Abstract
Chia seeds (Salvia hispanica L.) when immersed in water, produce a highly viscous solution due to the release of mucilage, high molecular weight complex carbohydrates with wide application in the food industry. Thus, this study involve development of method for extracting mucilage from chia seed based on mechanical process and low temperature. The method involve extraction by cold pressing and drying by freeze-drying, which was compared to the traditional hot extraction method. The chia seed mucilage cultivated in Brazil was extracted successfully using the previously mentioned extraction method. Rheological analysis including thixotropy, flow curve and frequency sweep of mucilage was done. Microstructure was examined by scanning electron microscopy. The optimal process at 27 °C gave yield of 8.46%. The rheograms showed that the apparent viscosity decreased with increase in shear rate and this effect was most notable in the dispersions obtained by cold extraction and with high concentrations. The gum obtained using CE presented higher values for thixotropic behavior. The storage modulus (G′) was consistently higher than the loss modulus (G″) and the data indicated formation of ‘weak gel’ structure of the dispersions. SEM indicated macroscopic fibrous structure of mucilage obtained through cold extraction process, indicating that the macromolecular network formed by fibrous material contained in mucilage maintained its structure in the process of deep freezing and freeze-drying.
Keywords: Extraction, Yield, Rheology, Chia, Salvia hispanica L
Introduction
Chia (Salvia hispanica L.) is an annual herbaceous plant belonging to the Lamiaceae family, is native to southern Mexico and northern Guatemala (Ixtaina et al. 2010). The seed Chia was an important food for the pre-Columbian peoples in America, including the Maya and Aztec peoples, assuming greater importance than corn, beans and other cereals (Ayerza and Coates 2004). In time its use was being abandoned, but at the end of the twenty century there has been renewed interest in their use, mainly due to its nutritional value.
It is noteworthy the high seed oil content (about 40% of the seed weight) with almost 60% fatty α-linolenic acid (omega-3) and also dietary fiber (more than 30% by weight) (Reyes-Caudillo et al. 2008; Ullah et al. 2016). Its seeds are a natural source of omega 3 fatty acids, antioxidants, proteins, vitamins, minerals and dietary fiber (Ixtaina et al. 2010; Capitani et al. 2013; Julio et al. 2016). Because it still contains a high nutritional value, the residues obtained in chia oil extraction process are considered by-products (Ramos 2013). Moreover, there is no evidence that the whole or ground chia seed cause adverse or allergenic effects (European Food Safety Authority—EFSA 2009), thus chia seed and its derivatives are promising sources of food (Muñoz et al. 2012). Due to its high nutritional value, the chia seed is being used as a nutritional supplement, as well as in the preparation of cereal bars, cereals consumed for breakfast and cookies in the US, Latin America and Australia (Dunn 2010; Muñoz et al. 2012). On the other hand, chia seed oil by-product obtained after extraction also exhibit some interesting functional properties with potential for broad use in the food industry.
When many seeds are immersed in water, a highly viscous solution is formed due to the release of mucilage by the outer cell wall of the epidermal cells. This is a complex carbohydrate of high molecular weight, is a major component of the seed due to their physiological role (Marin Flores et al. 2008). According to Muñoz et al. (2012), mucilage appears to be firmly attached to the seed, making its extraction difficult. Preliminary studies of mucilage extraction obtained through various processes found that yields varied from 5 to 15.5% (Ayerza and Coates 2001; Marin Flores et al. 2008; Muñoz et al. 2012; Reyes-Caudillo et al. 2008). According to Muñoz et al. (2012), these wide variations in yield are related to methods of extraction and hydration.
The extraction of the mucilage can be done directly from whole or ground seed, thus as the residue of oil extraction, among others. Optimum conditions using elevated temperature, alkaline pH adjustment, urea, salt addition have been used to optimize the gel extraction with yield ranging from approximately 5–7% (Lin et al. 1994; Ayerza and Coates 2001; Muñoz et al. 2012), reaching even 12.6% yield using Soxhlet and super critical fluid extraction methods (Segura-Campos et al. 2014). In the work of Goh et al. (2016), the polysaccharide gel layer surrounding hydrated chia seeds was extracted using water and isolated by ethanol precipitation. Both layers in the supernatant were recovered, mixed and freeze-dried. The yield based on freeze-dried sample with respect to dry seeds was 1.20 ± 0.05% w/w on a dry weight basis. The authors focused largely on the rheological characteristics of the gel layer stripped off from the hydrated chia seeds. Thus, this work proposes the development of a method for extracting mucilage of chia seed based on mechanical processes to low temperatures and evaluating its rheological behavior and microstructure.
Materials and methods
Chia seeds
The seeds are from the city of Entre-Ijuís/Rio Grande do Sul, Brazil, crop 2013. These were cleaned manually to remove foreign materials such as stones, motes and broken seeds. The seeds were stored in hermetically sealed plastic containers and stored at 5 °C until further use.
