Abstract
In this study, the effect of icing medium containing different concentration of pistachio (Pistacia vera L. cv. Akbari) green hull extract (PHE) was investigated on the chemical stability of rainbow trout. The fishes were stored for 12 days in flaked ice containing 0, 0.1, 0.2 and 0.3% PHE and the parameters of pH, total volatile base nitrogen (TVB-N), histamine, free fatty acids, peroxide and thiobarbituric acid index (TBA-i) were periodically evaluated. In all experiments, a significant difference was evident between control and fish muscles stored in ice containing PHE. Fish icing with 0.3% PHE could considerably retard the oxidative and hydrolytic rancidity. In addition, it had significant effect on prevention of pH increase and production of volatile basic nitrogen during 12 days storage. PHE could diminish histamine accumulation in fish muscle and improve safety of rainbow trout. Consequently, PHE could have potential of application in icing medium for preservation of fish quality especially fatty ones.
Keywords: Rainbow trout, Pistachio green hull extract, Icing, Chemical stability
Introduction
Rainbow trout (Onhcorynchus mykiss) is one of the important cultivated fish which is farmed in Iran and other Middle East countries for meeting consumer demand toward fresh fish (Aubourg et al. 2010; Mexis et al. 2009). The rainbow trout is susceptible to biochemical and microbial changes, especially lipid oxidation during storage (Rezaei et al. 2008). In order to retain the quality of fresh fish and reduce the rate of spoilage during handling and storage, a wide number of preservation methods have been applied. Refrigerated sea water, flake ice or ice slurries as well as exposure to chemical agents are known as important strategies to attain this purpose (Rey et al. 2012). Among them, storage in ice is one of the most noteworthy and efficient way to prolong the shelf-life of fish. Nevertheless, food industry is still looking for new preservation approaches with the aim of supplying high quality and safe fresh products to consumers (Özyurt et al. 2012).
Due to the both consumer demand and economic reasons, mildly-preserved high quality products have been attracted much attention in recent years. To reach this goal, the combination of icing system with other preserving methods has been developed and flaked ice along with some physical treatments like high-pressure processing, irradiation or packaging as well as some chemical compounds (bisulphites, phenolic compounds, polyphosphates, etc.) was applied (Ashie et al. 1996; Briones et al. 2010; García-Soto et al. 2013). Recently, the addition of preservative compounds such as organic acids (ascorbic, citric and lactic acid; Rey et al. 2012), ozone (Pastoriza et al. 2008) and plant extracts in icing medium has been also investigated. Bensid et al. (2014) evaluated the quality changes of anchovy stored in ice containing thyme, oregano and clove extracts. Additionally, the effect of icing with rosemary (Özyurt et al. 2012), pomegranate peel, tea leaf and grape seed (Shinde and Patange 2015) in fresh fish as well as Chinese red pepper leaf extracts (Li et al. 2015) in salted fish was evaluated on the oxidative stability and quality characteristics of some fishes.
Since the health problems and detrimental effects of chemical preservatives are arguable, much attention has been attracted to natural extract of plants as biopreservative (Shinde and Patange 2015). The plant extracts show their preservative capacity due to the presence of a wide range of food phytochemicals such as phenolic acids, flavonoids and glycosides (Quitral et al. 2009).
Pistachio (Pistacia vera) green hull is known as a by-product of pistachio production which is separated industrially from the nut. This part of pistachio is 10% of the total weight of shelled nut and could pollute environment if it is discarded (Tomaino et al. 2010). Some researchers reported that pistachio green hull could be introduced as a good source of phenolic compounds (Goli et al. 2005; Rajaei et al. 2010).
Thus, the aim of this work was to evaluate the effect of pistachio green hull extract (PHE) on the quality changes and oxidative stability of rainbow trout during ice storage.
Materials and methods
Materials
Pistachio (Pistacia vera L. cv. Akbari) green hulls were obtained from Kerman agricultural research center of Iran. Hulls were dried and ground to give 40-mesh size powder and stored in a freezer at −18 °C until extraction. All chemicals and solvents were of analytical grade and obtained from Merck Co. (Darmstadt, Germany).
Extraction and determination of phenolic compounds
Dried powders of hulls (2.5 g) were mixed with 25 ml water at 60 °C for 4 h using an orbital shaker. The extract was filtered through Whatman No. 42 filter paper for removal of solid particles. The extraction was conducted twice and the extracts were stored at 4 °C until use for preparation of ice. Then, the total phenolic content was determined in the extract to know the exact phenolic compound concentration in each treatment. The total phenolic content was assayed colorimetrically using Folin-Ciocalteu reagent as described by Pinelo et al. (2004). A 2.5 ml of ten-fold diluted reagent, 2 ml of 7.5% sodium carbonate, and 0.5 ml of phenolic extract were mixed well. The absorbance was measured at 765 nm after 30 min holding in darkness. The content of phenolics in PHE was determined as gallic acid equivalent (GAE).
