Abstract
Objective
To examine the contributions of individual‐ and neighborhood‐level spatial access to care to the utilization of emergency departments (EDs) for preventable conditions through implementation of novel local spatial access measures.
Data Sources/Study Setting
Emergency department admissions data are from four HealthLNK member hospitals in Chicago from 2007 to 2011. Primary care physician office and clinic locations were obtained from the American Medical Association and the City of Chicago.
Study Design
Multilevel logit regression was used to model the relationship between individual‐ and neighborhood‐level attributes and preventable ED use.
Data Collection/Extraction Methods
Emergency department admissions data were classified based on the primary diagnosis for each encounter. Spatial access to care indices were generated in ArcGIS, and values were extracted at each ZIP code centroid to match patients' ZIP codes.
Principal Findings
Beyond sociodemographic factors such as gender and race, patients living in medically underserved areas (MUAs) and areas with lower spatial access to primary care clinics had higher odds of preventable ED use.
Conclusions
Preventable ED use can be associated with sociodemographic characteristics, as well as spatial access to primary care services. This study reveals potential for using local measures of spatial accessibility for preventable ED analyses.
Keywords: Spatial access, GIS, preventable ED admissions
Use of hospital emergency departments (EDs) for conditions that are treatable in a primary care setting is a significant concern in the United States, leading to high costs, ED overcrowding, and in some cases poor health outcomes for patients. Providing care in the ED for these primary care treatable conditions (referred to as PCED) diverts staff and resources from patients whose conditions require immediate attention, representing a misallocation of health care services (Institute of Medicine, 2006; Newton et al. 2008). Although the causes of PCED are complex, PCED fundamentally reflects health care access issues—barriers that prevent patients from obtaining health services that are appropriate for their needs. Research shows that patients who face economic, geographic, and social barriers to primary care are more likely to use the ED for primary care treatable conditions than are other patients (Billings et al. 1993; Laditka, Laditka, and Probst 2005; Mathison et al. 2013). Such barriers are rooted in the local geographic contexts in which people live and interact.
This research examines the role of geographic factors in PCED utilization by developing and applying detailed measures of spatial accessibility to diverse primary health care services. Combining a large dataset on ED encounters at a subset of Chicago hospitals with geospatial data on primary care physicians, clinics, hospitals, and medically underserved areas (MUAs), we analyze spatial and socioeconomic factors in PCED utilization. We implement and test local spatial statistical measures of service accessibility that show fine‐grained variation in access to services across the city. Local measures are ones that reveal spatial differences within a study region (Lloyd 2011; Wang 2015), thus capturing important geographic variation within large areas such as counties and metropolitan regions. These measures are included in a multilevel random effects logit regression model of PCED use.
Background
Although most people admitted to the ED do require emergency care, a significant portion—anywhere from 8 percent (Johnson et al. 2012) to nearly one‐third (DeLia 2006)—visit the ED for visits which could be managed at a lower acuity setting. Preventable visits by Medicaid enrollees have grown significantly and implementation of the Affordable Care Act in 2014 is expected to stimulate even more growth (Johnson et al. 2012). To improve outcomes and care quality, limiting intensive, high‐cost, ED‐based care for primary care treatable conditions is critically important (Agency for Healthcare Research and Quality, 2011; Johnson et al. 2012).
Why do people use hospital EDs to obtain care for primary care treatable conditions? Researchers argue that both patient demand factors and health services supply and availability factors are important. Demand factors include patient‐level predisposing and enabling factors that influence people's knowledge of and ability to access appropriate primary care services for routine conditions. Education, demographics, financial and insurance resources, and access to transportation are among the demand/patient‐level influences on PCED utilization. Supply‐side factors refer to characteristics of local health care systems that impact people's ability to use appropriate health services when needed. These include the locations, number, quality, waiting times, and hours of operation of primary care physicians, clinics, and other health care providers.
With respect to demand factors, variables such as education, income, race, and insurance status have been examined. Analyzing data from the National Hospital Ambulatory Care Survey, Johnson et al. (2012) observed higher preventable ED use among those who were female, non‐Hispanic black or Hispanic, older, or publically insured. Areas with large concentrations of ethnic and racial minority populations have been shown to have high rates of preventable use (McWilliams et al. 2011; Johnson et al. 2012). Additionally, low rates of high school graduation have been associated with high rates of preventable ED utilization (Bindman et al. 1995; Laditka, Laditka, and Probst 2005).
Studies also observe strong and complex associations between economic factors such as income and health insurance coverage and preventable ED use. Poverty and low‐income population concentration are important predictors of high rates of both nonemergent ED use and hospitalizations for conditions potentially responsive to timely outpatient management (Billings et al. 1993; Jiang et al. 2014). At the same time, however, high‐income populations may also exhibit high preventable ED use because they face few financial barriers to care (Laditka, Laditka, and Probst 2005). Health insurance is also important, as those who have insurance coverage, especially public insurance coverage, were found in some studies to be more likely to use the ED for primary care treatable conditions than are those who lack coverage (Alpern et al. 2014; Oster and Bindman 2003).
Evidence indicates that supply‐side factors are also important in preventable ED utilization (Rust et al. 2008). Spatial accessibility of health services is a key supply‐side factor that may influence people's likelihood of PCED. Spatial accessibility refers to geographical factors that affect the ability to use health services, including the location and number of health providers and the distances and transportation networks linking them to local populations (Wang and Luo 2005). In the case of PCED, we hypothesize that people with poor spatial access to care—that is, people who live in neighborhoods with few primary care providers available and who face long travel distances and times to obtain care—are more likely to rely on hospital EDs for needed services.