Extraction of mucilage
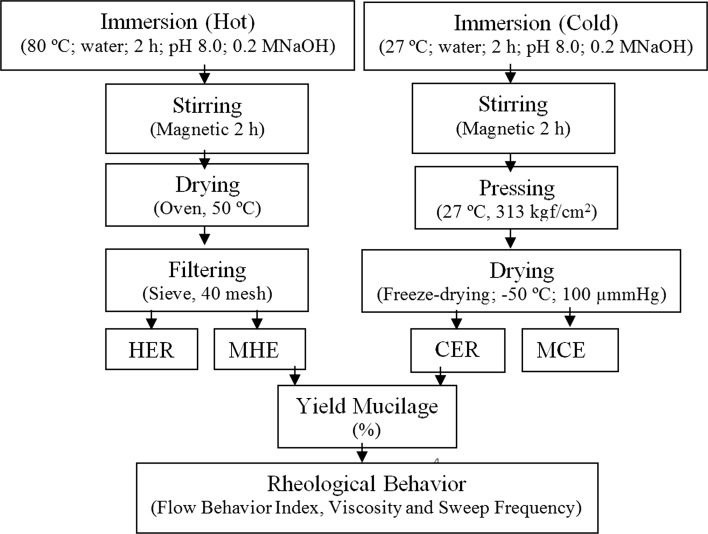
The process of extraction and characterization of mucilage and byproducts can be seen in Fig. 1. The hot extraction of mucilage was based on the methodology proposed by Muñoz et al. (2012), following the parameters obtained by the authors for maximum extraction yield. Samples of 100 g of whole seeds were added to containers containing distilled water in the seed:water ratio of 1:40. The pH was adjusted and maintained at the value 8, and the temperature maintained at 80 ± 1.5 °C using a temperature controller. The mixtures were magnetically stirred and hydrated for 2 h. Then the water suspension was spread onto a drying tray and exposed to temperatures of 50 °C for 48 h. The seed mucilage was separated by sieving on a 40 mesh screen, and then obtaining the weight of the pulp.
Fig. 1.
Flowchart for extraction and characterization of chia seed mucilage (MHE mucilage hot extraction, HER hot extraction residue, MCE mucilage cold extraction, CER cold extraction residue)
For the cold extraction, the samples of 100 g of whole seeds were added to containers containing distilled water in the seed:water ratio of 1:10, 1:20, 1:30 and 1:40. The pH was adjusted and maintained at 8.0 and the temperature value at 27 °C using a temperature controller. The mixtures were magnetically stirred and hydrated for 2 h. The separation of the seed mucilage was made by pressing controlled at 313 kgf/cm2. After pressing, the freezing of the samples was performed in a ultrafreezer (Sanyo VIP ™ Series, Model MDF-U53VA) at − 86 °C and dried in freeze-drier (Liobras Ltda., Model L101, São Carlos/SP, Brazil). All the tests were performed with three repetitions. The freeze-drier operating temperature was − 50 °C. The finish of freeze-drying process was established after the device reaches the 100 μmmHg pressure that occurred after 72 h of operation. The freeze-dried mucilage was then weighed to calculate the yield.
Proximate composition of mucilage
The moisture was determined by gravimetric method with drying in an oven at 65 °C for 48 h, time taken to reach the constant weight (AOAC 1990). The lipid content, protein content and ash content was determined using (AOAC 1990). Crude fiber was extracted by acid hydrolysis and quantified by the gravimetric method proposed by Kamer and Ginkel (1952). Carbohydrate content was estimated as nitrogen-free extract (NFE) by difference using Eq. (1) (Capitani et al. 2015).
| 1 |
Yield of mucilage
The extraction yield (YM) of the mucilage obtained by cold extraction and the hot processes were determined by Eq. 2.
| 2 |
Reconstitution of mucilage
After obtained the mucilage by the two methods of extraction, they were reconstituted in distilled water at different concentrations for rheology analysis. With the mucilage obtained by the cold extraction method in which a seed:water ratio of 1:20 was used, mucilage dispersions at five different concentrations were prepared: 1.00; 2.00; 3.00; 4.00 and 5.00 (g powder/100 g gum). The gum obtained from hot extraction process, seed:water ratio of 1:40 was prepared at a concentration of 1.00 g powder/100 g of gum. The gums were prepared with distilled water at 80 °C and agitated for 30 min with a magnet bar on a magnetic stirrer and maintained under growth chamber at 4 °C for 24 h for complete hydration.
Rheology
HAAKE RheoStress Rheometer 6000 (Thermo Scientific) connected to UTM temperature controller (Thermo Scientific) was used to perform rheological measurements. The oscillatory shear tests were performed with a parallel plate geometry having diameter of 34.997 mm and 1 mm gap. All analyses were performed in duplicate. The oscillatory tests were conducted at 1 Hz frequency stress sweep to determine the linear viscoelastic range. The frequency sweep tests (from 1 to 10 Hz) were performed by selecting a strain within the linear viscoelastic range. The influence of temperature on apparent viscosity of dispersions, was studied between temperature 10 to 90 °C.
Flow curves
The shear stress was measured as a function of the shear rate to generate three curves (0–300 s−1, 300–0 s−1; 0–300 s−1). The data of the third curve were fitted to the rheological models of Newton Law, Power Law and Herschel-Buckley.
Thixotropy
The verification of the existence of thixotropy was made by evaluating the flow curves obtained by varying the shear rate (γ) for a period of time, keeping the temperature constant at 20 °C. The programming parameters used to quantify the thixotropy value were: rise ramp of shear rate test: 0–300 (s−1) for a period of 2 min; ramp down shear rate test: 300–0 (s−1) for a period of 2 min. The thixotropic behavior of the dispersions was evaluated by hysteresis between the first and second curve in which the shear rate ranged from 0–300 s−1 to 300–0 s−1, respectively. Thixotropic behavior of the dispersions was verified and quantified by the difference of the areas between the climbing curves (increased shear stress) and descent (reduction of shear stress).
Frequency sweep
The frequency sweep was carried out using an oscillatory test with a fixed shear stress of 0.01 Pa varying the frequency between 1 and 10 Hz. The shear stress of 0.01 Pa was determined by pre-testing checking the linear viscoelasticity. The linear viscoelasticity test shows the maximum shear stress which can be applied to a material without encountering disruption of the internal structure. In the linear viscoelastic region is not observed dependence of shear stress in the frequency.
