Abstract
Objective
To find clinically relevant combinations of chronic conditions among patients with diabetes and to examine their relationships with six diabetes quality metrics.
Data Sources/Study Setting
Twenty‐nine thousand five hundred and sixty‐two adult patients with diabetes seen at eight Midwestern U.S. health systems during 2010–2011.
Study Design
We retrospectively evaluated the relationship between six diabetes quality metrics and patients' combinations of chronic conditions. We analyzed 12 conditions that were concordant with diabetes care to define five mutually exclusive combinations of conditions (“classes”) based on condition co‐occurrence. We used logistic regression to quantify the relationship between condition classes and quality metrics, adjusted for patient demographics and utilization.
Data Collection
We extracted electronic health record data using a standardized algorithm.
Principal Findings
We found the following condition classes: severe cardiac, cardiac, noncardiac vascular, risk factors, and no concordant comorbidities. Adjusted odds ratios and 95 percent confidence intervals for glycemic control were, respectively, 1.95 (1.7–2.2), 1.6 (1.4–1.9), 1.3 (1.2–1.5), and 1.3 (1.2–1.4) compared to the class with no comorbidities. Results showed similar patterns for other metrics.
Conclusions
Patients had distinct quality metric achievement by condition class, and those in less severe classes were less likely to achieve diabetes metrics.
Keywords: Diabetes, quality, multiple chronic conditions, multimorbidity, public reporting
Diabetes care quality, as measured by achievement of quality metrics that were derived from clinical care guidelines, is suboptimal (American Diabetes Association 2016a). Many factors can and likely do influence suboptimal care, including a patient's specific comorbidities, socioeconomic factors, insurance status, health beliefs, and other patient contextual factors (Bayliss et al. 2014). The role of comorbidities in diabetes care cannot be overlooked as over 80 percent of patients with diabetes have multiple chronic conditions (diabetes plus at least one comorbid condition) (Bae and Rosenthal 2008; Magnan et al. 2015c). Conceptual and early analytic studies have suggested that patients' comorbid conditions can alter their care quality and morbidity, even after controlling for patient demographics and health care utilization (Piette and Kerr 2006; Lee et al. 2007; Magnan et al. 2015d). Certain conditions have care that overlaps with diabetes to such a degree that the same care can benefit both conditions and might be more likely to be completed (e.g., cholesterol care for heart disease and diabetes). Other conditions, or combinations of conditions, might lead to inappropriate medication combinations or increased adverse effects (e.g., falls with tight blood pressure control and peripheral neuropathy). For patients with these conditions, not achieving metrics might be appropriate. The American Diabetes Association recommends patient‐centered care that considers individual patient health needs; however, we still lack the necessary evidence to target interventions to patients based on the burden of their coexisting chronic conditions (American Diabetes Association 2016b). For example, patients with early cardiac conditions are likely to benefit from more focus on cardiovascular risk reduction, while patients with limited life expectancy due to advanced comorbidities might appropriately have less tight glycemic control. Understanding the relationship between a patient's combination of chronic conditions and diabetes care quality is a vital step toward improving care for patients with diabetes and multiple chronic conditions.
It is unclear how best to describe the patterns of multiple chronic conditions among patients with diabetes, and how to operationalize the concept to allow for meaningful measurement of outcomes. Past work has shown that simply counting a patient's chronic conditions is not sufficient. Although studies have suggested higher diabetes quality of care in patients with more chronic conditions (Bae and Rosenthal 2008; Millett et al. 2009), a study of veterans examining combinations of diabetes and other chronic conditions found significant variation in mortality across patients with different specific conditions but the same number of conditions, suggesting that condition burden is due to more than the number of chronic conditions (Lee et al. 2007). The U.S. Department of Health and Human Services has recommended identification of similar subgroups among the population of patients with multiple chronic conditions as a critical step to improve the health status of the total population (Kronick et al. 2007; Parekh et al. 2011). Past work shows that it is not practical to consider every possible combination of conditions separately as there are over 2 million combinations in the Medicare population (Sorace et al. 2011). Therefore, it is necessary to find a manageable number of combinations of comorbidities in diabetes to measure the relationship between multiple chronic conditions and diabetes care outcomes in a clinically meaningful way.
Stratifying patients into mutually exclusive subgroups based on their combinations of conditions will be most meaningful if the combinations of conditions are clinically relevant. A conceptual model of comorbidity in diabetes suggests that concordant conditions (conditions that share care goals and/or pathophysiology with diabetes) improve diabetes care, and patients with more diabetes‐concordant conditions have been shown to be more likely to achieve diabetes metrics (Piette and Kerr 2006; Magnan et al. 2015a). In addition, diabetes‐concordant conditions are relatively common in diabetes, adding to their clinical importance as combinations of these common conditions will lead to prevalent subgroups of patients. From our past work, we have identified 12 diabetes‐concordant conditions that vary from being asymptomatic risk factors for diabetes complications (e.g., hypertension) to severe and often symptomatic complications (e.g., heart failure) (Magnan et al. 2015b). The pattern of co‐occurrence of these conditions among patients with diabetes is related to how the conditions develop in diabetes, leading to clinically relevant combinations of conditions.
Our objective was to identify clinically relevant combinations of chronic conditions occurring among patients with diabetes and to examine the patterns of variation in the relationships of these combinations to six diabetes quality metrics. We hypothesized that diabetes‐concordant conditions would tend to co‐occur in patients according to the progression of diabetes complications. Further, patients with less severe comorbidities would be less likely to achieve diabetes metrics than those with more severe conditions combinations. Identifying the relationships between patients' combinations of chronic conditions and their quality outcomes is a first step toward understanding appropriate and inappropriate variations in diabetes quality metric achievement and identifying patients for future targeted interventions in diabetes (Fortin et al. 2007; Kronick et al. 2007).