Fish and ice preparation
56 fresh rainbow trouts with (Onchorynchus mykiss) weight of 410.53 ± 44.26 g were purchased and transferred to the laboratory on ice. Immediately after arrival to the laboratory (within 3 h), three individual fishes were selected as fresh fish to be analyzed at day 0. Remaining fishes were divided into four lots and each lot was divided into three batches. Icing in one lot was done with pure ice (control) while fishes in the other three lots were surrounded with ice containing 0.1% (v/v), 0.2% (v/v) and 0.3% (v/v) PHE. The fish to ice ratio was 1:1 in all treatments. After preparation of lots, all boxes were transferred to 4 °C and sampling was accomplished at days 4, 8 and 12.
Proximate analysis
In order to determine the proximate composition of fish muscles, AOAC methods were applied. Lipid content was determined by the Soxhlet method. Kjeldahl method was applied for measuring the crude protein (% total nitrogen × 6.25), and moisture content was measured by evaporation of water from fish muscles in an oven at 105 °C until the weight of sample became constant. Total crude ash was analyzed by incineration in a muffle furnace at 550 °C for 5 h (AOAC 1996). All chemical components were expressed as percentage of fish flesh.
Chemical assessment of microbial activity
Determination of pH
Five grams fish muscle were homogenized with 45 mL distilled water for 30 s using Ultra-Turrax homogenizer (IKA, Germany) and then pH value was measured using a pH meter (JENWAY, USA).
Determination of total volatile base nitrogen (TVB-N)
TVB-N was determined by homogenization of 10 g fish flesh with 300 mL distilled water and 2 g MgO. After distillation of 150 mL of this mixture in 50 mL boric acid and methyl red indicator, the boric acid solution was titrated using 0.1 N hydrochloric acid. The TVB-N was reported as mgN/100 g fish muscle (Goulas and Kontominas 2005).
Histamine
Histamine content was measured according to the method reported by Patange et al. (2005). Briefly, after homogenization of 5 g fish samples in 20 mL 0.85% NaCl solution for 2 min, the mixture was centrifuged at 12,000 g for 10 min at 4 °C. The 0.85% saline was added to the supernatant until the volume reaches to 25 mL. Then, 6.25 g of anhydrous sodium sulfate was mixed with 1 g tri-sodium phosphate monohydrate, and 0.5 g of this mixture was added to 1 mL of the extract, diluted to 2 mL with saline and shacked thoroughly. After addition of 2 mL n-butanol, it was centrifuged at 3100 g for 10 min and 1 mL of the upper layer was separated and evaporated using nitrogen. The residue was dissolved in 1 mL distilled water and shacked with 5 mL 1.1% sodium carbonate solution and 2 mL chilled p-phenyldiazonium sulfonate reagent. The absorbance of the solution was measured after 5 min at 496 nm.
For preparation of p-phenyldiazonium sulfonate reagent, 1.5 mL of 0.9% (w/v) chilled sulfanilic acid in 4% hydrochloric acid was added to 1.5 mL 5% (w/v) sodium nitrite and kept on ice for 5 min. Again, 6 mL 5% sodium nitrite solution was poured in the chilled reagent and the volume was reached to 50 mL with chilled distilled water after 5 min.
Histamine concentration in fish flesh was estimated using the following equation.
where A is the value of histamine obtained in μg/mL from the standard curve.
Lipid damage
Free fatty acids
The lipid was extracted from fish using chloroform–methanol solvent based on the method of Bligh and Dyer (1959). Free fatty acids (FFA) in extracted lipid which is expressed as percentage of oleic acid, was measured by the acidimetric titration of fish oil mixed with ethanol and using phenolphthalein as an indicator following AOCS method of Cd 3d-63 (2004a).
Peroxide value
Peroxide value was determined by iodometric titration according to AOCS method of Cd 8-53 (2004b) and was expressed in milliequivalents of peroxide oxygen per kilogram of fish oil.
Determination of thiobarbituric acid index (TBA-i)
Ten grams of fish flesh were firstly homogenized (IKA, Germany) with 25 mL of 20% trichloroacetic acid (TCA) for 2 min. Then, 25 mL of distilled water was blended with homogenized fish muscle and filtered through a Whatman filter paper (No. 1). Five milliliters of filtered fish sample were mixed with 5 ml of 0.01 M 2-thiobarbitoric acid solution in 90% glacial acetic acid and incubated for 1 h in boiling water. The TBA value was reported as mg malonaldehyde per kilogram fish flesh (Strange et al. 1977).
