these results indicate that collaborative regulation of many mRNAs is possible, but the extent depends on the importance of proximity of the RBP binding sites. Interestingly, only 63 mRNAs have a BBS located upstream (< 13 nucleotides between BBS and PRE) of a NBS+PRE (a perfect NRE), and, most surprisingly, the only target with more than one such perfect NRE motif is hunchback, perhaps making it the most sensitive to Nos-Pum-Brat cooperative regulation (Table S1). The other genes in the perfect NRE category are enriched for gene ontologies of signal transduction (such as tolloid, rhomboid and Ric) and transcription (such as knirps, sex combs reduced, clock, and drop). Indeed, knirps mRNA is bound and repressed by Pum and Brat.29,56 The tolloid mRNA has 3 BBS motifs in the context of a perfect NRE and encodes a metalloprotease that promotes Decapentaplegic (Dpp) signaling, which controls dorsal embryonic development and germline stem cell maintenance.90-92 In addition, the pum mRNA contains a perfect NRE, supported by binding data, suggesting a means of feedback to regulate the regulator.23,29 Overall, these results suggest that combinatorial regulation by Pum, Nos and Brat could impact many transcripts and biological processes, but functional analysis is essential to determine if cooperative RNA binding and synergistic repression are widespread.
These binding site predictions are informative and can stimulate future investigations, but have limitations that are important to acknowledge. Regulation will be affected by parameters that we cannot yet integrate, including the level, timing, and cell type expression of each RBP in vivo. Nos is a prime example. Nos is predominantly expressed in the adult ovary and early embryo,93 though it also has documented roles in neurons.13,39,82,94 Regulation of Cyclin B mRNA provides an example of cell type specific regulation.31 Nos and Pum repress Cyclin B in primordial germ cells, which have a high concentration of Nos. The Cyclin B 3´UTR has no perfect PRE, but instead it possesses 5 NBS+rPRE motifs that confer regulation. Cyclin B also has 7 BBS motifs, but since Brat is absent in primordial germ cells, they are irrelevant for regulation in this cell type. Mei-P26 mRNA is another example of cell-type specific regulation.46 As noted above, its 3´UTR contains both perfect PREs and many NBS+rPREs, and it is repressed by Pum and Nos in germline stem cells. Despite its many BBS elements, mei-P26 mRNA is not likely affected by Brat in germline stem cells, as brat mRNA itself is repressed in this cell type by Nos and Pum via multiple NBS+rPREs.80 The expression pattern of the predicted targets will also determine whether they are regulated by Brat, Pum, and/or Nos, dictated by coincidence of target and regulator expression. In the example of hunchback, its mRNA is most highly expressed in early embryo and adult female ovary, coincident with high expression of Pum, Nos and Brat.65,66,68,73,93
The effect of the number, affinity, location, and spacing of each binding site is also not fully known. For our survey, we required that at least one binding site is present in the putative target. Indeed, for Nos and Pum, reporter assays indicate that one PRE or NBS+rPRE is sufficient to confer repression, and increased number and affinity of binding sites correlates with stronger repression.19 For Brat, one binding motif can by recognized by the protein, but 2 BBS motifs in the same RNA were bound more tightly.55 Moreover, in cellular repression assays, multiple BBS motifs conferred regulation by Brat, with 2 motifs being the minimum tested.29,55 The relative orientation of the binding sites is also likely relevant. For Nos-Pum targets, the NBS must be directly upstream of the PRE.19 For Brat-Pum targets, we allowed up to 13 nucleotides of separation between BBS and PRE, a parameter that is consistent with validated targets. The impact of the proximity of the BBS to an NBS or PRE is not known, although it is likely to affect collaboration. Based on cellular repression assays with reporter mRNAs, other spacing and arrangements of BBS and PRE motifs may be permissible for Brat or Pum-mediated repression,56 but no data are available regarding cooperative RNA binding or synergistic repression.