Although several studies include mapping of preventable ED use rates to understand the neighborhoods and populations most at risk of such utilization (Gresenz, Ruder, and Lurie 2009; Dulin et al. 2010), only a few studies have taken the next step to examine if high rates are associated with poor spatial access to primary care. Chen et al. (2015) found that patients living further from an ED were less likely to use the ED for nonurgent conditions than those living nearby. Examining PCED rates by ZIP code in Houston, Begley et al. (2006) uncovered an inverse association between PCED and the Index of Medical Underservice score, a multivariate index that includes the ratio of primary care physicians to population as one of its components. Finally, using kernel density estimation (KDE), Mathison et al. (2013) found that a low spatial density of primary care physicians in Washington, D.C., was associated with high rates of nonurgent pediatric ED utilization.
The Mathison et al. (2013) study illustrates the insights gained from utilizing detailed local measures of spatial access to services in analyzing PCED. Past research has often relied on access measures for discrete geographic zones such as counties or census tracts. Such measures are limited by their failure to incorporate flows of patients from one zone to another and to model variation in access within zones (Luo and Wang 2003). In contrast, local spatial methods involve estimating values and associations within subareas of a study region (Lloyd 2011). The subareas comprise spatial windows or filters which reveal fine‐grained patterns of spatial variation. Innovative local methods, including the two‐step floating catchment area method and KDE, have been developed for measuring spatial accessibility to health care (Mao and Nekorchuk 2013; Wang 2015).
This study develops and estimates detailed local measures of spatial accessibility to primary care clinics and physicians, along with designation as a medically underserved or primary care shortage area, to analyze the statistical associations between PCED use and spatial access to services. A large clinical dataset comprising all ED visits for four Chicago hospitals over a 5‐year period is used for illustrative purposes. Associations between patient demographic characteristics and insurance status, and ZIP code–level measures of spatial accessibility to primary care and socioeconomic deprivation are estimated via multilevel modeling.
Data and Methods
Clinical Data
The clinical data come from the Chicago HealthLNK Data Repository (HealthLNK), a fully operational health data exchange composed of both merged and deduplicated patient electronic health records. HealthLNK includes patient demographic and ED visit data from three academic medical centers and one safety‐net hospital in the City of Chicago.
The complete dataset includes all ED patient encounters (visits) and associated diagnoses at the four contributing hospitals over 5 years, 2007–2011, totaling over 560,000 encounters from billing records of patients aged 18–87 whose home address was in the City of Chicago. For each individual patient encounter at the ED, it is possible to have multiple diagnoses; however, only the primary diagnosis was used as the indicator of PCED. Additionally, if a patient visited the ED several times over the 5‐year span, each encounter was used as a separate observation in the statistical models. Joined by a common patient ID variable, the dataset is composed of two merged data tables: a patient demographics table and a diagnosis table, including month and year of encounter, International Classification of Diseases Ninth Revision diagnosis code, and a flag to denote which diagnosis was the primary diagnosis.
PCED conditions were classified based on the ED classification algorithm developed by Billings and colleagues (Billings et al. 2000; New York University, n.d.). The Billings algorithm has been used widely across research studies examining the types of conditions presenting to EDs (Begley et al. 2006; DeLia 2006; Weinick, Burns, and Mehrotra 2010) and has been statistically validated as accurately differentiating ED visits based on the need for hospitalization or mortality risk (Ballard et al. 2010). Although some previous studies have used different classifications (e.g., ambulatory care sensitive conditions) to study preventable hospital and ED utilization, the diagnoses identified in the Billings algorithm have the advantage that they are specific to the ED setting and are well validated.
For each diagnosis, the algorithm gives percentage values referring to the likelihood that the diagnosis is one of four different categories: (i) Non‐Emergent/Primary Care treatable; (ii) Emergent/Primary Care Treatable; (iii) Emergent, ED Care Needed, Preventable/Avoidable; and (iv) Emergent, ED Care Needed, Not Preventable/Avoidable (New York University, n.d.). Percentage scores indicate the likelihood that a diagnosis falls within each of the four groups.
We defined PCED diagnoses as all diagnoses considered to be 100 percent Non‐Emergent/Primary Care Treatable by the algorithm. We chose to identify PCED diagnoses only as those with 100 percent Nonemergent status and to model those against only 100 percent Not Preventable/Avoidable status, respectively, so as to reduce any ambiguity in potential severity of the condition. Only observations from patients residing in the City of Chicago were included in the analysis. Overall, 144 conditions from the algorithm were used to flag diagnoses as PCED.
Spatial and Socioeconomic Data
Predictors of PCED included measures of spatial accessibility to diverse health services and socioeconomic characteristics of ZIP code populations. To model spatial accessibility to primary care physicians, locations of primary care physician offices in the Chicago metro area were obtained from the 2008 American Medical Association Physician's Masterfile. Physicians were geocoded to point locations based on the office address, and separate points were assigned to physicians in shared offices. Approximately 6 percent of physicians did not report an office address and therefore were geocoded to the mailing address location (McLafferty et al. 2012). Physicians whose offices were within 10 km of the Cook County boundary were included in the dataset to account for potential use of services in neighboring counties. Primary care physicians were classified as any physician with the specialty of Internal Medicine, Family Medicine, or General Practitioner.