Statistical analysis
All measurements were replicated three times for each lot and mean values ± standard deviation were reported for each case. The split-plot analysis of variance, with confidence intervals set for a level of significance of p < 0.05 on Statistical Analysis System (SAS) to evaluate the significance of differences among mean values.
Results and discussion
Total phenolic content of pistachio green hull
Total phenolic content of pistachio green hull was measured about 87.00 ± 4.39 mg GAE per dry weight which was higher than the value reported by Rajaei et al. (2010) (49.32 mg GAE) and Goli et al. (2005) (34.7 mg GAE/g PHE) in aqueous extracts. This difference might be described by the variation in pistachio variety, extraction method and the assay of TPC determination.
Proximate composition
Moisture, protein, fat and ash content of the rainbow trout were 73.23 ± 0.05%, 21.33 ± 0.15%, 4.44 ± 0.14% and 0.97 ± 0.03%, respectively. Bureau et al. (2003) obtained the chemical composition of 28 rainbow trout fed with six different diets. The estimated amount of moisture, lipid, protein and ash was approximately 60–77%, 4–16%, 12–17% and 1.5–2.5%, respectively. The wide variation in proximate composition can be originated from fish size, nutrition, living area, catching season, sexual variation and the other environmental conditions (González-Fandos et al. 2005). The presence of high amount of lipid makes rainbow trout susceptible to oxidation. Therefore, prevention of lipid oxidation is an important issue during cold and frozen storage of fish.
Chemical assessment of microbial activity
pH
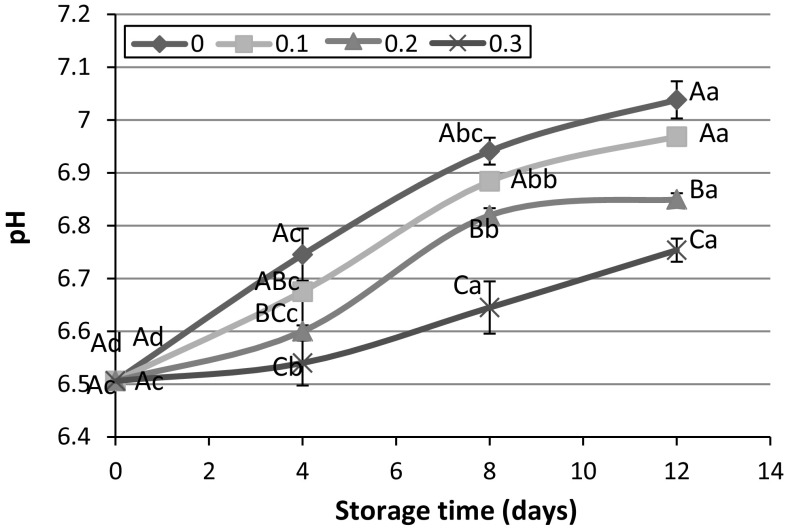
The results indicated that pH value of fresh rainbow trout was approximately 6.5. This amount was significantly increased (p < 0.05) in all treatments during storage at ice (Fig. 1). By incorporating PHE in icing medium, an inhibitory effect on pH increase was observed. So that, with elevation in extract concentration, the rate of pH increase was diminished. Although addition of 0.1% PHE to icing medium did not have any significant effect on pH value, 0.2% PHE could significantly prevent increasing pH at 4th day of icing. At the end of storage, control sample had pH ≈ 7.0; however, this value for fishes stored in ice containing 0.3% PHE was significantly (p < 0.05) lower (pH ≈ 6.7). Several studies reported the impact of natural compounds and their extracts in icing medium against pH increase during chilled storage. Miranda et al. (2016) used an icing medium containing alga Fucus spiralis for preservation of megrim. Özyurt et al. (2012) reported the lower pH of sardine during 12 days storage in icing medium containing rosemary extract in comparison with those stored in ice without the extract (control sample). Also, the role of oregano and rosemary leave extract on prevention of pH increase in Chilean jack mackerel during storage was reported by Quitral et al. (2009). Enhancement of pH might be the result of formation of nitrogenous compounds such as TMA and ammonia. This compounds mainly derived from microbial and enzymatic activity and protein decomposition (Mexis et al. 2009; Özyurt et al. 2012).
Fig. 1.
