Abstract
Objective
To investigate effects of a novel dementia care coordination program on health services utilization.
Data Sources/Study Setting
A total of 303 community‐dwelling adults aged ≥70 with a cognitive disorder in Baltimore, Maryland (2008–2011).
Study Design
Single‐blind RCT evaluating efficacy of an 18‐month care coordination intervention delivered through community‐based nonclinical care coordinators, supported by an interdisciplinary clinical team.
Data Collection/Extraction Methods
Study partners reported acute care/inpatient, outpatient, and home‐ and community‐based service utilization at baseline, 9, and 18 months.
Principal Findings
From baseline to 18 months, there were no significant group differences in acute care/inpatient or total outpatient services use, although intervention participants had significantly increased outpatient dementia/mental health visits from 9 to 18 months (p = .04) relative to controls. Home and community‐based support service use significantly increased from baseline to 18 months in the intervention compared to control (p = .005).
Conclusions
While this dementia care coordination program did not impact acute care/inpatient services utilization, it increased use of dementia‐related outpatient medical care and nonmedical supportive community services, a combination that may have helped participants remain at home longer. Future care model modifications that emphasize delirium, falls prevention, and behavior management may be needed to influence inpatient service use.
Keywords: Dementia, care coordination
The care of over 5 million Americans living with dementia represents a growing challenge to the health care system (Bynum 2014; Alzheimer's Association 2015). The estimated formal and informal care costs of dementia range from $175 to $215 billion in the United States (Hurd et al. 2013). The cost of dementia care, already higher than heart disease and cancer (Kelley et al. 2015), will increase as dementia prevalence increases (Hebert et al. 2013). Health service utilization accounts for an important segment of formal care cost, with high rates of hospitalization, nursing home stays, long‐term care placement, emergency department (ED) visits, and outpatient care (Bynum et al. 2004; Zhao et al. 2008; Phelan et al. 2012; Feng et al. 2014). Despite growing costs, quality of care for people with dementia is generally poor (Chodosh et al. 2007; Odenheimer et al. 2014), and people with dementia are at high risk of adverse events during the course of acute medical care (McCloskey 2004; Mecocci et al. 2005; Pedone et al. 2005; Inouye 2006). Dementia remains a particularly challenging condition for clinicians working in a fragmented health care system (Boustani, Sachs, and Callahan 2007; Hinton et al. 2007; Harris et al. 2009).
To tackle these issues, the National Plan to Address Alzheimer's Disease seeks to explore the effectiveness of new models of care for people with dementia with the goal of enhancing care quality and efficiency (US Department of Health and Human Services 2015). However, home‐ and community‐based services (HCBS) that support primary care providers, improve quality of care, and reduce caregiver burden are underused (Weber, Pirraglia, and Kunik 2011), and Medicare Advantage and Accountable Care Organizations (ACOs) are not currently incentivized to manage care beyond an individual's medical needs. The integration of medical and long‐term services and supports through Medicaid managed care programs is showing positive results for reducing hospital stays and emergency department visits among high‐need dual‐eligible populations (Anderson, Feng, and Long 2016). As a result, private managed care plans may be inclined to expand coverage of services to include some home‐ and community‐based services for high‐need populations like people with dementia.
Dementia care management is a promising approach for improving quality of care and linking people with dementia to appropriate medical and community services that could be incorporated through alternative payment models, like ACOs, with a broader accountability for medical and long‐term services and supports (Davis et al. 2015; Davis, Willink, and Schoen 2016). Care management may help alleviate fragmentation of medical care and services, bridge resources, and, potentially, reduce cost of formal care for people with dementia by delaying costly nursing home placement (Callahan et al. 2006; Vickrey et al. 2006; Samus et al. 2014).
Existing studies of dementia care management interventions largely focus on long‐term care placement as the primary resource utilization outcome. Evaluation of the effect of such programs on other forms of health services, such as hospitalization, is more limited (Hickam et al. 2013; Tam‐Tham et al. 2013; Reilly et al. 2015). The few studies that have examined hospitalization or ED use demonstrate mixed results (Clark et al. 2004; Callahan et al. 2006; Duru et al. 2009), while pooled analysis shows no difference between intervention and usual care (Tam‐Tham et al. 2013; Reilly et al. 2015). HCBS use remained similar or increased in the studies that examined their use (Newcomer et al. 1999; Chu et al. 2000; Lam et al. 2010). A recent review concluded that there is not yet a robust evidence base to determine whether dementia care management meets the health care needs of this population, primarily because high‐quality trials are lacking (e.g., few randomized controlled trials, with heterogeneous interventions and outcomes, and small sample sizes). The majority of studies were also conducted outside the United States, and differences in health care delivery and culture may impact effectiveness (Reilly et al. 2015). Despite a lack of empirical data, current economic pressures and the impact of health care reform pushing value‐based care are creating an environment where care management programs are emerging through recent health care reforms and alternative payment models, including patient‐centered medical homes and ACOs (Longworth 2011). Thus, this study is timely and important as major decisions are being made swiftly regarding health care delivery for dementia.
In a randomized controlled trial, a team of nonclinical community workers, linked to an interdisciplinary team of mental health clinicians, provided a dementia care coordination program (Maximizing Independence [MIND] at Home) through community‐based agencies to a heterogeneous dementia population living at home over 18 months. The primary goals of the intervention were to delay transition from the home and reduce unmet care needs; secondary goals included improving quality of life, neuropsychiatric symptoms, and depression. We previously reported that the intervention significantly reduced unmet safety and legal dementia care needs, decreased the percentage of persons transitioning out of their homes, delayed time to leaving the home, and improved participant quality of life (Samus et al. 2014). The objective of this study was to determine the effect of MIND at Home on the use of a range of health and supportive services. We hypothesized that persons receiving the intervention would have reductions in use of acute care or inpatient medical services and increases in use of outpatient and HCBSs relative to persons receiving augmented usual care over the 18‐month period. Table 1 provides details on the care needs assessed in MIND at Home, examples of care strategies, and hypothesized effects of care strategies on health service use. Effects on outpatient and HCBSs were more likely to be direct, resulting from referrals, while effects on acute care/inpatient medical service use were hypothesized to be mediated by other factors, such as reduced hospitalization due to addressing polypharmacy, neuropsychiatric symptoms, fall risk, or caregiver burden.
Table 1.