Scanning electron microscopy (SEM)
Samples of chia seed mucilage were fixed with double-sided carbon tape onto an aluminum support (stubs) that was sputter-coated under vacuum with a thin film of metallic gold using a Bal-Tec model SCD 050 evaporator (Balzers, Liechtenstein). A Nano Technology Systems (Carl Zeiss, Oberkochen, Germany) model Evo® 40 VP scanning electron microscope was used with an accelerating voltage of 20 kV and a working distance of 9 mm to obtain the digital images using the Leo User Interface software at varying magnifications. The images were processed using Corel Draw 14 Photo paint Software.
Statistical analysis
Statistical analyzes were performed using the Scott-Knott or regression testing, depending on the purpose of analysis, adopting a 5% critical level of probability for the occurrence of errors Type I using the statistical software SAS licensed by the Federal University of Lavras (UFLA).
Results and discussion
Proximate composition of mucilage
The proximate composition presented respectively for mucilage obtained from hot extraction method (HE) and cold extraction method (MCE) was moisture 3.7 (MCE) and 12.3 (MHE); ash 8.71 (CE) and 17.30 (MHE); lipid 3.35 (MCE) and 4.96 (MHE); protein 6.98 (MCE) and 21.12 (MHE); crude fiber 6.44 (CE) and 9.02 (HE); carbohydrate 73.48 (MCE) and 47.68 (MHE). All the results except for moisture content were expressed on dry base.
The mucilage moisture content from HE process was higher than 9.32% observed by Segura-Campos et al. (2014). The moisture content of gum extracted from the CE was lower than those extracted from the HE process. This observation was justified by the differences in the composition of the matrices formed in the drying process which influences the water withdrawal rate. Low humidity level is desirable for physical chemical and microbiological stability of mucilage in storage conditions. The ash content found in the mucilage obtained by CE process was similar to those seen by Segura-Campos et al. (2014) for chia mucilage without lipids (8.28%), and higher for chia mucilage with lipids (5.48%). The mucilage obtained by HE process presented higher ash content (17.3%), exceeding all above. The lipid content of mucilage did not differ statistically between the methods, and were similar to those found by Capitani et al. (2013) (3.1%), but lower than those observed by Segura-Campos et al. (2014) for chia mucilage (26.4%) with lipids and without lipids (10.90%). The protein content observed for mucilage from the HE process (21.12%) was higher than observed in mucilage obtained by CE process (6.98%).
The mucilage obtained by CE process is predominantly composed of NFE. With carbohydrate content of 73.84%, the mucilage obtained by CE was superior to the mucilage obtained from HE process (47.65%) and those obtained by Segura-Campos et al. (2014) in mucilage obtained from chia seeds ground (29.3–47.7%), however, relatively close to those obtained by these authors with whole grain (59.8–65.7%) and those found by Capitani et al. (2013) also from whole seeds (63.7%). According to Marin Flores et al. (2008) reported, the chia mucilage as a high molecular weight complex carbohydrate, which was extracted when the seed comes in contact with water, generating high viscosity solutions. Lin et al. (1994) have proposed a provisional structure of the basic unit of polysaccharide as a waste of 4-O-methil-α-d-glucoronopyranosil occurring as β-d-glucuronic acid in the main chain. The monosaccharides β-d-Xylose, α-d-glucose and 4-O-methyl-α-d-glucuronic were obtained by acid hydrolysis in the ratio of 2:1:1, respectively. In this sense, it would be coherent to say that the more efficient the mucilage extraction process when higher would be the levels of NFE.
Yield of mucilage extraction
Table 1 presents the results for the extraction of mucilage yield versus seed: water ratio in different processes. CE method did not show, statistical differences between treatments with seed:water ratio of 1:20, 1:30 and 1:40, which showed the highest yield, 8.46, 8.65 and 8.31%, respectively. These optimal values were higher to those observed by Reyes-Caudillo et al. (2008), 6%, Ayerza and Coates (2001), 5% and Muñoz et al. (2012), 6.97%, but lower than those observed by Marin Flores et al. (2008) 15.1% (all have at least one hot stage).
Table 1.
Values of yield, flow index (n) and apparent viscosity values under shear rate of 100 s−1; and thixotropy of mucilage extracted using different extraction methods, water:seed ratio and concentrations
| Extraction method | Seed:waterratio | Yield (%) | n | Apparent viscosity (Pa.s) | Thixotropy (Pa.s−1) | Concentration (g powder/100 g gum) | Thixotropy (Pa.s−1) |
|---|---|---|---|---|---|---|---|
| MHE | 1:40 | 6.42b | 0.9448a | 0.0031a | 10a* | MCE 1.0 | 105a* |
| MCE | 1:10 | 4.76a | 0.7788b | 0.0480b | 83b | MCE 2.0 | 995b |
| MCE | 1:20 | 8.46c | 0.7599b | 0.0573b | 104b | MCE 3.0 | 1120b |
| MCE | 1:30 | 8.65c | 0.7147b | 0.0615b | 238c | MCE 4.0 | 1628b |
| MCE | 1:40 | 8.31c | 0.6904b | 0.0768b | 333d | MCE 5.0 | 3097c |
MCE Mucilage cold extraction, MHE mucilage hot extraction
* Means followed by the same letter do not differ (p > 0.05)
a–c different letters in the same column indicate statistical differences (p < 0.05) using Scott-Knott test
The results suggested that seed:water ratio of 1:20 did not show any significant improvement in the yield. This effect was due to separation of the seed mucilage that was made by pressing controlled between 281.7 and 313kgf/cm2. In these treatments, pressing separated the mucilage from the seed and was more effective compared to the screening method of the seeds.
Muñoz et al. (2012) studied the extraction yield of chia seed mucilage and found optimum yield of 6.97% at temperature of 80 °C, pH 8.0, and seed:water ratio of 1:40 where the separation of the mucilage was made by sieving. Under the same process conditions, we observed yield of 6.42%, similar to these obtained by the authors. The seed:water ratio of 1:10 was the one with the lowest yield, with mean yield of 4.76%. This may be associated with the less release of mucilage when in contact with water which was dependent on the hydration level.
