Abstract
Purpose
The purpose of the study is to describe the role of human metapneumovirus (hMPV) infection in critical illness and acute respiratory distress syndrome (ARDS).
Materials and methods
We collected clinical and demographic information from a retrospective chart review, comparing patients with and without an intensive care unit (ICU) admission. Among patients admitted to the ICU, we assessed whether hMPV was “unlikely,” “possibly,” or “likely” the reason for ICU admission, based on a prespecified definition, and whether the patient met criteria for ARDS.
Results
We identified 128 hospitalized adults with hMPV infection. Forty hospitalized patients (31%) with hMPV infection required admission to the ICU. Among patients cared for in the ICU, hMPV was “possibly” the reason for ICU admission in 55% of patients and “likely” the reason in 38%. Forty-eight percent of ICU patients met criteria for ARDS. Although most patients admitted to the ICU had significant comorbidities or were immunosuppressed, 6 patients requiring ICU admission had more minor comorbidities and no underlying immunosuppression.
Conclusions
Although most patients hospitalized with hMPV had chronic cardiac or pulmonary disease, hMPV can also be associated serious respiratory illness and ARDS in adult patients without significant comorbidities or immunosuppression.
Keywords: Viral pneumonia, ARDS
1. Introduction
Human metapneumovirus (hMPV) was first identified in 2001, although serologic data indicate that it has been present in human populations as early as the 1950s. It is a member of the paramyxovirus family and genetically similar to respiratory syncytial virus (RSV) [1]. Human metapneumovirus infections typically occur between January and April, account for anywhere from 3 to 11% of respiratory tract infections [2–5], and are commonly unidentified triggers in asthma and chronic obstructive pulmonary disease (COPD) in adults [2–4,6–8]. Presenting symptoms are virtually indistinguishable from infection with RSV and include fever, cough, rhinorrhea, and wheezing [9]. Although severity of illness associated with acute hMPV infection varies considerably, some patients may manifest with severe respiratory illness requiring admission to an intensive care unit (ICU) and mechanical ventilation [4,9].
Studies characterizing patients with hMPV who develop severe illness have primarily been limited to children, whereas those characterizing adults are infrequent and have limited data [10,11]. The rate of ICU admission among hospitalized patients with hMPV in 1 study was 12%, with 11% of patients requiring ventilator support [4]. The existing literature consists of case reports and retrospective cohort studies and suggests that elderly individuals as well as those with significant comorbidities are at greatest risk for severe disease and even death secondary to hMPV infection [5,12,13]. This has shaped the prevailing wisdom among adult providers that only patients who are immunocompromised are at risk for serious illness due to hMPV infection.
We performed a large retrospective cohort study of adults hospitalized with an associated hMPV infection. We sought to better describe the association between hMPV infection, critical illness, and acute respiratory distress syndrome (ARDS) as well as characterize the comorbidities of adults with hMPV infection admitted to the ICU.
2. Methods
We identified all patients admitted to the University of Michigan Health System with laboratory-confirmed hMPV infection between January 2009 and May 2013. We defined a positive hMPV result as a positive reverse transcription–polymerase chain reaction (RT-PCR) or viral culture. Samples were obtained either in the emergency department or in the inpatient setting, although only the samples belonging to patients who required inpatient admission were included in the analysis. All diagnostic testing was performed within the University of Michigan Health System. For patients who had multiple positive hMPV results within a 30-day period, only the index result was counted.
Among adult patients with confirmed hMPV infection, we performed a retrospective review of their hospitalizations records, collecting demographic and comorbidity data, as well as details of the hospitalization. We defined a viral coinfection as the identification of influenza A or B or RSV by polymerase chain reaction or immunofluorescence assay from a nasopharyngeal swab, throat swab, or bronchoalveolar lavage during the same admission.
For all patients, we used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), codes associated with the hospitalization to evaluate the presence of chronic pulmonary disease, including cystic fibrosis (ICD 277), asthma (ICD 493), and COPD (ICD 490, 491, 492, 496), and heart disease including congestive heart failure (ICD 428) and pulmonary hypertension (ICD 416).