Unmet Memory Care Needs, Care Strategies, and Hypothesized Links to Health Services Use
| Memory Care Needs Domains | Abbreviated Care Option/Strategy Examplesa | Hypothesized Effect on Participant Health Services Use |
|---|---|---|
| Evaluation/diagnosis | PCP or specialist referral for dementia evaluation; neurologic evaluation; substance abuse referral. | ↑ outpatient medical services |
| Treatment of cognitive symptoms | Evaluate whether medication might be indicated and refer to PCP or specialist for discussion/evaluation. | ↑ outpatient medical services |
| Treatment of neuropsychiatric symptoms | In‐depth review and characterization of concerning symptoms; assessment of potential causes (e.g., infection, constipation, pain); refer to PCP or specialist for discussion/evaluation of possible medication indications. | ↓ acute care/inpatient medical services |
| ↑ outpatient medical services | ||
| Behavior management | In‐depth review and characterization of concerning symptoms; provide instruction on specific behavior management/caregiver skills counseling; assessment of potential causes (e.g., infection, constipation, pain); refer to Alzheimer's Association. | ↓ acute care/inpatient medical services |
| ↑ outpatient medical services | ||
| ↑ home and community‐based services | ||
| Medication management | Initial review of medications; request PCP or prescribing physician to evaluate polypharmacy or regimen adjustment; assist in coordination of multiple prescribing physicians/pharmacies. | ↓ acute care/inpatient medical services |
| ↑ outpatient medical services | ||
| Medication administration | Create medication administration routine that promotes compliance; coordinate second party supervision or medication administration; recommend specific devices or reminder tools. | ↓ acute care/inpatient medical services |
| ↑ home and community‐based services | ||
| General medical/health care | Referral to PCP, medical specialist, or geriatric care manager; recommend family and PCP consider hospice care. | ↓ acute care/inpatient medical services |
| ↑ outpatient medical services | ||
| Allied health specialist care | Referral to PCP; recommend referral by PCP to therapy (physical, occupational, or speech), home health care agency. | ↑ outpatient medical services |
| ↑ home and community‐based services | ||
| Safety | Identify possible environmental hazards (e.g., scatter rugs, expired food, fall risks, fire risks, wander risks, guns/power tools) and make plans to address each; referral to driving evaluation; home safety evaluation; recommend asking PCP for physical or occupational therapy referral. | ↓ acute care/inpatient medical services |
| ↑ home and community‐based services | ||
| Assistance with daily activities | Arrange for informal or formal assistance for needed service; provide caregiver skills counseling. | ↑ home and community‐based services |
| Meaningful activities | Evaluate and develop a list of activities that match preferences, personality, and lifestyle and help caregiver implement; provide caregiver skills counseling for help with creating a daily routine structure; refer to friendly visitor programs, senior center, adult day, transportation service, etc. | ↑ home and community‐based services |
| Legal issues/advance care planning | Recommend patient and family engage in end‐of‐life care discussions with PCP and family; referral to eldercare attorney or state attorney office about power of attorney, will, advance directives. | ↓ acute care/inpatient medical services |
| Assistance with health insurance | Review current medical needs, medications and referral to Medicare or Medicaid, US Veterans Affairs, AARP, etc. | ↔ acute care/inpatient medical services |
| ↑ outpatient medical services | ||
| Patient education | Refer to PCP for discussion of illness; refer to Alzheimer's Association support group. | ↑ outpatient medical services |
| ↑ home and community‐based services | ||
| Caregiver availability | Identify and arrange for someone to take responsibility for phone checks, in‐person visits, supervision. | ↑ home and community‐based services |
| Caregiver education | Educate on dementia course and impact; provide written learning material; inform of educational events or local resources (health fairs, clinicians, senior centers, day care/home care services, support groups); instruct and counsel on care management issues (behavioral issues, ADLs, communication, family conflicts, planning, safety). | ↓ acute care/inpatient medical services |
| ↑ home and community‐based services | ||
| Caregiver resource referrals | Refer to local or national chapter of Alzheimer's Association, eldercare attorney (e.g., estate planning, will, power of attorney, advanced directives), Dept. of aging or local agency, private geriatric care management services, adult protective services. | ↑ home and community‐based services |
| Caregiver mental health care | Proactively monitor stress levels; provide informal counseling, help with coping skills, and emotional support; refer to licensed mental health professional; arrange and plan regular respite care periods. | ↔ acute care/inpatient medical services |
| ↑ home and community‐based services | ||
| Caregiver general medical/health care | Referral to PCP, specialist, other health care professional (e.g., dentist, optometrist, PT). | ↔ acute care/inpatient medical services |
Listed recommended interventions are not exhaustive. Actual recommendations based on individual's specific need within a category.
PCP, primary care provider.
Methods
Study Population
The process of recruitment and randomization has previously been described in detail (Samus et al. 2014; Tanner et al. 2015). Community‐dwelling adults were recruited from July 2008 to May 2010 in Baltimore, Maryland. Eligible participants were age ≥70, English‐speaking, community‐residing in northwest Baltimore (28 postal codes), had a reliable study partner, met Diagnostic and Statistical Manual, Fourth Edition, Text Revision criteria for dementia or Cognitive Disorder Not Otherwise Specified (American Psychiatric Association 2000), and had one or more unmet care needs on the Johns Hopkins Dementia Care Needs Assessment (JHDCNA; Black et al. 2008). Individuals in crisis, with signs of abuse, neglect, or danger to self or others, were excluded. A cohort of 303 older adults (265 with dementia, 38 with mild cognitive impairment) was randomized to intervention or augmented usual care. Randomization was stratified by whether the participant lived with a caregiver. Oral consent was obtained from participants during an initial telephone screen, and written consent was obtained from participants and study partners at the in‐home assessment. Participant capacity to consent for participation in research was assessed using a structured interview with questions to assess understanding of the study. For participants too impaired to provide consent, proxy consent was obtained from a legally authorized representative using the Maryland Health Care Decisions Act as a guide, and assent was obtained from the participant. The study was reviewed and overseen by a Johns Hopkins Medicine Institutional Review Board.
Intervention
A total of 193 participants were randomized to augmented usual care (control) with 110 randomized to care coordination (intervention). The Johns Hopkins Dementia Care Needs Assessment (JHDCNA) was administered to all participants and their caregivers, including control, during a home visit at baseline. Control participants, their study partners, and primary care physicians (PCP) received written results of the JHDCNA, including recommendations for each unmet need and a brief resource guide. Intervention participants, study partners, and PCPs received written JHDCNA results followed by up to 18 months of care coordination for participants through an interdisciplinary team of nonclinical memory care coordinators linked to a registered nurse and a geriatric psychiatrist. The care coordination protocol included individualized care planning based on unmet needs and patient/family priorities, dementia education and skill‐building, referrals and linkages to services, informal counseling, and care monitoring. Table 1 displays the 19 domains of care needs assessed in the 86‐item JHDCNA and examples of care strategies recommended to address unmet needs. After randomization, coordinators conducted an in‐home visit with the participant and study partner to review and prioritize needs and develop a care plan. The plan was implemented by study partners and/or participants with guidance from the coordinator. A menu of care strategies was available for each unmet need and consisted of linkage to resources/services, caregiver education and skill‐building, and informal counseling and problem‐solving. While intervention intensity and contact frequency varied by individual needs and circumstances, the protocol prespecified two in‐home visits (at baseline and 18 months) and at least one monthly contact (e.g., phone, in‐person). Coordinators were available to families without time restrictions. On average, coordinators made two contacts per month to participants/families (mean 1.8, standard deviation 24.1; Samus et al. 2014). In recognition of potentially changing needs and priorities, needs were re‐evaluated over time and the care plan and strategies adjusted as appropriate. When indicated, coordinators took direct roles to ensure implementation of recommended strategies (e.g., attending outpatient appointments, assisting with program applications). The three coordinators, employees of two community‐based social service agencies, did not have prior formal training in geriatric case management or dementia care. They were trained in dementia care management over 4 weeks and met with the intervention team weekly for case discussion and continuous case‐based training, clinical oversight, and protocol adherence.