RNA structure is likely to influence accessibility of the predicted binding sites, but how this parameter affects binding and regulation by Pum, Nos and Brat remains uncertain. Since each RBP binds a single-stranded RNA motif, structure may occlude or reduce binding affinity; however, evidence indicates that mammalian Pum proteins can disrupt double-stranded RNA to gain access to a PRE.95,96 RNA binding by the Brat NHL domain was reduced when the BBS motif was within a stem loop of an RNA ligand that also contained a PRE, and addition of Pum strengthened binding, perhaps promoted by Pum's ability to disrupt RNA structure.56 The ability of Nos and Pum to bind structured RNA cooperatively remains untested. Because of these remaining questions and lack of information on the effect of RNA structure on regulation in vivo, and the difficulties of accurately predicting RNA structure, we did not incorporate RNA structure predictions into our analysis at this time. Future research should address the relationship of RNA structure to binding and regulation of RNAs by Nos, Pum and Brat.
Alternative 3´ end processing of mRNAs could impact regulation by Brat, Nos and Pum in cases where their binding sites are altered. The hid mRNA, which is regulated by Nos and Pum in neurons,94 is an example where 2 mRNA isoforms are produced by alternative 3´ end processing (Table S1): the hid-RA mRNA has a long 3´UTR with multiple NBS+PREs that is regulated by Nos-Pum, whereas these sites are eliminated in the shorter hid-RB version. Alternative processing of para mRNA produces 3 3´UTR isoforms: the longest has one perfect PRE, 26 NBS+rPRE motifs and 19 BBS motifs; the medium length isoform has multiple NBS+rPREs but no PRE; and the short isoform lacks these sites altogether. Intriguingly, regulation of para depends on Brat in certain neuronal subtypes but not others, perhaps the result of alternative processing of the 3´UTR or on differential expression of Brat.39 In the case of hunchback mRNA, alternative 3´ end processing is developmentally regulated to produce 2 mRNA isoforms: a long 3´UTR present on the zygotically-expressed hunchback-RA mRNA and a short 3´UTR on the maternally-provided hunchback-RB isoform in early embryos. Importantly, each isoform contains both NRE elements.
Conclusion
With the revelation of many uncharacterized RBPs,1-4 future studies are necessary to analyze their individual regulatory activities, RNA-binding specificities and target mRNAs. However, as exemplified by Pum, Nos and Brat, to succeed in understanding post-transcriptional regulatory networks, it is imperative to address combinatorial control. Control by the many more uncharacterized RBPs will likely involve cooperative RNA binding, altered specificity, and the interplay of multiple regulatory mechanisms that contribute to synergistic regulation or even bifunctional switches.35,97 We have learned a great deal about the functions of Pum, Nos and Brat mediated regulation, but important challenges remain, including identification of combinatorially regulated mRNAs on a global scale, comprehensive dissection of the protein interaction network between the trio of RBPs and their corepressors, and interrogation of the multiple repression mechanisms in vivo. Future work should also extend the paradigms of Drosophila Pum, Nos and Brat to investigate the targets, RNA and protein interactions, and regulatory mechanisms of their mammalian homologs. Ultimately these efforts should uncover more of the underlying code of combinatorial regulation by RBPs.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by grant R01GM105707 from the National Institute of General Medical Sciences, National Institutes of Health (ACG), Research Scholar Grant from RSG-13–080–01-RMC from the American Cancer Society (ACG), and the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (TMTH). René M. Arvola is supported by graduate research fellowship DGE 1256260 from the National Science Foundation. We sincerely apologize to colleagues whose work we were unable to include due to space limitations.