Locations of publicly funded primary care clinics within Chicago were geocoded to addresses. Obtained from the City of Chicago, the clinic data include Federally Qualified Health Centers, and primary care clinics run by government agencies and other health care providers. HealthLNK hospitals were also geocoded to their address locations. Geographic boundaries of federally defined Primary Medical Care Health Professional Shortage Areas (HPSAs) and MUAs were downloaded from the U.S. Department of Health and Human Services. Data from the American Community Survey's 2009–2013 5‐year estimates (United States Census Bureau, 2011a,b,c) on vehicle ownership, median income, and percent bachelor's degrees were obtained at the ZIP code level to match the geography of the clinical dataset.
Statistical Methods
To analyze the contributions of individual‐ and neighborhood‐level (ZIP code) factors to PCED use, a two‐level random effects regression model was estimated with individual‐level and ZIP code‐level predictor variables. The ZIP code level was used, because, to protect privacy and confidentiality, the clinical data only include the patient's ZIP code of residence. The model is a logit regression, with random intercepts and fixed slopes and patient ZIP code as the grouping variable. The dependent variable was a binary variable indicating whether the presenting primary diagnosis for each patient encounter to the ED was 100 percent “Non‐Emergent/Primary Care Treatable” or 100 percent “Emergent, ED Care Needed, Not Preventable/Avoidable” according to the algorithm by Billings, Parikh, and Mijanovich (2000). Thus, the model identifies factors that differentiate PCED encounters from encounters for emergency, nonprimary care treatable conditions. In addition, because of moderate collinearity among the spatial access to primary care variables, we estimated a series of regression models that included each of the spatial accessibility variables separately.
Patient‐level characteristics included patient gender, race, ethnicity, age at encounter, and insurance coverage as derived from the HealthLNK dataset. For race, groups with small numbers—American Indian/Alaska Native, Native Hawaiian or Other Pacific Islander, Declined, and Other—were combined into the “Other” category, leaving four race categories: white, black, Asian, and other. Hispanics were removed from the race categories and classified separately in the ethnicity variable. Additionally, for insurance status, No Charge, and Other were combined into the “Other” category, leaving Private, Medicaid, Medicare, Self‐pay, and Other as the five insurance categories. Patient age was calculated as the year of ED encounter minus the year of birth.
At the ZIP code level, variables include median household income and percent of ZIP code adult population with bachelor's degree or higher, as well as measures describing spatial access to health services.
Spatial Access Measures
Several indicators of spatial access to health services were incorporated in the multilevel model. To measure spatial access to primary care physicians, we utilized two local spatial methods: (i) the enhanced two‐step floating catchment area method (E2SFCA) (Luo & Qi 2009), and (ii) KDE (Guagliardo 2004).
The E2SFCA is a two‐stage spatial accessibility method that generates estimates of the local match between available physicians (or other health services) and population in need of those services (Luo & Qi 2009). A distance decay effect—a decline in access with increasing distance or time from physician services—is incorporated in this approach. Overlapping spatial windows are used to compute values in each of the method's two stages. The E2SFCA begins by identifying the region within a fixed travel time or distance of each physician office location and subdividing that region into zones based on travel time. We used zones of 0–10, 10–20, and 20–30 minutes. Populations within each zone are weighted based on a chosen distance decay function and summed, and the physician to (weighted) population ratio is computed for that office location. In the second step, the E2SFCA shifts to each population location and computes the weighted sum of physician to population ratios within the fixed travel time radius of that location. The resulting values represent the travel‐time‐weighted physician to population ratios for each population zone. Using Luo and Qi's (2009) distance decay weights, we computed these ratios for census tracts in Chicago and then determined the population‐weighted average of the census tract values for each ZIP code. An important feature of the E2SFCA is that it incorporates travel by car via the road network, reflecting typical speeds and network barriers.
The second local method, KDE, is a method for estimating the intensity or density of points (in this case, physicians) per unit area. Density is represented as a continuous field in which peaks correspond to areas of high physician density and valleys areas of low density. Density at location s (λ(s)) is estimated as:
where, d i = distance from physician i to location s; τ = bandwidth; k( ) = kernel weighting function.
The density of points is calculated within a specified radius distance (bandwidth), and a larger radius results in a more generalized surface. To estimate physician to population ratios, we divided the physician density surface by a similar surface representing population density (based on population data by census tract), resulting in a smooth surface that shows geographic variation in local physician to population ratios. Kernel estimation has been used in evaluating spatial access to health services such as physicians and clinics (Guagliardo 2004; McLafferty and Grady 2005).
Because access to transportation, especially automobile transit, strongly influences people's ability to obtain primary care, we modified the kernel density method to incorporate vehicle ownership. Our index assumes that people with cars can more easily visit primary care physicians located farther away from their homes as compared to people without cars. The index is created first by producing primary care provider to car owning and noncar owning population ratios at different kernel density bandwidths, 8 km and 3 km, respectively, to represent greater mobility for people with cars than for those without cars. Then, the two provider to population ratios are multiplied by the proportion of tract population that does or does not own cars, respectively, and then added together to form a composite accessibility surface. The following equation models the composite index.