Effect of icing with 0, 0.1, 0.2 and 0.3% PHE extract on pH value during 12 days storage at 4 °C. Capital and lowercase letters indicate significant difference between different fish muscles in each time and between different storage times in each treatment, respectively
TVB-N
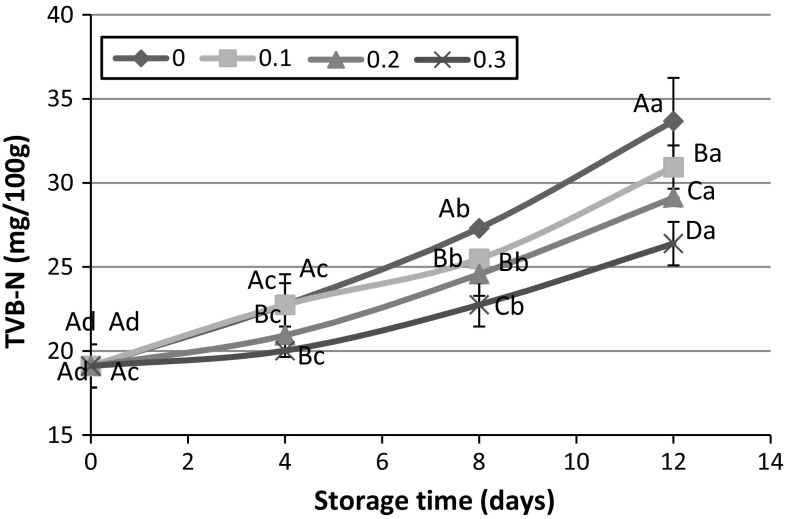
One of the most important indicators of freshness for aquatic animals is amount of basic volatile compounds which measured by extracting or distilling fish muscle under alkaline conditions (Howgate 2010). In fact, the hydrolysis of trimethylamine oxide (TMAO) to trimethylamine (TMA) by reductive enzymes present in microorganisms leads to the production of volatile basic nitrogens (Lu 2009). Figure 2 indicates the effect of icing with different concentrations of PHE on TVB-N value of rainbow trout during 12 days storage at 4 °C. The amount of TVB-N in fresh fish was measured 19.11 mgN/100 g that was in the range of 10–20 mgN/100 g TVB-N for freshwater fish muscle (Alçiçek 2011). After 4 days storage of fishes in icing medium, TVB-N value increased in all treatments except medium containing 0.3% PHE. The volatile basic nitrogen continually enhanced in next days. Although, the rate of volatile nitrogen production in control sample was higher than fishes stored in medium containing PHE. The acceptable TVB-N value for fresh fish is 25–30 mgN/100 g (Lopez-Caballero et al. 2000). The findings revealed that after 12 days storage, icing of fish without PHE or 0.1% PHE led to the unacceptable levels of TVB-N. The presence of 0.2 and 0.3% PHE could maintain the freshness of fish and prevent enhancement of TVB-N value. Rajaei et al. (2010) applied PHE against some gram positive and gram negative bacteria. They found this extract could have inhibitory effect against bacteria specifically gram positive types. Therefore, the lower TVB-N value at higher concentration of PHE could be attributed to the diminishing oxidative deamination of non-protein nitrogenous compounds by enzymes. Furthermore, the reduction of microbial population by PHE might be considered for this event (Song et al. 2011).
Fig. 2.
Effect of icing with 0, 0.1, 0.2 and 0.3% PHE extract on TVB-N during 12 days storage at 4 °C. Capital and lowercase letters indicate significant difference between different fish muscles in each time and between different storage times in each treatment, respectively
Histamine
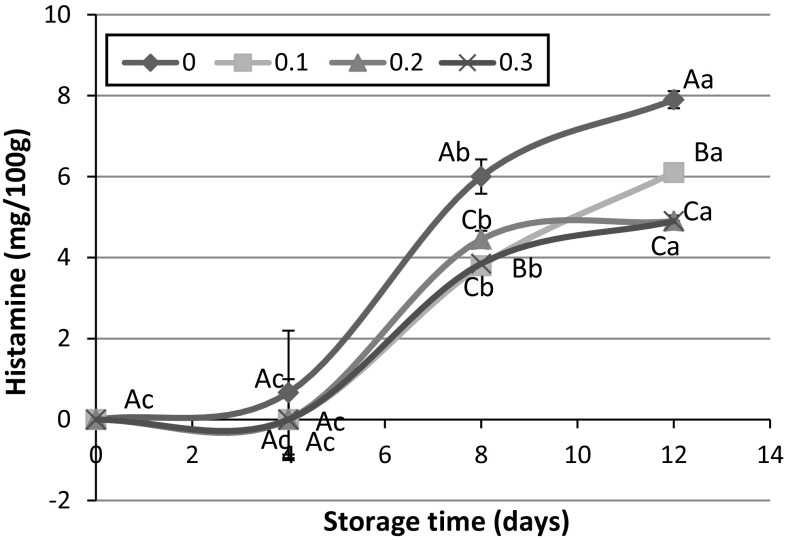
Biogenic amines, which are low in fresh fish and form due to the microbial spoilage, can be used as a factor for estimation of fish freshness and degree of its spoilage. Factors affecting the amount and type of produced amines are fish species, microbial flora, and some factors like packaging, temperature and antimicrobial agents which determine microbial growth (Özyurt et al. 2012). Histamine as one of the biogenic amines is toxic and causes histamine poisoning (Rezaei et al. 2008). In this work, histamine content was not detectable until day 4 over cold storage. Rezaei et al. (2008) also could not measure histamine in rainbow trout until 9th day during cold storage. They related this finding to low level of bacteria as well as low histidine content as a precursor of histamine in rainbow trout (Rezaei et al. 2008). As can be seen in Fig. 3, icing in natural ice led to the production of higher amount of histamine during 12 days storage. Although, addition of PHE to the ice used for cold storage of fish could prevent the rapid increase of histamine content. So that, with enhancement in extract concentration, lower histamine formed in fish flesh (p < 0.05). Özyurt et al. (2012) evaluated the effect of icing with rosemary extract on the formation of biogenic amines in sardine during 15 days cold storage. They also found that the presence of rosemary extract could significantly prevent the formation of histamine in sardine (Özyurt et al. 2012). It seems that the antimicrobial and antioxidant properties of PHE could decrease the formation of histamine during 12 days icing. The amount of 10 mg/100 g, declared by EU (1991) (Directive 1991) as the legal limit for histamine content, was not observed in all fish muscles during storage.