Outcome Measures
A research team masked to intervention assignment assessed service utilization through in‐person, self‐report interviews administered to the study partner at baseline, 9 months, and 18 months. Three categories of services were considered as follows: (1) acute care/inpatient medical services including ED visits, hospital admissions, hospital nights, nursing home nights (rehabilitation and short‐term skilled care), and respite residential care nights; (2) outpatient medical services including total outpatient clinician visits and dementia/mental health or general medical/surgical related; and (3) home and community‐based services (HCBS). Hospital, nursing home, and respite residential care stays were specifically elicited and the number of separate admissions and nights per admission ascertained. Outpatient visits specifically for dementia, mental health, or for other physical or medical conditions were also specifically elicited alongside the number of visits of each type. ED visits were elicited within outpatient service use during the interview.
Home‐ and community‐based services represent 15 different types of medical or nonmedical services delivered in the home or community through home health or community‐based agencies. HCBS use was examined in total and a priori stratified into health (care management, visiting nurse services, physical therapy, speech/occupational therapy, transportation to health services, adaptive/assistive supplies), social (social day care, congregate meals, companion services, patient support group), and support services (in‐home respite care, home delivered meals, homemaker/housekeeping, live‐in paid caregiver, client education training services). The use of each of the 15 specific HCBS types (e.g., physical therapy, day care) was assessed as yes/no rather than counting individual services or providers within specific service type (total HCBS value ranged between 0 and 15). For example, if a participant attended two different day care facilities, it was measured as 1 HCBS. Baseline interviews specifically assessed services used in the prior 12 months, while 9‐ and 18‐month interviews measured use over the prior 9 months. Service utilization over 12 months at baseline was converted to utilization over 9 months by calculating mean monthly utilization and multiplying by 9 to allow for direct comparisons of mean utilization at each time point.
Covariates
Additional participant characteristics assessed included demographics (age, sex, race, education), living arrangement (with or without a caregiver), Mini‐Mental State Exam (Folstein, Folstein, and McHugh 1975), and Psychogeriatric Dependency Rating Scale‐Physical subscale (PGDRS) (Wilkinson and Graham‐White 1980) to assess functional status. Prescription and over‐the‐counter medications were recorded from pill bottles; any medication taken regularly was considered a routine medication. The study partner reported medical comorbidities. Dementia, hypertension, diabetes, peripheral vascular disease, heart disease, coronary bypass or stenting, hip fracture, recurrent urinary tract infections, stroke, psychiatric disease, traumatic brain injury, and Parkinson's were specifically elicited, while other medical conditions were elicited through targeted questioning linked to medications and open‐ended questioning. Insurance coverage, including Medicare, Medicaid, and other insurance, was reported by the study partner. Neuropsychiatric and depressive symptoms were assessed using the Neuropsychiatric Inventory‐Q (Kaufer et al. 2000) and the Cornell Scale for Depression in Dementia (Alexopoulos et al. 1988), respectively.
Statistical Analysis
We first examined group differences in baseline characteristics using simple inferential statistics (Pearson's chi‐square or t‐test). An intention‐to‐treat approach was used in analyses, with participants included as randomized. Linear mixed‐effects regression models estimated the effect of the intervention relative to control on change in each service utilization outcome, or difference in slopes, from baseline to 18 months and in each 9‐month intervention period. The use of a mixed‐effects model allowed for missing observations and correlation among time points, or repeated measures, for the same subject. Covariates included living with a caregiver (the randomization stratification variable), time interval, total medications, and PGDRS score. PGDRS scores were included due to association with dropout/attrition and total medications due to differences between intervention and control groups at baseline. Given post hoc hypotheses that intervention effect may be greater in the second 9 months of the intervention as coordinator–participant relationships are strengthened and recommendations implemented over time, a group × time interval interaction term was included. Sensitivity analyses compared mixed‐effects models with and without random effects, negative binomial models, and mixed‐effect models without random effect that incorporated a time‐varying PGDRS × time interaction term to better account for attrition. Analyses were conducted using SAS V9.3 (SAS Institute Inc, Cary, NC, USA). Tests were considered statistically significant at α ≤0.05.
Results
Description of Participants
Table 2 displays baseline characteristics of participants. The average age was 84 years, with 64 percent women and 29 percent non‐whites. Eighty‐eight percent of participants had dementia, with remaining participants having mild cognitive impairment. Intervention and control groups were similar on all characteristics except that intervention participants were taking significantly more routine medications than control participants. Participants were also similar on the proportion reporting ED visits or hospitalization and average HCBS programs used in the prior year. The intervention group had more hospital nights in the prior year (mean 4.1 vs. 2.2, p = .03). There were no significant baseline group differences on caregiver characteristics, including age (mean 66.7 years), gender (approximately 75 percent female), relationship to participant (91 percent spouse or child), time elapsed in caregiving (mean 38 months), and employment (47 percent employed). In the intervention group, 87 older adults (79 percent) were available for 9‐month follow‐up; 74 (67 percent) were available at 18 months. In the control group, 136 older adults (70 percent) were available for 9‐month follow‐up; 114 (59 percent) were available at 18 months. Remaining participants transitioned permanently from the home or died (n = 101) or were not available due to drop out (n = 11), loss to follow‐up (n = 1), or temporary nursing home stay (n = 3). Differences in attrition between groups were not statistically significant at either time point (p = .10 at 9 months and p = .16 at 18 months).
Table 2.
