Abstracts
This study aimed to explore ideal processing condition for black garlic based on the change of nutritional and active components and antioxidant capacity. Fresh garlic was processed under the condition of constant temperature (65, 75 and 85 °C) and relative humidity (70, 75, 80 and 85%) for 16 days. The sensory scores, contents of nutritional and active components, and antioxidant capacity were monitored. The sensory scores reached the maximum on the 8th day at 85% humidity and 75 °C. The contents of nutritional components were significantly affected by humidity and temperature, and 85% humidity and 75 °C were appropriate. The polyphenol content increased with increase in temperature and decrease in humidity. The reducing sugars and total sugars, total acids and 5-HMF were higher at 75 °C than at 65 and 85 °C. Reducing sugar and protein contents and sensory scores decreased on the 8th day. Maintaining the temperature of 75 °C and relative humidity of 85% for 8 days were ideal for black garlic to retain antioxidant capacity and abundant nutrients.
Keywords: Black garlic, Nutritional components, Allicin, Thiosulfinates, Polyphenols, Antioxidant capacity
Introduction
Thermal processing is not only important for improving the taste of garlic but also critical to generate a functional food (Liang et al. 2015). Black garlic (Allium sativum L.), a food with wonderfully complex flavor, is produced by thermal processing of fresh garlic. Maillard reaction occurs during this process and produces amount of compounds such as early MR products (MRP), Amadori compounds, 5-hydroxymethylfurfural (5-HMF) and furfural (Delgado-Andrade et al. 2010). Besides, other components, including alliin, saccharide contents (Zhang et al. 2015) and S-allyl cysteine (Bae et al. 2014) have been identified in the black garlic under thermal processing, which have multiple bioactivities.
It is known that black garlic extracts can boost immunity (Wang et al. 2010; Salman et al. 1999; Purev et al. 2012). Recently, black garlic or its extracts have been reported to have antioxidant activities (Queiroz et al. 2009) and anticancer activities (Wang et al. 2012, Sasaki et al. 2007). Maillard reaction during the thermal processing on one hand enhances the production of aroma, taste, and color; however, on the other hand induces a reduction of nutritive value (Ledl and Schleicher 1990). Liang et al. using a comprehensive nuclear magnetic resonance (NMR) analysis identified the changes of a sum of 38 components of black garlic such as reducing sugars, amino acids, organic acids, cycloalliin, and 5-HMF during thermal processing within 90 days (Liang et al. 2015). However, there is not an ideal processing condition for the black garlic with more nutrients and biofunctional compounds and higher antioxidant capacity.
Therefore, the present study was conducted to observe the changes of sensory scores, nutritional constituents, bioactive components and antioxidant capacity with heating time, temperature and humidity and to explore an appropriate processing method for black garlic.
Materials and methods
Black garlic processing and sensory evaluation
Fresh garlic, purchased from Xuzhou agricultural market (Jiangsu, China), was processed at a thermostat with 65, 75 or 85 °C and relative humidities of 70, 75, 80 or 85% for 16 days. The color and sensory scores were determined every 2 days. Sensory evaluation was carried out based on the color, taste, smell and appearance according to the scoring criteria in Table 1.
Table 1.
Scoring standards of sensory evaluation
| Items | Scoring standard | Scores |
|---|---|---|
| Color (40%) | Black, color of section uniform | 32–40 |
| Brown, color of section uniform | 16–31 | |
| Black, color of section not uniform | 0–15 | |
| Taste (30%) | Sweet taste fresh and long, slightly sour, appropriate sour and sweet ratio; taste delicate, soft, no piquancy | 25–30 |
| Sweet prominent, slightly bitter, acceptable | 14–24 | |
| Sweet not prominent, obviously bitter, unacceptable | 0–14 | |
| Smell (20%) | Aromatic flavor, coordinate, no spicy or foreign flavor | 16–20 |
| General smell, garlic smell | 9–15 | |
| No obvious smell | 0–8 | |
| Appearance (10%) | Garlic granule full, not shrink, not sticky | 8–10 |
| Garlic granule full, not shrink, sticky | 5–7 | |
| Garlic granule shrink, not sticky | 0–4 |
Color parameters
The color of black garlic was determined by color difference method using a WSC colorimeter (Shanghai precision scientific instrument co., LTD, Shanghai, China) basing on the CIELab scale (CIE Colorimetric Committee, 1974). Two discs of black garlic were taken out and sliced. The L*, a* and b* values of the cross section was determined. Total color difference (E*) was calculated according to the formula:
where L* from 0 to 100 indicates burnish brightness from black to white; a* indicates the red and green with higher positive value representing enhanced red and lower negative value representing enhanced green; b* indicates the yellow and blue with higher positive value representing enhanced yellow and lower negative value representing enhanced blue.