For the subset of patients admitted to the ICU, chart review was performed by 1 investigator (JH) to identify the presence of additional comorbidities that have the potential to be associated with a more severe illness. These included chronic respiratory insufficiency (neuromuscular disease and/or significant sleep apnea), immunocompromised state (active malignancy or previous bone marrow or solid organ transplant), end-stage renal disease requiring dialysis, and cirrhosis. We identified clinically relevant parameters on admission to the ICU including temperature, respiratory rate, applied fraction of inspired oxygen, partial pressure of arterial oxygen, white blood cell count, and duration of mechanical ventilation. Some patients, not all, had blood and/or sputum cultures performed. Of those patients, only a subset had a satisfactory sputum specimen (positive Gram stain as well as the presence of polymononuclear cells with minimal epithelial cells). Patients who had either a positive blood culture or a positive sputum culture from a satisfactory specimen were identified as having bacterial coinfection. This infection was identified as a ventilator-associated pneumonia if it occurred within 72 hours after intubation.
We defined whether hMPV was the reason for ICU admission in a prespecified manner: “Unlikely”—the patient was admitted to the ICU for a non-respiratory reason. “Possible”—the patient was admitted for respiratory failure. Patients with bacterial super infections were placed in this category, as were patients who did not have any sputum cultures performed. “Likely”—the patient was admitted for hypoxic respiratory failure, not explained by cardiogenic pulmonary edema, and bacterial cultures were sent on admission from sputum or bronchoalveolar lavage and were negative.
Two investigators (JH and MS) independently reviewed all charts of patients admitted to the ICU to determine whether hMPV was the reason for ICU admission and whether patients met criteria for ARDS using the Berlin definition [14]. Cases in which the reviewers’ findings were discordant were discussed at greater length until a consensus determination was reached.
2.1. Statistical analysis
Continuous variables were compared by t test or Wilcoxon rank sum test as appropriate. Categorical variables were compared by the Fisher exact test. All data analysis was performed using Stata version 13.0 (StataCorp, College Station, TX). The institutional review board at the University of Michigan approved this study (HUM00077714).
3. Results
3.1. Epidemiology
We identified 335 cases of hMPV by either RT-PCR or viral culture between December 2009 and May 2013 among adult and pediatric patients (Fig. 1). Most hMPV-positive results were detected via RT-PCR on a specimen obtained via nasopharyngeal or throat swab or, less commonly, bronchoalveolar lavage. A minority of the positive results (13) were detected via viral culture of throat specimens. Of these, 245 results occurred within 30 days of a hospital admission.
Fig. 1.
Flow diagram of selection of eligible subjects for the study between January 2009 and May 2013.
The number of hMPV infections varied considerably from year to year (Fig. 2). Most cases occurred in late winter/early spring, with counts peaking between February and March; however, positive hMPV results were reported in the summer and fall months as well. After excluding patients younger than 18 years, there were 128 adult patients with a positive hMPV result included in the primary analysis.
Fig. 2.
Cases identified during study period (adult and pediatric, associated with hospitalization, n = 245), by year (A) and month (B).
3.2. Patient characteristics
We compare the characteristics of hospitalized patients with and without ICU admission in Table 1. The mean age of hospitalized adults was 55 years, and 30% of patients required admission to the ICU. Roughly half of all hospitalized patients had chronic lung disease, whereas 40% had underlying cardiac disease. Studied comorbidities were not statistically different between the ICU and non-ICU cohorts (P > .05 for all). There was 1 patient who had coinfection with influenza. Ten patients died during hospitalization; as expected, the mortality rate of patients admitted to the ICU was significantly higher than those cared for on the floor (18% vs 3%; P = .01).
Table 1.
Characteristics of patients admitted positive for hMPV
| All | Non-ICU | ICU | P | |
|---|---|---|---|---|
| No. of patients | 128 | 88 | 40 | |
| Age | ||||
| Mean, y (SD) | 54.9 (19.1) | 55.4 (18.9) | 54.0 (20.0) | |
| Race | ||||
| White | 102 (81%) | 74 (86%) | 28 (70%) | |
| African American | 14 (11%) | 6 (7%) | 8 (20%) | |
| Asian | 4 (3%) | 3 (4%) | 1 (2%) | |
| American Indian | 1 (2%) | 0 (0%) | 1 (1%) | |
| Unknown | 4 (3%) | 2 (2%) | 2 (5%) | |
| Coinfection | ||||
| Influenza | 1 (1%) | 1 (1%) | 0 (0%) | 1.00 |
| Comorbidities | ||||
| COPD | 44 (34%) | 29 (33%) | 15 (38%) | .69 |
| Cystic fibrosis | 12 (9%) | 10 (11%) | 2 (5%) | .34 |
| Asthma | 32 (25%) | 22 (25%) | 10 (25%) | 1.00 |
| Lung disease | 69 (54%) | 47 (53%) | 22 (55%) | 1.00 |
| CHF | 43 (34%) | 26 (30%) | 17 (43%) | .16 |
| Pulmonary hypertension | 11 (9%) | 8 (9%) | 3 (8%) | 1.00 |
| Heart disease | 47 (37%) | 30 (34%) | 17 (42%) | .43 |
| Either heart or lung disease | 89 (70%) | 60 (68%) | 29 (73%) | .68 |
| Length of stay, total median, days (IQR) | 5 (3–9) | 4 (2–5) | 9 (6–17) | <.01 |
| Deaths | 10 (8%) | 3 (3%) | 7 (18%) | .01 |
CHF indicates congestive heart failure.
