Abstract
Background
Acute high-risk abdominal (AHA) surgery is associated with high mortality, multiple postoperative complications and prolonged hospital stay. Further development of strategies for enhanced recovery programs following AHA surgery is needed. The aim of this study was to describe physical performance and barriers to independent mobilization among patients who received AHA surgery (postoperative days [POD] 1–7).
Methods
Patients undergoing AHA surgery were consecutively enrolled from a university hospital in Denmark. In the first postoperative week, all patients were evaluated daily with regards to physical performance, using the Cumulated Ambulation Score (CAS; 0–6 points) to assess basic mobility and the activPAL monitor to assess the 24-hour physical activity level. We recorded barriers to independent mobilization.
Results
Fifty patients undergoing AHA surgery (mean age 61.4 ± 17.2 years) were included. Seven patients died within the first postoperative week, and 15 of 43 (35%) patients were still not independently mobilized (CAS < 6) on POD-7, which was associated with pulmonary complications developing (53% v. 14% in those with CAS = 6, p = 0.012). The patients lay or sat for a median of 23.4 hours daily during the first week after AHA surgery, and the main barriers to independent mobilization were fatigue and abdominal pain.
Conclusion
Patients who receive AHA surgery have very limited physical performance in the first postoperative week. Barriers to independent mobilization are primarily fatigue and abdominal pain. Further studies investigating strategies for early mobilization and barriers to mobilization in the immediate postoperative period after AHA surgery are needed.
Abstract
Contexte
La chirurgie abdominale d’urgence à risque élevé est associée à un fort taux de mortalité, à des complications postopératoires multiples et à des hospitalisations prolongées. Il est donc nécessaire d’élaborer de nouvelles stratégies pour améliorer le rétablissement après ce type de chirurgie. La présente étude visait à décrire le fonctionnement physique et les obstacles aux déplacements autonomes chez les patients ayant subi une chirurgie de ce type (jours postopératoires 1 à 7).
Méthodes
Nous avons recruté successivement les patients subissant une chirurgie abdominale d’urgence à risque élevé dans un hôpital universitaire du Danemark. Durant la première semaine postopératoire, tous les patients ont subi quotidiennement une évaluation visant à vérifier leur fonctionnement physique. Nous nous sommes servis du Cumulated Ambulation Score (CAS; de 0 à 6 points) pour évaluer la mobilité de base et du moniteur activPAL pour évaluer le niveau d’activé physique 24 heures par jour. Nous avons noté les obstacles aux déplacements autonomes.
Résultats
Cinquante patients (âge moyen: 61,4 ans ± 17,2) ont été retenus. Sept sont décédés durant la première semaine postopératoire, et 15 des 43 patients restants (35 %) ne se déplaçaient pas encore de façon autonome (CAS < 6) le septième jour, une situation associée à l’apparition de complications pulmonaires (53 % c. 14 % de ceux qui avaient un CAS de 6, p = 0,012). Les patients étaient couchés ou assis pendant une durée médiane de 23,4 heures par jour durant la première semaine postopératoire, et les principaux obstacles aux déplacements autonomes étaient la fatigue et la douleur abdominale.
Conclusion
Les patients qui subissent une chirurgie abdominale d’urgence à risque élevé ont un fonctionnement physique très faible durant la première semaine postopératoire. Les obstacles aux déplacements autonomes sont principalement la fatigue et la douleur abdominale. Il faudra d’autres études sur les stratégies de mobilisation précoces et les obstacles aux déplacements peu après une chirurgie abdominale d’urgence à risque élevé.
Acute high-risk abdominal (AHA) surgery is associated with high mortality, multiple postoperative complications and prolonged hospital stay, especially in elderly patients.1–6 It is defined as immediate emergency laparoscopy or laparotomy primarily to treat intestinal obstruction, perforated viscus or bowel ischemia.7 A focus on enhanced recovery programs specifically for patients following AHA surgery is needed to improve the postoperative outcome.8–15 Possible interventions include reducing time before surgery, the early use of antibiotics, optimized fluid management, pain treatment, early nutrition and early mobilization.8,10,12
Early mobilization and exercise are known to play important roles in postoperative care following abdominal surgery and are associated with less postoperative reduction of fitness and fewer postoperative complications in patients undergoing elective surgery.9,15–20
There are, to our knowledge, no published data on postoperative physical performance, level of 24-hour physical activity, or barriers to independent mobilization within the first week following AHA surgery. Such findings are crucial when organizing strategies for enhanced recovery programs after AHA surgery.
The aim of the present study was to describe physical performance and barriers to independent mobilization (on postoperative days [POD] 1–7) in patients who underwent AHA surgery.
Methods
Study population
This single-centre prospective cohort study included consecutive patients who underwent AHA surgery between April 27 and June 18, 2014, at the Copenhagen University Hospital, in Hvidovre, Denmark. This study is an embedded part of a larger cohort study, initiated in 2013, investigating the effect of an enhanced recovery program after AHA surgery in 600 patients (Clinicaltrials.gov: NCT01899885).7 The larger study does not include the aim and outcomes reported in the present study.