Nutritional components
Moisture contents were determined by gravimetric method according to the standard AOAC-925.10. Total acid was tested by acid–base titration according to the standard of Chinese GB/T 12456-2008. Total sugar and reducing sugar were determined by the Phenol–sulfuric acid method and the DNS method, respectively. Protein content was measured using Kjeldah method.
The content of 5-HMF was determined based on the reports of Muangthai et al. (Bozkurt et al. 1999). Briefly, 5 g sample was mixed with 0.5 mL 15% potassium ferrocyanide solution and 0.5 mL 30% zinc acetate solution and then diluted to 25 mL. The mixture was filtrated with filter paper. Initial 5 mL filter liquor was removed and the remaining filter liquor was used for determination of 5-HMF. A volume of 2 mL 5-HMF standard (Sigma, USA) or sample was mixed with 5 mL paratoluidine solution and 1 mL barbituric acid. Then the absorbance at 550 nm was measured immediately after mixing.
Active components
Superoxide dismutase (SOD) activity
A weight of 1 g black garlic sample was grinded for 5 min and placed in a centrifuge tube. It was then diluted with 0.2 mmol/L phosphate buffer saline (PBS) (pH = 7.2) to a final volume of 4 mL. The tube was heated at 60 °C water bath for 20 min and then centrifuged at 12,000 r/s for 20 min. The crude enzyme was prepared by removing the liquid supernatant. Pyrogallol autoxidation method was used for SOD activity determination as previously described (Liu and Zhang 2008).
Total polyphenol content
A weight of 1 g sample was grinded and mixed with 10 mL 70% methyl alcohol in a centrifuge tube. They were vibrated in an ultrasonic bath for 30 min and then centrifuged at 4000 r/min for 20 min. The liquid supernatant was taken out and the precipitate was centrifuged again. The second liquid supernatant was merged with the first liquid supernatant and diluted with 70% methyl alcohol to a volume of 100 mL. Total polyphenol content was determined according to previously reported procedures (Wootton-Beard et al. 2011) with gallic acid as a standard. Simply, 0.5 mL sample or gallic acid solution (gradient concentration 0–500 mg/L) was mixed with 30 mL diluted water, 2.5 mL Folin-Phenol reagents and 7.5 mL 20% sodium carbonate solution and then diluted to 50 mL. The reaction was maintained for 2 h at 20 °C then the absorbance at 765 nm was determined.
Thiosulfinates and allicin
Sample (2 g) were grinded and mixed with 8 mL 95% ethyl alcohol solutions. They were then vibrated in an ultrasonic bath for 20 min and centrifuged at 4000 r/min for 15 min. The supernatants were used for the determination of thiosulfinates and allicin basing on the report of Han et al. (1995). Briefly, 0.25 mL cysteine solution (1 mmol/L) was mixed with 1 mL 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB, 2 mmol/L) and 0.25 mL sample or HEPES buffer (50 mmol/L, as control) and diluted with HEPES buffer to 6.0 mL and maintained for 15 min at room temperature. The absorbance at 412 nm was tested as A0 (control) or A (sample). Blank control was the same mixture above with the cysteine solution substituted by HEPES buffer. Contents of thiosulfinates and allicin were calculated according to the following formulas:
among which, d = dilution times; 162.26 = the molecular weight of allicin; 14,150 = molar extinction coefficient of 2-nitro 5-thio-benzoic acid (NTB) (product of DTNB and allicin) at 412 nm, 1 cm optical path.
Antioxidant capacity
Sample preparation
Mashed black garlic samples (2 g) were placed in 10 mL 80% ethyl alcohol solutions and shocked at ultrasonic bath for 20 min. The mixture was then centrifuged at 4000 r/min for 20 min. The liquid supernatant was poured in a 25 mL brown glass volumetric flask. The precipitate was further shocked for 20 min and then centrifuged for 20 min. Then the supernatant was added to the above flask and diluted to 25 mL with 70% ethyl alcohol solution. The sample was placed at 4 °C for determination of the antioxidant capacity.