A limitation of KDE compared to the E2SFCA is that the former uses Euclidean distance, while the latter uses network travel distance. However, our modified KDE incorporates transportation access, which is important in an urban setting. Although both methods have strengths and limitations (Neutens 2015), their resulting local physician to population ratios are likely to be highly correlated because they estimate the spatial match between physicians and population based on the same data inputs. In this research, results were almost identical for each method, so for brevity, only the kernel method results are reported.
The map of the kernel‐based primary care physician spatial accessibility index (Figure 1) reveals uneven geographical availability of physicians across Chicago. Using GIS, values of the index were extracted at the population‐weighted centroids of each ZIP code for use as ZIP code–level variables in the multilevel regression model.
Figure 1.
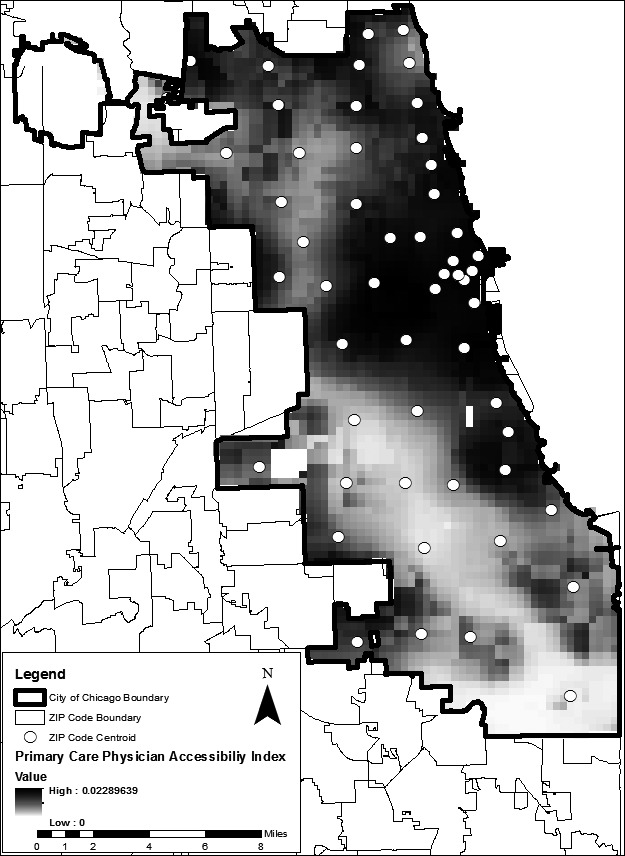
Primary Care Physician Spatial Accessibility Index Based on Kernel Estimation, Chicago, IL
To measure spatial accessibility to primary health care clinics, a KDE surface was created from locations of Federally Qualified Health Centers and other publically funded primary health clinics in the City of Chicago, all geocoded to the clinic address. The kernel bandwidth for the clinic index, 5 km, was determined manually, based on fit and overall coverage within the study area. As in the case of physician spatial accessibility, values of the clinic density surface were extracted to the population‐weighted centroid of each ZIP code to be included in the multilevel regression model. Higher values of the clinic density index indicate greater spatial availability of publicly funded clinics.
GIS was used to create variables measuring the percent of each ZIP code area designated as MUA and the percent designated as HPSA. MUAs and HPSAs are designated at the census tract level and therefore it was necessary to calculate the percent of each ZIP code that consisted of MUA‐ and HPSA‐designated census tracts. These percentages were included in the random effects logit regression as predictor variables. Finally, distance to hospital was calculated as the distance from the population‐weighted centroid of each ZIP code to the nearest HealthLNK hospital.
Results
Spatial and Social Characteristics of PCED Encounters
For the four hospitals studied, there were many more PCED encounters than non‐PCED encounters (Table 1). In terms of demographics, women made up a higher portion of all ED visits compared to men, but an even higher percentage of PCED visits. Additionally, the youngest cohort of patients made up a higher proportion of PCED visits, while the older age cohorts were more strongly represented among non‐preventable visits (Table 2).
Table 1.
Descriptive Statistics of ED Use for Categorical Variables
| Variable | Group | PCED % (n = 22,221) | Nonprev % (n = 9,787) | Total ED % (n = 567,672) |
|---|---|---|---|---|
| Overall | 3.91 | 1.72 | 100 | |
| Gender | Male | 23.43 | 43.33 | 38.46 |
| Female | 76.42 | 56.44 | 61.36 | |
| Race | White | 18.06 | 20.62 | 22.63 |
| Black | 58.34 | 53.30 | 53.13 | |
| Asian | 1.27 | 1.42 | 1.58 | |
| Other | 8.72 | 10.05 | 9.32 | |
| Ethnicity | Non‐Hispanic | 78.68 | 77.32 | 78.42 |
| Hispanic | 16.80 | 18.25 | 16.99 | |
| Insurance | Private | 36.52 | 28.89 | 35.95 |
| Medicaid | 27.37 | 20.81 | 20.43 | |
| Medicare | 19.33 | 34.62 | 24.03 | |
| Self‐pay | 12.78 | 11.84 | 14.79 | |
| Other | 3.42 | 2.82 | 3.91 | |
| Age | 18–30 | 41.53 | 22.50 | 30.85 |
| 31–45 | 29.92 | 23.68 | 29.78 | |
| 46–64 | 20.16 | 32.35 | 27.25 | |
| 65 or older | 8.19 | 21.07 | 11.84 |
Table 2.