References
- 1.Baltz AG, Munschauer M, Schwanhäusser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al.. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell 2012; 46(5):674-90; PMID:22681889; https://doi.org/22658674 10.1016/j.molcel.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 2.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, et al.. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 2012; 149(6):1393-406; PMID:22658674; https://doi.org/ 10.1016/j.cell.2012.04.031 [DOI] [PubMed] [Google Scholar]
- 3.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet 2014; 15(12):829-45; PMID:25365966; https://doi.org/23912277 10.1038/nrg3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon SC, Yi H, Eichelbaum K, Föhr S, Fischer B, You KT, Castello A, Krijgsveld J, Hentze MW, Kim VN, et al.. The RNA-binding protein repertoire of embryonic stem cells. Nat Struct Mol Biol 2013; 20(9):1122-30; PMID:23912277; https://doi.org/ 10.1038/nsmb.2638 [DOI] [PubMed] [Google Scholar]
- 5.Sonoda J, Wharton RP. Drosophila Brain Tumor is a translational repressor. Genes Dev 2001; 15(6):762-73; PMID:11274060; https://doi.org/11336677 10.1101/gad.870801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards TA, Pyle SE, Wharton RP, Aggarwal AK. Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell 2001; 105(2):281-9; PMID:11336677; https://doi.org/ 10.1016/S0092-8674(01)00318-X [DOI] [PubMed] [Google Scholar]
- 7.Edwards TA, Wilkinson BD, Wharton RP, Aggarwal AK. Model of the brain tumor-Pumilio translation repressor complex. Genes Dev 2003; 17(20):2508-13; PMID:14561773; https://doi.org/ 10.1101/gad.1119403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet 2002; 18(3):150-7; PMID:11858839; https://doi.org/ 10.1016/S0168-9525(01)02616-6 [DOI] [PubMed] [Google Scholar]
- 9.Lehmann R, Nusslein-Volhard C. Involvement of the pumilio gene in the transport of an abdominal signal in the Drosophila embyro. Nature 1987; 329:167-170; https://doi.org/ 10.1038/329167a0 [DOI] [Google Scholar]
- 10.Nusslein-Volhard C, Frohnhofer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science 1987; 238(4834):1675-81; PMID:3686007; https://doi.org/14972682 10.1126/science.3686007 [DOI] [PubMed] [Google Scholar]
- 11.Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 1998; 125(4):679-90; PMID:943528814972682 [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 1997; 124(12):2463-76; PMID:919937214972682 [DOI] [PubMed] [Google Scholar]
- 13.Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol 2004; 14(4):314-21; PMID:14972682; https://doi.org/ 10.1016/j.cub.2004.01.052 [DOI] [PubMed] [Google Scholar]
- 14.Schweers BA, Walters KJ, Stern M. The Drosophila melanogaster translational repressor pumilio regulates neuronal excitability. Genetics 2002; 161(3):1177-85; PMID:12136020 12593794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al.. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol 2003; 13(4):286-96; PMID:12593794; https://doi.org/ 10.1016/S0960-9822(03)00064-2 [DOI] [PubMed] [Google Scholar]
- 16.Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol 1999; 1(7):431-7; PMID:10559987; https://doi.org/ 10.1038/15666 [DOI] [PubMed] [Google Scholar]
- 17.Edwards TA, Trincao J, Escalante CR, Wharton RP, Aggarwal AK. Crystallization and characterization of Pumilo: a novel RNA binding protein. J Struct Biol 2000; 132(3):251-4; PMID:11303521; http://doi.org/ 10.1006/jsbi.2000.4319 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, McLachlan J, Zamore PD, Hall TM. Modular recognition of RNA by a human pumilio-homology domain. Cell 2002; 110(4):501-12; PMID:12202039; https://doi.org/ 10.1016/S0092-8674(02)00873-5 [DOI] [PubMed] [Google Scholar]