Methods
Study Design
For this observational study, we used patient‐level retrospective electronic health record data from 2 years; a baseline year, 2010; and a public reporting year, 2011. The Minimal Risk Health Sciences Institutional Review Board at the University of Wisconsin determined the project was exempt from IRB review.
Population/Study Setting
We included 29,562 adults patients aged 18–75 with type 1 or type 2 diabetes who were current patients in 2010–2011 at any of eight health systems that participate in a Midwestern quality reporting collaborative, the Wisconsin Collaborative for Healthcare Quality (WCHQ).
Wisconsin Collaborative for Healthcare Quality defines the presence of diabetes as patients having at least two face‐to‐face ambulatory visits (using CPT‐4 outpatient evaluation and management or E&M codes) with any provider (MD, DO, PA, NP) on different dates of service with an ICD‐9‐CM diagnosis code of 250.XX, 357.2, 362.XX, 366.41, or 648.XX over the 2 years of data (Wisconsin Collaborative for Healthcare Quality 2011). The 18–75 age limit reflects the standard age range for public reporting of quality metrics nationally and for WCHQ (National Committee for Quality Assurance 2011; Wisconsin Collaborative for Healthcare Quality 2011). The eight health systems include academic and community systems in rural, suburban, and urban settings (Wisconsin Collaborative for Healthcare Quality 2011) ranging in adult diabetic patient population size from 907 to 5,881. To be attributed to a health system as a current patient for diabetes public reporting, WCHQ requires patients to have had at least two E&M office visits on different dates of service in the past 2 years to either a primary care provider or to both a primary care provider and an endocrinologist, regardless of diagnostic codes, with at least one ambulatory care visit in the reporting year, 2011 (Wisconsin Collaborative for Healthcare Quality 2011). We obtained patient‐ and encounter‐level data from the eight health systems from WCHQ. Health systems submit electronic health record data to WCHQ annually; WCHQ uses standardized algorithms to identify patients with diabetes who qualify for public reporting and to calculate diabetes quality metrics (Wisconsin Collaborative for Healthcare Quality 2011). We included in our analyses all patients who qualified for public reporting of their diabetes quality metrics during 2011 from these eight systems (Wisconsin Collaborative for Healthcare Quality 2011). We employed no additional exclusion criteria.
Outcome Variables
We used six individual diabetes quality metrics that are currently publicly reported by WCHQ, and that are commonly used in national public reporting; the American Diabetes Association clinical practice guidelines for both type 1 and type 2 diabetes list them as important to reduce macrovascular and microvascular complications (American Diabetes Association 2011; Wisconsin Collaborative for Healthcare Quality 2011). Each metric was a binary outcome variable for achievement or not during the reporting year. These included three testing metrics: HbA1c testing two or more times/year, LDL cholesterol testing in the past year, and kidney function testing (urine microalbumin test in past year or documented evidence of nephropathy); and three control metrics: HbA1c control <7 percent at last measurement of the year (or <8 percent if 65–75 years old or having guideline‐specified comorbidities), LDL cholesterol control <100 mg/dL at last measurement of the year, and blood pressure control at <130/80 mmHg at last measurement (American Diabetes Association 2011; Wisconsin Collaborative for Healthcare Quality 2011). The goal levels for these metrics are from 2011, the reporting year for our study.
Primary Explanatory Variables
We created indicator variables for the presence or absence of 12 diabetes‐concordant chronic conditions. Diabetes‐concordant conditions are those with similar pathophysiology and/or treatment to diabetes, and they are often complications of or risk factors for diabetes, as determined from previous work (Magnan et al. 2015b). These conditions included hypertension, hyperlipidemia, obesity, cerebrovascular disease, peripheral vascular disease, chronic renal failure, thrombosis and embolism, retinopathy, and other chronic eye conditions, coronary artery disease, myocardial infarction within the past 2 years, congestive heart failure (CHF), and cardiomyopathy. To identify each patient's concordant conditions, we used our previously developed set of chronic conditions, identified from AHRQ Clinical Classification Software condition categories using the Chronic Condition Indicator, and modified to incorporate ICD‐9‐CM updates (Hwang et al. 2001; Healthcare Cost and Utilization Project 2011; Magnan et al. 2015a). Each patient's concordant chronic conditions were identified by ICD‐9‐CM codes billed at one or more clinical encounters during the baseline year. We used conditions from the baseline year only to ensure that the conditions were actively managed, rather than historical diagnoses, and were present prior to the reporting year, so that they had the opportunity to influence care from the start of the reporting year. The mapping from ICD‐9‐CM code to chronic conditions is available at no cost online with development details through HIPxChange at www.hipxchange.org (Algorithm for Identifying Patients with Multiple Chronic Conditions (Multimorbidity)) (Magnan et al. 2015b).
Covariates
We included the following descriptive categorical variables: age, gender, race/ethnicity, insurance, number of visits in the baseline year, and health system. We defined the age categories to represent young (18–34), younger middle‐age (35–49), older middle‐age (50–64), and Medicare‐age with a cap at 75 (65–75) per public reporting standards. The categories for number of visits represent those patients with diabetes with few visits (0–2), a moderate number (3–4), a high number (5–7), and a very high number (eight or more), with the expectation that patients with diabetes are seen twice a year for diabetes, plus a wellness exam, when in good health.