The key elements of the enhanced recovery program include the use of a preoperative nasogastric tube, an arterial catheter and antibiotics, surgery within 6 hours after decision to operate, perioperative fluid and pain management (thoracic epidural with local anesthetics and oral nonsteriodal anti-inflammatories [NSAIDs]), and early nutrition and mobilization.7 In addition to early mobilization, every patient received daily physiotherapy until POD-7 and was evaluated by an occupational therapist. The physiotherapy sessions (10–30 min per session based on the capability of the patient) progressed on an individual level and consisted of exercises in bed and practising basic mobility activities, including transfer in and out of bed, sitting to standing from a chair, walking and stair climbing. Additionally, walking aids were changed to less supportive aids as needed, as decribed by Münter and colleagues21 in a similar study of patients with hip fracture. The goal of physiotherapy was for the patients to achieve independent mobility. When independent, patients were instructed in self-training and the importance of daily and frequent physical activity. If required (e.g., because of shallow breathing, coughing, secretion and atelectasis), patients received specific respiratory therapy on a daily basis.
To be included in the study, patients had to be 18 years or older and undergoing emergency laparotomy or laparoscopy (including reoperations after elective surgery) for intestinal obstruction, perforated viscus or bowel ischemia.7 Patient characteristics and information about comorbidities; day of admission; type and duration of operation; presence of epidural, preoperative sepsis and postoperative pulmonary complications; Eastern Cooperative Oncology Group (ECOG) scores; American Society of Anesthesiologists (ASA) classification; and 30-day mortality were all extracted from a central database from the larger cohort study. Postoperative pulmonary complications were defined as Clavien–Dindo classification higher than grade I, and preoperative sepsis was evaluated using the predisposition, insult, response, organ dysfunction (PIRO) classification.22,23 The ECOG score is a preoperative physical performance status on general well-being and activities of daily living, with a score of 0 indicating perfect health and a score of 5 indicating death.24 The ASA classification assesses physical status before surgery, with a score of 1 indicating normal health and a score of 5 indicating a moribund patient.25 The prehospital functional performance, assessed using the New Mobility Score (NMS), was obtained from the electronic patient records supplemented with information from the patient and/or relatives when necessary. The NMS, developed in 1993, is used to assess the functional level in patients with decreased mobility.26 It assesses 3 activities: walking indoors, walking outdoors and shopping. Each activity is scored from 0 to 3 (0 = not at all able, 1 = able with help from another person, 2 = able with a walking aid, 3 = no difficulty and no aid), giving a total score of 0–9 for overall mobility.27,28
The regional ethical committee waived the need for written informed consent in this study (H-2–2014-FSP31). This study was preregistered with Clinicaltrials.gov: NCT02124356. We used the Strengthening The Reporting of OBservational Studies in Epidemiology (STROBE) checklist for observational studies for the reporting of the study.29
Physical performance
We assessed postoperative physical performance using the Cumulated Ambulation Score (CAS) and with a 24-hour activity monitor (activPAL, PAL Technologies Ltd.).
The CAS was first described in a 2006 validation paper to investigate independence in ambulation following hip fracture surgery.30 In 2012, the CAS was validated for use in geriatric patients.31 Three basic mobility activities are evaluated:
getting in and out of bed (from supine in bed to sitting at the side of the bed to standing or transfer to sitting in chair placed beside the bed, and return to the supine position in bed)
sitting to standing to sitting from a chair with armrests
indoor walking, with an appropriate aid allowed in transfer and walking if necessary32
Each activity is scored from 0 to 2 (0 = not able despite human assistance and verbal cueing, 1 = able with verbal cueing and/or human assistance from 1 or more individuals, 2 = able safely without human assistance or verbal cueing), making up a total score of 0–6 reflecting the scale from bedbound to independent mobilization.32,33 In addition to routine treatment, a physiotherapist tested the patients after AHA surgery using CAS on PODs 1–7. A total of 8 skilled physiotherapists participated in data collection. They all had experience using the CAS and were calibrated before the present study.
The 24-hour physical activity level was assessed on PODs 1–8 using the activPAL. The activPAL is a uniaxial accelerometer that registers time spent, in hours, in sitting/lying and standing/walking positions. It also includes an inclinometer that can register the number of transitions made from sitting to standing and from standing to sitting.34,35 The validity of the activPAL has been confirmed in both healthy and hospitalized elderly people.36,37 The monitor was attached to the skin on the anterior of the mid thigh. Every day, a physiotherapist checked the battery and recording indicator and ensured that the monitor was firmly attached. ActivPAL results are presented for PODs 2, 4 and 7 in order to present a picture of the 24-hour activity level immediately after surgery, midweek and 1 week postoperatively. Results from PODs 1 and 8 were omitted because the monitor could not record the full 24-hour period for these days. The decision to omit these data was made during data quality checks before any statistical analyses were run. Results are presented as time (hours) each day spent inactive (sitting/lying) and active (standing/walking) in addition to the number of transitions from sitting to standing. The categories of walking and standing were combined because the activPAL sensor has been known to underestimate the time spent walking at low walking speed.36,37
Barriers to independent mobilization
Patients with a CAS score of 6 are by definition independently mobile with or without a walking aid, thus, we used a CAS score of 6 as a cut-off point for independently/nonindependently mobile patients during hospitalization. Every day, patients who were not independently mobile were asked what factors/barriers had restricted their mobilization, and the physiotherapists registered the primary barrier for independent mobilization from a predefined list: pain, motor blockade, dizziness, exhaustion/fatigue, nausea and vomiting, acute cognitive dysfunction, respiratory problems, unconsciousness, patient declines, logistics (e.g., examination at other hospital ward), monitoring equipment, or other. The list of barriers was predefined by the research group before study initiation.