- 19.Weidmann CA, Qiu C, Arvola RM, Lou TF, Killingsworth J, Campbell ZT, Tanaka Hall TM, Goldstrohm AC. Drosophila Nanos acts as a molecular clamp that modulates the RNA-binding and repression activities of Pumilio. Elife 2016; Aug 2; 5:pii:e17096; PMID:27482653; https://doi.org/ 10.7554/eLife.17096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wharton RP, Sonoda J, Lee T, Patterson M, Murata Y. The Pumilio RNA-binding domain is also a translational regulator. Mol Cell 1998; 1(6):863-72; PMID:9660969; https://doi.org/ 10.1016/S1097-2765(00)80085-4 [DOI] [PubMed] [Google Scholar]
- 21.Zamore PD, Bartel DP, Lehmann R, Williamson JR. The PUMILIO-RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry 1999; 38(2):596-604; PMID:9888799; https://doi.org/11336708 10.1021/bi982264s [DOI] [PubMed] [Google Scholar]
- 22.Zamore PD, Williamson JR, Lehmann R. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. Rna 1997; 3(12):1421-33; PMID:940489311336708 [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber AP, Luschnig S, Krasnow MA, Brown PO, Herschlag D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc Natl Acad Sci U S A 2006; 103(12):4487-92; PMID:16537387; https://doi.org/11336708 10.1073/pnas.0509260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall TM. De-coding and re-coding RNA recognition by PUF and PPR repeat proteins. Curr Opin Struct Biol 2016; 36:116-21; PMID:26874972; https://doi.org/11336708 10.1016/j.sbi.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zamore PD, Hall TM. Crystal structure of a Pumilio homology domain. Mol Cell 2001; 7(4):855-65; PMID:11336708; https://doi.org/ 10.1016/S1097-2765(01)00229-5 [DOI] [PubMed] [Google Scholar]
- 26.Hall TM. Expanding the RNA-recognition code of PUF proteins. Nat Struct Mol Biol 2014; 21(8):653-5; PMID:25093524; https://doi.org/24942623 10.1038/nsmb.2863 [DOI] [PubMed] [Google Scholar]
- 27.Weidmann CA, Goldstrohm AC. Drosophila Pumilio protein contains multiple autonomous repression domains that regulate mRNAs independently of Nanos and brain tumor. Mol Cell Biol 2012; 32(2):527-40; PMID:22064486; https://doi.org/24942623 10.1128/MCB.06052-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidmann CA, Raynard NA, Blewett NH, Van Etten J, Goldstrohm AC. The RNA binding domain of Pumilio antagonizes poly-adenosine binding protein and accelerates deadenylation. RNA 2014; 20(8):1298-319; PMID:24942623; https://doi.org/ 10.1261/rna.046029.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laver JD, Li X, Ray D, Cook KB, Hahn NA, Nabeel-Shah S, Kekis M, H1 Luo, AJ12 Marsolais, Fung KY, et al.. Brain tumor is a sequence-specific RNA-binding protein that directs maternal mRNA clearance during the Drosophila maternal-to-zygotic transition. Genome Biol 2015; 16:94; PMID:25962635; https://doi.org/ 10.1186/s13059-015-0659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem 2012; 287(43):36370-83; PMID:22955276; https://doi.org/ 10.1074/jbc.M112.373522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadyrova LY, Habara Y, Lee TH, Wharton RP. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 2007; 134(8):1519-27; PMID:17360772; https://doi.org/ 10.1242/dev.002212 [DOI] [PubMed] [Google Scholar]
- 32.Goldstrohm AC, Hook BA, Seay DJ, Wickens M. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol 2006; 13(6):533-9; PMID:16715093; https://doi.org/ 10.1038/nsmb1100 [DOI] [PubMed] [Google Scholar]
- 33.Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 2008; 9(4):337-44; PMID:18334997; https://doi.org/19221201 10.1038/nrm2370 [DOI] [PubMed] [Google Scholar]