Analytic Approach
Creating Clinically Relevant Combinations of Chronic Conditions
We used factor analysis to reduce patterns of co‐occurrence for the 12 diabetes‐concordant conditions among our sample of patients with diabetes to a smaller number of condition combinations. We implemented exploratory factor solutions ranging from 1 to 5 factors, attending to both statistical criteria and interpretability of the factors as a basis for selecting an appropriate solution. The factor analytic approach applied to the condition co‐occurrence matrix attends to the categorical nature of the variables by assuming an underlying latent bivariate normal distribution for each pair of conditions, of which the observed binary condition variable is considered a discrete manifestation (Muthen 1997). We applied a robust weighted least squares estimator followed by Geomin rotation using the Mplus software (Los Angeles, CA, USA; Muthen & Muthen, 1998–2015). We used the Geomin rotation to allow the factors to correlate. The factor analysis was supportive of multiple factors, with the first four eigenvalues exceeding 1 (4.283, 1.323, 1.179, and 1.024), supporting four factors. Moreover, across all solutions, the four‐factor solution also yielded factors with greatest interpretability following rotation. Therefore, we used a four‐factor solution, yielding four factors of co‐occuring diabetes‐concordant chronic conditions.
Relationship with Diabetes Quality Metrics
We then fit logistic regression models for each of the six diabetes quality metrics (three testing and three control) to assess the relationship between the achievement of each quality metric and the patient's concordant condition class. We estimated the models both unadjusted and adjusted for the covariates above. There were no missing data for any covariates or outcome variables in the data from WCHQ as we included “unreported” in our payor variable and “other” in our race field. We report our results as predicted probabilities and as odds ratios with 95 percent confidence intervals. We conducted descriptive statistics and regression analyses using Stata 13.0 (Stata‐Corp, College Station, TX, USA).
Results
Patient Sample Characteristics
The sample of 29,562 patients with diabetes was 48 percent female, 74 percent white, and the vast majority had either commercial insurance or Medicare (Table 1). The average age was 57 years (standard deviation 11). On average, patients had 2.3 (1.3) diabetes‐concordant conditions. Hyperlipidemia was the most common (80 percent), thrombosis and embolism the least common (0.1 percent), and 6 percent had no concordant comorbidities. Patients were more likely to achieve testing metrics than control metrics.
Table 1.
Characteristics of Patients with Diabetes Aged 18–75 (%) (Total n = 29,562)
| Age | Diabetes‐concordant comorbidities | ||
| 18–34 | 4 | Hypertension | 77 |
| 35–49 | 17 | Hyperlipidemia | 80 |
| 50–64 | 47 | Obesity | 21 |
| 65–75 | 31 | Cerebrovascular disease | 4 |
| Sex (female) | 48 | Peripheral vascular disease | 4 |
| Race/ethnicity | Chronic renal failure | 11 | |
| White | 74 | Thrombosis and embolism | 0.1 |
| All others, including unreported | 26 | Retinal and other eye disease | 9 |
| Insurance | Coronary artery disease | 14 | |
| Commercial | 48 | Myocardial infarction within 2 years | 0.5 |
| Medicare | 30 | Congestive heart failure | 5 |
| Medicaid | 6 | Cardiomyopathy | 3 |
| Uninsured/unreported | 16 | Diabetes quality metrics | |
| Number of visits in baseline year | HbA1c testing | 73 | |
| 0–2 | 29 | LDL cholesterol testing | 88 |
| 3–4 | 25 | Kidney testing | 81 |
| 5–7 | 22 | HbA1c control | 62 |
| 8 or more | 24 | LDL cholesterol control | 58 |
| Blood pressure control | 50 |
Factor Analysis of Diabetes‐Concordant Conditions
We found four factors to underlie diabetes‐concordant chronic conditions that clinically span the spectrum of diabetes complications (Table 2). Based on the pattern of rotated pattern loadings, we clinically interpret these factors as Risk Factors for Diabetes and Its Complications (hypertension, hyperlipidemia, and obesity), Non‐Cardiac Vascular Disease (cerebrovascular disease, peripheral vascular disease, chronic renal failure, thrombosis and embolism, and retinal disease and other eye conditions), Cardiac Disease (coronary artery disease and myocardial infarction within 2 years), and Advanced Cardiac Disease (heart failure and cardiomyopathy).
Table 2.
Four Factors Representing Co‐Occurring Diabetes‐Concordant Chronic Conditions
| Condition | Risk Factors for Diabetes and Its Complications | Noncardiac Vascular Disease | Cardiac Disease | Advanced Cardiac Disease |
|---|---|---|---|---|
| Hypertension | 0.801 a | 0.218a | −0.017 | 0.025a |
| Hyperlipidemia | 0.670 a | −0.007 | 0.223a | −0.244a |
| Obesity | 0.343 a | −0.177a | 0.004 | 0.197a |
| Cerebrovascular disease | 0.055 | 0.463 a | 0.147a | 0.017 |
| Peripheral vascular disease | −0.035 | 0.660 a | 0.169a | −0.018 |
| Chronic renal failure | 0.238a | 0.416 a | −0.018 | 0.352a |
| Thrombosis and embolism | −0.012 | 0.540 a | 0.033 | −0.013 |
| Retinal disease and other eye conditions | 0.133a | 0.287 a | −0.033 | 0.094a |
| Coronary artery disease | 0.051 | 0.137a | 0.757 a | 0.003 |
| Myocardial infarction within 2 years | −0.011 | −0.001 | 0.826 a | 0.004 |
| Congestive heart failure | −0.038a | 0.037a | 0.512a | 0.725 a |
| Cardiomyopathy | 0.049a | −0.059 | 0.457a | 0.523 a |
Bold identifies factor on which condition displays loading of greatest magnitude.
p < .05.