Vásques-Ovando et al. (2009) observed maximal absorption capacity of water into a fiber fraction extracted from chia seed of 11.73 g water/g sample. According to the authors, the hydration is primarily a surface phenomenon, but higher levels of moisture absorption may occur inside the structure, which leads to swelling and eventual solubilization of fibrous material. In this sense, the seed:water ratio of 1:10 may not be sufficient to complete seed hydration, thus limiting the extraction process. According to Muñoz et al. (2012), the mucilage is present inside of the integument of mature chia seed epidermal cells, which when in contact with water, expands immediately causing the breakdown of primary cell layer protruding from these neighboring epidermal cells thus encircling the seed. For Windsor et al. (2000), possibly mucilage is located outside the cells that form the seed coat, called mucilage cells. In the hot extraction process was difficult to separate the seed of the tray, which adhered strongly, as in screening the seed. The freeze-dried does not adhere to the surface of the drying vessel and is readily removed without any effort.
Rheological analysis of dispersions
Characterization of the rheological behavior
The flow curve data were adjusted to Law of Newton, Power Law and Herschel-Buckley rheological models. All rheological parameters of Newton’s law and Power Law models were significant for all treatments (p < 0.01) (data not shown). But the model of Herschel-Buckley showed no significant parameters for all treatments. Thus, analyzing the correlation coefficients, the value of the residue mean square and the significance of the parameters, the model that best explained the experimental data was the Power Law, since it had the highest correlation coefficients (R2 > 98.58), the smallest mean square values of the residues and all significant parameters (data not shown).
The parameters of Power Law model, consistency index (k) and flow behavior index (n) were analyzed by analysis of variance and the parameters that showed significant differences between treatments (p < 0.05) were analyzed by the average test Scott-Knott. It was observed from the statistical analysis that the ways of extracting the mucilage hot and cold and the different concentrations influenced the consistency index (k). The flow index (n) showed significant difference between the mucilage extracted from different methods. The hot extracted gum had the highest value of n, showing the behavior closer to the Newtonian behavior in which the flow behavior index was 1. From the statistical analysis it was observed that the concentrations used in the dispersion preparations have a significant influence on the parameters k and n in Power Law model. The increase in the concentration of the dispersions reduced the flow index values. Fitting the curves with a power law equation for data at concentrations from 1 to 5 g powder/g gum indicated strong shear-thinning with pseudoplasticity indexes (n) ranging from 0.78 to 0.33 for MCE and 0.92 for MHE. Thus, it was found that increasing the concentration, gums departs from the Newtonian behavior. The consistency index (k) increased with the concentration used in the preparation of dispersions.
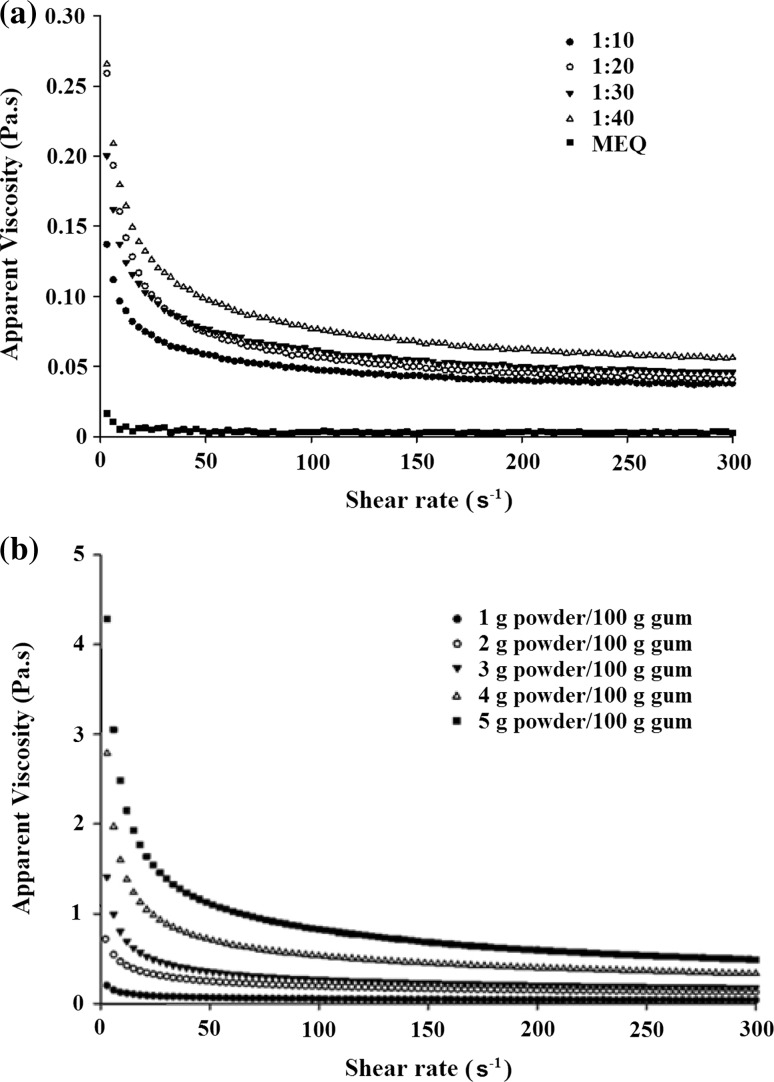
The relationship between the shear stress and the shear rate form a convex curve which, according Sharma et al. (1999) characterized a pseudoplastic fluid. The pseudoplastic fluids constitute the majority of non-Newtonian fluids. The vast majority of the gums have pseudoplastic behavior. According Samavati and Skandari (2014), the pseudoplasticity was the result of an orientation effect. As shear rate was increased, the molecules of the polymers, which were long randomly positioned strings, became increasingly aligned in the direction of flow, resulting in less interaction between adjacent polymer chains. Capitani et al. (2015) observed that the values of the consistency index (k) and flow behavior index(n) of the chia mucilage dispersions for the upward and downward was associated with the higher viscosity of the dispersions formulated since the consistency index is indicative of the viscous nature of the system. The rheograms shown in Fig. 2a, b show the behavior of the apparent viscosity as function of shear rate on the gum extracted with varying proportions of seed:water, and of gum that were prepared at different concentrations, respectively.