Ferric reducing antioxidant capacity
The ferric reducing antioxidant capacity was measured according to a previously described protocol (Ak and Gülçin 2008). Briefly, a mixture of 1 mL 2.5 mmol/L phen, 2 mL PBS (pH = 7.40), 1 mL distilled water (control group 1 and control group 2) or sample solution (test group), 1 mL 2.5 mmol/L ferrous sulphate solution and 1 mL distilled water (control group 1) or 0.01% hydrogen peroxide solution (control group 2 and test group) was incubated at 37 °C water bath for 1 h and the absorbance at 536 nm was recorded. The ferric reducing capacity was calculated as the following formula:
Superoxide anion radical (·O2−) scavenging capacity
The ·O2 − scavenging capacity was evaluated by using pyrogallic acid autoxidation method as described previously with slight modification (Qingming et al. 2010). A volume of 0.5 mL sample solution and 0.5 mL distilled water was mixed in a test tube with 3 mL Tris–HCl (pH = 8.2, containing 0.1 mmol/L ethylene diamine tetraacetic acid (EDTA)). As a blank control, 1 mL distilled water was added to the tube. After sufficient mixing, the tubes were placed in a 25 °C water bath for 25 min and then 40 μL pyrogallic acid (45 mmol/L) were added. The absorbance at 325 nm was monitored every 30 s for 4 min. The ·O2 − scavenging capacity was calculated according to the following formula:among which, A0 = A 325 nm of the blank control and A1 = A 325nm of the sample.
DPPH assay
Free radical DPPH (1,1-diphenyl-2-picryl-hydrazyl) scavenging capacity was determined by using the previously described methods (Garzón and Wrolstad 2009). Briefly, a volume of 5 mL DPPH (Sigma, USA) solution (120 μmol/L) in 70% ethyl alcohol was mixed with 0.2 mL sample solution, and was standing for 30 min in dark place. The absorbance at 517 nm (A sample) was recorded. In the control group, 5 mL DPPH solution was replaced by 5 mL 70% ethyl alcohol. In the blank group, 0.2 mL sample solution was substituted by 0.2 mL distilled water. Trolox (Sigma) was used as a standard. DPPH scavenging capacity was calculated according to the following formula:
ABTS assay
ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging ability was determined according to the trolox equivalent antioxidant capacity (TEAC) methods (Arts et al. 2004). ABTS (Sigma) work solution was prepared according to the instructions of the kit. Trolox was used as a standard. A volume of 10 μL samples or Trolox solution was added to each well of a 96-well plate. Then 200 μL ABTS work solutions were added to the sample or Trolox. The plate was vibrated for 10 s in a Multiscan Spectrum (SynergyH1, Biotek, USA) and the absorbance at 734 nm was recorded. TEAC was used to represent the capacity of ABTS radical scavenging. TEAC was calculated according to the standard curve by Trolox (van den Berg et al. 1999) with a standard curve y = − 0.3114x + 0.4745 (R2 = 0.9969). TEAC (mmol/L) = (A 734nm− 0.4745)/(− 0.3114 × 2).
Statistical analysis
All experiments were repeated for three times and the data were presented as the mean ± standard deviation (SD). The data were analyzed using SPSS (version 19.0; SPSS Inc., IL, USA).
Results and discussion
Sensory score, color, moisture contents
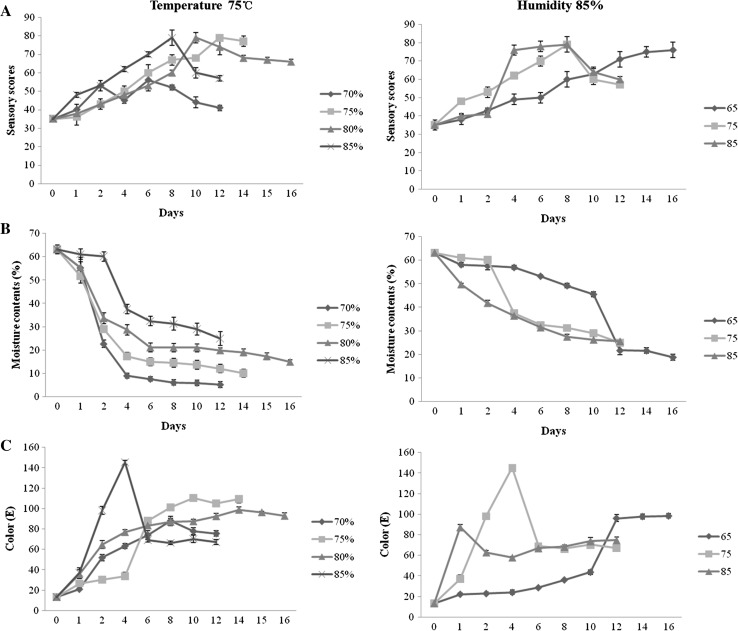
Changes in the sensory score, color, moisture content of black garlic during thermal processing at various temperatures and humidities are shown in Fig. 1. At 75 °C, the sensory scores under different humidity increased over time and then began to decrease when reached to a maximum (Fig. 1a). The higher the humidity and temperature resulted in higher sensory scores within 8 days. Moisture contents decreased with humidity decreasing and temperature increasing (Fig. 1b). The color of black garlic became dark over time but was not significantly influenced by humidity (Fig. 1c). The humidity 85% might be optimal for better sense and higher moisture contents. The sensory scores reached the maximum on the 8th day at 85% humidity and 75 °C (Fig. 1a).
Fig. 1.
Sensory scores (a), moisture contents (b) and color parameters (c) of the black garlic during the processing
Nutritional components
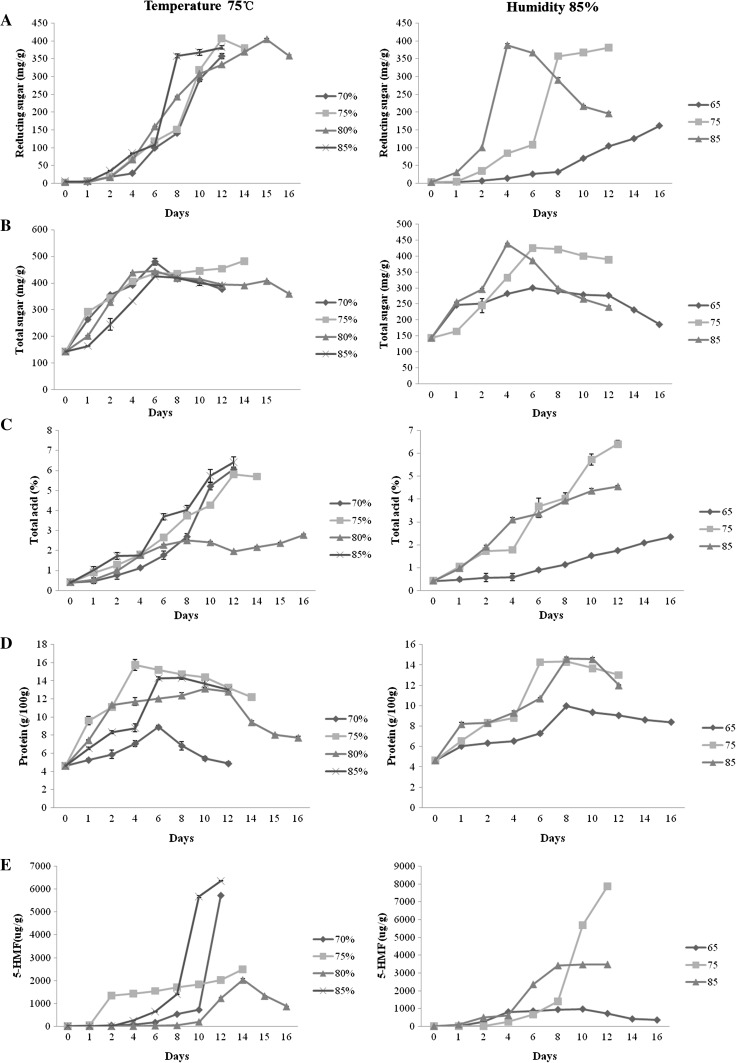
At 75 °C, the contents of reducing sugar and total sugar were increased with time prolonging and reached the peak at different time under different humidity (Fig. 2a, b). The content of reducing sugar under 75 and 80% humidity reached the peak on the 12th and 15th day, respectively. The total sugar content increased with time and reached the maximum on the 6th day. The total acid content was lower at 80% humidity and the highest at 85% humidity (Fig. 2c). The protein content dramatically increased within 6 days and then decreased. The protein contents at 75 and 85% humidity were higher than those at 80 and 85% (Fig. 2d). The 5-HMF content markedly increased after the 8th day at 70, 80, 85% humidity and it was highest at 85% humidity. These results indicated that humidity significantly affected the contents of nutritional components and 85% was appropriate.
Fig. 2.