Fig. 3.
Effect of icing with 0, 0.1, 0.2 and 0.3% PHE extract on Histamine during 12 days storage at 4 °C. Capital and lowercase letters indicate significant difference between different fish muscles in each time and between different storage times in each treatment, respectively
Lipid damage
FFA
The fish lipid hydrolysis was measured by means of free fatty acids (FFA) content. FFA could be released from triacylglycerols, diacylglycerols and phospholipids when lipid is hydrolyzed by endogenous enzyme of lipase and other factors (Özyurt et al. 2012). Although the formation of FFA itself does not indicate nutritional loss, high FFA is pertained to some extent to lack of acceptability. FFA has detrimental effect on fish muscle texture and accelerates lipid oxidation and off-flavor development (Miranda et al. 2016; Sanjuás-Rey et al. 2011). Initial FFA content of fish sample was 0.97% (based on oleic acid). As seen in Table 1, a remarkable increment in FFA was observed in all groups during ice storage. At the end of storage, the highest FFA content (6.58%) belonged to the control sample which was not significantly different with the fish flesh stored in ice containing 0.1% PHE (5.73%; p > 0.05). The lower hydrolytic activity obtained in fish covered with 0.3% PHE ice (3.16%). The results revealed that with increasing in PHE concentration in ice, FFA content of fish lipids reduced.
Table 1.
Free Fatty Acid (FFA) changes of rainbow trout during 12 days storage in ice containing 0, 0.1, 0.2 and 0.3% pistachio green hull extract (PHE)
| PHE Concentration (%) | Time (days) | |||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| 0 | 0.97Ac ± 0.01 | 5.00ABb ± 1.34 | 3.61ABb ± 0.24 | 6.58Aa ± 0.67 |
| 0.1 | 0.97Ac ± 0.01 | 4.87Bc ± 0.11 | 4.74Ab ± 0.17 | 5.73ABa ± 0.60 |
| 0.2 | 0.97Ac ± 0.01 | 6.07Aa ± 0.02 | 3.82ABb ± 0.61 | 5.32Ba ± 0.29 |
| 0.3 | 0.97Ab ± 0.01 | 3.67Ca ± 0.24 | 3.49Ba ± 0.07 | 3.10Ca ± 0.03 |
The values are expressed as mean ± standard deviation, n = 3. Capital letters in each column and lowercase letters in each row represent significant different (p < 0.05)
The high FFA content in control fish could be explained on the basis of the marked pH increase (Fig. 1) and approaching towards the optimum activity pH range of lipases (López-Amaya and Marangoni 2000a) and phospholipases (López-Amaya and Marangoni 2000b). In agreement with this work, a lower lipid hydrolysis development was observed in chilled Chilean jack mackerel stored with ice prepared from aqueous extracts of rosemary or oregano (Quitral et al. 2009). Moreover, Bensid et al. (2014) reported that the employment of icing systems containing thyme (0.04%), oregano (0.03%) and clove (0.02%) extracts was successful in reducing FFA formation in anchovy over 12 days storage. However, in contrast with the present results, an inhibitory effect of rosemary extract on lipid hydrolysis in chilled sardine was not observed by Özyurt et al. (2012).
Peroxide value
Oil rancidity limits the shelf life of oily fish species and can be followed by measuring the increase in hydroperoxides, as primary oxidation products, and by determining the formation of thiobarbituric acid reactive substances, mainly malondialdehyde, as secondary types (Özyurt et al. 2012).