Pain was assessed using a visual analogue scale (VAS) on PODs 1–7. Patients were asked to indicate their level of pain at rest and during physical activity on a 10 cm line indicating “no pain” to “worst possible pain.” The physiotherapist registered the corresponding measurement on the back of the VAS scale and noted the location of the pain.38,39
Statistical analysis
No previous data existed in this patient group from which a sample size could be made. No formal sample size estimation was done for this descriptive study, but we considered a consecutive sample of 50 patients undergoing AHA surgery to be representative.
Data were tested for normal distribution using the Kolmogorov–Smirnov test and visual inspection of Q-q plots. For descriptive data, continuous, normally distributed data are presented as means ± standard deviations (SD), categorical and non-normally distributed data are presented as medians and interquartile ranges (IQR), and nominal data are presented as numbers and percentages. We analyzed differences between groups using the independent t test for continuous, normally distributed data, the Mann–Whitney U test for non-normally distributed data and the χ2 or Fisher exact test for categorical and nominal data. We used univariable logistic regression to evaluate the association between the development of a pulmonary complication and the CAS level at POD-7. All analyses were performed using SPSS software version 19. We considered results to be significant at p < 0.05.
Results
Study population
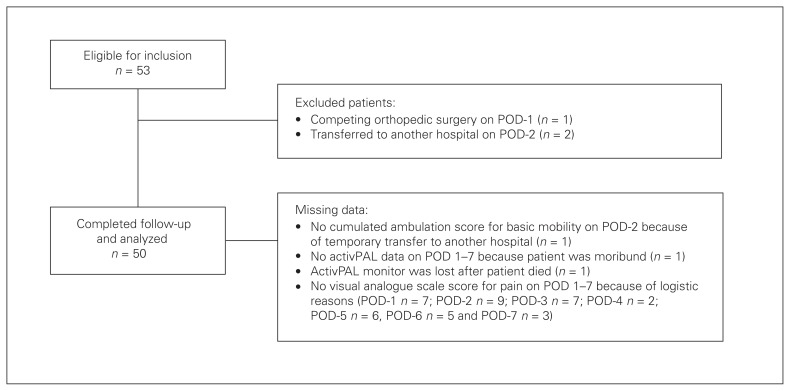
Fifty-three patients underwent AHA surgery during the study period and were eligible for inclusion. Three patients were excluded: 1 because of competing orthopedic surgery on PODs 1 and 2 because they were permanently transferred to another hospital on POD-2 (Fig. 1). The total number of included patients was 50. Missing data in the first postoperative week are clarified in Figure 1.
Fig. 1.
Flow of patients through the study. POD = postoperative day.
Patient characteristics are presented in Table 1. The mean age of patients was 61.4 ± 17.2 years, and almost all patients were admitted from their own homes and with a high preoperative functional level. Furthermore, most patients were generally healthy, with a high physical status before surgery when evaluated with the ECOG score (74% had a score of 0–1) and ASA classification (66% were ASA class 1–2). The median length of stay of the cohort was 12 (IQR 7–22) days from the day of AHA surgery to discharge. Ten patients were admitted to the intensive care unit postoperatively for a median of 3.0 (IQR 1.0–5.5) days. During hospitalization, 17 (34%) patients experienced a pulmonary complication within a mean of 3.3 ± 2.5 days after surgery.
Table 1.
Characteristics of patients undergoing AHA surgery (n = 50)
| Characteristic | No. (%)* |
|---|---|
| Age, mean ± SD, yr | 61.4 ± 17.2 |
| Female sex | 24 (48) |
| BMI, mean ± SD [range]† | 25.0 ± 5.8 [15.3–40.6] |
| Diagnosis | |
| Obstruction | 15 (30) |
| Perforation | 25 (50) |
| Other‡ | 10 (20) |
| Type of surgery | |
| Emergency laparotomy | 42 (84) |
| Emergency laparoscopy | 8 (16) |
| Duration of surgery, mean ± SD, h | 2.1 ± 1.3 |
| Thoracic epidural | 45 (90) |
| Preoperative sepsis§ | 26 (54) |
| Postoperative pulmonary complications | 17 (34) |
| Comorbidities | |
| Cardiovascular diseases | 22 (44) |
| Respiratory diseases | 9 (18) |
| Diabetes mellitus (type 1 and 2) | 1 (2) |
| ECOG score | |
| 0–1 points | 37 (74) |
| 2–4 points | 13 (26) |
| ASA classification | |
| 1–2 | 33 (66) |
| 3–4 | 17 (34) |
| LOS after surgery, median (IQR), d¶ | 12 (7–22) |
| Admitted to ICU | 10 (20) |
| LOS in the ICU, median (IQR), d | 3.0 (1.0–5.5) |
| Residential status, own home | 47 (94) |
| NMS score, median (IQR) | 9 (6–9) |
| Walking aid† | 11 (22) |
| Home care | 11 (22) |
AHA = acute high-risk abdominal; ASA = American Society of Anesthesiologists; BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay in hospital; NMS = New Mobility
Score; SD = standard deviation.