Baseline Characteristics of Participants
| Characteristic | Augmented Care Group (N = 193) | Intervention Group (N = 110) | p‐Valuea |
|---|---|---|---|
| Age, mean (SD) | 83.9 (5.9) | 84.0 (5.8) | .840 |
| Female, n (%) | 120 (62.2) | 73 (66.4) | .466 |
| Black/African American or other race, n (%) | 55 (28.5) | 32 (29.1) | .913 |
| Education, mean (SD), years | 13.2 (3.9) | 13.0 (3.1) | .668 |
| Living with caregiver, n (%) | 131 (67.9) | 80 (72.7) | .377 |
| Dementia, n (%) | 166 (86.0) | 99 (90) | .313 |
| Number of routine medications, mean (SD) | 6.1 (2.9) | 6.9 (3.4) | .023 |
| Cardiovascular disease, n (%)b | 154 (79.8) | 96 (87.3) | .099 |
| Pulmonary disease, n (%)b | 12 (6.2) | 7 (6.4) | .650 |
| Endocrine disease, n (%)b | 104 (53.9) | 66 (60.0) | .302 |
| Medicare Part A, n (%) | 192 (99.5) | 109 (99.1) | .686 |
| Medicare Part B, n (%) | 189 (97.9) | 109 (99.1) | .445 |
| Medicare Part D, n (%) | 79 (40.9) | 44 (40.0) | .874 |
| Medical assistance, n (%) | 11 (5.7) | 9 (8.2) | .403 |
| Supplemental insurance, n (%) | 167 (86.5) | 91 (82.7) | .371 |
| Hospitalized in past year, n (%) | 67 (34.7) | 37 (33.6) | .849 |
| ED visit in past year, n (%) | 99 (51.6) | 50 (45.5) | .307 |
| Number of HCBS used, mean (SD) | 3.2 (1.7) | 3.2 (1.7) | .912 |
| MMSE, mean (SD)c | 19.2 (7.7) | 19.0 (7.9) | .815 |
| NPI‐Q, mean (SD)c | 7.1 (6.2) | 7.2 (5.7) | .920 |
| CSDD, mean (SD)c | 6.1 (4.6) | 6.5 (4.8) | .569 |
| PGDRS‐P, mean (SD)c | 9.5 (8.0) | 10.3 (7.8) | .406 |
| Total % unmet JHDCNA needs, mean (SD)d | 10.2 (6.5) | 9.8 (5.3) | .580 |
p‐Value calculated by Pearson's chi‐square or t‐test.
Cardiovascular disease includes hypertension, congestive heart failure, coronary artery disease, arrhythmia, valvular disease, aortic aneurysm, peripheral vascular disease, and atrial fibrillation. Pulmonary disease includes chronic obstructive pulmonary disease and asthma. Endocrine disease includes adrenal insufficiency, diabetes mellitus, hyperthyroidism, hypothyroidism, hyperlipidemia, and hyperparathyroidism.
MMSE score range 0–30, higher scores are better. NPI‐Q score range 0–36, CSDD score range 0–38, PGDRS‐P score range 0–39; higher scores are worse.
The 86‐item JHDCNA assesses 19 common care need categories for participants and caregivers.
CSDD, Cornell Scale for Depression in Dementia; ED, emergency department; HCBS, home‐ and community‐based services; JHDCNA, Johns Hopkins Dementia Care Needs Assessment. MMSE, Mini‐Mental State Examination; NPI‐Q, Neuropsychiatric Inventory‐Questionnaire; PGDRS‐P, Psychogeriatric Dependency Rating Scale‐Physical; SD, standard deviation;
Acute Care/Inpatient Medical Services Utilization
Adjusted average inpatient and outpatient health services utilization at baseline, 9, and 18 months are in Table 3. From baseline to 18 months, both intervention and control groups had an increase in ED visits, hospital admissions, and nursing home nights; there were no significant differences in change of acute care/inpatient medical services use between groups. There was, however, a decrease in hospital nights in the intervention group compared to an increase in controls. In examining the potential influence of time on intervention effect, the decrease in hospital nights in the intervention group was significant compared to control from baseline to 9 months, but this trend was attenuated and no longer statistically significant at 18 months. There was also a nonsignificant increase in respite residential care nights in the intervention group compared to a decrease in control from baseline to 9 months. There was a greater though nonsignificant increase in ED visits among controls from baseline to 9 months.
Table 3.
Medical Services Utilization at Baseline, 9, and 18 Months
| Outcome | Estimated Mean (Standard Error)a | Δ in Intervention—Δ in Augmented Usual Care from Baseline to 9 or 18 months (95% CI) | p‐Valueb | |
|---|---|---|---|---|
| Augmented Usual Care (N = 193) | Intervention (N = 110) | |||
| Emergency department visits | ||||
| Baseline | 0.63 (0.09) | 0.59 (0.11) | — | |
| 9 month | 0.79 (0.11) | 0.61 (0.13) | −0.14 (−0.53 to 0.25) | .479 |
| 18 month | 0.80 (0.12) | 0.77 (0.14) | 0.01 (−0.40 to 0.42) | .960 |
| Hospital admission | ||||
| Baseline | 0.39 (0.06) | 0.45 (0.08) | — | |
| 9 month | 0.55 (0.08) | 0.46 (0.09) | −0.15 (−0.44 to 0.14) | .301 |
| 18 month | 0.57 (0.08) | 0.56 (0.10) | −0.06 (−0.37 to 0.24) | .683 |
| Hospital nights | ||||
| Baseline | 1.90 (0.43) | 3.09 (0.57) | — | |
| 9 month | 3.07 (0.52) | 1.80 (0.64) | −2.46 (−4.45 to −0.46) | .016 |
| 18 month | 2.80 (0.57) | 2.96 (0.69) | −1.04 (−3.15 to 1.08) | .337 |
| Nursing home nights | ||||
| Baseline | 1.63 (0.51) | 1.76 (0.67) | — | |
| 9 month | 1.74 (0.61) | 1.58 (0.76) | −0.29 (−2.76 to 2.17) | .815 |
| 18 month | 2.16 (0.68) | 2.85 (0.82) | 0.56 (−2.05 to 3.17) | .673 |
| Respite care nights | ||||
| Baseline | 0.64 (0.37) | 0.68 (0.49) | — | |
| 9 month | 0.45 (0.45) | 1.81 (0.55) | 1.32 (−0.47 to 3.11) | .149 |
| 18 month | 0.33 (0.50) | 0.44 (0.60) | 0.06 (−1.83 to 1.96) | .946 |
| Outpatient visits total | ||||
| Baseline | 14.48 (1.04) | 13.47 (1.38) | — | |
| 9 month | 13.43 (1.20) | 13.92 (1.49) | 1.50 (−2.64 to 5.65) | .477 |
| 18 month | 14.45 (1.32) | 17.63 (1.61) | 4.20 (−0.22 to 8.61) | .062 |
| Outpatient dementia/mental health visits | ||||
| Baseline | 1.39 (0.31) | 1.90 (0.42) | — | |
| 9 month | 1.57 (0.35) | 1.56 (0.44) | −0.51 (−1.61 to 0.58) | .357 |
| 18 month | 1.16 (0.38) | 2.40 (0.47) | 0.73 (−0.44 to 1.91) | .220 |
| Outpatient medical/surgical visits | ||||
| Baseline | 13.04 (0.94) | 11.42 (1.26) | — | |
| 9 month | 11.91 (1.12) | 12.38 (1.39) | 2.08 (−1.77 to 5.94) | .288 |
| 18 month | 13.36 (1.23) | 15.38 (1.50) | 3.63 (−0.48 to 7.75) | .084 |
Means reflect utilization over previous 9 months. Adjusted for living with a caregiver, total medications, and Psychogeriatric Dependency Rating Scale‐Physical score.
p‐Value of difference between slopes (Δ in intervention—Δ in augmented usual care from baseline to 9 or 18 months).