Fig. 2.
Curves of apparent viscosity as function of shear rate obtained using a different extraction methodsand, b using different powder concentrations in the cold extraction process andseed:water ratio of 1:20
It can be clearly seen from the rheograms that the apparent viscosity decreases with increase shear rate. Only in gum extracted by heat treatment the behavior was not evident due to degradation of protein and polysaccharide chains. The demonstrated behavior in the rheograms is because these fluids have disordered molecules when they are at rest, which begin to orient with the applied stress. Thus, the higher the applied stress, the greater was the ordering and hence lowered the apparent viscosity. Furthermore, with increasing shear rate applied, polymers can disintegrate and align with the flow, offering less resistance to this, or the possible break of colloidal aggregates, would also result in a decrease in apparent viscosity values (Campanella et al. 1995).
Capitain et al. (2015) also verified that the apparent viscosity of the chia dispersions increased with the increase in mucilage concentration from 0.25 to 1.00% (w/v). This could be attributed to the higher content of total solids in the dispersion, which caused an increase in viscosity due to increased restriction of intermolecular motion caused by hydrodynamic forces and the formation of an interfacial film. According to Goh et al. (2016), the strong pseudoplastic behavior may have caused by the presence of these soft microgel particles which were highly sensitive to shear and rapidly orientate in the direction of the flow even at very low shear and concentration.
The apparent viscosity of the gum was analyzed fixing shear rate of 100 s−1. The parameter that was evaluated for shear rate is typical for food processing, such as flow through tubes in the industry, stirring and chewing processes (McClements 2005). Table 1 shows the observed apparent viscosity mean at 100 s−1 for gum obtained from different extraction methods. It can be seen that gums obtained using the cold extraction method did not presented significant difference in the apparent viscosity, showing the amount of extracted solids did not change when the amount of water used in the gum extraction was varied. The hot extracted gum has the lowest apparent viscosity under shear rates studied.
The gum concentrations influenced the apparent viscosity, studied the shear rate of 100 s−1 and can be observed from the Fig. 2b that the apparent viscosity increases with increased concentration of gums, this is because at higher concentrations has a higher water retention and an increased amount of intermolecular bonds, which results in a higher viscosity.
Thixotropy
The existence of thixotropy reveals an internal structure that after the destruction is subjected to the shear stress. In this case the structure is not renewed in the same way in which it existed initially considered the analysis times. This behavior reveals elasticity of the materials and that the amount of energy stored and dissipated by the system as a result of deformation is not equal (Alves 2003).
The thixotropic behavior analysis was performed applying to the gum an increase and the subsequent reduction in shear stress under a constant shear rate. The thixotropic behavior of the gums was quantified by the area between the curves rise (increase of shear stress) and decrease (reduction of shear stress). The thixotropic values obtained by the difference between the areas are shown in Table 1. The statistical analysis indicated that comparing the extraction methods, the gum obtained using cold extraction presented higher values for thixotropic behavior. These values were proportional to powder concentrations. Thixotropic fluids are known to contain small particles (crystals or biopolymer) which are held together by weak forces. The material shearing provide the separation of aggregated particles and so there is a lower flow resistance and viscosity decreases with time until a constant value is reached (McClements 2005).
Frequency sweep
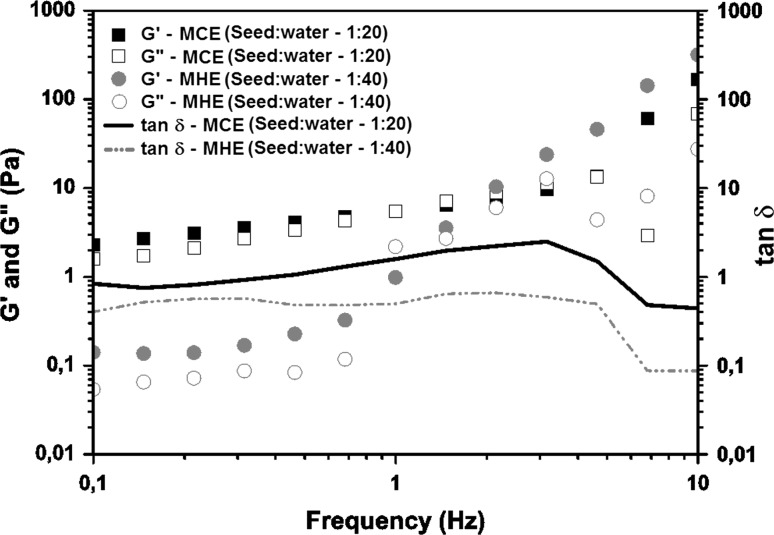
Frequency sweep test is the most common oscillatory test where the storage modulus G′ describes the elastic properties and the loss modulus G″ describes the viscous properties. The frequency sweep was performed to determine the viscoelastic behavior of the gums in the range of 0.01–10 Hz, according to Figs. 3 and 4.
Fig. 3.
Storage modules (G′) and loss modulus (G″) as function of frequency for cold extraction method and the seed:water ratio of 1:20 and hot extraction method and seed:water ratio of 1:40
Fig. 4.