We found low‐to‐moderate positive correlations between the four factors, suggesting a clear separation of the factors, with higher scores on one factor tending toward higher scores on the other factors. The highest correlation was 0.442 between Cardiac Disease and Non‐Cardiac Vascular Disease. The next highest correlations were 0.319 between Non‐Cardiac Vascular Disease and 0.308 between Cardiac Disease and Risk Factors. The correlations between Advanced Cardiac Disease and Non‐Cardiac Vascular Disease, and Advanced Cardiac Disease and Risk Factors, are 0.239 and 0.191, respectively.
Classes of Diabetes‐Concordant Chronic Conditions
The factors that emerged under the four‐factor solution possess a clear ordering of conditions in terms of stages of severity and care needs. Consequently, we grouped patients into five mutually exclusive diabetes‐concordant condition classes, based on their combinations of conditions, including a class for patients with no diabetes‐concordant comorbidities. These classes were chosen to be clinically relevant, based on patient severity and care needs, and to represent the spectrum of diabetes complications.
Each patient is assigned to the class that corresponds to their most severe diabetes complication. Class 1 includes patients who have no diabetes‐concordant conditions (1,826 patients, 6.4 percent). Class 2 includes patients with Risk Factors for Diabetes and Its Complications conditions only (17,699; 60 percent). Class 3 includes patients with Non‐Cardiac Vascular Disease conditions, with or without Risk Factors, and without Cardiac or Advanced Cardiac conditions (4,718; 16 percent). Class 4 includes patients with Cardiac Disease conditions but without Advanced Cardiac Disease conditions, with or without Risk Factors or Non‐Cardiac Vascular Disease (3,326; 11 percent). Finally, Class 5 includes patients with Advanced Cardiac Disease conditions, with or without any other diabetes‐concordant conditions (1,927; 6.5 percent). We put patients into Classes 2–5 regardless of whether they had the Risk Factors diagnoses, as it is likely that patients with more advanced diabetes complications also have Risk Factor conditions that are no longer routinely coded at health care encounters in favor of coding for the more severe diagnoses.
Predictive Probabilities by Condition Class
Patients were less likely to achieve quality metrics if they are in Class 1 (do not have concordant conditions) than if they are in any other Class, with the exception of blood pressure control (Figure 1). Note that patients in Class 1 do not have a diagnosis of hypertension, while most patients in the other Classes have hypertension. Patients in Class 2, those with Risk Factors conditions only, have lower or similar metric achievement compared to Class 3 for all metrics, and lower metric achievement compared to Class 4 and 5 for all metrics except of HbA1c and LDL testing.
Figure 1.
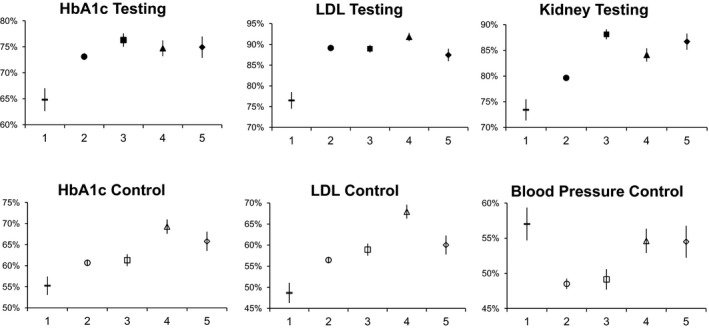
Diabetes‐Concordant Condition Classes (1–5) and Their Relationships to Each Diabetes Quality Metric, Adjusted, Predicted Probabilities (95% CI), among Patients with Diabetes 18–75 Years Old
- Notes. Class 1: No diabetes‐concordant conditions. Class 2: Risk factor conditions only. Class 3: Non‐cardiac vascular disease without cardiac disease, with or without risk factors. Class 4: Cardiac disease without advanced cardiac disease, with or without risk factors. Class 5: Advanced cardiac disease, with or without other diabetes‐concordant conditions.
In general, Classes 3–5 have better metric achievement than Class 1 or Class 2, but this is metric dependent. Class 3 (contains chronic renal failure and excludes any cardiac disease) patients are more likely to achieve kidney testing than other classes. Otherwise, this Class has lower or similar metric achievement compared to Classes 4 and 5. Class 4 patients have among the highest metric achievement for all metrics except kidney testing and blood pressure control. Class 5 (Advanced Cardiac Disease) patients have better or similar metric achievement than Classes 1–3 on all metrics, with similar metric achievement to Class 4 with the exception of less LDL testing and control.
Relationship of Covariates to Testing and Control Metrics
Odds ratios and 95 percent confidence intervals for covariates in our models (Tables 3 and 4) show that patients with Medicaid or self‐pay/unreported as their payor are less likely to achieve testing metrics than those with commercial insurance. A higher number of visits is significantly related to achieving HbA1c testing, but it has borderline or no significant relationship with other testing metrics. Medicaid or self‐pay/unreported insurance was not related to control metrics, and the relationship between number of visits and metric achievement is small for all three control metrics. Health system had variable patterns of significance and direction in relationship with both testing and control metrics across the eight systems (data not shown).
Table 3.