The initial peroxide value of fish oil was obtained 0.54 meq/kg of fat. As indicated in Table 2, the peroxide value of the oil extracted from fish muscles increased as storage time prolonged. At the end of storage, the highest content of hydroperoxide (15.14 meq/kg) belonged to the control sample while icing with PHE reduced peroxide formation. By increasing in concentration of PHE, the inhibitory effect of icing was elevated as the lowest peroxide value (3.81 meq/kg) found in the sample of 3% PHE. It can be concluded that the presence of PHE in the chilling medium could be effective at delaying lipid peroxidation in rainbow trout stored on ice. This ability is probably related to its phenolic compounds which react with oxygen in autoxidation process, thus reducing formation of hydroperoxides (Bensid et al. 2014). In agreement with this work, the inhibitory effect of oregano and rosemary extracts on Chilean jak mackerel (Quitral et al. 2009) and thyme, oregano and clove extracts on anchovy (Bensid et al. 2014) during chilled storage is also reported.
Table 2.
Peroxide value changes of rainbow trout during 12 days storage in ice containing 0, 0.1, 0.2 and 0.3% pistachio green hull extract (PHE)
| PHE Concentration (%) | Time (days) | |||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| 0 | 0.54Ad ± 0.00 | 13.04Ac ± 0.56 | 13.44Ab ± 0.13 | 15.14Aa ± 0.22 |
| 0.1 | 0.54Ad ± 0.00 | 5.02Bc ± 0.08 | 9.21Bb ± 0.85 | 11.60Ba ± 0.27 |
| 0.2 | 0.54Ad ± 0.00 | 3.89Cc ± 0.07 | 7.61Cb ± 0.11 | 8.72Ca ± 0.14 |
| 0.3 | 0.54Ad ± 0.00 | 4.60Bc ± 0.47 | 6.35Db ± 0.07 | 3.81 Da ± 0.16 |
The values are expressed as mean ± standard deviation, n = 3. Capital letters in each column and lowercase letters in each row represent significant different (p < 0.05)
TBA-i
In developed oxidation, secondary products of oxidation would result from degradation of hydroperoxides. One of the most important secondary oxidation products is malondialdehyde (MDA). This compound uses as an indicator for propagation of oxidation and measures using TBA-i (Fernández et al. 1997).
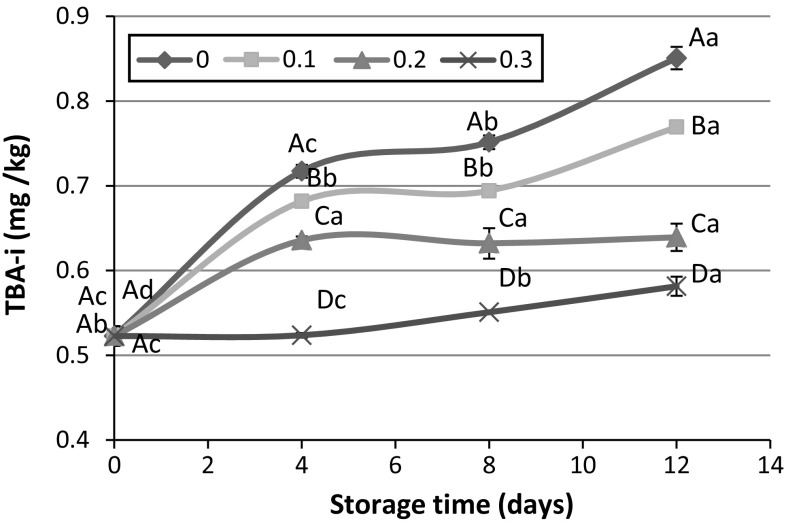
Based on the obtained results, both storage time and concentration of PHE had significant effect on TBA-i (p < 0.05). The initial thiobarbituric acid reactive substances (TBARs) of rainbow trout was approximately 0.52 mg MDA/kg. This amount increased to 0.85 mg MDA/kg in control sample after 12 days icing in flaked ice, while in sample containing 0.3% PHE, TBA-i only increased to 0.06 mg MDA/kg at the end of storage (Fig. 4). In all testing days, TBA-i value in the presence of PHE was significantly lower than control sample and with enhancement of the extract concentration in icing medium, TBARs concentration decreased. It can be concluded that the presence of high amount of phenolic compounds in pistachio green hull can prevent the propagation of oxidation process or retard it. Hence, the rate of increasing TBA-i decreased with enhancement of PHE in ice used for storage of rainbow trout.
Fig. 4.
Effect of icing with 0, 0.1, 0.2 and 0.3% PHE extract on TBARs during 12 days storage at 4 °C. Capital and lowercase letters indicate significant difference between different fish muscles in each time and between different storage times in each treatment, respectively
Conclusion
This study revealed that icing with pistachio hull extract could significantly improve chemical quality and prolong shelf-life of rainbow trout throughout cold storage. The presence of phenolic compounds in PHE could protect unsaturated fatty acids against oxidation and partially prevented the formation of volatile basic nitrogen and biogenic amine, histamine. However, the potential application of such plant residual extracts should be carefully studied specifically in terms of sensorial acceptability of fish; since, these extracts might impart unpleasant attributes (bitter taste and/or strong odor) on fish flesh.