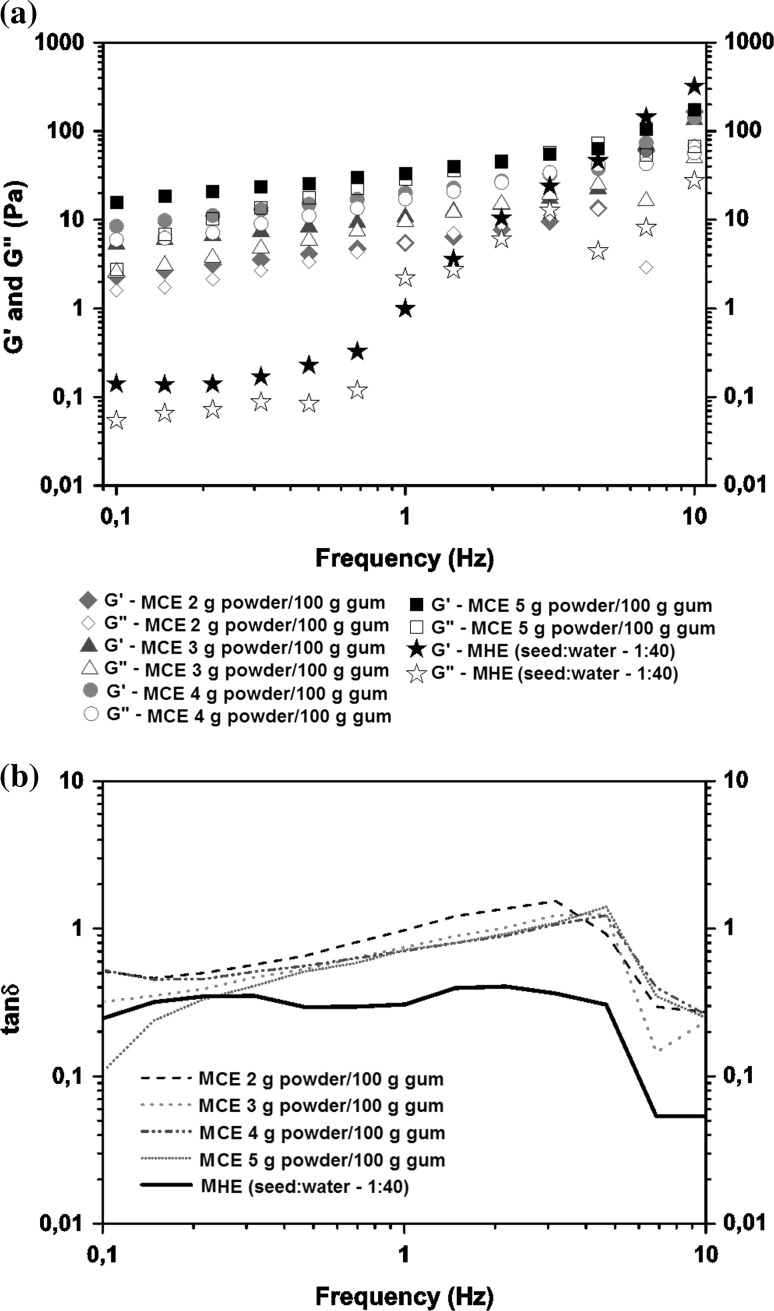
a Storage modules (G′) and loss modulus (G″) as function of frequency for mucilage obtained using cold extraction method (MCE), seed:water ratio of 1:20, concentrations of 2, 3, 4, and 5 g powder/100 g gum and hot extraction method (MHE), seed:water ratio of 1:40. b tan (G″/G′) as function of frequency
The frequency sweeps for the dispersions show the typical form of a macromolecular solution. In general, for all dispersions the storage modulus (G’) were greater than the loss modulus (G”) at low frequencies, whereas G' was predominant at highest frequencies (Figs. 3, 4). It can be observed that for both methods of CE, the storage modulus (G’) and the loss modulus (G”) showed dependence on frequency, and this effect was more marked in the dispersions prepared by HE. In all the dispersions G′ was larger than G″, indicating a prevalent elastic behavior of the samples, similar to that chia mucilage’s described by Capitani et al. (2015). The mucilage obtained by cold extraction procedure behaved similarly with increasing frequency, behaving as intersection point between 4 and 6 Hz.
For the dispersion of the mucilage obtained in the HE process, the point of intersection occured at lower frequency (2 Hz). Figure 4 illustrates the viscoelastic behavior of chia mucilage dispersions at different gum concentrations obtained from CE method (seed:water ratio of 1:20) compared to HE method (seed:water ratio of 1:40). It was observed that various cross-over points between G′ and G″ occurred and the intersection frequency was dependent of mucilage concentration in the dispersion, and can have more than one intersection. With increasing concentration, the solutions showed atypical behavior, which gradually came to have more than one intersection point (above 2% concentrations).
In this regard, at frequencies less than 2 Hz, the dispersions have predominantly elastic properties (G′ > G″). The gums show predominantly viscous behavior (G″ > G′) between the frequency of 2 and 6 Hz. From 6 Hz, again show elastic properties (G′ > G″). The chia mucilage dispersions exhibited complex behavior by switching between viscous and elastic properties as a function of concentration and frequency. It can also be observed that at higher mucilage concentrations, the difference between both modules was greater. This behavior indicated that at high frequencies the structure of the mucilage broke, showing a slightly viscous behavior. As the frequency increases, after the cross-over points between G’ and G”, G’ becomes greater than G”. In this case, the time is insufficient to break the entanglement and the dispersions behave as a network with cross-links (Moraes et al. 2011). The values of the storage and modulus modules were higher as mucilage concentration increased, presenting the highest values in the dispersions prepared by CE.