Diabetes‐Concordant Condition Classes (1–5) and Their Relationships to Each Diabetes Quality Testing Metric, Adjusted, Odds Ratios (95% CI), among Patients with Diabetes 18–75 Years Old
| Diabetes‐Concordant Condition Class | Testing Metric Achieved | |||||
|---|---|---|---|---|---|---|
| HbA1c Testing | LDL Cholesterol Testing | Kidney Testing | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 1‐No diabetes‐concordant conditions (reference) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 2‐Risk factor conditions only | 1.5 | 1.4–1.7 | 2.6 | 2.3–2.99 | 1.4 | 1.3–1.6 |
| 3‐Noncardiac vascular disease without cardiac disease, with or without risk factors | 1.8 | 1.6–2.03 | 2.6 | 2.2–3.02 | 2.7 | 2.4–3.2 |
| 4‐Cardiac disease without serious cardiac disease, with or without risk factors | 1.6 | 1.4–1.9 | 3.6 | 3.03–4.4 | 1.9 | 1.7–2.3 |
| 5‐Advanced cardiac disease, with or without other concordant conditions | 1.7 | 1.4–1.9 | 2.2 | 1.8–2.7 | 2.4 | 2.01–2.9 |
| Age category (ref: 18–34) | ||||||
| 35–49 | 1.0 | 0.8–1.1 | 1.6 | 1.3–1.8 | 1.1 | 0.9–1.3 |
| 50–64 | 1.1 | 0.99–1.3 | 2.0 | 1.7–2.3 | 1.2 | 1.1–1.4 |
| 65–75 | 1.3 | 1.2–1.6 | 2.4 | 1.98–2.8 | 1.4 | 1.2–1.7 |
| Gender (ref: male) | 0.9 | 0.9–0.9 | 0.9 | 0.8–0.97 | 1.0 | 0.9–1.1 |
| Race (ref: non‐white) | 1.1 | 0.98–1.2 | 1.2 | 1.1–1.4 | 1.0 | 0.9–1.1 |
| Payor (ref: commercial) | ||||||
| Medicare | 1.1 | 0.99–1.1 | 0.9 | 0.8–1.05 | 1.0 | 0.9–1.1 |
| Medicaid | 0.8 | 0.7–0.9 | 0.7 | 0.6–0.8 | 0.9 | 0.8–0.98 |
| Self‐pay or unreported | 0.7 | 0.7–0.8 | 0.7 | 0.6–0.8 | 0.8 | 0.7–0.9 |
| Number of visits in baseline year (ref: 0–2) | ||||||
| 3–4 | 1.4 | 1.3–1.5 | 1.1 | 1.03–1.3 | 1.0 | 0.9–1.1 |
| 5–7 | 1.7 | 1.6–1.8 | 1.1 | 0.97–1.2 | 1.1 | 0.99–1.2 |
| 8 or more | 2.0 | 1.8–2.1 | 1.02 | 0.9–1.1 | 1.2 | 1.1–1.3 |
Also adjusted for health system.
Table 4.
Concordant Condition Classes (1–5) and Their Relationships to Each Diabetes Quality Control Metric, Adjusted, Odds Ratios (95% CI), among Patients with Diabetes 18–75 Years Old
| Diabetes‐Concordant Condition Class | Control Metric Achieved | |||||
|---|---|---|---|---|---|---|
| HbA1c Control | LDL Cholesterol Control | Blood Pressure Control | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 1‐No diabetes‐concordant conditions (reference) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 2‐Risk factor conditions only | 1.3 | 1.2–1.4 | 1.4 | 1.2–1.5 | 0.7 | 0.6–0.8 |
| 3‐Noncardiac vascular disease without cardiac disease, with or without risk factors | 1.3 | 1.2–1.5 | 1.5 | 1.4–1.7 | 0.7 | 0.6–0.8 |
| 4‐Cardiac disease without serious cardiac disease, with or without risk factors | 1.95 | 1.7–2.2 | 2.3 | 2.0–2.6 | 0.9 | 0.8–1.02 |
| 5‐Advanced cardiac disease, with or without other concordant conditions | 1.6 | 1.4–1.9 | 1.6 | 1.4–1.8 | 0.9 | 0.8–1.03 |
| Age category (ref: 18–34) | ||||||
| 35–49 | 1.5 | 1.3–1.7 | 1.4 | 1.3–1.6 | 0.7 | 0.6–0.8 |
| 50–64 | 2.3 | 1.99–2.6 | 2.0 | 1.8–2.3 | 0.7 | 0.7–0.8 |
| 65–75 | 7.8 | 6.8–8.9 | 2.3 | 2.0–2.7 | 0.8 | 0.7–0.9 |
| Gender (ref: male) | 1.1 | 1.05–1.2 | 0.8 | 0.7–0.8 | 1.2 | 1.1–1.2 |
| Race (ref: non‐white) | 1.2 | 1.1–1.3 | 1.3 | 1.2–1.4 | 1.2 | 1.2–1.3 |
| Payor (ref: commercial) | ||||||
| Medicare | 1.2 | 1.1–1.2 | 1.0 | 0.98–1.1 | 1.1 | 0.96–1.1 |
| Medicaid | 0.99 | 0.9–1.1 | 0.8 | 0.7–0.9 | 1.0 | 0.9–1.1 |
| Self‐pay or unreported | 0.9 | 0.9–1.0 | 0.9 | 0.8–0.9 | 0.9 | 0.9–0.99 |
| Number of visits in baseline year (ref: 0–2) | ||||||
| 3–4 | 1.1 | 1.04–1.2 | 1.2 | 1.1–1.2 | 1.1 | 1.1–1.2 |
| 5–7 | 1.1 | 1.04–1.2 | 1.1 | 1.1–1.2 | 1.1 | 1.1–1.2 |
| 8 or more | 1.1 | 0.99–1.2 | 1.2 | 1.1–1.3 | 1.2 | 1.1–1.3 |
Also adjusted for health system.