Acknowledgements
The authors would like to acknowledge the Scientific Association of Isfahan University of Technology for financial support of this research.
References
- Alçiçek Z. The effects of thyme (Thymus vulgaris L.) oil concentration on liquid-smoked vacuum-packed rainbow trout (Oncorhynchus mykiss Walbaum, 1792) fillets during chilled storage. Food Chem. 2011;128(3):683–688. doi: 10.1016/j.foodchem.2011.03.087. [DOI] [Google Scholar]
- AOAC (1996) Official methods of analysis of AOAC international, 16th edn. (AOAC Vols II). AOAC, Virginia
- AOCS (2004a) Acid value. In: Official methods and recommended practices of the AOCS (Vol. Official method Cd 3d-63). AOCS Press, Champaign
- AOCS (2004b) Peroxide value. In: Official methods and recommended practices of the AOCS (Vol. Official method Cd 8-53). AOCS Press, Champaign
- Ashie I, Smith J, Simpson B, Haard NF. Spoilage and shelf-life extension of fresh fish and shellfish. Crit Rev Food Sci Nutr. 1996;36(1–2):87–121. doi: 10.1080/10408399609527720. [DOI] [PubMed] [Google Scholar]
- Aubourg SP, Álvarez V, Pena J. Lipid hydrolysis and oxidation in farmed gilthead seabream (Sparus aurata) slaughtered and chilled under different icing conditions. Grasas Aceites. 2010;61(2):183–190. doi: 10.3989/gya.108909. [DOI] [Google Scholar]
- Bensid A, Ucar Y, Bendeddouche B, Özogul F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chem. 2014;145:681–686. doi: 10.1016/j.foodchem.2013.08.106. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Briones LS, Reyes JE, Tabilo-MunizagaGE Pérez-Won MO. Microbial shelf-life extension of chilled Coho salmon (Oncorhynchus kisutch) and abalone (Haliotis rufescens) by high hydrostatic pressure treatment. Food Control. 2010;21(11):1530–1535. doi: 10.1016/j.foodcont.2010.04.027. [DOI] [Google Scholar]
- Bureau DP, Gunther SJ, Cho CY. Chemical composition and preliminary theoretical estimates of waste outputs of rainbow trout reared in commercial cage culture operations in Ontario. N Am J Aquac. 2003;65(1):33–38. doi: 10.1577/1548-8454(2003)065<0033:CCAPTE>2.0.CO;2. [DOI] [Google Scholar]
- Directive E. Council Directive 91/493/EEC of 22 July 1991 laying down the health conditions for the production and the placing on the market of fishery products. Off J L. 1991;268(24/09):0015–0034. [Google Scholar]
- Fernández J, Pérez-Álvarez JA, Fernández-López JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59(3):345–353. doi: 10.1016/S0308-8146(96)00114-8. [DOI] [Google Scholar]
- García-Soto B, Aubourg SP, Calo-Mata P, Barros-Velázquez J. Extension of the shelf life of chilled hake (Merluccius merluccius) by a novel icing medium containing natural organic acids. Food Control. 2013;34(2):356–363. doi: 10.1016/j.foodcont.2013.05.007. [DOI] [Google Scholar]
- Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005;92(3):521–525. doi: 10.1016/j.foodchem.2004.08.020. [DOI] [Google Scholar]
- González-Fandos E, Villarino-Rodrıguez A, Garcıa-Linares M, Garcıa-Arias M, Garcıa-Fernández M. Microbiological safety and sensory characteristics of salmon slices processed by the sous vide method. Food Control. 2005;16(1):77–85. doi: 10.1016/j.foodcont.2003.11.011. [DOI] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93(3):511–520. doi: 10.1016/j.foodchem.2004.09.040. [DOI] [Google Scholar]
- Howgate P. A critical review of total volatile bases and trimethylamine as indices of freshness of fish. Part 1. Determination. Electron J Environ Agric Food Chem. 2010;9(1):29–57. [Google Scholar]
- Li J, Hui T, Wang F, Li S, Cui B, Cui Y. Chinese red pepper (Zanthoxylum bungeanum Maxim.) leaf extract as natural antioxidants in salted silver carp (Hypophthalmichthys molitrix) in dorsal and ventral muscles during processing. Food Control. 2015;56:9–17. doi: 10.1016/j.foodcont.2015.03.001. [DOI] [Google Scholar]
- López-Amaya C, Marangoni A. Lipases. In: Haard N, Simpson B, editors. Seafood enzymes. New York: Marcel Dekker; 2000. pp. 121–146. [Google Scholar]