- 34.Temme C, Simonelig M, Wahle E. Deadenylation of mRNA by the CCR4-NOT complex in Drosophila: molecular and developmental aspects. Front Genet 2014; 5:143; PMID:24904643; https://doi.org/19221201 10.3389/fgene.2014.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J. FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 2009; 181(4):1249-60; PMID:19221201; https://doi.org/ 10.1534/genetics.108.099440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chritton JJ, Wickens M. A role for the poly(A)-binding protein Pab1p in PUF protein-mediated repression. J Biol Chem 2011; 286(38):33268-78; PMID:21768112; https://doi.org/11562474 10.1074/jbc.M111.264572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chagnovich D, Lehmann R. Poly(A)-independent regulation of maternal hunchback translation in the Drosophila embryo. Proc Natl Acad Sci U S A 2001; 98(20):11359-64; PMID:11562474; https://doi.org/ 10.1073/pnas.201284398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann R, Nusslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development 1991; 112(3):679-91; PMID:193568418305244 [DOI] [PubMed] [Google Scholar]
- 39.Muraro NI, Weston AJ, Gerber AP, Luschnig S, Moffat KG, Baines RA. Pumilio binds para mRNA and requires Nanos and Brat to regulate sodium current in Drosophila motoneurons. J Neurosci 2008; 28(9):2099-109; PMID:18305244; https://doi.org/ 10.1523/JNEUROSCI.5092-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis D, Treiber DK, Tao F, Zamore PD, Williamson JR, Lehmann R. A CCHC metal-binding domain in Nanos is essential for translational regulation. Embo J 1997; 16(4):834-43; PMID:9049312; https://doi.org/ 10.1093/emboj/16.4.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto H., Hara K, Hishiki A, Kawaguchi S, Shichijo N, Nakamura K, Unzai S, Tamaru Y, Shimizu T, Sato M. Crystal structure of zinc-finger domain of Nanos and its functional implications. EMBO Rep 2010; 11(11):848-53; PMID:20948543; https://doi.org/ 10.1038/embor.2010.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wharton RP, Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell 1991; 67(5):955-67; PMID:1720354; http://doi.org/9247343 10.1016/0092-8674(91)90368-9 [DOI] [PubMed] [Google Scholar]
- 43.Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development 1997; 124(15):3015-23; PMID:9247343 [DOI] [PubMed] [Google Scholar]
- 44.Raisch T, Bhandari D, Sabath K, Helms S, Valkov E, Weichenrieder O, Izaurralde E. Distinct modes of recruitment of the CCR4-NOT complex by Drosophila and vertebrate Nanos. EMBO J 2016; 35(9):974-90; PMID:26968986; https://doi.org/ 10.15252/embj.201593634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhandari D, Raisch T, Weichenrieder O, Jonas S, Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4-NOT complex and translational repression. Genes Dev 2014; 28(8):888-901; PMID:24736845; https://doi.org/ 10.1101/gad.237289.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joly W, Chartier A, Rojas-Rios P, Busseau I, Simonelig M. The CCR4 deadenylase acts with Nanos and Pumilio in the fine-tuning of Mei-P26 expression to promote germline stem cell self-renewal. Stem Cell Reports 2013; 1(5):411-24; PMID:24286029; https://doi.org/ 10.1016/j.stemcr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics 1991; 129(4):1119-36; PMID:178329510949924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arama E, Dickman D, Kimchie Z, Shearn A, Lev Z. Mutations in the beta-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene 2000; 19(33):3706-16; PMID:10949924; https://doi.org/ 10.1038/sj.onc.1203706 [DOI] [PubMed] [Google Scholar]
- 49.Woodhouse E, Hersperger E, Shearn A. Growth, metastasis, and invasiveness of Drosophila tumors caused by mutations in specific tumor suppressor genes. Dev Genes Evol 1998; 207(8):542-50; PMID:951054916549393 [DOI] [PubMed] [Google Scholar]