Discussion
We found that patients with diabetes can be grouped into mutually exclusive classes that represent their clinically relevant diabetes‐concordant chronic conditions, and these classes appear to have some distinct relationships to diabetes care quality metrics. First, these results are consistent with how comorbidities develop in patients with diabetes, with separate classes for those with and without cardiac disease. Second, we observed relevant differences in quality metric achievement between condition classes, especially comparing classes with and without cardiac disease. Diabetes quality metric achievement was worse in patients with less severe or no diabetes‐concordant comorbidities. These classes demonstrate the variation in quality of diabetes care between patients when grouped by their diabetes‐concordant chronic conditions, and highlight opportunities to deliver care that considers the impact of a patient's combination of chronic conditions. These clinically meaningful classes can be used to stratify patients for targeted interventions, including targeting healthier patients to prevent the development of future complications.
Our results suggest that comorbidities beyond risk factor conditions group separately into noncardiac vascular, cardiac, and advanced cardiac conditions. One interpretation of this condition grouping is that noncardiac conditions develop separately from cardiac conditions. Among cardiac conditions, the conditions included in the advanced cardiac conditions group clinically are often complications of risk factors and other cardiac conditions. The separate grouping of noncardiac and cardiac conditions parallels previous findings that glycemic control is more important for microvascular outcomes than macrovascular outcomes (American Diabetes Association 2016a). Within the noncardiac vascular conditions group are vascular conditions that are traditionally microvascular (e.g., chronic renal failure) or might be considered either macro or microvascular (e.g., stroke) (Castilla‐Guerra and Fernandez‐Moreno Mdel 2007). Overall, we found that conditions in each class demonstrate distinct symptomatologies, long‐term morbidity, and, to some degree, treatments, including aggressiveness of glycemic management, from conditions in other groups (American Diabetes Association 2016a). These distinctions between conditions in each group, combined with separate pathophysiologic pathways in the development of the conditions in each class, could explain the variations in quality metric achievement between condition groups we found and highlight opportunities to separately target patients for future care based on their combination of chronic conditions.
Our results show distinct diabetes metric achievement between patients with and without cardiac disease. Patients with any cardiac conditions (Classes 4 and 5) show more control metric achievement compared to patients without cardiac conditions (Classes 2 and 3) in general. This could be appropriate, as patients with cardiac disease might benefit from tighter blood pressure control targets, and LDL control has been a major focus among those with known coronary disease (Skyler et al. 2009; American Diabetes Association 2016a). LDL control was less likely in patients in Class 5 compared to Class 4, and similar to Classes 2–3. This could be appropriate clinically if the Class 5 patients have more advanced heart failure (Stone et al. 2014). The higher likelihood of metric achievement in general for patients with cardiac conditions compared to those without cardiac disease was present even after adjusting for patient demographics and health care utilization (number of office visits and payor) that could affect a patient's exposure to and opportunities for care. Our results demonstrate the importance of a patient's combination of chronic conditions on the quality of care he or she receives, above and beyond other important health care modifiers.
The pattern in quality metric achievement between condition classes across the six diabetes metrics suggests that patients with no diabetes‐concordant conditions, or less severe conditions, have worse metric achievement than patients who have more severe conditions. Patients in Class 1, those without any comorbidities, have the lowest achievement on all metrics (excluding blood pressure control, but note blood pressure is unlikely to be an issue in patients without diagnosed hypertension). This pattern is seen after adjusting for number of clinic visits to account for infrequently seen patients who might be less likely to have diagnoses recorded, or to benefit from opportunistic care. There are similarities in metric achievement between Classes 2 and 3, that is, for patients with risk factors only and patients with noncardiac vascular disease conditions; however, testing metric achievement is more likely for patients with noncardiac vascular disease conditions (Class 3). Once a patient has cardiac disease diagnosed (Classes 4 and 5), the patient is even more likely to have his or her metrics achieved. This pattern suggests that patients who have more advanced conditions have better quality metric achievement. Previous work supports the finding that patients with more chronic conditions have better quality metric achievement (Ricci‐Cabello et al. 2015). This could be due to greater effort or attention to diabetes management in patients who have more comorbidities and appear sicker. This pattern also highlights the potentially inappropriate lack of metric achievement for patients with fewer or less severe comorbidities, who would be most able to benefit from treatment to prevent future comorbidities. Risk factor conditions, including hypertension and hyperlipidiemia, were extremely prevalent in our sample and represent a large group of patients where there is an opportunity to intervene prior to the development of further complications.
Limitations
This study has several limitations. First, the population was from a single U.S. state that may not be fully reflective of all patients across the United States. However, the sample included a wide range of ages, race/ethnicities, and payors, and patients from rural, urban, and suburban settings. We adjusted for several patient demographic and health care utilization factors, including payor. Our race data were limited by low sample size for the non‐white races, and so we were unable to use race as a multilevel variable. We were unable to adjust for more nuanced contextual factors, such as household socioeconomic status, health literacy, and health care behaviors (Bayliss et al. 2014). Second, the study sample received care at institutions that participate in public reporting, and this reporting has the potential to influence the care the patients received. However, diabetes care goal achievement in the study population was similar to results from a national sample in 2011 (National Committee for Quality Assurance 2011) and as our objective was to assess the relationship between chronic conditions and publicly reported quality metrics, a public reporting population is appropriate. The health systems are independently operated and do not share treatment protocols. Third, we identified chronic conditions by ICD‐9‐CM codes billed at office visits, an approach that could under‐represent certain conditions, such as obesity (Bleich, Pickett‐Blakely, and Cooper 2011). Detailed assessment data on these conditions, including BMI, were not available.