Unless indicated otherwise.
n = 49.
Primarily diverticulitis Hinchey grade 3, mesenteric ischemia and surgically treated gastrointestinal bleeding.
n = 48.
n = 43.
Seven patients died and 12 were discharged during the first postoperative week. From PODs 8 to 30, no more patients died. The patients who died were older (mean age 79.7 ± 5.5 yr v. 58.4 ± 16.6 yr, p = 0.002) and had a lower median NMS score (5 [IQR 1–5] v. 9 [IQR 9–9], p < 0.001) than the rest of the cohort. There was no significant difference in sex distribution between those who died and those who survived (p = 0.24), but the 7 patients who died had significantly higher ECOG (≥ 2) and ASA (5 of 7 patients had ASA ≥ 3) scores. Five of the 7 patients who died experienced a postoperative pulmonary complication.
Physical performance
During PODs 1–7, 28 of the 43 (65%) patients who survived became independently mobile (CAS = 6) after a median of 3 (IQR 1–8) days. Patients who were nonindependently mobile (CAS < 6) on POD-7 were significantly older, were more often admitted to the ICU, more often experienced a pulmonary complication, and had a longer stay in hospital after surgery than those who were independently mobile (Table 2). Correspondingly, the odds of a pulmonary complication occurring in the independent (CAS = 6) group was 85% less (odds ratio 0.15, 95% confidence interval [CI] 0.03–0.63).
Table 2.
Differences in patient characteristics between independently (CAS = 6) and nonindependently (CAS < 6) mobile patients within the first postoperative week (n = 43)
| Characteristic | Group; no. (%)* | p value | |
|---|---|---|---|
| CAS < 6 (n = 15) | CAS = 6 (n = 28) | ||
| Age, mean ± SD, yr | 67.2 ± 15.5 | 53.7 ± 15.5 | 0.009 |
| NMS score, median (IQR) | 9 (4–9) | 9 (9–9) | 0.08 |
| Sex | 0.69 | ||
| Female | 6 (40) | 13 (46) | |
| Male | 9 (60) | 15 (54) | |
| Admitted to ICU | 6 (40) | 1 (4) | 0.005 |
| Type of surgery | |||
| Emergency laparotomy | 13 (87) | 22 (79) | 0.69 |
| Emergency laparoscopy | 2 (13) | 6 (21) | |
| Duration of surgery, mean ± SD, h | 2.2 ± 1.5 | 2.0 ± 1.3 | 0.67 |
| Preoperative sepsis | 8 (57)† | 15 (56)‡ | 0.92 |
| Postoperative pulmonary complication | 8 (53) | 4 (14) | 0.012 |
| LOS, median (IQR), d | 22 (13–28) | 8 (5–14.5) | 0.001 |
CAS = Cumulated Ambulation Score; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; NMS = New Mobility Score; SD = standard deviation.
Unless indicated otherwise.
n = 14.
n = 27.
The patients lay or sat for a median of 23.8 (IQR 22.8–24.0) hours on POD-2, 23.5 (IQR 22.5–24.0) hours on POD-4, and 23.4 (IQR 22.3–23.8) hours on POD-7. Patients who were independently mobile on different postoperative days stood or walked significantly more minutes, and had more transitions from sitting to standing than those who were not independent (Table 3).
Table 3.