Descriptive Statistics of ED Use by Socioeconomic and Spatial Variables
| City of Chicago | |||
|---|---|---|---|
| Nonemergent (PCED), Mean (SD) | Nonpreventable, Mean (SD) | All Diagnoses, Mean (SD) | |
| Median Household Income (dollars) | 43,525.8 (17,960.48) | 43,973.16 (17,806.9) | 45,052.28 (18,559.55) |
| Percent Bachelor's Attained | 28.55 (23.25) | 28.65 (23.09) | 30.69 (24.20) |
| Primary Care Physician Accessibility Index | 0.004437 (0.0033) | 0.004453 (0.0033) | 0.004623 (0.0033) |
| Primary Care Clinic Index | 0.05465 (0.032) | 0.05313 (0.031) | 0.05507 (0.032) |
| Percent Medically Underserved Area (%) | 28.47 (18.83) | 27.35 (18.64) | 27.47 (18.71) |
| Percent Health Professional Shortage Area (%) | 46.58 (26.13) | 45.36 (26.16) | 44.55 (26.85) |
| Distance to Hospital (m) | 5,453.37 (3,375.25) | 5,422.96 (3,454.62) | 5,361.359 (3,468.21) |
The ratio of PCED conditions to nonpreventable conditions by ZIP code varies considerably across the City of Chicago (Figure 2). The highest ratios are found in the western and northern ZIP codes of the city, while individual ZIP codes with low ratios were found near the center of the study area, south and west of downtown and on the south side of the city. ZIP codes with no data or low non‐preventable utilization are left blank on the map because their ratio values are not statistically valid due to small numbers.
Figure 2.
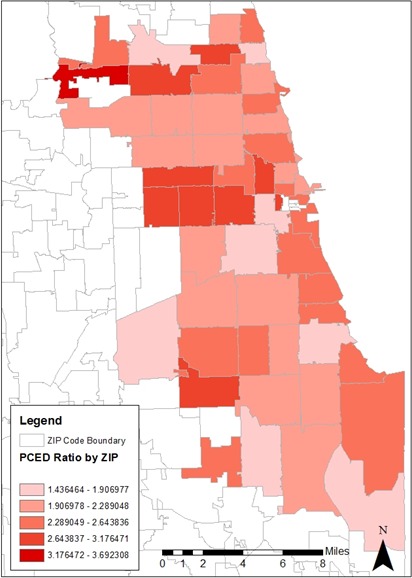
Ratio of PCED Encounters to Nonpreventable Encounters by ZIP Code
Individual‐Level Results
All logit coefficient estimates presented in Table 3 were transformed to odds ratios for ease of interpretation and 90 percent confidence intervals were used to determine statistical significance. Among the individual‐level variables, being female was associated with higher PCED utilization: Women were more than twice as likely as men to use the ED for PCED conditions. Race was also significantly associated with PCED utilization for certain groups. In comparison to White patients, Black patients were more likely to have PCED diagnoses. Ethnicity was also significantly associated with PCED utilization. Hispanic patients had a 37 percent higher odds of PCED use compared to non‐Hispanic patients.
Table 3.
Multilevel Regression Results—All Variables
| Variable | Factor | Odds Ratio | Z | P > z |
|---|---|---|---|---|
| Gender | Male | (ref.) | ||
| Female | 2.075 | 23.53 | <.001*** | |
| Race | White | (ref.) | ||
| Black | 1.214 | 4.32 | <.001*** | |
| Asian | 0.909 | −0.8 | .424 | |
| Other | 0.771 | −3.39 | .001** | |
| Ethnicity | Non‐Hispanic | (ref.) | ||
| Hispanic | 1.375 | 4.04 | <.001*** | |
| Insurance | Private | (ref.) | ||
| Medicaid | 0.808 | −5.06 | <.001*** | |
| Medicare | 0.702 | −8.91 | <.001*** | |
| Self‐pay | 0.881 | −2.64 | .01** | |
| Other | 0.908 | −1.02 | .269 | |
| Age at Encounter | 0.973 | −28.5 | <.001*** | |
| Median Household Income | 0.999 | −1.73 | .084* | |
| Percent Bachelor's Degree Attained | 1.008 | 2.97 | .003** | |
| Kernel Primary Care Accessibility Index (log) | 1.042 | 0.67 | .504 | |
| Primary Care Clinic Index (log) | 0.945 | −1.65 | .098* | |
| Percent Designated MUA | 1.002 | 1.82 | .064* | |
| Percent Designated HPSA | 0.999 | −0.97 | .331 | |
| Distance to Hospital (log) | 1.051 | 1.36 | .175 | |
| Constant | 3.316 | 3.55 | <.001*** | |
| Regression with only one area‐level spatial access variable | ||||
| Kernel Primary Care Accessibility Index (log) | 1.060 | 1.04 | .297 | |
| E2SFCA Primary Care Accessibility Index (log) | 1.073 | 0.70 | .483 | |
| Primary Care Clinic Index (log) | 0.954 | −1.33 | .184 | |
*p < .1, **p < .01, ***p < .001.
Insurance status also played a significant role in PCED utilization for nearly every category. Compared to patients with private insurance, patients covered under Medicaid were less likely to visit the ED for PCED conditions, as were patients covered under Medicare who had the lowest odds ratio of any insurance type. The self‐pay category also had a reduced odds of PCED utilization compared to privately insured patients. Finally, being younger was associated with higher PCED utilization.