Implications
The clinically relevant combinations of conditions created in this work will support the identification of patient subgroups to target for future interventions that are both urgently needed and supported by Medicare policy changes (Smith et al. 2012; Edwards and Landon 2014). Current interventions, such as registries or disease management programs, generally have focused on patients with the worst control or targeted all patients with diabetes equally, regardless of comorbidities (Bojadzievski and Gabbay 2011; Stellefson, Dipnarine, and Stopka 2013). However, early research suggests that there are subtypes of diabetes with different comorbidity risks and that a patient's specific comorbidity profile influences his or her diabetes quality of care (Li et al. 2015; Ricci‐Cabello et al. 2015). Using a clinically relevant comorbidity‐based grouping, rather than considering all patients with diabetes equal, regardless of comorbidity, might help better target interventions to fit specific patients needs. For example, patients with cardiac conditions could have more emphasis on their cardiac needs and less on glucose control, since they are at high risk for future cardiovascular events and in our study they generally did well with glucose control. On the other side of the complication spectrum, patients with fewer or no comorbidities could be directly targeted to improve their diabetes care to reduce their risk of long‐term adverse outcomes and to keep them healthier longer. Improving diabetes care for the presumed healthier patients is especially important as many current interventions target the least healthy patients, missing the opportunity to prevent complications in the healthier patients who were also the least likely to have good diabetes control in our study. Future work should asses the role of patient socioeconomic and contextual factors on diabetes care quality. Future interventions will likely need to consider the combined influences of comorbidities, patient characteristics, and patient priorities for care.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This project was funded by grants R21 HS021899 and R01 HS018368 from the Agency for Healthcare Research and Quality. Additional support was provided by the Health Innovation Program, the University of Wisconsin School of Medicine and Public Health from The Wisconsin Partnership Program, and the Community‐Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR) through the National Center for Advancing Translational Sciences (NCATS) grant UL1TR000427. The work presented here was partially carried out while Dr. Magnan was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine, and partially while Dr. Magnan was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant #UL1 TR000002 and linked award KL2 TR000134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHRQ. The authors wish to thank Katie Ronk and Dave Beam for data management, and the Wisconsin Collaborative for Healthcare Quality (WCHQ) for its collaboration in this project.
Disclosures: None.
Disclaimers: None.
References
- American Diabetes Association . 2011. “Standards of Medical Care in Diabetes–2011.” Diabetes Care 34 (Suppl 1): S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association . 2016a. “Cardiovascular Disease and Risk Management (Standards of Diabetes Care).” Diabetes Care 39 (Suppl 1): S60–7. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . 2016b. “Strategies for Improving Care (Standards for Diabetes Care).” Diabetes Care 39 (Suppl 1): S6–12. [DOI] [PubMed] [Google Scholar]
- Bae, S. , and Rosenthal M. B.. 2008. “Patients with Multiple Chronic Conditions Do Not Receive Lower Quality of Preventive Care.” Journal of General Internal Medicine 23 (12): 1933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss, E. A. , Bonds D. E., Boyd C. M., Davis M. M., Finke B., Fox M. H., Glasgow R. E., Goodman R. A., Heurtin‐Roberts S., Lachenmayr S., Lind C., Madigan E. A., Meyers D. S., Mintz S., Nilsen W. J., Okun S., Ruiz S., Salive M. E., and Stange K. C.. 2014. “Understanding the Context of Health for Persons with Multiple Chronic Conditions: Moving from What Is the Matter to What Matters.” Annals of Family Medicine 12 (3): 260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich, S. N. , Pickett‐Blakely O., and Cooper L. A.. 2011. “Physician Practice Patterns of Obesity Diagnosis and Weight‐Related Counseling.” Patient Education and Counseling 82 (1): 123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojadzievski, T. , and Gabbay R. A.. 2011. “Patient‐Centered Medical Home and Diabetes.” Diabetes Care 34 (4): 1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla‐Guerra, L. , and Fernandez‐Moreno Mdel C.. 2007. “Stroke in Diabetic Patients: Is It Really a Macrovascular Complication?” Stroke; A Journal of Cerebral Circulation 38 (10): e106. [DOI] [PubMed] [Google Scholar]
- Edwards, S. T. , and Landon B. E.. 2014. “Medicare's Chronic Care Management Payment–Payment Reform for Primary Care.” New England Journal of Medicine 371 (22): 2049–51. [DOI] [PubMed] [Google Scholar]
- Fortin, M. , Soubhi H., Hudon C., Bayliss E. A., and van den Akker M.. 2007. “Multimorbidity's Many Challenges.” British Medical Journal 334 (7602): 1016–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthcare Cost and Utilization Project . 2011. HCUP Chronic Condition Indicator (Release). Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Hwang, W. , Weller W., Ireys H., and Anderson G.. 2001. “Out‐of‐Pocket Medical Spending for Care of Chronic Conditions.” Health Affairs (Project Hope) 20 (6): 267–78. [DOI] [PubMed] [Google Scholar]