The determination of viscoelastic behavior of a sample can be done using of the phase angle (tan δ), which is the ratio of the loss modulus G″ to the storage modulus G′. A tan δ < 1 indicates a predominantly elastic behavior, whereas tan δ > 1 indicates a predominantly viscous behavior (Capitani et al. 2015). Figures 3e and 4b show the variation of tan δ as a function of frequency for the different extraction methods and chia mucilage concentrations obtained from CE method compared to HE method, respectively. In all the dispersions, the values of this parameter exhibited an increasing tendency as frequency increased to achieve the cross-over points after which showed sharp reductions. These values were higher in the dispersions with lower concentrations. Thus, as mucilage concentration increased, the values of tan δ decreased to values below 1, maintaining G′ > G″, which would indicate that at high concentrations the samples may display a weak elastic gel-like behavior (Capitani et al. 2015).
Goh et al. (2016) also evaluated the effect of concentration on the viscoelastic properties of chia seed polysaccharide gel dispersions. The results showed that the storage modulus (G′) is consistently higher than the loss modulus (G″) and the data indicated the presence of a ‘weak gel’ structure of the polysaccharide dispersions. This ‘weak gel’ behavior is believed to be caused by the elasticity of the swollen microgel particles dispersed in a soluble polysaccharide fraction somewhat forming a weak transient gel network connected by the physical contact of neighboring particles.
Scanning electron microscopy (SEM)
The analysis of the surface of the chia particles derived from the CE (freeze-dried) and the HE (dried at 50 °C) was performed. The electron micrographs are shown in Fig. 5. Figure 5a illustrates the surface of chia seed mucilage derived from CE process, using pH of 8.0 and seed:water ratio of 1:20.
Fig. 5.
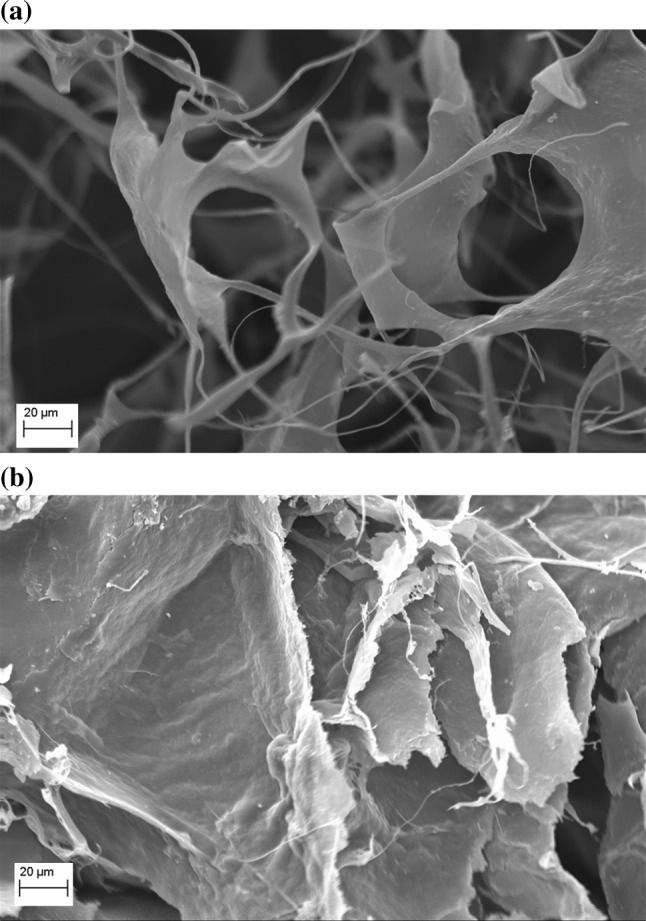
Scanning electron microscopy (SEM): a MCE mucilage cold extraction, b MHE mucilage hot extraction
The macroscopic porous structure of network composed mainly of carbohydrates, arranged evenly can be seen in figure. These features indicate that the macromolecular structure formed in the mucilage matrix preserved its structure during the process of deep freezing and freeze-drying. Figure 5b illustrates the surface of the mucilage obtained after HE process, the formations of laminar plate’s aggregates with brittle aspect and less uniformity compared to mucilage obtained through CE process can been seen. Capitani et al. (2013) evaluated fresh and freeze-dried crude mucilage of chia seeds using SEM. They observed that there was association between different components mucilage forms a network structure of open pores, which provide interesting rheological properties (gel formation). The extraction, purification, drying and/or further modification processes can significantly affect the chemical composition and molecular structure of natural plant-based biopolymers. The freeze-dried gum showed the highest porosity, solubility and viscoelastic parameters. This behavior was justified by the reduction of thermal degradation which probably resulted in less compact structure than samples dried in oven at 50 °C.
Conclusions
The mucilage was extracted from chia seed at low temperatures. The optimal extraction of 8.46% was possible room temperature using seed:water ratio of 1:20.
The increase in the concentration of the dispersions obtained from CE reduced the n values, increased k values and indicated strong shear-thinning. The apparent viscosity decreased with increases shear rate and this effect was most notable in the dispersions at higher concentration and for those obtained by method of CE. The gum obtained using CE presented higher values for thixotropic behavior that were proportional to powder concentrations. The storage modulus (G′) were higher than the loss modulus (G″) and the data indicated the presence of a ‘weak gel’ structure of the dispersions.
The macroscopic fibrous structure was observed for mucilage obtained by CE process, indicating that the macromolecular network formed during of deep freezing and freeze-drying.
Acknowledgements
The authors are grateful for the financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Conselho Nacional de desenvolvimento científico e tecnológico (CNPQ).