Level of 24-hour physical activity between independently (CAS = 6) and nonindependently (CAS < 6) mobile patients within the first postoperative week
| Activity | CAS < 6 | CAS = 6 | p value | ||
|---|---|---|---|---|---|
|
|
|
||||
| Median (IQR) | n* | Median (IQR) | n* | ||
| Sit/lie, h | |||||
|
| |||||
| POD-2 | 23.9 (23.8–24.0) | 28 | 22.5 (22.3–23.3) | 16 | < 0.001 |
|
| |||||
| POD-4 | 24.0 (23.7–24.0) | 21 | 22.7 (21.2–23.2) | 22 | < 0.001 |
|
| |||||
| POD-7 | 23.8 (23.5–24.0) | 15 | 22.5 (21.6–23.2) | 15 | < 0.001 |
|
| |||||
| Stand/steps, h | |||||
|
|
|||||
| POD-2 | 0.1 (0.0–0.2) | 28 | 1.5 (0.8–1.7) | 16 | < 0.001 |
|
| |||||
| POD-4 | 0.0 (0.0–0.3) | 21 | 1.3 (0.8–2.8) | 22 | < 0.001 |
|
| |||||
| POD-7 | 0.2 (0.1–0.5) | 15 | 1.5 (0.8–2.4) | 15 | < 0.001 |
|
| |||||
| No. of transitions from sitting to standing | |||||
|
| |||||
| POD-2 | 3.0 (2.0–5.0) | 28 | 15.5 (8.8–26.8) | 16 | < 0.001 |
|
| |||||
| POD-4 | 2.0 (0.5–7.5) | 21 | 24.5 (18.5–36.5) | 22 | < 0.001 |
|
| |||||
| POD-7 | 8.0 (4.0–14.0) | 15 | 33.0 (26.0–46.0) | 15 | < 0.001 |
CAS = Cumulated Ambulation Score; IQR = interquartile range; POD = postoperative day.
Number of patients still alive and not discharged.
Barriers to independent mobilization
Patients who were not independently mobile on POD-1 were primarily restricted by monitoring equipment (12 of 38) and fatigue (11 of 38). On PODs 2–7 the primary barrier to mobilization was fatigue (42%–70%), followed by pain (13%–20%; Table 4). On POD-1, 11 of 42 (26%) patients experienced moderate to severe pain (VAS 5–10), primarily in the abdominal area; the degree and location of pain are presented in Table 5.
Table 4.
Barriers for independent mobilization (CAS < 6) in the first postoperative week
| Barrier | No. (%) of patients | |||
|---|---|---|---|---|
| POD-1 (n = 38) | POD-2 (n = 31) | POD-4 (n = 23) | POD-7 (n = 15) | |
| Pain | 3 (8) | 6 (19) | 3 (13) | 3 (20) |
| Motor blockade | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Dizziness | 3 (8) | 2 (6) | 0 (0) | 0 (0) |
| Fatigue | 11 (29) | 13 (42) | 16 (70) | 8 (53) |
| Nausea and vomiting | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| Acute cognitive dysfunction | 0 (0) | 0 (0) | 0 (0) | 1 (7) |
| Respiratory problems | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Unconsciousness | 3 (8) | 3 (10) | 1 (4) | 1 (7) |
| Patient declines | 2 (5) | 0 (0) | 0 (0) | 0 (0) |
| Logistics | 0 (0) | 3 (10) | 2 (9) | 1 (7) |
| Monitoring equipment | 12 (32) | 1 (3) | 0 (0) | 0 (0) |
| Other | 2 (5) | 1 (3) | 1 (4) | 1 (7) |
CAS = Cumulated Ambulation Score; POD = postoperative day.
Table 5.
Degree and location of pain during physical performance on PODs 1, 2, 4 and 7
| Pain during physical activity | No. (%) of patients | |||
|---|---|---|---|---|
| POD-1 (n = 42) | POD-2 (n = 39) | POD-4 (n = 43) | POD-7 (n = 31) | |
| VAS 0–4* | 31 (74) | 26 (67) | 33 (77) | 25 (81) |
| VAS 5–10† | 11 (26) | 13 (33) | 10 (23) | 6 (19) |
| Location of pain (primarily) | POD-1 (n = 24) | POD-2 (n = 28) | POD-4 (n = 26) | POD-7 (n = 16) |
| Abdomen | 23 (96) | 26 (93) | 22 (85) | 14 (88) |
| Other‡ | 1 (4) | 2 (7) | 4 (15) | 2 (13) |
POD = postoperative day; VAS = visual analogue scale.
No pain to mild pain.
Moderate to severe pain.
Columna lumbalis/cervicalis and lower extremity.
Discussion
The main findings of the present study were the low level of physical performance in the first postoperative week in patients undergoing AHA surgery despite early mobilization in addition to standardized physiotherapy, occupational therapy assessments and training. Patients who were nonindependently mobile within the first postoperative week more often experienced a pulmonary complication than patients who were independently mobile. The primary barriers to independent mobilization within the first week were fatigue and abdominal pain.