- Kronick, R. G. , Bella M., Gilmer T. P., and Somers S.. 2007. The Faces of Medicaid II: Recognizing the Care Needs of People with Multiple Chronic Conditions. Hamilton, NJ: Center for Health Care Strategies Inc. [Google Scholar]
- Lee, T. A. , Shields A. E., Vogeli C., Gibson T. B., Woong‐Sohn M., Marder W. D., Blumenthal D., and Weiss K. B.. 2007. “Mortality Rate in Veterans with Multiple Chronic Conditions.” Journal of General Internal Medicine 22 (Suppl 3): 403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Cheng W. Y., Glicksberg B. S., Gottesman O., Tamler R., Chen R., Bottinger E. P., and Dudley J. T.. 2015. “Identification of Type 2 Diabetes Subgroups through Topological Analysis of Patient Similarity.” Science Translational Medicine 7 (311): 311ra174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan, E. , Palta M., Johnson H., Bartels C., Schumacher J., and Smith M.. 2015a. “The Impact of a Patient's Concordant and Discordant Chronic Conditions on Diabetes Care Quality Measures.” Journal of Diabetes and Its Complications 29 (2): 288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan, E. M. , Gittelson R., Bartels C. M., Johnson H. M., Pandhi N., Jacobs E. A., and Smith M. A.. 2015b. “Establishing Chronic Condition Concordance and Discordance with Diabetes: A Delphi Study.” BMC Family Practice 16: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan, E. M. , Palta M., Johnson H. M., Bartels C. M., Schumacher J. R., and Smith M. A.. 2015c. “The Impact of a Patient's Concordant and Discordant Chronic Conditions on Diabetes Care Quality Measures.” Journal of Diabetes and Its Complications 29 (2): 288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan, E. M. , Palta M., Mahoney J., Pandhi N., Bolt D., Fink J., Greenlee R. T., and Smith M. A.. 2015d. “The Relationship of Individual Comorbid Chronic Conditions to Diabetes Care Quality.” BMJ Open Diabetes Research and Care 16 (1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett, C. , Netuveli G., Saxena S., and Majeed A.. 2009. “Impact of Pay for Performance on Ethnic Disparities in Intermediate Outcomes for Diabetes: A Longitudinal Study.” Diabetes Care 32 (3): 404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen, B. 1997. “Latent Variable Modeling of Longitudinal and Multilevel Data.” Sociological Methodology 27: 453–80. [Google Scholar]
- National Committee for Quality Assurance . 2011. The State of Health Care Quality 2011: Continuous Improvement and the Expansion of Quality Measurement. Washington, DC: The State of Health Care Quality. [Google Scholar]
- Parekh, A. K. , Goodman R. A., Gordon C., and Koh H. K.. 2011. “Managing Multiple Chronic Conditions: A Strategic Framework for Improving Health Outcomes and Quality of Life.” Public Health Reports 126 (4): 460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette, J. D. , and Kerr E. A.. 2006. “The Impact of Comorbid Chronic Conditions on Diabetes Care.” Diabetes Care 29 (3): 725–31. [DOI] [PubMed] [Google Scholar]
- Ricci‐Cabello, I. , Violan C., Foguet‐Boreu Q., Mounce L. T., and Valderas J. M.. 2015. “Impact of Multi‐Morbidity on Quality of Healthcare and Its Implications for Health Policy, Research and Clinical Practice. A Scoping Review.” The European Journal of General Practice 21 (3): 192–202. [DOI] [PubMed] [Google Scholar]
- Skyler, J. S. , Bergenstal R., Bonow R. O., Buse J., Deedwania P., Gale E. A., Howard B. V., Kirkman M. S., Kosiborod M., Reaven P., and Sherwin R. S.. 2009. “Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials: A Position Statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.” Diabetes Care 32 (1): 187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Soubhi H., Fortin M., Hudon C., and O'Dowd T.. 2012. “Interventions for Improving Outcomes in Patients with Multimorbidity in Primary Care and Community Settings.” Cochrane Database Systematic Review 4: CD006560. [DOI] [PubMed] [Google Scholar]
- Sorace, J. , Wong H. H., Worrall C., Kelman J., Saneinejad S., and MaCurdy T.. 2011. “The Complexity of Disease Combinations in the Medicare Population.” Population Health Management 14 (4): 161–6. [DOI] [PubMed] [Google Scholar]
- Stellefson, M. , Dipnarine K., and Stopka C.. 2013. “The Chronic Care Model and Diabetes Management in US Primary Care Settings: A Systematic Review.” Preventing Chronic Disease 10: E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, N. J. , Robinson J. G., Lichtenstein A. H., Bairey Merz C. N., Blum C. B., Eckel R. H., Goldberg A. C., Gordon D., Levy D., Lloyd‐Jones D. M., McBride P., Schwartz J. S., Shero S. T., Smith S. C. Jr, Watson K., Wilson P. W., Eddleman K. M., Jarrett N. M., LaBresh K., Nevo L., Wnek J., Anderson J. L., Halperin J. L., Albert N. M., Bozkurt B., Brindis R. G., Curtis L. H., DeMets D., Hochman J. S., Kovacs R. J., Ohman E. M., Pressler S. J., Sellke F. W., Shen W. K., and Tomaselli G. F.. 2014. “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.” Circulation 129 (25 Suppl 2): S1–45. [DOI] [PubMed] [Google Scholar]
- Wisconsin Collaborative for Healthcare Quality . 2011. WCHQ Ambulatory Measure Specification: Diabetes Care Performance Measures. Middleton, WI: Wisconsin Collaborative for Healthcare Quality. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