References
- Alves MMM. A reologia. In: De Castro AG, editor. A química e a reologia no processamento de alimentos. Lisboa: Instituto Piaget; 2003. [Google Scholar]
- Association of Official Analytical Chemistry . Official methods of analysis. 15. Arlington: AOAC international; 1990. p. 1298. [Google Scholar]
- Ayerza R, Coates W. Protein content, oil content and fatty acid profiles as potencial criteria to determine the origin of commercially grown chia (Salvia hispanica L.) Ind Crops Prod. 2001;34:1366–1371. doi: 10.1016/j.indcrop.2010.12.007. [DOI] [Google Scholar]
- Ayerza R, Coates W. Protein and oil content, peroxide index and fatty acid composition of chia (Salvia hispanica L.) grown in six tropical and sub tropical ecosystems of South America. Trop Sci. 2004;44:131–135. doi: 10.1002/ts.154. [DOI] [Google Scholar]
- Campanella OH, Dorward NM, Singh H. A study of the rheological properties of concentrated food emulsions. J Food Eng. 1995;25:427–440. doi: 10.1016/0260-8774(94)00000-Y. [DOI] [Google Scholar]
- Capitani M, Ixtaina V, Nolasco S, Tomás M. Microstructure, chemical composition and mucilage exudation of chia (Salvia hispanica L.) nutlets from Argentina. J Sci Food Agric. 2013;93(15):3856–3862. doi: 10.1002/jsfa.6327. [DOI] [PubMed] [Google Scholar]
- Capitani M, Corzo-Rios LJ, Chel-Guerrero L, Betancur-Ancona D, Nolasco SM, Tomás M. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J Food Eng. 2015;149:70–77. doi: 10.1016/j.jfoodeng.2014.09.043. [DOI] [Google Scholar]
- Dunn J (2010) The chia company seeks entry into european market. Disponível em: http://www.ausfoodnews.com.au/2010/02/08/the-chia-companyseeks-entry-into-european-market.html. Accessed 9 Jul 2015
- European Food Safety Authority Scientific opinion of the panel on dietetic products nutrition and allergies on a request from the European Commission on the safety of ‘chia seed (Salvia hispanica) and ground whole chia seed’ as a food ingredient. EFSA J. 2009;996:1–2. [Google Scholar]
- Goh KKT, Matia-Merino L, Chiang JH, Quek R, Soh SJB, Lentle RG. The physico-chemical properties of chia seed polysaccharide and its microgel dispersion rheology. Carbohydr Polym. 2016;149:297–307. doi: 10.1016/j.carbpol.2016.04.126. [DOI] [PubMed] [Google Scholar]
- Ixtaina VY, Nolasco SM, Tomás MC. Characterization of chia (Salvia hispanica L.) white and dark seeds and oils. In: Tomás MC, editor. Advances in fats and oils research. Kerala: Transworld Research Network; 2010. pp. 135–147. [Google Scholar]
- Julio LM, Ixtaina VY, Fernández M, Sánchez RMT, Nolasco SM, Tomás MC. Development and characterization of functional O/W emulsions with chia seed (Salvia hispanica L.) by-products. J Food Sci Technol. 2016;53:3206–3214. doi: 10.1007/s13197-016-2295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KY, Daniel JR, Whistler RL. Structure of chia seed polysaccharide exudate. Carbohydr Polym. 1994;23:13–18. doi: 10.1016/0144-8617(94)90085-X. [DOI] [Google Scholar]
- Marin Flores FM, et al. Method for obtaining mucilage from Salvia hispânica L. Heidelberg: Springer; 2008. [Google Scholar]
- McClements DJ. Critical review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr. 2005;47:611–649. doi: 10.1080/10408390701289292. [DOI] [PubMed] [Google Scholar]
- Moraes ICF, Fasolin LH, Cunha RL, Menegalli FC. Dynamic and steady-shear rheological properties of xanthan and guar gums dispersed in yellow passion fruit pulp (Passiflora edulis f. flavicarpa) Braz J Chem Eng. 2011;28:483–494. doi: 10.1590/S0104-66322011000300014. [DOI] [Google Scholar]
- Muñoz LA, et al. Chia seeds: microstructure, mucilage extraction and hydration. J Food Eng. 2012;108:216–224. doi: 10.1016/j.jfoodeng.2011.06.037. [DOI] [Google Scholar]
- Ramos SCF. Avaliação das propriedades gelificantes da farinha de chia (Salvia hispanica L.). Desenvolvimento de novas aplicações culinárias. Lisboa: ISA; 2013. [Google Scholar]
- Reyes-Caudillo E, Tecante A, Valdivia-López MA. Dietary fiber content and antioxi-dant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008;107:656–663. doi: 10.1016/j.foodchem.2007.08.062. [DOI] [Google Scholar]
- Samavati V, Skandari F. Recovery, chemical and rheological characterization of gum from Assyrian pulm. Int J Biol Macromol. 2014;67:172–179. doi: 10.1016/j.ijbiomac.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Segura-Campos MR, Ciau-Solis N, Rosado-Rubio G, Chel-Guerrero L, Betancur-Ancona D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int J Food Sci. 2014 doi: 10.1155/2014/241053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Mulvaney SJ, Rizvi SSH. Food processing engineering: theory and laboratory experiments. Hoboken: Wiley; 1999. [Google Scholar]
- Ullah R, Nadeem M, Khalique A, Imran M, Mehmood S, Javid A, Hussain J. Nutritional and therapeutic perspectives of chia (Salvia hispanica L.): a review. J Food Sci Technol. 2016;53:1750–1758. doi: 10.1007/s13197-015-1967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásques-Ovando A, Rosado-Rubio G, Chel-Guerrero L, Betancur-Ancona D. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.) LWT Food Sci Technol. 2009;42:168–217. doi: 10.1016/j.lwt.2008.05.012. [DOI] [Google Scholar]
- Windsor JB, et al. Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J. 2000;22:483–493. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]