- 50.Lee CY, Wilkinson BD, Siegrist SE, Wharton RP, Doe CQ. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev Cell 2006; 10(4):441-9; PMID:16549393; https://doi.org/ 10.1016/j.devcel.2006.01.017 [DOI] [PubMed] [Google Scholar]
- 51.Shi W, Chen Y, Gan G, Wang D, Ren J, Wang Q, Xu Z, Xie W, Zhang YQ. Brain tumor regulates neuromuscular synapse growth and endocytosis in Drosophila by suppressing mad expression. J Neurosci 2013; 33(30):12352-63; PMID:23884941 DOI: 10.1523/JNEUROSCI.0386-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchetti G, Reichardt I, Knoblich JA, Besse F. The TRIM-NHL protein Brat promotes axon maintenance by repressing src64B expression. J Neurosci 2014; 34(41):13855-64; PMID:25297111; https://doi.org/ 10.1523/JNEUROSCI.3285-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho PF, Gamberi C, Cho-Park YA, Cho-Park IB, Lasko P, Sonenberg N. Cap-dependent translational inhibition establishes two opposing morphogen gradients in Drosophila embryos. Curr Biol 2006; 16(20):2035-41; PMID:17055983; https://doi.org/ 10.1016/j.cub.2006.08.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tocchini C, Ciosk R. TRIM-NHL proteins in development and disease. Semin Cell Dev Biol 2015; 47-48:52-9; PMID:26514622; https://doi.org/24696456 10.1016/j.semcdb.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 55.Loedige I., Stotz M, Qamar S, Kramer K, Hennig J, Schubert T, Löffler P, Längst G, Merkl R, Urlaub H, et al.. The NHL domain of BRAT is an RNA-binding domain that directly contacts the hunchback mRNA for regulation. Genes Dev 2014; 28(7):749-64; PMID:24696456; https://doi.org/ 10.1101/gad.236513.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loedige I, Jakob L, Treiber T, Ray D, Stotz M, Treiber N, Hennig J, Cook KB, Morris Q, Hughes TR, et al.. The Crystal Structure of the NHL Domain in Complex with RNA Reveals the Molecular Basis of Drosophila Brain-Tumor-Mediated Gene Regulation. Cell Rep 2015; 13(6):1206-20; PMID:26527002; https://doi.org/ 10.1016/j.celrep.2015.09.068 [DOI] [PubMed] [Google Scholar]
- 57.Laver JD, Marsolais AJ, Smibert CA, Lipshitz HD. Regulation and Function of Maternal Gene Products During the Maternal-to-Zygotic Transition in Drosophila. Curr Top Dev Biol 2015; 113:43-84; PMID:26358870; https://doi.org/ 10.1016/bs.ctdb.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 58.Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development 2009; 136(18):3033-42; PMID:19700615; https://doi.org/20504953 10.1242/dev.033183 [DOI] [PubMed] [Google Scholar]
- 59.Temme C, Zhang L, Kremmer E, Ihling C, Chartier A, Sinz A, Simonelig M, Wahle E. Subunits of the Drosophila CCR4-NOT complex and their roles in mRNA deadenylation. Rna 2010; 16(7):1356-70; PMID:20504953; https://doi.org/ 10.1261/rna.2145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tautz D. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature 1988; 332(6161):281-4; PMID:2450283; https://doi.org/1568245 10.1038/332281a0 [DOI] [PubMed] [Google Scholar]
- 61.Hulskamp M, Pfeifle C, Tautz D. A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Kruppel and knirps in the early Drosophila embryo. Nature 1990; 346(6284):577-80; PMID:2377231; https://doi.org/1568245 10.1038/346577a0 [DOI] [PubMed] [Google Scholar]
- 62.Irish V, Lehmann R, Akam M. The Drosophila posterior-group gene nanos functions by repressing hunchback activity. Nature 1989; 338(6217):646-8; PMID:2704419; https://doi.org/1568245 10.1038/338646a0 [DOI] [PubMed] [Google Scholar]
- 63.Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell 1992; 69(2):237-249; PMID:1568245; http://doi.org/ 10.1016/0092-8674(92)90405-2 [DOI] [PubMed] [Google Scholar]
- 64.Lehmann R, Nusslein-Volhard C. hunchback, a gene required for segmentation of an anterior and posterior region of the Drosophila embryo. Dev Biol 1987; 119(2):402-17; PMID:3803711; http://doi.org/26027925 10.1016/0012-1606(87)90045-5 [DOI] [PubMed] [Google Scholar]