Of the 41 patients admitted to the ICU, the majority had an underlying risk factor for severe respiratory illness such as preexisting pulmonary disease or a compromised immune system (Table 2). Eight patients were organ transplant recipients, 6 patients had malignancy, 8 had significant COPD, and 5 had neuromuscular disease resulting in respiratory insufficiency.
Table 2.
Characteristics of patients admitted to the ICU and positive for hMPV
| n | % | |
|---|---|---|
| No. of patients | 40 | 100 |
| Female | 22 | 55 |
| Temperature, mean (SD) | 39 (1.2) | |
| Respiratory rate, mean (SD) | 22 (18–33) | |
| PaO2/FIO2 ratio, median (IQR) | 157 (81–208) | |
| WBC (total), median (IQR) | 11.5 (7.4–17.4) | |
| Leukocyte percentage, median (IQR) | 82.8 (65.6–86.9) | |
| Lymphocyte percentage, median (IQR) | 11.5 (7.4–17.4) | |
| Comorbidities | ||
| COPD | 8 | 20 |
| Chronic respiratory failure | 5 | 13 |
| ESRD | 4 | 10 |
| Cirrhosis | 3 | 8 |
| CHF | 10 | 25 |
| Malignancy | 6 | 15 |
| Organ transplant | 8 | 20 |
| hMPV cause of ICU admission | ||
| No | 3 | 8 |
| Possible | 22 | 55 |
| Likely | 15 | 38 |
| Characteristics of ICU stay | ||
| APACHE score, mean (SD) | 64 (26) | |
| Mechanical ventilation | 22 | 55 |
| Required pressors | 9 | 23 |
| Bacterial coinfection | 8 | 20 |
| Developed ventilator-associated pneumonia | 1 | 2 |
| Duration of ICU stay, median (IQR) | 4 (1–10) | |
| Mechanical ventilation duration, median (IQR) | 3 (2–7) | |
| Met definition of ARDS | 19 | 48 |
| Mild | 5 | 13 |
| Moderate | 7 | 18 |
| Severe | 7 | 18 |
Fio2 indicates fraction of inspired oxygen; IQR, interquartile range; WBC, white blood cell; ESRD, end-stage renal disease; APACHE, Acute Physiology and Chronic Health Evaluation.
The mean Acute Physiology and Chronic Health Evaluation IV score for patients admitted to the ICU was 64 (SD, 26). We found that hMPV was the likely reason for admission in 38%, the possible reason in 55%, and not the reason in 8%. Fifty-five percent of patients admitted to the ICU required mechanical ventilation, 23% required vasopressor support within the first 48 hours of admission, and 48% met criteria for ARDS. Twenty percent of patients had a documented bacterial coinfection.
Patients whose reason for ICU admission was “likely” due to hMPV more often met criteria for ARDS compared to those whose reason for reason for ICU admission was “possibly” due to hMPV (Table 3), 63% vs 37% (P = .004). However, among those who met criteria for ARDS, the difference in the severity of ARDS was not statistically significant (P = .84). Compared to patients with “possible” hMPV, patients with “likely” hMPV had an odds ratio for in hospital death of 1.13 (confidence interval, 0.21–5.95), although this also was not statistically significant (P = .89). We were unable to make a comparison to the patients with “unlikely” hMPV, as there were only 3 in this group and no deaths.
Table 3.
Acute respiratory distress syndrome among patients with hMPV as the likely or possible cause of ICU admission
| Likely | Possible | P | |
|---|---|---|---|
| Met ARDS criteria (n = 19) | 80 | 31 | .007 |
| Severity among patients with ARDS | |||
| Mild | 25 | 28 | |
| Moderate | 42 | 28 | |
| Severe | 33 | 42 | |
Data are presented as percentages; P values are from Fisher exact test.