Outpatient Medical Services Utilization
From baseline to 18 months, there was a nonsignificant increase of 4.2 total outpatient visits and increases in both dementia/mental health and general medical/surgical visits among intervention participants relative to control. While the increase in dementia/mental health visits was not significant when comparing baseline to 18 months, there was a significant increase by 1.25 visits in the intervention group relative to control from 9 months to 18 months (p = .04).
Home and Community‐Based Services Utilization
Home‐ and community‐based services use, total and health, social, and support HCBSs, were similar between groups at baseline. From baseline to 18 months, HCBS utilization increased in both intervention and control groups. The increase in total HCBS use was significantly greater in the intervention group, as mean HCBS use increased by 1.77 in the intervention group compared to 1.07 in the control group (p = .022). At 18 months, the intervention group, on average, used 0.59 more service types compared to control (p = .033). This difference translates to intervention participants using one additional type of HCBS on average relative to controls. The change in health, social, and support services used over the intervention period is shown in Figure 1. From baseline to 18 months, the increase in health HCBS use was similar between groups. The change in social and support HCBS utilization from baseline to 18 months was significantly different between groups, with a greater increase in use among intervention participants. While the mean types of social HCBSs used was nonsignificantly higher in the intervention group at 18 months (p = .087), the rate of increase was significantly higher in the intervention group (p = .026). The mean types of support HCBSs used were significantly greater at 18 months in the intervention group (p = .005).
Figure 1.
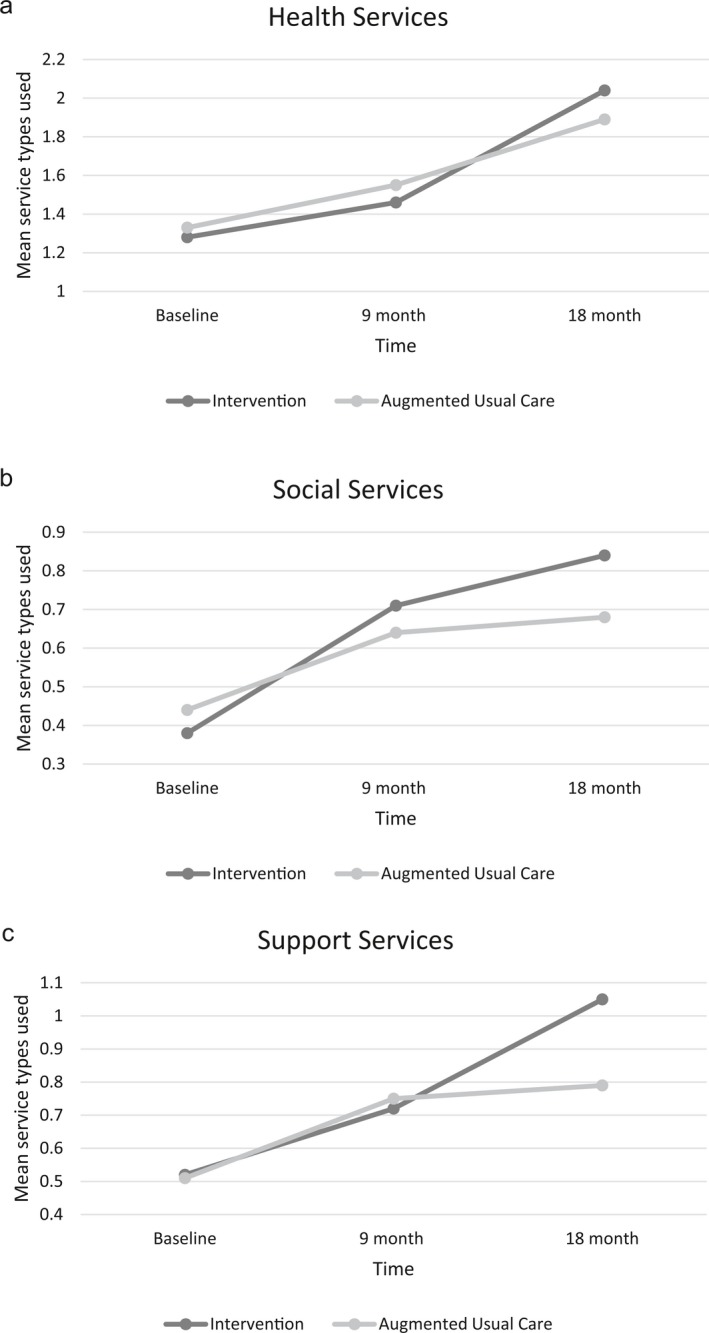
(a–c) Home‐ and Community‐Based Services (HCBS) Use in Intervention and Augmented Usual Care Groups over Time, Adjusted for Living with a Caregiver, Total Medications, and Psychogeriatric Dependency Rating Scale‐Physical Score
Notes. (a) Categories of HCBS health use include Care Management, Visiting Nurse Services, Physical Therapy, Speech/Occupational Therapy, Transportation to Health Services, Adaptive/Assistive Supplies. The rate of increase in mean categories of HCBS health used from baseline to 18 months was similar between groups (p = .383). (b) Categories of HCBS social service use include Social Day Care, Congregate Meals, Companion Services, and Patient Support Groups. The change in mean categories of social services used was similar between groups from baseline to 9 months (p = .139), but the increase in the intervention group was significantly higher from baseline to 18 months. (p = .026). (c) Categories of HCBS support service use include In‐Home Respite Care, Home Delivered Meals, Homemaker/Housekeeping, Live‐in Paid Caregiver, and Client Education Training Services. The change in mean categories of support services used was similar between groups from baseline to 9 months (p = .561), but the increase in the intervention group was significantly higher from baseline to 18 months (p = .008).
Linear mixed‐effect models with random effects yielded similar results. Negative binomial models also yielded similar results though with marginal changes in statistical significance. Increases in outpatient dementia/mental health visits from 9 months to 18 months and in total HCBS use from baseline to 18 months in intervention relative to control was no longer significant (p = .09 for both comparisons). When mixed‐effect models included PGDRS × time interaction, total HCBS use from baseline to 18 months again trended toward greater increase in the intervention group (p = .09), but the increase in outpatient dementia/mental health visits was statistically significant (p = .04). Greater support HCBS use among intervention participants was significant in all models.