- López-Amaya C, Marangoni A. Phospholipases. In: Haard N, Simpson B, editors. Seafood enzymes. New York: Marcel Dekker; 2000. pp. 91–119. [Google Scholar]
- Lopez-Caballero M, Pérez-Mateos M, Montero P, Borderías AJ. Oyster preservation by high-pressure treatment. J Food Prot. 2000;63(2):196–201. doi: 10.4315/0362-028X-63.2.196. [DOI] [PubMed] [Google Scholar]
- Lu S. Effects of bactericides and modified atmosphere packaging on shelf-life of Chinese shrimp (Fenneropenaeus chinensis) LWT. 2009;42(1):286–291. doi: 10.1016/j.lwt.2008.03.004. [DOI] [Google Scholar]
- Mexis SF, Chouliara E, Kontominas MG. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 C. Food Microbiol. 2009;26(6):598–605. doi: 10.1016/j.fm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Miranda JM, Trigo M, Barros-Velázquez J, Aubourg SP. Effect of an icing medium containing the alga Fucus spiralis on the microbiological activity and lipid oxidation in chilled megrim (Lepidorhombus whiffiagonis) Food Control. 2016;59:290–297. doi: 10.1016/j.foodcont.2015.05.034. [DOI] [Google Scholar]
- Özyurt G, Kuley E, Balikçi E, Kaçar Ç, Gökdogan S, Etyemez M. Effect of the icing with rosemary extract on the oxidative stability and biogenic amine formation in sardine (Sardinella aurita) during chilled storage. Food Bioprocess Tech. 2012;5(7):2777–2786. doi: 10.1007/s11947-011-0586-7. [DOI] [Google Scholar]
- Pastoriza L, Bernárdez M, Sampedro G, Cabo ML, Herrera JJR. The use of water and ice with bactericide to prevent onboard and onshore spoilage of refrigerated megrim (Lepidorhombus whiffiagonis) Food Chem. 2008;110(1):31–38. doi: 10.1016/j.foodchem.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Patange S, Mukundan M, Kumar KA. A simple and rapid method for colorimetric determination of histamine in fish flesh. Food Control. 2005;16(5):465–472. doi: 10.1016/j.foodcont.2004.05.008. [DOI] [Google Scholar]
- Pinelo M, Rubilar M, Sineiro J, Nunez M. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster) Food Chem. 2004;85(2):267–273. doi: 10.1016/j.foodchem.2003.06.020. [DOI] [Google Scholar]
- Quitral V, Donoso ML, Ortiz J, Herrera MV, Araya H, Aubourg SP. Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): effect of a plant-extract icing system. LWT. 2009;42(8):1450–1454. doi: 10.1016/j.lwt.2009.03.005. [DOI] [Google Scholar]
- Rajaei A, Barzegar M, Mobarez AM, Sahari MA, Esfahani ZH. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food Chem Toxicol. 2010;48(1):107–112. doi: 10.1016/j.fct.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Rey MS, García-Soto B, Fuertes-Gamundi JR, Aubourg S, Barros-Velázquez J. Effect of a natural organic acid-icing system on the microbiological quality of commercially relevant chilled fish species. LWT. 2012;46(1):217–223. doi: 10.1016/j.lwt.2011.10.003. [DOI] [Google Scholar]
- Rezaei M, Hosseini SF, Langrudi HE, Safari R, Hosseini SV. Effect of delayed icing on quality changes of iced rainbow trout (Onchorynchus mykiss) Food Chem. 2008;106(3):1161–1165. doi: 10.1016/j.foodchem.2007.07.052. [DOI] [Google Scholar]
- Sanjuás-Rey M, Barros-Velázquez J, Aubourg SP. Effect of different icing conditions on lipid damage development in chilled horse mackerel (Trachurus trachurus) muscle. Grasas Aceites. 2011;62(4):436–442. doi: 10.3989/gya.033611. [DOI] [Google Scholar]
- Shinde P, Patange S. Quality of Indian mackerel as affected by pomegranate peel and tea leaf extracts during ice storage. SAARC J Agric. 2015;13(1):109–122. doi: 10.3329/sja.v13i1.24185. [DOI] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2011;22(3):608–615. doi: 10.1016/j.foodcont.2010.10.012. [DOI] [Google Scholar]
- Strange E, Benedict R, Smith J, Swift C. Evaluation of rapid tests for monitoring alterations in meat quality during storage: I. Intact meat. J Food Prot. 1977;40:843–847. doi: 10.4315/0362-028X-40.12.843. [DOI] [PubMed] [Google Scholar]
- Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. 2010;92(9):1115–1122. doi: 10.1016/j.biochi.2010.03.027. [DOI] [PubMed] [Google Scholar]