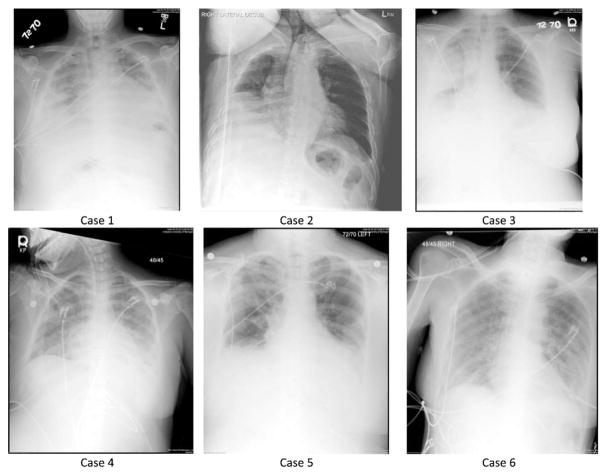
Six patients in the ICU cohort had more minor comorbidities and were not immunosuppressed. Two of these patients were transfers from other institutions for extracorporeal membrane oxygenation treatment; however, neither required extracorporeal membrane oxygenation. Three met criteria for ARDS, and 2 were found to have concurrent methicillin-resistant Staphylococcus aureus (MRSA) pneumonia. Although these patients constitute a relatively heterogeneous group, each presented with a typical constellation of symptoms, invariably consisting of some combination of fever, chills, wheezing, dyspnea, or cough. The characteristics of this group are summarized in Table 4, and their chest x-rays are shown in Fig. 3.
Table 4.
Case vignettes: patients with hMPV admitted to the ICU without significant comorbidities
| Case | Presentation | Chest radiograph | Bacterial pneumonia | ARDS |
|---|---|---|---|---|
| 1 | 44-year-old man with remote Hodgkin disease | Diffuse bilateral airspace disease | No | Did not require PPV |
| 2 | 84-year-old man with diabetes mellitus, dementia | Small right pleural effusion, bibasilar atelectasis | No | Did not require PPV |
| 3 | 31-year-old woman, otherwise healthy | Air space opacification in right upper and middle lobes | MRSA pneumonia | Unilateral CXR |
| 4 | 27-year-old woman with type 1 diabetes mellitus | Extensive bilateral airspace opacification | No | Moderate ARDS |
| 5 | 54-year-old woman with mild intermittent asthma | Opacification of right upper lobe and left lower lobe | MRSA pneumonia | Moderate ARDS |
| 6 | 58-year-old woman with COPD (severity unknown) | Diffuse bilateral air space opacification | No | Severe ARDS |
PPV indicates positive pressure ventilation; CXR, chest x-ray.
Fig. 3.
Chest x-rays of patients described in vignettes: cases 3 and 5 had MRSA pneumonia.
4. Discussion
Our study is the first to characterize the severity of illness in a large sample of adult patients with hMPV infection. We determined that a significant proportion of patients with hMPV required admission to the ICU, the majority of whom required mechanical ventilation and approximately half of whom met criteria for ARDS. To our knowledge, this is the largest case series of patients who developed ARDS potentially caused by hMPV. We also found that hMPV has the potential to produce significant respiratory disease in patients with minor comorbidities and no underlying immune compromise.
Fifteen percent of patients developed ARDS in the context of a positive hMPV, and 6 patients with only minor comorbidities developed respiratory failure requiring ICU admission. Only 1 patient had coinfection with influenza, and among ICU cohort, only a minority had documented bacterial coinfection.
The rate of ICU admission was 31%, which is higher than in other studies in patients with viral pneumonia [4]. Seven percent of patients whose hospitalization was associated with a positive hMPV result died during their hospital stay. Solid organ transplant recipients, patients with chronic respiratory insufficiency, and patients with severe COPD comprised most patients who required critical care. Three of the solid organ transplant recipients were lung transplant patients. These results compliment previous studies that suggest immunosuppressed patients and patients with underlying lung disease are at high risk for severe disease in hMPV infection [4,5,12,13]. We also identified a handful of patients with relatively few comorbidities who developed severe respiratory illness in the setting of hMPV infection, which is a departure from conventional wisdom regarding this pathogen.
Historically, our understanding of the clinical impact of specific respiratory viruses has been hampered by low-yield detection techniques such as viral culture which is cumbersome and direct fluorescent antibody tests or immunoassays which are easier to perform but have poor sensitivity. The increasing availability and broadened scope of viral respiratory polymerase chain reaction panels is reshaping our understanding of viral pathogens, and hMPV is no exception [15]. Our previously limited understanding of hMPV in critical illness is also due in part to its relatively recent discovery in 2001; routine testing for hMPV at our institution began in 2009 (the start of our study period).