Discussion
Maximizing Independence at Home, a home‐based dementia care coordination intervention, previously shown to delay transition from the home, improve patient quality of life, and reduce unmet dementia care needs (Samus et al. 2014), led to a greater increase in utilization of home‐ and community‐based services compared to control in these analyses. Community‐based services and support are increasingly recognized as key components of high‐quality dementia care (National Quality Forum 2014); increased HCBS use therefore provides evidence for additional benefit resulting from this novel care coordination program. This intervention did not, however, impact ED visits, hospital admissions, or short‐term nursing home stays. Acute care/inpatient medical service use thus remains an important target to understand and address in dementia care interventions and models of care that emphasize care coordination. Relative to control, the intervention does seem to increase outpatient visits related to dementia and mental health, and there was a trend toward decreased hospital nights. As hypothesized, effects were generally more pronounced from 9 to 18 months, supporting the idea that longer‐term care coordination programs may lead to greater effectiveness as care coordinators establish relationships with patients/families and recommendations are implemented.
The lack of significant impact on hospital admissions and ED visits is consistent with meta‐analyses of previous dementia care management RCTs that have examined these outcomes (Tam‐Tham et al. 2013; Reilly et al. 2015). Length of hospital stay, represented by hospital nights and rarely examined in other studies, may be a metric worth further investigation as shorter length of stay may decrease the risk of adverse events during hospitalization even when admission itself is not preventable. Thirty‐day hospital readmission rates, not measured in the current study, are another potentially important metric, particularly given the evolution of bundled payments. The trend toward decreased hospital nights in this study differs from previous studies which report increased nights (Callahan et al. 2006; Jansen et al. 2011; Reilly et al. 2015). MIND at Home may have created a home environment and support system that supported timely admissions, with less severe or complicated illness, and/or earlier discharge. It is also possible that, because these patients were already identified as having dementia by family members and clinicians, recognition of the condition somehow impacted inpatient care decisions and treatment. The effect on outpatient and HCBS use is also less studied, but prior studies suggest increased social care service use with care management (Newcomer et al. 1999; Chu et al. 2000; Lam et al. 2010; Reilly et al. 2015). Pooled data suggest similar overall health care service use but greater outpatient physician or nurse visits in people receiving care management interventions compared to control, which is also consistent with our findings (Reilly et al. 2015).
While MIND at Home did not reduce acute medical services use as hypothesized, the intervention does seem to achieve the desired effect of linking persons with dementia to needed outpatient and home‐ and community‐based support services that may have enhanced their ability to remain at home. The lack of effect on hospitalization and ED visits is perhaps not surprising given the structure of the intervention, with nonclinical community workers serving as care coordinators and implementation through community‐based agencies. Letters regarding the findings of the dementia care needs assessment, recommendations, and resources were sent to the PCPs of both intervention and control participants. While the MIND care coordinators for intervention participants attempted to establish contacts with the respective health providers as indicated over the course of the intervention, this was often difficult. Better integration, communication, and collaboration between the MIND care team and patients’ health providers may have a greater effect on acute medical services use. More flexible coordinator access to clinical consultation in the field might also increase early detection and prevention of problems leading to hospital admission. Emerging low‐cost technologies such as telemedicine and live clinical video‐teleconsultation could facilitate timely access. While such changes would require increased program capacity as well as clinician acceptance and buy‐in, open communication between traditional medical providers and community care providers will ultimately be necessary to provide high‐quality dementia care (National Quality Forum 2014). Targeted emphasis on caregiver education and skill‐building around medical care (e.g., management of comorbid medical conditions) and behavioral symptoms management, early recognition of delirium or acute medical problems, falls prevention, and navigation of care transitions likely represent other mechanisms to decrease acute medical services use. While we did not explicitly examine cost and potential cost‐saving, at first blush, our findings suggest that formal costs of care may actually increase in participants. However, our previous finding of reduced and delayed nursing home placement (Samus et al. 2014) may portend cost benefit, with the increased cost of outpatient and HCBS use offset by decreased spending on long‐term care. The cost of the program itself requires further study, but given novel use of nonclinical frontline care coordination staff, MIND at Home likely has greater implementation potential and lower costs compared to prior interventions that have used professional case managers, including nurses, occupational therapists, and social workers (Reilly et al. 2015). Future evaluation of the effects on cost, including shifted costs, is warranted to better anticipate the impact of such programs.
In addition to limited PCP involvement in the intervention, this study has other limitations. First, service utilization was assessed by study partner self‐report rather than medical records or administrative claims. Statistical power to detect differences in these outcomes was also limited and may account for shifts in statistical significance in sensitivity analyses. Attrition in the sample by 18 months may have obscured effects of the intervention as service utilization may be greater prior to death or institutionalization; however, though the absolute rate of attrition was higher in the control group, attrition rates were statistically similar between groups. We also accounted for PGDRS score, the only significant predictor of attrition when we compared those who remained in the study to those who did not. Generalizability is limited given that we recruited individuals from a single, large urban catchment area, and participants and caregivers were, on average, highly educated. Lastly, between‐group differences may be underestimated because control participants, caregivers, and PCPs received the results of the care needs assessment, recommendations, and a list of resources. The increase in HCBS use in both groups may reflect this augmented usual care in an educated population.
Our individualized dementia care coordination intervention, unique in its delivery by nonclinical community‐based care coordinators, increased outpatient medical visits for dementia and mental health care and the use of home and community‐based services, particularly support services, important but often overlooked components of dementia care. While use of acute care/inpatient medical services was unchanged, the increased cost of care and services may be offset by savings from reduced and delayed transition to long‐term care. Further intervention enhancement, integration with medical care, and/or interventions targeting primary care and clinicians may improve the ability of dementia care coordination programs and health systems to reduce hospitalization and ED visits in this vulnerable population. Patient‐centered medical homes and ACOs in particular may represent opportunities in which care coordination programs such as MIND at Home may be embedded to complement existing care and services for patients with dementia.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The MIND at Home project sponsors and donors were The Hoffberger Family Fund; LeRoy Hoffberger; The Harry and Jeannette Weinberg Foundation; Rosenberg Foundation; Hirschhorn Foundation; Stulman Charitable Foundation; Meyerhoff Foundation; Marc and Leonor Blum; Baltimore County Department of Aging; Blum Family; Lowell Glazer Greif Family Fund; Marvin Schapiro Family Foundation; Lois and Phillip Macht Eliasberg Family Foundation; Richard and Rosalee Davison; Alison and Arnold Richman; Moser Family Philanthropic Fund; Richard Lansburgh; Anonymous; and other supporting contributions. Substantial support for this study was also provided by The Associated Jewish Community Federation of Baltimore, the National Institute of Mental Health and National Institute on Aging (K01 MH085142), the MSTAR (Medical Student Training in Aging and Research) program grant through the American Federation for Aging Research and National Institute on Aging, and the Pearl M. Stetler Research Fund. Under an agreement between DEMeasure and Drs. Black and Rabins, Dr. Black and Dr. Rabins are entitled to a share of income received by DEMeasure from sales of the Alzheimer's Disease Related Quality of Life questionnaire and scale used in the study described in this article. Drs. Black and Rabins have ownership interests in DEMeasure. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Lyketsos has grant support from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo‐Smith‐Kline, Eisai, Pfizer, Astra‐Zeneca, Lilly, Ortho‐McNeil, Bristol‐Myers, Novartis, National Football League, Elan, Functional Neuromodulation Inc., and Janssen. He is a consultant/advisor for Astra‐Zeneca, Glaxo‐Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genetech, Elan, NFL Players Association, NFL Benefits office, Avanir, Zinfandel, and BMS. He has received honorarium or travel support from Pfizer, Forest, Glaxo‐Smith Kline, and Health Monitor. All other authors have no potential conflicts of interest.