Physical performance
Most patients undergoing AHA surgery in this study had a high preoperative functional level and were generally healthy. Thus, a relatively high level of physical performance could be expected after surgery, but this was not the case, as 35% of the patients were nonindependently mobile and had low levels of 24-hour physical activity 1 week after surgery. The remaining patients, although independently mobile, still stood or walked for fewer than 1.5 hours per day in the first postoperative week. This degree of inactivity is associated with a high risk of sarcopenia, loss of muscle strength and decreased functional performance due to immobilization.18,40–44
Other studies have also reported a low degree of mobilization following abdominal surgery.19,45 In the study by Haines and colleagues,19 only 48% of patients undergoing abdominal surgery were able to walk more than 10 m away from the bed on POD-1.19 Their study included both emergent (22%) and elective surgery (78%) patients, and the primary surgeries were hepatobiliary (60%) and colorectal (31%).19 A study by Browning and colleagues45 including patients undergoing elective upper abdominal surgery also reported a low level of physical activity within the first 4 days.45
Correspondingly, a recent study by Bailey and colleagues46 found that 22.6% of older people (≥ 70 yr) undergoing nonelective abdominal surgery experienced a loss of independence, and this was associated with increased health care cost. Our results show that patients who were nonindependently mobile 1 week postoperatively were significantly older, more inactive and had an increased risk of longer hospital stay. These results confirm that, following AHA surgery, elderly people are vulnerable and at risk of losing physical performance when hospitalized. Furthermore, the loss of physical performance after discharge in elderly patients is associated with an increased risk of falls, readmission, social isolation, home care replacement and, in the worst case scenario, death.40,41,43,47–49 Therefore, intervention strategies, such as early mobilization and more intensive mobilization, should be prioritized for elderly patients. A recent systematic review by Castelino and colleagues16 reports little available evidence to guide clinicians in strategies of early mobilization following abdominal and thoracic surgery. This reveals the need for studies investigating the effect of early and standardized mobilization in patients following AHA surgery in association with physical performance.
Among the most frequent postoperative complications following major abdominal surgery are pulmonary complications, 2,17,50 which are associated with a low degree of physical activity and delayed or dependence in mobilization.19,46,51–53 In the present study 34% of patients experienced postoperative pulmonary complications, which corresponds to the 36% reported for the total cohort of 600 patients who received AHA surgery following a multidisciplinary perioperative protocol.7 Results of the present study showed that a higher proportion of patients who were nonindependently mobile on POD-7 experienced a pulmonary complication. Nonindependent patients were also more sedentary (lay or sat more) than patients who were independently mobile on POD-7. Evidence suggests that early mobilization may reduce the incidence of pulmonary complications after abdominal surgery, but there is limited knowledge on the frequency and intensity of mobilization needed to achieve this outcome.16,19,52,54–56 This reveals the importance of interventions aimed at reducing the risk of postoperative pulmonary complications, where early mobilization and respiratory therapy could play a role.
Barriers to independent mobilization
On POD-1, patients and hospital staff reported monitoring equipment (e.g., blood pressure, heart rate, saturation) and therapeutic equipment (e.g., oxygen tubing and intravenous therapy) as the main barrier to mobilization, preventing movement out of the bed in the early stage. In the study by Haines and colleagues,19 hypotension was the most common barrier to mobilization on POD-1 after acute and elective major abdominal surgery. Hypotension was not by itself an indication for nonindependent mobilization in the present study, and dizziness, which could be a result of orthostatic hypotension, was seldom reported on POD-1.
Throughout PODs 2–7, fatigue was the main barrier to independent mobilization, especially on POD-4 (70%), but it was not possible to explain the reason for patients feeling fatigued based on the data collected in the present study. Fatigue is pronounced after major abdominal surgery, probably because of inflammatory response.15,20,56
Despite a multimodal analgesia regime, including thoracic epidural with local anesthetics and oral NSAIDs, a large proportion of patients experienced moderate to severe pain in the abdominal area during mobilization. Thus, 13%–20% of the patients reported pain as the main barrier to independent mobilization on PODs 2–7. Correspondingly, other studies found that patients reporting more pain or inadequate pain relief were less physically active after elective abdominal surgery.45,51 Overall, fatigue and abdominal pain are the primary barriers to independent mobilization and may inhibit early ambulation, therefore a focus on these barriers and interventions aiming to deal with them, should be considered. Possible interventions are the use of intraoperative high-dose glucocorticoids, which attenuate inflammatory response and could reduce both fatigue and pain, as well as the use of psychotropic drugs that reduce fatigue.57,58
Limitations
A limitation to the present study might be that, even though all the included patients attempted mobilization on POD-1, it was not possible to conduct this assessment at the same time point postoperatively, so some patients were tested a few hours after surgery and others were tested up to 24 hours after surgery. This may have affected the comparability of CAS and VAS scores on POD-1. Another limitation is that postoperative pain assessments were missing in some patients on each of the first 7 days after surgery. This was mainly because of difficulties for patients to specify their level of pain using a VAS. Therefore, a VAS may not be the most appropriate assessment tool of pain in this population.
Conclusion
Following AHA surgery, patients have very limited physical performance in the first postoperative week, with a low level of 24-hour physical activity, as well as a higher risk of pulmonary complications. Barriers to independent mobilization within the first week after AHA surgery appear to be fatigue and abdominal pain. When developing enhanced recovery programs for patients undergoing AHA surgery, further studies investigating strategies for early mobilization and barriers to mobilization in the immediate postoperative period are urgently needed.
Acknowledgements
The authors thank the staff at the Copenhagen University Hospital Hvidovre, Denmark, for the recruitment and testing of the participants. A special thanks to Janne Orbæk, Trine Rehfeld Lind, Maria Stage, Sofie Dill, Robert-Jan Nienhuis, Jette Christensen and Morten Bay-Nielsen for practical support and guidance.