- 65.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 1989; 98(2):81-5; PMID:2476281; https://doi.org/26027925 10.1007/BF00291041 [DOI] [PubMed] [Google Scholar]
- 66.Barker DD, Wang C, Moore J, Dickinson LK, Lehmann R. Pumilio is essential for function but not for distribution of the Drosophila abdominal determinant Nanos. Genes Dev 1992; 6(12A):2312-26; PMID:1459455; https://doi.org/26027925 10.1101/gad.6.12a.2312 [DOI] [PubMed] [Google Scholar]
- 67.Hulskamp M, Schröder C, Pfeifle C, Jäckle H, Tautz D. Posterior segmentation of the Drosophila embryo in the absence of a maternal posterior organizer gene. Nature 1989; 338(6217):629-32; PMID:2704418; https://doi.org/26027925 10.1038/338629a0 [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Lehmann R. Nanos is the localized posterior determinant in Drosophila. Cell 1991; 66(4):637-47; PMID:1908748; http://doi.org/26027925 10.1016/0092-8674(91)90110-K [DOI] [PubMed] [Google Scholar]
- 69.Zaessinger S, Busseau I, Simonelig M. Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 2006; 133(22):4573-83; PMID:17050620; https://doi.org/26027925 10.1242/dev.02649 [DOI] [PubMed] [Google Scholar]
- 70.Gavis ER, Lehmann R. Translational regulation of nanos by RNA localization. Nature 1994; 369(6478):315-8; PMID:7514276; https://doi.org/26027925 10.1038/369315a0 [DOI] [PubMed] [Google Scholar]
- 71.Smibert CA, Wilson JE, Kerr K, Macdonald PM. smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes Dev 1996; 10(20):2600-9; PMID:8895661; https://doi.org/26027925 10.1101/gad.10.20.2600 [DOI] [PubMed] [Google Scholar]
- 72.Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD, Smibert CA. Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila embryo. Curr Biol 2005; 15(4):284-94; PMID:15723788; https://doi.org/26027925 10.1016/j.cub.2005.01.048 [DOI] [PubMed] [Google Scholar]
- 73.Macdonald PM. The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development 1992; 114(1):221-32; PMID:157696226027925 [DOI] [PubMed] [Google Scholar]
- 74.Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 1995; 80(5):747-56; PMID:7889568; http://doi.org/26027925 10.1016/0092-8674(95)90353-4 [DOI] [PubMed] [Google Scholar]
- 75.Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev 1999; 13(20):2704-12; PMID:1054155626027925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lou TF, Weidmann CA, Killingsworth J, Tanaka Hall TM, Goldstrohm AC, Campbell ZT. Integrated analysis of RNA-binding protein complexes using in vitro selection and high-throughput sequencing and sequence specificity landscapes (SEQRS). Methods 2016; pii:S1046-2023(16)30338-3; PMID:27729296; https://doi.org/26027925 10.1016/j.ymeth.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waghray S, Williams C, Coon JJ, Wickens M. Xenopus CAF1 requires NOT1-mediated interaction with 4E-T to repress translation in vivo. RNA 2015; 21(7):1335-45; PMID:26015597; https://doi.org/26027925 10.1261/rna.051565.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishimura T, Padamsi Z, Fakim H, Milette S, Dunham WH, Gingras AC, Fabian MR. The eIF4E-Binding Protein 4E-T Is a Component of the mRNA Decay Machinery that Bridges the 5′ and 3′ Termini of Target mRNAs. Cell Rep 2015; 11(9):1425-36; PMID:26027925; https://doi.org/ 10.1016/j.celrep.2015.04.065 [DOI] [PubMed] [Google Scholar]
- 79.Ozgur S, Basquin J, Kamenska A, Filipowicz W, Standart N, Conti E. Structure of a Human 4E-T/DDX6/CNOT1 Complex Reveals the Different Interplay of DDX6-Binding Proteins with the CCR4-NOT complex. Cell Rep 2015; 13(4):703-11; PMID:26489469; https://doi.org/ 10.1016/j.celrep.2015.09.033 [DOI] [PubMed] [Google Scholar]
- 80.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bisTable Switch that restricts BMP signaling. Dev Cell 2011; 20(1):72-83; PMID:21238926; https://doi.org/ 10.1016/j.devcel.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G, Li W, Zhang QS, Regulski M, Sinha N, Barditch J, Tully T, Krainer AR, Zhang MQ, Dubnau J. Identification of synaptic targets of Drosophila pumilio. PLoS Comput Biol 2008; 4(2):e1000026; PMID:18463699; https://doi.org/ 10.1371/journal.pcbi.1000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon KP, Andrews S, Murthy M, Gavis ER, Zinn K. The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition. J Neurosci 2009; 29(17):5558-72; PMID:19403823; https://doi.org/ 10.1523/JNEUROSCI.0520-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menon KP, Sanyal S, Habara Y, Sanchez R, Wharton RP, Ramaswami M, Zinn K. The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E. Neuron 2004; 44(4):663-76; PMID:15541314; https://doi.org/ 10.1016/j.neuron.2004.10.028 [DOI] [PubMed] [Google Scholar]
- 84.Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 2006; 133(14):2639-48; PMID:16774999; https://doi.org/18342578 10.1242/dev.02429 [DOI] [PubMed] [Google Scholar]
- 85.Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 2006; 124(6):1241-53; PMID:16564014; https://doi.org/18342578 10.1016/j.cell.2006.01.038 [DOI] [PubMed] [Google Scholar]
- 86.Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell 2008; 14(4):535-46; PMID:18342578; https://doi.org/ 10.1016/j.devcel.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature 2013; 499(7457):172-7; PMID:23846655; https://doi.org/ 10.1038/nature12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gamberi C, Peterson DS, He L, Gottlieb E. An anterior function for the Drosophila posterior determinant Pumilio. Development 2002; 129(11):2699-710; PMID:12015297 [DOI] [PubMed] [Google Scholar]
- 89.Mee CJ, Pym EC, Moffat KG, Baines RA. Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci 2004; 24(40):8695-703; PMID:15470135; https://doi.org/ 10.1523/JNEUROSCI.2282-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harris RE, Ashe HL. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep 2011; 12(6):519-26; PMID:21546910; https://doi.org/ 10.1038/embor.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development 1992; 114(3):583-97; PMID:161813020858238 [DOI] [PubMed] [Google Scholar]
- 92.Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell 1992; 71(3):451-61; PMID:1423606; http://doi.org/20858238 10.1016/0092-8674(92)90514-D [DOI] [PubMed] [Google Scholar]
- 93.Thomsen S, Anders S, Janga SC, Huber W, Alonso CR. Genome-wide analysis of mRNA decay patterns during early Drosophila development. Genome Biol 2010; 11(9):R93; PMID:20858238; https://doi.org/ 10.1186/gb-2010-11-9-r93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhogal B, Plaza-Jennings A, Gavis ER. Nanos-mediated repression of hid protects larval sensory neurons after a global switch in sensitivity to apoptotic signals. Development 2016; 143(12):2147-59; PMID:27256879; https://doi.org/21572425 10.1242/dev.132415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filipovska A, Razif MF, Nygård KK, Rackham O. A universal code for RNA recognition by PUF proteins. Nat Chem Biol 2011; 7(7):425-7; PMID:21572425; https://doi.org/ 10.1038/nchembio.577 [DOI] [PubMed] [Google Scholar]
- 96.Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol 2010; 12(10):1014-20; PMID:20818387; https://doi.org/ 10.1038/ncb2105 [DOI] [PubMed] [Google Scholar]
- 97.Richter JD. CPEB: a life in translation. Trends Biochem Sci 2007; 32(6):279-85; PMID:17481902; https://doi.org/ 10.1016/j.tibs.2007.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