Disclaimer: None.
References
- Alexopoulos, G. S. , Abrams R. C., Young R. C., and Shamoian C. A.. 1988. “Cornell Scale for Depression in Dementia.” Biological Psychiatry 23 (3): 271–84. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association . 2015. “2015 Alzheimer's Disease Facts and Figures.” Alzheimer's & Dementia: The Journal of the Alzheimer's Association 11 (3): 332–84. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 2000. DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision, pp. 739–99. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson, W. L. , Feng Z., and Long S. K.. 2016. “Minnesota Managed Care Longitudinal Data Analysis” [accessed on August 11, 2016]. Available at https://aspe.hhs.gov/report/minnesota-managed-care-longitudinal-data-analysis
- Black, B. , Johnston D., Handel S., Morrison A., Robbins B., and Rye R.. 2008. Manual for the Johns Hopkins Dementia Care Needs Assessment (JHDCNA). Baltimore, MD: Johns Hopkins University. [Google Scholar]
- Boustani, M. , Sachs G., and Callahan C. M.. 2007. “Can Primary Care Meet the Biopsychosocial Needs of Older Adults with Dementia?” Journal of General Internal Medicine 22 (11): 1625–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynum, J. P. 2014. “The Long Reach of Alzheimer's Disease: Patients, Practice, and Policy.” Health Affairs (Project Hope) 33 (4): 534–40. [DOI] [PubMed] [Google Scholar]
- Bynum, J. P. , Rabins P. V., Weller W., Niefeld M., Anderson G. F., and Wu A. W.. 2004. “The Relationship between a Dementia Diagnosis, Chronic Illness, Medicare Expenditures, and Hospital Use.” Journal of the American Geriatrics Society 52 (2): 187–94. [DOI] [PubMed] [Google Scholar]
- Callahan, C. M. , Boustani M. A., Unverzagt F. W., Austrom M. G., Damush T. M., Perkins A. J., Fultz B. A., Hui S. L., Counsell S. R., and Hendrie H. C.. 2006. “Effectiveness of Collaborative Care for Older Adults with Alzheimer Disease in Primary Care: A Randomized Controlled Trial.” Journal of the American Medical Association 295 (18): 2148–57. [DOI] [PubMed] [Google Scholar]
- Chodosh, J. , Mittman B. S., Connor K. I., Vassar S. D., Lee M. L., DeMonte R. W., Ganiats T. G., Heikoff L. E., Rubenstein L. Z., Della Penna R. D., and Vickrey B. G.. 2007. “Caring for Patients with Dementia: How Good Is the Quality of Care? Results from Three Health Systems.” Journal of the American Geriatrics Society 55 (8): 1260–8. [DOI] [PubMed] [Google Scholar]
- Chu, P. , Edwards J., Levin R., and Thomson J.. 2000. “The Use of Clinical Case Management for Early Stage Alzheimer's Patients and Their Families.” American Journal of Alzheimer's Disease and Other Dementias 15 (5): 284–90. [Google Scholar]
- Clark, P. , Bass D., Looman W., McCarthy C., and Eckert S.. 2004. “Outcomes for Patients with Dementia from the Cleveland Alzheimer's Managed Care Demonstration.” Aging & Mental Health 8 (1): 40–51. [DOI] [PubMed] [Google Scholar]
- Davis, K. , Willink A., and Schoen C.. 2016. “Medicare Help at Home.” Health Affairs Blog [accessed on July 7, 2016]. Available at http://healthaffairs.org/blog/2016/04/13/medicare-help-at-home/
- Davis, K. , Buttorff C., Leff B., Samus Q. M., Szanton S., Wolff J. L., and Bandeali F.. 2015. “Innovative Care Models for High‐Cost Medicare Beneficiaries: Delivery System and Payment Reform to Accelerate Adoption.” The American Journal of Managed Care 21 (5): e349–56. [PubMed] [Google Scholar]
- Duru, O. K. , Ettner S. L., Vassar S. D., Chodosh J., and Vickrey B. G.. 2009. “Cost Evaluation of a Coordinated Care Management Intervention for Dementia.” The American Journal of Managed Care 15 (8): 521–8. [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. , Coots L. A., Kaganova Y., and Wiener J. M.. 2014. “Hospital and ED Use among Medicare Beneficiaries with Dementia Varies by Setting and Proximity to Death.” Health Affairs (Project Hope) 33 (4): 683–90. [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein S. E., and McHugh P. R.. 1975. ‘Mini‐Mental State’: A Practical Method for Grading the Cognitive State of Patients for the Clinician.” Journal of Psychiatric Research 12 (3): 189–98. [DOI] [PubMed] [Google Scholar]
- Harris, D. P. , Chodosh J., Vassar S. D., Vickrey B. G., and Shapiro M. F.. 2009. “Primary Care Providers’ Views of Challenges and Rewards of Dementia Care Relative to Other Conditions.” Journal of the American Geriatrics Society 57 (12): 2209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, L. E. , Weuve J., Scherr P. A., and Evans D. A.. 2013. “Alzheimer Disease in the United States (2010‐2050) Estimated Using the 2010 Census.” Neurology 80 (19): 1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickam, D. H. , Weiss J. W., Guise J., Buckley D., Motu'apuaka M., Graham E., Wasson N., and Saha S.. 2013. “Outpatient Case Management for Adults With Medical Illness and Complex Care Needs.” Comparative Effectiveness Review No. 99. (Prepared by the Oregon Evidence‐based Practice Center under Contract No. 290‐2007‐10057‐I.) AHRQ Publication No. 13‐EHC031‐EF. Rockville, MD: Agency for Healthcare Research and Quality [accessed on January 25, 2016]. Available at www.effectivehealthcare.ahrq.gov/reports/final.cfm
- Hinton, L. , Franz C. E., Reddy G., Flores Y., Kravitz R. L., and Barker J. C.. 2007. “Practice Constraints, Behavioral Problems, and Dementia Care: Primary Care Physicians’ Perspectives.” Journal of General Internal Medicine 22 (11): 1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd, M. D. , Martorell P., Delavande A., Mullen K. J., and Langa K. M.. 2013. “Monetary Costs of Dementia in the United States.” The New England Journal of Medicine 368 (14): 1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye, S. K. 2006. “Delirium in Older Persons.” The New England Journal of Medicine 354 (11): 1157–65. [DOI] [PubMed] [Google Scholar]
- Jansen, A. P. , van Hout H. P., Nijpels G., Rijmen F., Dröes R., Pot A., Schellevis F. G., Stalman W. A., and van Marwijk H. W.. 2011. “Effectiveness of Case Management Among Older Adults With Early Symptoms of Dementia and Their Primary Informal Caregivers: A Randomized Clinical Trial.” International Journal of Nursing Studies 48 (8): 933–43. [DOI] [PubMed] [Google Scholar]
- Kaufer, D. I. , Cummings J. L., Ketchel P., Smith V., MacMillan A., Shelley T., Lopez O. L., and DeKosky S. T.. 2000. “Validation of the NPI‐Q, a Brief Clinical Form of the Neuropsychiatric Inventory.” The Journal of Neuropsychiatry and Clinical Neurosciences 12(2): 233–9. [DOI] [PubMed] [Google Scholar]
- Kelley, A. S. , McGarry K., Gorges R., and Skinner J. S.. 2015. “The Burden of Health Care Costs for Patients With Dementia in the Last 5 Years of Life.” Annals of Internal Medicine 163 (10): 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, L. C. , Lee J. S., Chung J. C., Lau A., Woo J., and Kwok T. C.. 2010. “A Randomized Controlled Trial to Examine the Effectiveness of Case Management Model for Community Dwelling Older Persons with Mild Dementia in Hong Kong.” International Journal of Geriatric Psychiatry 25 (4): 395–402. [DOI] [PubMed] [Google Scholar]
- Longworth, D. L. 2011. “Accountable Care Organizations, the Patient‐Centered Medical Home, and Health Care Reform: what Does It All Mean?” Cleveland Clinic Journal of Medicine 78 (9): 571–82. [DOI] [PubMed] [Google Scholar]
- McCloskey, R. M . 2004. “Caring for Patients with Dementia in an Acute Care Environment.” Geriatric Nursing (New York, N.Y.) 25 (3): 139–44. [DOI] [PubMed] [Google Scholar]
- Mecocci, P. , von Strauss E., Cherubini A., Ercolani S., Mariani E., Senin U., Winblad B., and Fratiglioni L.. 2005. “Cognitive Impairment Is the Major Risk Factor for Development of Geriatric Syndromes during Hospitalization: Results from the GIFA Study.” Dementia and Geriatric Cognitive Disorders 20 (4): 262–9. [DOI] [PubMed] [Google Scholar]
- National Quality Forum . 2014. “Priority Setting for Healthcare Performance Measurement: Addressing Performance Measure Gaps for Dementia, Including Alzheimer's Disease” [accessed on April 1, 2016]. Available at http://www.qualityforum.org/priority_setting_for_healthcare_performance_measurement_alzheimers_disease.aspx
- Newcomer, R. , Spitalny M., Fox P., and Yordi C.. 1999. “Effects of the Medicare Alzheimer's Disease Demonstration on the Use of Community‐Based Services.” Health Services Research 34 (3): 645–67. [PMC free article] [PubMed] [Google Scholar]
- Odenheimer, G. , Borson S., Sanders A. E., Swain‐Eng R. J., Kyomen H. H., Tierney S., Gitlin L., Forciea M. A., Absher J., Shega J., and Johnson J.. 2014. “Quality Improvement in Neurology: Dementia Management Quality Measures.” Journal of the American Geriatrics Society 62 (3): 558–61. [DOI] [PubMed] [Google Scholar]
- Pedone, C. , Ercolani S., Catani M., Maggio D., Ruggiero C., Quartesan R., Senin U., Mecocci P., Cherubini A., and GIFA Study Group . 2005. “Elderly Patients with Cognitive Impairment Have a High Risk for Functional Decline during Hospitalization: The GIFA Study.” The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 60 (12): 1576–80. [DOI] [PubMed] [Google Scholar]
- Phelan, E. A. , Borson S., Grothaus L., Balch S., and Larson E. B.. 2012. “Association of Incident Dementia with Hospitalizations.” JAMA 307 (2): 165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, S. , Miranda‐Castillo C., Malouf R., Hoe J., Toot S., Challis D., and Orrell M.. 2015. “Case Management Approaches to Home Support for People with Dementia.” Cochrane Database of Systematic Reviews Art. No. CD008345. doi: 10.1002/14651858.CD008345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samus, Q. M. , Johnston D., Black B. S., Hess E., Lyman C., Vavilikolanu A., Pollutra J., Leoutsakos J., Gitlin L. N., and Rabins P. V.. 2014. “A Multidimensional Home‐Based Care Coordination Intervention for Elders with Memory Disorders: The Maximizing Independence at Home (MIND) Pilot Randomized Trial.” The American Journal of Geriatric Psychiatry 22 (4): 398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam‐Tham, H. , Cepoiu‐Martin M., Ronksley P. E., Maxwell C. J., and Hemmelgarn B. R.. 2013. “Dementia Case Management and Risk of Long‐Term Care Placement: A Systematic Review and Meta‐Analysis.” International Journal of Geriatric Psychiatry 28 (9): 889–902. [DOI] [PubMed] [Google Scholar]
- Tanner, J. A. , Black B. S., Johnston D., Hess E., Leoutsakos J., Gitlin L. N., Rabins P. V., Lyketsos C. G., and Samus Q. M.. 2015. “A Randomized Controlled Trial of a Community‐Based Dementia Care Coordination Intervention: Effects of MIND at Home on Caregiver Outcomes.” The American Journal of Geriatric Psychiatry 23 (4): 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services . 2015. “National Plan to Address Alzheimer's Disease: 2015 Update.” US Department of Health and Human Services, Washington, DC [accessed on March 30, 2016]. Available at https://aspe.hhs.gov/national-plan-address-alzheimer's-disease-2015-update
- Vickrey, B. G. , Mittman B. S., Connor K. I., Pearson M. L., Della Penna R. D., Ganiats T. G., DeMonte R. W., Chodosh J., Cui X., and Vassar S.. 2006. “The Effect of a Disease Management Intervention on Quality and Outcomes of Dementia Care: A Randomized, Controlled Trial.” Annals of Internal Medicine 145 (10): 713–26. [DOI] [PubMed] [Google Scholar]
- Weber, S. R. , Pirraglia P. A., and Kunik M. E.. 2011. “Use of Services by Community‐Dwelling Patients with Dementia: A Systematic Review.” American Journal of Alzheimer's Disease and Other Dementias 26 (3): 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, I. M. , and Graham‐White J.. 1980. “Psychogeriatric Dependency Rating Scales (PGDRS): A Method of Assessment for Use by Nurses.” The British Journal of Psychiatry: The Journal of Mental Science 137: 558–65. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Kuo T. C., Weir S., Kramer M. S., and Ash A. S.. 2008. “Healthcare Costs and Utilization for Medicare Beneficiaries with Alzheimer's.” BMC Health Services Research 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