Footnotes
Presented in part at the Danish Surgical Society’s annual meeting, November 2014, Frederiksberg, Denmark; at the congress of the Danish Physical Therapy Association, March 2015, Odense, Denmark; and at Hvidovre Hospital researcher’s day, April 2015, Hvidovre, Denmark.
Competing interests: None declared.
Contributors: L.R. Jønsson, L.T. Tengberg, T. Bandholm, N.B. Foss and M.T. Kristensen designed the study. L.R. Jønsson, L.H. Ingelsrud and L.T. Tengberg acquired the data, which L.R. Jønsson, L.H. Ingelsrud and M.T. Kristensen analyzed. L.R. Jønsson and L.H. Ingelsrud wrote the article, which all authors reviewed and approved for publication.
References
- 1.Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:1–6. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenal JJ, Bengoechea-Beeby M. Mortality with emergency abdominal surgery in the elderly. Can J Surg. 2003;46:111–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Svenningsen P, Manoharan T, Foss NB, et al. Increased mortality in the elderly after emergency abdominal surgery. Dan Med J. 2014;61:5–8. [PubMed] [Google Scholar]
- 4.Saunders DI, Murray D, Pichel AC, et al. Variations in mortality after emergency laparotomy: the first report of the UK Emergency Laparotomy Network. Br J Anaesth. 2012;109:368–75. doi: 10.1093/bja/aes165. [DOI] [PubMed] [Google Scholar]
- 5.Vester-Andersen M, Lundstrøm LH, Møller MH, et al. Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study. Br J Anaesth. 2014;112:860–70. doi: 10.1093/bja/aet487. [DOI] [PubMed] [Google Scholar]
- 6.Barrow E, Anderson ID, Varley S, et al. Current UK practice in emergency laparotomy. Ann R Coll Surg Engl. 2013;95:599–603. doi: 10.1308/003588413X13629960048433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tengberg LT, Bay-Nielsen M, Bisgaard T, et al. Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg. 2017;104:463–71. doi: 10.1002/bjs.10427. [DOI] [PubMed] [Google Scholar]
- 8.Møller MH, Adamsen S, Thomsen RW, et al. Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. Br J Surg. 2011;98:802–10. doi: 10.1002/bjs.7429. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–98. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H, Mythen M. Why is the surgical high-risk patient still at risk? Br J Anaesth. 2011;106:289–91. doi: 10.1093/bja/aeq408. [DOI] [PubMed] [Google Scholar]
- 11.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–41. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 12.Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–40. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg. 2013;30:70–8. doi: 10.1159/000350701. [DOI] [PubMed] [Google Scholar]
- 14.Peden C, Scott MJ. Anesthesia for emergency abdominal surgery. Anesthesiol Clin. 2015;33:209–21. doi: 10.1016/j.anclin.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen DH, Sonne E, Andreasen J, et al. Convalescence after colonic surgery with fast-track vs conventional care. Color Dis. 2006;8:683–7. doi: 10.1111/j.1463-1318.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 16.Castelino T, Fiore JF, Niculiseanu P, et al. The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: a systematic review. Surgery. 2016;159:991–1003. doi: 10.1016/j.surg.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence VA, Hilsenbeck SG, Mulrow CD, et al. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10:671–8. doi: 10.1007/BF02602761. [DOI] [PubMed] [Google Scholar]
- 18.Suetta C, Magnusson SP, Beyer N, et al. Effect of strength training on muscle function in elderly hospitalized patients. Scand J Med Sci Sports. 2007;17:464–72. doi: 10.1111/j.1600-0838.2007.00712.x. [DOI] [PubMed] [Google Scholar]
- 19.Haines KJ, Skinner EH, Berney S. Association of postoperative pulmonary complications with delayed mobilisation following major abdominal surgery: an observational cohort study. Physiotherapy. 2013;99:119–25. doi: 10.1016/j.physio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Bautmans I, Njemini R, De Backer J, et al. Surgery-induced inflammation in relation to age, muscle endurance, and self-perceived fatigue. J Gerontol A Biol Sci Med Sci. 2010;65:266–73. doi: 10.1093/gerona/glp145. [DOI] [PubMed] [Google Scholar]
- 21.Münter KH, Clemmesen CG, Foss NB, et al. Fatigue and pain limit independent mobility and physiotherapy after hip fracture surgery. Disabil Rehabil. 2017 Apr;17:1–9. doi: 10.1080/09638288.20171314556. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 23.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 25.Owens WD, Felts JA, Spitznagel EL. ASA physical status clssifications: a study of consistency of ratings. Aneshesiology. 1978;49:239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. J Bone Jt Surg [Br] 1993;755:797–8. doi: 10.1302/0301-620X.75B5.8376443. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen MT, Kehlet H. Most patients regain prefracture basic mobility after hip fracture surgery in a fast-track programme. Dan Med J. 2012;59:A4447. [PubMed] [Google Scholar]
- 28.Kristensen MT, Bandholm T, Foss NB, et al. High inter-tester reliability of the new mobility score in patients with hip fracture. J Rehabil Med. 2008;40:589–91. doi: 10.2340/16501977-0217. [DOI] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Foss NB, Kristensen MT, Kehlet H. Prediction of postoperative morbidity, mortality and rehabilitation in hip fracture patients: the cumulated ambulation score. Clin Rehabil. 2006;20:701–8. doi: 10.1191/0269215506cre987oa. [DOI] [PubMed] [Google Scholar]