Neighborhood‐Level Results
At the neighborhood (ZIP code) level, several spatial and socioeconomic variables were significantly associated with PCED utilization (Table 3). Spatial access to clinics had the expected relationship with PCED: a high density of publicly funded primary care clinics was associated with lower odds of PCED utilization. This finding suggests that patients living closer to primary care clinics are less likely to go to the ED for primary care treatable conditions. The MUA variable also was significantly associated with PCED: people residing in ZIP codes with a higher percentage of land designated as MUA had higher odds of PCED utilization. Beyond spatial factors, the two socioeconomic variables affected PCED. As ZIP code median household income increases, the odds of PCED utilization decrease; while for education, a higher percent of residents with bachelor's degrees was associated with a higher odds of PCED utilization—an unexpected finding. The variables for spatial access to primary care physicians (both kernel and E2SFCA), HPSA designation, and distance from HealthLNK hospital were nonsignificant in all regression models conducted.
Discussion
Use of EDs for primary care treatable conditions is related to a complex set of socioeconomic and health care access factors. Through development and implementation of novel local measures of spatial access to care, this research has identified some of the factors important for the patients visiting a sample of EDs in Chicago. Our findings reveal certain specific indicators of local spatial access to primary care services that are moderately associated with PCED use. Patients living in MUAs were more likely to utilize the ED for PCED conditions, as was expected from previous research suggesting that low physician availability is linked with high rates of preventable hospitalizations (Laditka, Laditka, and Probst 2005). Moreover, patients living in areas with high spatial access to primary care clinics were less likely to go to the ED for PCED visits, a finding similar to that reported in Mathison et al. (2013). Unexpectedly, the two local measures of spatial access to primary care physicians had no statistical association with PCED. This may reflect the fact that we estimated access based on all office‐based primary care physicians, without taking into account the kinds of insurance they accept, their hours of operation, languages spoken, and other dimensions of access. Still, the importance of spatial access to clinics, as compared to primary care physicians, suggests that locally accessible clinics that offer a range of preventive health services may be crucial for reducing use of hospital EDs for PCED conditions.
Beyond spatial access to primary care, we found that women, non‐White racial and ethnic minorities, and younger patients were more likely to come to the ED with a condition that could be treated in a clinic. These findings support the results of previous studies related to gender and race/ethnicity (Billings, Parikh, and Mijanovich 2000; Laditka, Laditka, and Mastanduno 2003; Johnson et al. 2012; Oster and Bindman 2003); however, our findings differ from those presented in the literature with respect to age (Johnson et al. 2012). The preponderance of younger patients in our PCED population may reflect the types of diagnoses used in identifying PCED in this study. Specifically, our PCED diagnoses include certain types of conditions that are not relevant for elderly people, for example, issues related to childbirth.
Our findings suggest that socioeconomic characteristics of the neighborhoods in which people live and access care also affect PCED rates. Patients living in areas with higher median incomes were less likely to go to the ED with PCED conditions, while a higher overall educational attainment of an area was linked with higher PCED utilization. The inverse relationship between income and PCED is supported by previous research (Billings et al. 1993); however, the finding that higher levels of education attainment were associated with higher odds of PCED utilization was unexpected. It may reflect a mismatch in the model between the patients actually using the HealthLNK EDs and the overall average educational attainment derived from census data.
This research contributes to the literature on primary care preventable ED visits by developing and applying novel local measures of spatial access to primary care. In combination, these measures represent the local medical services landscapes—including physicians, clinics, and hospitals—that people encounter in making decisions about where to obtain health care. The measures have distinct advantages: the E2SFCA incorporates estimated travel times from residential populations to physician office locations, and the kernel method incorporates disparities in access between populations with and without access to cars. Furthermore, recent advances in local spatial analysis enable researchers to make the methods even more realistic. For example, a recent version of the E2SFCA models access for populations with multiple transportation modes (Mao and Nekorchuk 2013), while innovative new kernel methods incorporate network travel times and distances (Xie and Yan 2013). These methods offer exciting opportunities not just for PCED research but for work in many areas of health services research.
While many of our findings were expected, we nevertheless encountered some unexpected results that may partly reflect the study design. Specifically, our use of diagnoses only treatable in the ED as the control group may have affected the model results. Such diagnoses are truly emergent in nature and include events such as heart attacks, stroke, and trauma. In modeling the dichotomy between diagnoses only treatable in the ED and PCED, we may be capturing factors that are more associated with the control group—nonpreventable, emergency ED visits—than with specific contexts or behaviors associated with PCED. Another general limitation is that due to privacy restrictions, the geographical analyses were limited to ZIP codes which are relatively large and heterogeneous areal units. As a result, the spatial access measures are less spatially accurate than they would be if census tracts, or even individual addresses were used.
There are also limitations from the clinical data perspective. A more complete study would utilize data from all EDs in the Chicago area and would have patients' full ED records to model spatial predictors of PCED utilization beyond the HealthLNK hospitals. We were unable to obtain access to ED data from any other hospitals, therefore we cannot fully capture the full extent of PCED utilization in Chicago. Still, despite having only a fraction of the data from Chicago EDs, the very large sample size of over 500,000 patient encounters over 5 years facilitates exploratory statistical analysis.