We recognize this study has several limitations. First, we are unable to determine to what extent hMPV was responsible for the symptoms or decompensation for the patients in our cohort. In fact, several patients in our cohort were hospitalized for reasons other than respiratory tract infection. Human metapneumovirus in these patients was likely more of a bystander than a true pathogen. However, among the patients admitted to the ICU, we carefully reviewed charts to evaluate whether hMPV was the reason for respiratory decompensation and ICU admission. Twenty percent of patients were identified as having a bacterial co-infection; because blood and sputum cultures were not routinely performed, this is likely an underestimate. A substantial number of patients, either with or without proven bacterial coinfection, received antibiotic therapy targeted at treating bacterial pneumonia. Procalcitonin measurements were not routinely performed at our institution during the study period but could potentially help discriminate patients who have an isolated viral respiratory infection from those with a serious concurrent bacterial process. This would be an interesting area for further research [16]. Second, by limiting our cohort to patients testing positive for hMPV, we are likely selecting for a more severely ill sample. Physicians may be more likely to perform viral studies in patients with severe pulmonary illness requiring mechanical ventilation or bronchoscopy. Third, our use of ICD-9-CM codes for identifying comorbid illness in the larger hospitalized cohort may not accurately reflect the true burden of disease among the patients studied. Third, our use of ICD-9-CM codes for identifying comorbid illness in the larger hospitalized cohort may not accurately reflect the true burden of disease among the patients studied. For example, many patients carried an ICD-9 code diagnosis of both COPD and asthma, which is why the aggregate variable of any lung disease in Table 1 is larger than the sum of the diagnoses listed. Patients coded as having illness by ICD-9 codes might not meet criteria for the diagnosis clinically. Furthermore, patients may have comorbidities not captured by ICD-9 because the provider did not think it was relevant for billing purposes that hospitalization.
Human metapneumovirus is an important and previously underappreciated cause of critical illness and ARDS in adult hospitalized patients. Although most patients were immunosuppressed or had significant comorbidities, we identified a number of relatively healthy patients with hMPV as the only cause of severe respiratory illness. Treatment for hMPV remains supportive—bronchodilator therapy should be instituted in patients with obstructive lung disease, and patients for whom a bacterial process cannot be convincingly excluded should be treated with empiric antibiotic therapy. Droplet respiratory precautions are appropriate in preventing transmission of this pathogen. Clinicians should recognize that hMPV can produce severe respiratory illness alone or in conjunction with bacterial pathogens such as S aureus.
Acknowledgments
Drs Terri Stillwell and Michael Quasney gave significant feedback regarding the study design. Ms Lisa Sturm and Ms Lynn Holevinski performed the data extraction.
Footnotes
Funding source: Dr Cooke is supported by a grant from the Agency for Healthcare Research and Quality (K08HS020672).
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;6:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoogen, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Criddle MC, Walsh EE. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol. 2006;35(1):46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168(22):2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey AR, Erdman D, Anderson LJ, Wash EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187(5):785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 6.Williams JV, Crowe JE, Jr, Enriquez R, Minton P, Stokes Peebles R, Jr, Hamilton R, et al. Human metapneumovirus plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192(2):1149–53. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamelin ME, Cotul S, Laforge J, Lampron N, Bourbeau J, Weiss K, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41(4):498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 8.Martinello RA, Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53(4):248–54. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189(8):1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas LE, de Riik NX, Thiisen SF. Human metapneumovirus infections on the ICU: a report of three cases. Ann Intensive Care. 2012;2(1):30. doi: 10.1186/2110-5820-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi SH, Hong SB, Ko GB, Lee Y, Park HJ, Park WY, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–32. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 12.Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Côté S, et al. Virologic features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186(9):1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 13.Boivin G, De Serres G, Hamelin ME, Cote S, Arqouin M, Tremblay G, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44(9):1152–8. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 14.ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 15.Nichols G, Angela J, Boeckh M. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin Microbiol Rev. 2008;21(2):274–90. doi: 10.1128/CMR.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuquemelle E, Souis F, Villers D, Roche-Campo F, Ara Somohano C, Fartoukh M, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicenter study. Intensive Care Med. 2011;37(5):796–800. doi: 10.1007/s00134-011-2189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]