- 31.Kristensen MT, Jakobsen TL, Nielsen JW, et al. Cumulated Ambulation Score to evaluate mobility is feasible in geriatric patients and in patients with hip fracture. Dan Med J. 2012;59:A4464. [PubMed] [Google Scholar]
- 32.Kristensen MT, Andersen L, Bech-Jensen R, et al. High intertester reliability of the Cumulated Ambulation Score for the evaluation of basic mobility in patients with hip fracture. Clin Rehabil. 2009;23:1116–23. doi: 10.1177/0269215509342330. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen MT, Foss NB, Ekdahl C, et al. Prefracture functional level evaluated by the New Mobility Score predicts in-hospital outcome after hip fracture surgery. Acta Orthop. 2010;81:296–302. doi: 10.3109/17453674.2010.487240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan CG, Grant PM, Tigbe WW, et al. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med. 2006;40:992–7. doi: 10.1136/bjsm.2006.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellers C, Dall P, Grant M, et al. Gait & posture validity and reliability of the activPAL3 for measuring posture and stepping in adults and young people. Gait Posture. 2016;43:42–7. doi: 10.1016/j.gaitpost.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Taraldsen K, Askim T, Sletvold O, et al. Evaluation of a body-worn sensor system to measure physical activity in older people with impaired function. Phys Ther. 2011;91:277–85. doi: 10.2522/ptj.20100159. [DOI] [PubMed] [Google Scholar]
- 37.Grant PM, Granat MH, Thow M, et al. Analyzing free-living physical activity of older adults in different environments using body-worn activity monitors. J Aging Phys Act. 2010;18:171–84. doi: 10.1123/japa.18.2.171. [DOI] [PubMed] [Google Scholar]
- 38.Huskisson EC. Measurement of pain. Lancet. 1974;7:1127–31. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 39.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 40.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. JAGS. 2003;51:451–8. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 41.Gill TM, Allore HG, Zhenchao G. Restricted activity and functional decline among community-living older persons. Arch Intern Med. 2003;163:1317–22. doi: 10.1001/archinte.163.11.1317. [DOI] [PubMed] [Google Scholar]
- 42.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol. 2003;58:1012–7. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 43.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–8. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 44.Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65:208–13. doi: 10.1111/j.1753-4887.2007.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 45.Browning L, Denehy L, Scholes RL. The quantity of early upright mobilisation performed following upper abdominal surgery is low: an observational study. Aust J Physiother. 2007;53:47–52. doi: 10.1016/s0004-9514(07)70061-2. [DOI] [PubMed] [Google Scholar]
- 46.Bailey JG, Davis PJB, Levy AR, et al. The impact of adverse events on the health care costs for older adults undergoing nonelective abdominal surgery. Can J Surg. 2016;59:172–9. doi: 10.1503/cjs.013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–24. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 48.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–28. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lees MC, Merani S, Tauh K, et al. Perioperative factors predicting poor outcome in elderly patients following emergency general surgery: a multivariate regression analysis. Can J Surg. 2015;58:312–7. doi: 10.1503/cjs.011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khuri SF, Henderson WG, Depalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–43. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shea RA, Brooks JA, Dayhoff NE, et al. Pain intensity and postoperative pulmonary complications among the alderly after abdominal surgery. Heart Lung. 2002;31:440–9. doi: 10.1067/mhl.2002.129449. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence VA, Cornell JE, Smetana GW. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 53.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 54.Mackay MR, Ellis E, Johnston C. Randomised clinical trial of physiotherapy after open abdominal surgery in high risk patients. Aust J Physiother. 2005;51:151–9. doi: 10.1016/s0004-9514(05)70021-0. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen KG, Holte K, Kehlet H. Effects of posture on postoperative pulmonary function. Acta Anaesthesiol Scand. 2003;47:1270–6. doi: 10.1046/j.1399-6576.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 56.Havey R, Herriman E, O’Brien DO. Guarding the gut — early mobility after abdominal surgery. Crit Care Nurs Q. 2013;36:63–72. doi: 10.1097/CNQ.0b013e3182753237. [DOI] [PubMed] [Google Scholar]
- 57.Galvin E, Boesjes H, Hol J, et al. Modafinil reduces patient-reported tiredness after sedation/analgesia but does not improve patient psychomotor skills. Acta Anaesthesiol Scand. 2010;54:154–61. doi: 10.1111/j.1399-6576.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 58.de la Motte L, Kehlet H, Vogt K, et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair. Ann Surg. 2014;260:540–9. doi: 10.1097/SLA.0000000000000895. [DOI] [PubMed] [Google Scholar]