This research indicates that among the four hospitals sampled in the City of Chicago, PCED use is related to the sociodemographic characteristics of individuals and the neighborhoods in which they live, as well as their spatial access to primary care services. Linking hospital‐based data with GIS‐derived local spatial measures makes it possible to investigate influences on ED use for diverse populations in diverse geographic settings. Such approaches help determine not just what populations are at most risk for PCED utilization but also where patients live who are at highest risk, and what barriers they face in obtaining timely primary care that could ultimately decrease PCED utilization.
Supporting information
Appendix SA1: Author Matrix
Acknowledgements
Joint Acknowledgement/Disclosure Statement: The physician data for this project were obtained with the support of the American Cancer Society, under grant no. ACS RSGT‐09‐286‐01‐CPHPS, Dr. Vincent Freeman, Principal Investigator.
Disclosures: No other disclosures.
Disclaimers: None.
References
- Agency for Healthcare Research and Quality . 2011. “National Healthcare Disparities Report” [accessed December 30, 2014]. Available at http://www.ahrq.gov/research/findings/nhqrdr/nhdr11/nhdr11.pdf
- Alpern, E. R. , Clark A. E., Alessandrini E. A., Gorelick M. H., Kittick M., Stanley R. M., Dean J. M., Teach S. J., and Chamberlain J. M.. 2014. “Recurrent and High‐Frequency Use of the Emergency Department by Pediatric Patients.” Academic Emergency Medicine 21 (4): 365–73. doi:10.1111/acem.12347. [DOI] [PubMed] [Google Scholar]
- Ballard, D. W. , Price M., Fung V., Brand R., Reed M. E., Fireman B., Newhouse J. P., Selby J. V., and Hsu J.. 2010. “Validation of an Algorithm for Categorizing the Severity of Hospital Emergency Department Visits.” Medical Care 48 (1): 1–15. doi:10.1097/MLR.0b013e3181bd49ad.Validation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley, C. E. , Vojvodic R. W., Seo M., and Burau K.. 2006. “Emergency Room Use and Access to Primary Care: Evidence from Houston, Texas.” Journal of Health Care for the Poor and Underserved 17 (3): 610–24. doi:10.1353/hpu.2006.0098. [DOI] [PubMed] [Google Scholar]
- Billings, J. , Parikh N., and Mijanovich J.. 2000. “Emergency Department Use: The New York Story.” The Commonwealth Fund 434: 1–12. Available at http://www.commonwealthfund.org/usr_doc/billings_nystory.pdf [PubMed] [Google Scholar]
- Billings, J. , Zeitel L., Lukomnik J., Carey T. S., Blank A. E., and Newman L.. 1993. “Impact of Socioeconomic Status on Hospital Use in New York City.” Health Affairs 12 (1): 162–73. doi:10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- Bindman, A. B. , Grumbach K., Osmond D., Komaromy M., Vranizan K., Lurie N., Billings J., and Stewart A.. 1995. “Preventable Hospitalizations and Access to Health Care.” Journal of the American Medical Association 275 (4): 305–11. [PubMed] [Google Scholar]
- Chen, B. K. , Cheng X., Bennett K., and Hibbert J.. 2015. “Travel Distances, Socioeconomic Characteristics, and Health Disparities in Nonurgent and Frequent Use of Hospital Emergency Departments in South Carolina: A Population‐Based Observational Study.” BMC Health Services Research 15: 203. doi:10.1186/s12913‐015‐0864‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLia, D. 2006. “Potentially Avoidable Use of Hospital Emergency Departments in New Jersey” [accessed February 10, 2014]. Available at http://www.cshp.rutgers.edu/Downloads/6330.pdf
- Dulin, M. F. , Ludden T. M., Tapp H., Blackwell J., de Hernandez B. U., Smith H. A., and Furuseth O. J.. 2010. “Using Geographic Information Systems (GIS) to Understand a Community's Primary Care Needs.” Journal of the American Board of Family Medicine 23 (1): 13–21. doi:10.3122/jabfm.2010.01.090135. [DOI] [PubMed] [Google Scholar]
- Gresenz, C. R. , Ruder T., and Lurie N.. 2009. “Ambulatory Care Sensitive Hospitalizations and Emergency Department Visits in Baltimore City” [accessed February 10, 2014]. Available at http://www.rand.org/content/dam/rand/pubs/technical_reports/2009/RAND_TR671.pdf
- Guagliardo, M. F. 2004. “Spatial Accessibility of Primary Care: Concepts, Methods and Challenges.” International Journal of Health Geographics 3 (3): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . 2006. “The Future of Emergency Care: Key Findings and Recommendations” [accessed December 30, 2014]. Available at https://www.ncbi.nlm.nih.gov/books/NBK220327/
- Jiang, Y. , Novais A., Viner‐Brown S., and Fine M.. 2014. “Non‐Emergent Hospital Emergency Department Use and Neighborhood Poverty in Rhode Island, 2008‐2012.” Rhode Island Medical Journal July: 47–51. [PubMed] [Google Scholar]
- Johnson, P. J. , Ghildayal N., Ward A. C., Westgard B. C., Boland L. L., and Hokanson J. S.. 2012. “Disparities in Potentially Avoidable Emergency Department (ED) Care.” Medical Care 50 (12): 1. [DOI] [PubMed] [Google Scholar]
- Laditka, J. N. , Laditka S. B., and Mastanduno M. P.. 2003. “Hospital Utilization for Ambulatory Care Sensitive Conditions: Health Outcome Disparities Associated with Race and Ethnicity.” Social Science & Medicine 57 (8): 1429–41. doi:10.1016/S0277‐9536(02)00539‐7. [DOI] [PubMed] [Google Scholar]