Changes of nutritional constitutes of the black garlic during the processing. a Reducing sugar, b total sugar, c total acid, d protein, and e 5-HMF
Under 85% relative humidity, the content of reducing sugar increased markedly with increase in temperature during initial 6 days. At 85 °C, the content of reducing sugar reached the maximum on 4th day and then began to decrease. However it continuously increased with time at 75 and 65 °C within 16 days, and it was higher at 75 °C than at 65 °C (Fig. 2a). The total sugar reached the peak at 4–6 days and then the content was higher at 75 °C (Fig. 2b). Total acid increased with time at three temperatures, and 75 °C showed higher contents than 65 and 85 °C after the 6th day (Fig. 2c). The contents of protein reached the maximum on the 8th day at 65 and 85 °C and on the 6th day at 75 °C (Fig. 2d). There was a dramatic increase in the content of 5-HMF after the 8th day at 75 °C while it began to decrease on the 8th day at 65 and 85 °C (Fig. 2e). Therefore, the temperature of 75 °C might be the appropriate for more nutritional components.
Active components
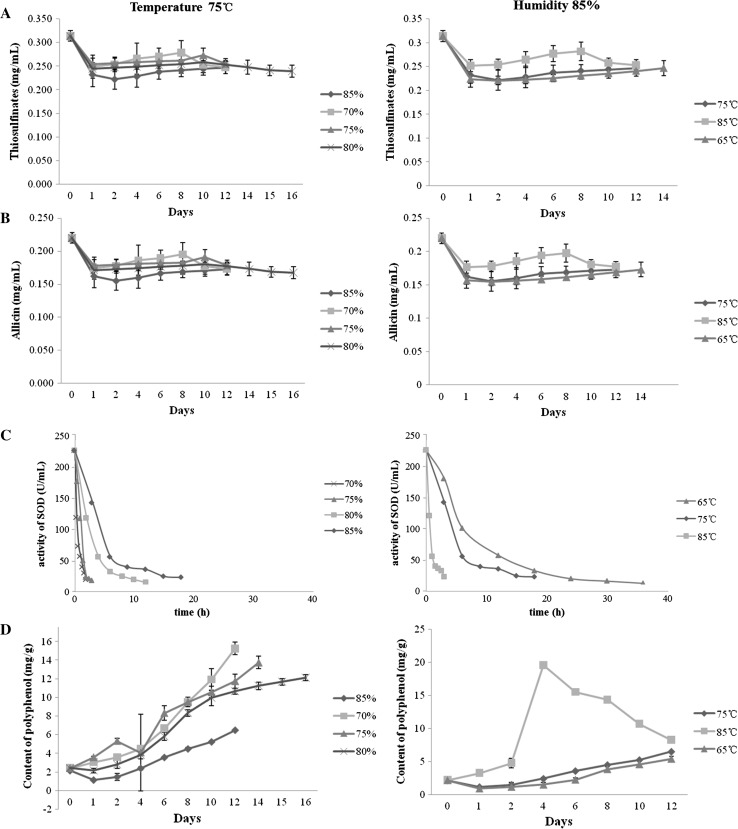
The contents of thiosulfinates, allicin and the activity of SOD decreased while the content of polyphenol increased with time prolonging (Fig. 3). The content of thiosulfinates decreased immediately after processing and then showed a gentle increase within 8 days (Fig. 3a). In addition, it increased with humidity decreasing (at 75 °C) and temperature increasing (at 85% humidity). Similar results were also found in the contents of allicin (Fig. 3b). The activities of SOD dramatically decreased below 50 U/mL within 36 h (Fig. 3c). It decreased with humidity decreasing (75 °C) and temperature increasing (85% humidity). The content of polyphenol increased with humidity decreasing (75 °C) and temperature increasing (85% humidity), however, when reached a certain level, it began to decompose and the content decreased (Fig. 3d). By comprehensive analysis, we considered that 75 °C, 85% humidity and 8 days might be the optimum conditions.
Fig. 3.
Changes of bioactive components of the black garlic during the processing. a Thiosulfinates, b allicin, c SOD activity and d polyphenol
Antioxidant capacity
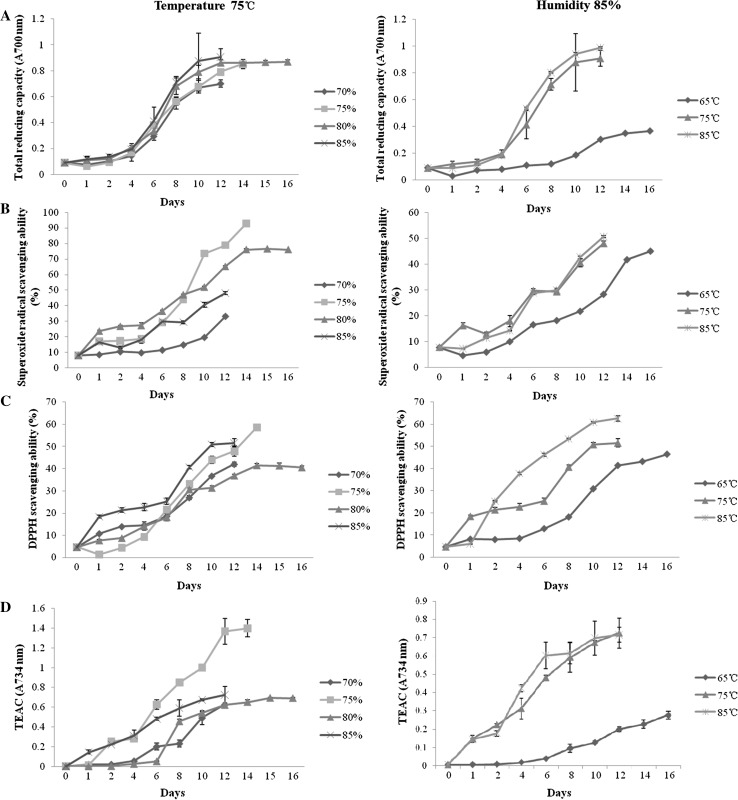
The antioxidant capacity of the black garlic increased over time within 16 days (Fig. 4). The ferric reducing capacity increased with time prolonging and humidity increasing. It was highest at 85% humidity (Fig. 4a). While with a humidity of 85%, the ferric reducing capacity of the black garlic increased with temperature increasing. Additionally, there was no further increase after the 12th day (postripeness process) at both 75 °C and 80% humidity. Thus, the subsequent study did not use the postripeness process. Superoxide radical scavenging ability was highest in 75% humidity followed by 80, 85, and 70%, besides it increased with temperature increasing (at 85% humidity) (Fig. 4b). DPPH scavenging ability increased with time prolonging and was highest in 85% humidity followed by 75, 70, and 85%, and increased with temperature increasing (85% humidity) (Fig. 4c). ABTS scavenging ability was represented by the TEAC value (Fig. 4d). It was highest at 75% humidity following by 85, 80 and 70%, and increased with temperature increasing (85% humidity). Therefore, antioxidant capacity increased with temperature increasing and 75 or 85% was the appropriate humidity.
Fig. 4.
Changes of antioxidant capacity of the black garlic during the processing. a Ferric reducing capacity, b superoxide radical scavenging ability, c DPPH scavenging ability and d ABTS scavenging ability indicated by TEAC
Optimization of processing conditions
By comprehensive analysis of the above results, 75 °C, 85% humidity and 8 days was the optimal condition for higher sensory scores, moisture contents, nutritional contents and antioxidant capacity. The contents of nutritional and active components and the antioxidant capacity of the black garlic under the optimal conditions were also determined. As shown in Table 2, the contents of nutritional and active components were: reducing sugar 357.946 mg/g, total sugar 420.893 mg/g, total acids 4.020%, protein content 14.326 g/100 g, 5-HMF 1397.813 μg/g, polyphenols 4.499 mg/g, allicin 0.169 mg/mL, and thiosulfinates 0.241 mg/mL. The ferric reducing capacity, superoxide radical scavenging ability, DPPH scavenging ability, and TEAC were 0.713, 29.296%, 40.708%, and 0.5917 mmol/L, respectively.
Table 2.
Contents of nutritional and active components and the antioxidant capacity of the black garlic at 75 °C, 85% humidity for 8 days (the optimal condition)
| Index | Content/capacity (n = 2) |
|---|---|
| Sensory scores | 79 ± 4.2 |
| Moisture content (%) | 31.28 ± 0.69 |
| Reducing sugar (mg/g) | 357.946 ± 5.812 |
| Total sugar (mg/g) | 420.893 ± 6.055 |
| Total acids (%) | 4.030 ± 0.235 |
| Protein content (g/100 g) | 14.326 ± 0.156 |
| 5-HMF (μg/g) | 1397.813 ± 7.621 |
| Polyphenols (mg/g) | 4.499 ± 0.140 |
| Thiosulfinates (mg/mL) | 0.241 ± 0.013 |
| Allicin (mg/mL) | 0.169 ± 0.009 |
| Total reducing capacity (A) | 0.713 ± 0.044 |
| Superoxide radical scavenging ability (%) | 29.296 ± 0.873 |
| DPPH scavenging ability (%) | 40.708 ± 0.843 |
| TEAC (mmol/L) | 0.5917 ± 0.081 |
This study reports the changes of nutritional components, active composites and antioxidant capacity of black garlic during thermal processing with different temperatures and humidities for 16 days and found an optimal processing condition of 75 °C, 85% humidity and 8 days for better taste and higher nutritive value and antioxidant capacity.
The taste of the black garlic improved with the increase of temperature within 8 days of processing, while after the 8th day, the sensory score of the black garlic began to reduce at 85 °C but that continuously increased with time at 75 °C. The reason may be that the higher temperature enhances the Maillard reaction and the production of flavor substances (Lan et al. 2010). Therefore, a moderate processing temperature should be employed. In addition, sensory scores and moisture increased with elevated humidity. Thus, 85% humidity was focused.
Nutritional components improved with temperature increasing in a certain degree and then decreased. At 85 °C and 85% humidity, the content of reducing sugar and total sugar reached to the maximum on the 4th day and began to decrease, while it continuously increased with time at 75 and 65 °C, and it was higher in 75 than 65 °C. Increasing of the reducing sugars also contributes to the sweet taste of the black garlic (Zhang et al. 2015). The total acid and protein were higher at 75 °C than those in 65 and 85 °C and the content of protein began to decline with time after a duration of increase within the first 6-8 days. In addition, the content of 5-HMF increased substantially at 75 °C than others, thus, we chose 75 °C as the optimal temperature. Initial heating promoted the hydrolysis of polysaccharide to reducing sugars (Zhang et al. 2015), while reached to a certain degree, the Maillard reaction consumed some reducing sugar and proteins and lead to the dramatically increasing of 5-HMF production.
The antioxidant capacity is highly correlated with total polyphenol and 5-HMF contents (Kwon et al. 2006). In addition, thiosulfinates and allicin are components of black garlic with antioxidant (Okada et al. 2005; Chung 2006; Xiao and Parkin 2002). In the present study, the content of total polyphenols, thiosulfinates and allicin increased with temperature increasing and humidity decreasing. However, when reached a certain level, polyphenols began to decompose and the content decreased. At 85 °C and 85% humidity, the content of polyphenols increased to the peak at the 4th day and began to decrease afterward. Contents of thiosulfinates and allicin decreased immediately after the processing and increased with time prolonging, humidity decreasing (at 75 °C) and temperature increasing (at 85% humidity). However, SOD activity decreased with temperature and humidity increasing, and it acutely declined below 50 U/mL within 36 h.
As expected, the antioxidant capacity increased with temperature increasing. DPPH radical scavenging ability was within the range of 6.21–44.77% and ferric reducing power was within the range of 0.08–3.13 increased with temperature (40–85 °C) during 0–14 days in a previous study (Bae et al. 2014), which were consistent with our study. In our study, higher DPPH radical scavenging (4–63%) and lower reducing power (0.08–0.983) were found compared with those in the previous study. In addition, we investigated the ABTS radical and superoxide radical scavenging ability which also showed similar trends.
Conclusion
The appropriate humidity for higher antioxidant capacity and better taste was 85%. Most of the indexes reached the peak or began to decrease on the 8th day, thus we chose 8 days as the processing time. A moderate temperature 75 °C was more appropriate for retaining the nutrients and taste, besides it could keep black garlic possessing a similar antioxidant capacity to that at 85 °C. Therefore, the conditions of 75 °C, humidity 85% and 8 days was recommended for processing to obtain better taste and antioxidant capacity and retain abundant nutrients.
Acknowlegements
This work was supported by National Natural Science Foundation of China (31301535), Qing Lan Project and Xuzhou science and technology plan (KC14NO069).
References
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Arts MJ, Dallinga JS, Voss H-P, Haenen GR, Bast A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004;88:567–570. doi: 10.1016/j.foodchem.2004.02.008. [DOI] [Google Scholar]
- Bae SE, Cho SY, Won YD, Lee SH, Park HJ. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT Food Sci Technol. 2014;55:397–402. doi: 10.1016/j.lwt.2013.05.006. [DOI] [Google Scholar]
- Bozkurt H, Göğüş F, Eren S. Nonenzymic browning reactions in boiled grape juice and its models during storage. Food Chem. 1999;64:89–93. doi: 10.1016/S0308-8146(98)00081-8. [DOI] [Google Scholar]
- Chung LY. The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food. 2006;9:205–213. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- Delgado-Andrade C, Seiquer I, Haro A, Castellano R, Navarro MP. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010;122:145–153. doi: 10.1016/j.foodchem.2010.02.031. [DOI] [Google Scholar]
- Garzón G, Wrolstad R. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus) Food Chem. 2009;114:44–49. doi: 10.1016/j.foodchem.2008.09.013. [DOI] [Google Scholar]
- Han J, Lawson L, Han G, Han P. Spectrophotometric method for quantitative determination of allicin and total garlic thiosulfinates. Anal Biochem. 1995;225:157–160. doi: 10.1006/abio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Kwon O-C, Woo K-S, Kim T-M, Kim D-J, Hong J-T, Jeong H-S. Physicochemical characteristics of garlic (Allium sativum L.) on the high temperature and pressure treatment. Korean J Food Sci Technol. 2006;38:331–336. [Google Scholar]
- Lan X, Liu P, Xia S, Jia C, Mukunzi D, Zhang X, Xia W, Tian H, Xiao Z. Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose–soybean peptide system: further insights into thermal degradation and cross-linking. Food Chem. 2010;120:967–972. doi: 10.1016/j.foodchem.2009.11.033. [DOI] [Google Scholar]
- Ledl F, Schleicher E. New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed Engl. 1990;29:565–594. doi: 10.1002/anie.199005653. [DOI] [Google Scholar]
- Liang T, Wei F, Lu Y, Kodani Y, Nakada M, Miyakawa T, Tanokura M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J Agric Food Chem. 2015;63:683–691. doi: 10.1021/jf504836d. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang J. Antioxidant activity of carboxymethylated and sulfated polysaccharides from pumpkin [J] Sheng Wu Jia Gong Guo Cheng. 2008;4:009. [Google Scholar]
- Okada Y, Tanaka K, Fujita I, Sato E, Okajima H. Antiodidant activity of thiosulfinates derived from garlic. Redox Rep. 2005;10:96–102. doi: 10.1179/135100005X38851. [DOI] [PubMed] [Google Scholar]
- Purev U, Chung MJ, Oh D-H. Individual differences on immunostimulatory activity of raw and black garlic extract in human primary immune cells. Immunopharmacol Immunotoxicol. 2012;34:651–660. doi: 10.3109/08923973.2011.649288. [DOI] [PubMed] [Google Scholar]
- Qingming Y, Xianhui P, Weibao K, Hong Y, Yidan S, Li Z, Yanan Z, Yuling Y, Lan D, Guoan L. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010;118:84–89. doi: 10.1016/j.foodchem.2009.04.094. [DOI] [Google Scholar]
- Queiroz YS, Ishimoto EY, Bastos DH, Sampaio GR, Torres EA. Garlic (Allium sativum L.) and ready-to-eat garlic products: in vitro antioxidant activity. Food Chem. 2009;115:371–374. doi: 10.1016/j.foodchem.2008.11.105. [DOI] [Google Scholar]
- Salman H, Bergman M, Bessler H, Punsky I, Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int J Immunopharmacol. 1999;21:589–597. doi: 10.1016/S0192-0561(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Sasaki JI, Lu C, Machiya E, Tanahashi M, Hamada K. Processed black garlic (Allium sativum) extracts enhance anti-tumor potency against mouse tumors. Energy (kcal/100 g) 2007;227:138. [Google Scholar]
- van den Berg R, Haenen GR, van den Berg H, Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. doi: 10.1016/S0308-8146(99)00089-8. [DOI] [Google Scholar]
- Wang D, Feng Y, Liu J, Yan J, Wang M, Sasaki J-i LuC. Black garlic (Allium sativum) extracts enhance the immune system. Med Aromat Plant Sci Biotechnol. 2010;4:37–40. [Google Scholar]
- Wang X, Jiao F, Wang Q-W, Wang J, Yang K, Hu R-R, Liu H-C, Wang H-Y, Wang Y-S. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Rep. 2012;5:66–72. doi: 10.3892/mmr.2012.745. [DOI] [PubMed] [Google Scholar]
- Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. 2011;44:217–224. doi: 10.1016/j.foodres.2010.10.033. [DOI] [Google Scholar]
- Xiao H, Parkin KL. Antioxidant functions of selected allium thiosulfinates and S-alk (en) yl-l-cysteine sulfoxides. J Agric Food Chem. 2002;50:2488–2493. doi: 10.1021/jf011137r. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lei M, Liu R, Gao Y, Xu M, Zhang M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J Food Biochem. 2015;39:39–47. doi: 10.1111/jfbc.12102. [DOI] [Google Scholar]