- Laditka, J. N. , Laditka S. B., and Probst J. C.. 2005. “More May Be Better: Evidence of a Negative Relationship between Physician Supply and Hospitalization for Ambulatory Care Sensitive Conditions.” Health Services Research 40 (4): 1148–66. doi:10.1111/j.1475‐6773.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, C. 2011. Local Models for Spatial Analysis, 2d Edition New York: CRC Press. [Google Scholar]
- Luo, W. , and Qi Y.. 2009. “An Enhanced Two‐Step Floating Catchment Area (E2SFCA) Method for Measuring Spatial Accessibility to Primary Care Physicians.” Health & Place 15 (4): 1100–7. doi:10.1016/j.healthplace.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Luo, W. , and Wang F.. 2003. “Measures of Spatial Accessibility to Health Care in a GIS Environment: Synthesis and a Case Study in the Chicago Region.” Environment & Planning B 30: 865–84. doi:10.1068/b29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , and Nekorchuk D.. 2013. “Measuring Spatial Accessibility to Healthcare for Populations with Multiple Transportation Modes.” Health and Place 24: 115–22. doi:10.1016/j.healthplace.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Mathison, D. J. , Chamberlain J. M., Cowan N. M., Engstrom R. N., Fu L. Y., Shoo A., and Teach S. J.. 2013. “Primary Care Spatial Density and Nonurgent Emergency Department Utilization: A New Methodology for Evaluating Access to Care.” Academic Pediatrics 13 (3): 278–85. doi:10.1016/j.acap.2013.02.006. [DOI] [PubMed] [Google Scholar]
- McLafferty, S. , and Grady S.. 2005. “Immigration and Geographic Access to Prenatal Clinics in Brooklyn, NY: A Geographic Information Systems Analysis.” American Journal of Public Health 95 (4): 638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty, S. , Freeman V. L., Barret R. E., Luo L., and Shockley A.. 2012. “Spatial Error in Geocoding Physician Location Data from the AMA Physician Masterfile: Implications for Spatial Accessibility Analysis.” Spatial and Spatio‐Temporal Epidemiology 3 (1): 31–8. doi:10.1016/j.sste.2012.02.004. [DOI] [PubMed] [Google Scholar]
- McWilliams, A. , Tapp H., Barker J., and Dulin M.. 2011. “Cost Analysis of the Use of Emergency Departments for Primary Care Services in Charlotte, North Carolina.” North Carolina Medical Journal 72 (4): 265–71. [PubMed] [Google Scholar]
- Neutens, T. 2015. “Accessibility, Equity and Health Care: Review and Research Directions for Transport Geographers.” Journal of Transport Geography 43: 14–27. [Google Scholar]
- New York University . n.d. “Background/Introduction” [accessed November 15, 2014]. Available at http://wagner.nyu.edu/faculty/billings/nyued-background
- Newton, M. F. , Keirns C. C., Cunningham R., Hayward R. A., and Stanley R.. 2008. “Uninsured Adults Presenting to US Emergency Departments: Assumptions vs Data.” Journal of the American Medical Association 300 (16): 1914–24. doi:10.1001/jama.300.16.1914. [DOI] [PubMed] [Google Scholar]
- Oster, A. , and Bindman A.. 2003. “Emergency Department Visits for Ambulatory Care Sensitive Conditions: Insights into Preventable Hospitalizations.” Medical Care 41 (2): 198–207. [DOI] [PubMed] [Google Scholar]
- Rust, G. , Ye J., Baltrus P., Daniels E., Adesunloye B., and Fryer G. E.. 2008. “Practical Barriers to Timely Primary Care Access: Impact on Adult Use of Emergency Department Services.” Archives of Internal Medicine 168 (15): 1705–10. doi:10.1001/archinte.168.15.1705. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau . 2011a. “Selected Economic Characteristics” [Data file] [accessed June 7, 2014]. Available at http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml
- United States Census Bureau . 2011b. “Selected Housing Characteristics” [Data file] [accessed December 4, 2014]. Available at http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml
- United States Census Bureau . 2011c. “Selected Social Characteristics in the United States” [Data file] [accessed June 1, 2014]. Available at http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml
- Wang, F. 2015. Quantitative Methods and Socio‐Economic Applications in GIS, 2d Edition New York: CRC Press. [Google Scholar]
- Wang, F. , and Luo W.. 2005. “Assessing Spatial and Nonspatial Factors for Healthcare Access: Towards an Integrated Approach to Defining Health Professional Shortage Areas.” Health & Place 11 (2): 131–46. [DOI] [PubMed] [Google Scholar]
- Weinick, R. M. , Burns R. M., and Mehrotra A.. 2010. “Many Emergency Department Visits Could Be Managed at Urgent Care Centers and Retail Clinics.” Health Affairs (Project Hope) 29 (9): 1630–6. doi:10.1377/hlthaff.2009.0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z. , and Yan J.. 2013. “Detecting Traffic Accident Clusters with Network Kernel Density Estimation and Local Spatial Statistics: An Integrated Approach.” Journal of Transport Geography 31: 64–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix


