Abstract
Physicochemical properties, trypsin inhibitory activity, and gelling properties of albumen from duck egg during 15 days of storage at 4 °C and room temperature (28–30 °C) were studied. As the storage time increased, Haugh unit and moisture content decreased, while the pH value increased (P < 0.05). The rate of changes was lower at 4 °C. Trypsin inhibitory activity in albumen from egg stored at 4 °C was higher than that kept at room temperature throughout the storage time (P < 0.05). Nevertheless, no differences in protein patterns were observed during the storage. Based on texture profile analysis, the highest hardness, gumminess, and chewiness were found at day 3 for room temperature and at day 6 for 4 °C. Higher values were attained for eggs kept at 4 °C. Conversely, albumen gels made from eggs stored at room temperature exhibited higher cohesiveness, adhesiveness, springiness, resilience than those kept at 4 °C. The gels had the lowered whiteness when eggs were stored for a longer time, particularly at room temperature. Thus, storage condition directly affected the quality of albumen from duck egg.
Keywords: Duck egg, Albumen, Trypsin inhibitor, Storage condition, Gelling property
Introduction
Over decades, eggs have been used for human consumption as a part of nutritional diet, rich in proteins, lipids, all necessary vitamins (except vitamin C) and minerals (Lomakina and Mikova 2006). Apart from its nutrition value, egg possesses the functional properties of several foods including solubility, water holding capacity, emulsifying, fat binding, foaming and gelling properties (Zayas 1997). Amongst the commercially produced eggs, duck egg is one of the avian eggs, which has been consumed fresh and produced as the preserved form, especially salted egg, in some countries, e.g. China and South East Asia (Ganesan et al. 2014).
Egg consists of two major edible parts, egg white (albumen) and yolk. Albumen possesses several biological proteins such as lysozyme, cystatin, trypsin inhibitor, ovomucoid and ovoinhibitor (Kopeć et al. 2005). Both cysteine and serine protease inhibitors are present in albumen (Machleidt et al. 1989). Cystatin shows a very strong inhibitory capacity towards ficin, papain and cathepsins B, H and L, whereas ovomucoid has the inhibitory activity towards trypsin and chymotrypsin (Kopeć et al. 2005). Therefore, egg albumen has been employed in surimi to alleviate protein degradation caused by the endogenous proteinase, thus improving the properties of surimi gel (Benjakul et al. 2001). In general, hen egg is deteriorated during storage at room temperature and quality loss can be retarded when kept at refrigerated storage (Akter et al. 2014; Jin et al. 2011; Samli et al. 2005). Albumen pH and lipid oxidation of hen egg increased and Haugh unit decreased at higher rate at room temperature. Those changes were lower at refrigerated temperature up to 28 days of storage (Akter et al. 2014).
However, no information regarding quality, composition especially trypsin inhibitor and functional property, particularly gelation of duck albumen, has been reported. Apart from binding and gelling properties, protease inhibitors in duck egg albumen could play a role in prevention of protein degradation in surimi gel, prepared by conventional heating process. Therefore, the purpose of this study was to investigate the changes of some selected quality index and properties of albumen from duck egg as affected by storage conditions.
Materials and methods
Chemicals
All chemicals used in this study were of analytical grade. Na-Benzoyl-DL-arginine-ρ-nitroanilide (BAPNA), trypsin from bovine pancreas (Type I, ~10,000 BAEE units/mg protein), and bovine serum albumin (BSA) were obtained from Sigma Chemical Co. (St. Louis, MO), and high and low molecular protein markers were purchased from GE healthcare UK Limited (Buckinghamshire, UK). Sodium dodecyl sulfate (SDS), β-mercaptoethanol (β-ME), glutaraldehyde, ethanol and Coomassie blue R-250 were obtained from Merck (Darmstadt, Germany).
Preparation of duck egg albumen
Fresh eggs (totally 360 eggs) from the ducks (Anas platyrhynchos domesticus) with the age of 8–10 months within 24 h after laying were collected randomly at a farm house in Kantang, Trang province, Thailand. Eggs were stored at room temperature (28–30 °C) and 4 °C. Eggs were randomly taken at day 0, 3, 6, 9, 12 and 15. The eggs were then broken. Albumen was separated from egg yolk manually. Albumen was subjected to analyses.
Quality index of duck egg during storage
Haugh unit (HU) was used as quality index of duck egg during storage. HU was determined using Haugh unit tester (Technical Services and Supplies, Technical Services and Supplies Ltd (TSS), York city, England). Before testing, an egg was weighed and then broken onto a flat surface. A micrometer of Haugh unit tester was used to determine the height of the thick albumen surrounding the yolk. HU was then calculated from the recorded egg weights and albumen heights using the following formula (Samli et al. 2005):
where HU = Haugh unit, H = height of the albumen (mm), and W = egg weight (g).
Changes in pH and chemical composition of albumen during storage
Determination of moisture content, protein content and pH
Moisture and protein contents of albumen samples were determined using oven method (AOAC 2000) and the Biuret method (Robinson and Hogden 1940), respectively. The pH of albumen was measured directly using pH meter (pH 700, EUTECH Instruments, Singapore).
Determination of trypsin inhibitory activity
Trypsin inhibitory activity was measured according to the method of Benjakul et al. (2001). Albumen solution with 50-fold dilution (200 µL) was incubated with 200 µL of porcine pancreas trypsin (0.05 mg/mL) at 37 °C for 15 min. Then, 1000 µL of reaction buffer (50 mM Tris–HCl containing 20 mM CaCl2, pH 8.2) were added. Thereafter, 200 µL of BAPNA (2 mg/mL) was added and incubated at 37 °C for 15 min. To stop the reaction, 200 µL of 30% acetic acid (v/v) were added. The release of ρ-nitroalaniline was measured by a spectrophotometer at 410 nm (UV-16001, SHIMADZU, Kyoto, Japan). One unit of trypsin inhibitory activity was defined as the enzyme causing an increase of 0.01 absorbance unit/min under the assay condition. One unit of protease inhibitory activity was defined as the amount of inhibitor, which reduced trypsin activity by one unit.
SDS–PAGE
Protein compositions of albumen samples were visualized by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) according to the method of Laemmli (1970). To prepare protein sample, 2 mL of albumen were mixed with 12 mL of SDS 5% (w/v). The mixture was homogenized and heated at 85 °C for 40 min. Protein concentration was determined by the Biuret method (Robinson and Hogden 1940). Sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4% SDS, 20% glycerol, with and without 10% β-ME) was added in protein samples and boiled for 3 min. The prepared sample (15 µg protein) was loaded onto gel electrophoresis comprising 12% running gel and 4% stacking gel and subjected to electrophoresis at constant current of 15 mA/gel using electrophoresis unit (Mini-protein II; Bio-Rad Laboratories, Richmond, CA, USA). The gels were stained with Coomassie Brilliant Blue R-125 (0.125%) in 25% methanol and 10% acetic acid. Destaining was performed using 40% methanol and 10% acetic acid. Molecular weight (MW) of protein bands was estimated from the plot of MW standards and Rf.
Changes in gel properties of albumen during the storage
Preparation of albumen gel
Albumen gel was prepared following the method of Mmadi et al. (2014) with slight modification. Albumen was added with distilled water to obtain the final solid content of 10%. The mixture was stirred gently. Then, the solution was poured into a casing (diameter of 25 mm). Both ends were sealed tightly, and heated at 90 °C for 30 min. Thereafter, the gel was cooled immediately at 4 °C and kept overnight. Finally, gel samples were cut into cylinders (diameter 25 mm, height 30 mm) prior to analyses.
Texture profile analysis
Texture profile analysis of gel was performed using a texture analyzer (Model TA-XT2i, Stable Micro System, Surrey, England). The samples were compressed twice to 40% of their original height with a compression cylindrical aluminum probe (50 mm diameter). Force-distance deformation curve was recorded at a cross head speed of 3 mm/s and the recording speed was 3 mm/s. Hardness (N), adhesiveness (N s), springiness (cm), cohesiveness, resilience, chewiness (N cm) and gumminess (N) were evaluated. These parameters were recorded using the MicroStable software version 6 (Surrey, England).
Determination of color
The color of gel samples was determined by a colorimeter (ColorFlex, Hunter Lab, Reston, VA, USA) and reported in CIE system. L*, a*, b* and ΔE*, representing lightness, redness/greenness, yellowness/blueness and total difference of color, respectively. The whiteness of gel was calculated (Kaewmanee et al. 2011) using the following equation:
The ΔE* was also calculated by the following formulation:
where ΔL*, Δa*, Δb* are the difference between color parameter of the samples and the color parameter of the white standard (L* = 92.82, a* = − 1.24, b* = 0.50).
Determination of microstructure
Microstructure of albumen gels stored for the selected time at both storage temperatures was visualized by a scanning electron microscopy as described by Kaewmanee et al. (2011). Albumen gels were cut into small pieces (1 × 1 × 1 mm3). Samples were fixed with 2.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 12 h. Then, fixed samples were rinsed with distilled water for 1 h. Subsequently, samples were dehydrated using ethanol with various concentrations (25, 50, 70, 80, 90 and 100%) for 15 min at each concentration. The dehydrated samples were subjected to critical point drying (CPD). The samples were coated with 100% gold (sputter coater SPI-Module, West Chester, PA, USA). The gel microstructure was visualized by a scanning electron microscope (JEOL JSM-5800LV, Tokyo, Japan).
Statistical analysis
All the experiments were conducted in triplicate using three lots of samples. The model included the main effects of the storage times and temperatures and the two-way interactions between these factors. Significant differences between means were determined by the Duncan’s multiple range tests at P < 0.05 level using the statistical program (SPSS 11.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Effect of storage temperature and time on quality index of duck egg during storage
Haugh unit (HU) of duck eggs stored at 4 °C and room temperature up to 15 days is shown in Table 1. HU is a standard quality index of egg and is considered to be a visually appearance measurement describing the height of egg albumen, when it is broken onto a flat surface (Jones and Musgrove 2005; Samli et al. 2005). HU of fresh duck egg in this study was approximate 81 and graded as AA quality based on USDA standard. Eggs are graded AA with HU index in the range of > 72. For grade A, HU is 71–60, and HU of grade B and C are in the range of 59–31 and < 30, respectively (Department of Agriculture 2000). HU of egg decreased after 3 days of storage (P < 0.05) at both storage temperatures. Higher HU was found in eggs kept at lower temperature (P < 0.05). After 9 days, HU of eggs decreased from 81 to 68, which was considered as grade AA to grade A. Both storage time and temperature were major factors significantly associated with HU. Interactions between storage time and temperature were significant (P < 0.05). HU decreased with the increases in both storage time and temperature. The decrease in HU was suggested to be related with the destruction of the ovomucin–lysozyme complex. This was associated with the reduction of thick albumen height during storage (Akter et al. 2014). These results were in accordance with Akter et al. (2014); Jin et al. (2011); Siyar et al. (2007) who reported that HU of hen eggs was appreciably affected by storage time and temperature.
Table 1.
Effect of storage temperature and time on moisture content, total trypsin inhibitory activity, pH value and Haugh unit of albumen from duck egg
| Moisture content (%) | Total trypsin inhibitory activity (kunits/mg solid) | pH | Haugh unit (HU) | |
|---|---|---|---|---|
| Storage time (day) | ||||
| 0 | 87.93a* | 10.47a | 8.66a | 80.72a |
| 3 | 87.82ab | 9.55b | 9.11b | 74.77b |
| 6 | 87.75b | 9.41b | 9.28d | 72.20b |
| 9 | 87.72b | 8.81c | 9.24c | 68.63c |
| 12 | 87.26c | 8.42d | 9.24c | 63.56d |
| 15 | 86.79d | 7.13e | 9.22c | 63.35d |
| Storage temperature | ||||
| 28–30 °C | 87.35b | 8.78b | 9.22a | 63.93b |
| 4 °C | 87.76a | 9.22a | 9.03b | 76.25a |
| P value | ||||
| Temperature | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Day | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Temperature × day | < 0.05 | NS | < 0.05 | < 0.05 |
| MSE | 0.016 | 0.085 | 0.001 | 1.9 |
MSE mean square error, NS not significant
* Means within storage time or temperature with different superscripts are different at P < 0.05
Effect of storage temperature and time on pH and chemical compositions of albumen during storage
Change in moisture content
Moisture content of albumen from duck egg kept at 4 °C and room temperature are presented in Table 1. Moisture content of albumen of egg stored at room temperature decreased from 87.9% at day 0 to 86.8% after 15 days of storage. Nevertheless, higher change in moisture content was observed in duck eggs stored at room temperature (P < 0.05). During the extended storage, particularly at room temperature, water was lost from albumen during storage through the shell pores (Bell 1996; Ren et al. 2010).
Change in pH
pH value of albumen of duck eggs increased dramatically (P < 0.05) after 3 days from 8.66 to 9.11–9.28 after 6 days of storage (Table 1). Thereafter, the pH decreased gradually and remained constant up to day 15 (P > 0.05). Carbon dioxide (CO2) from albumen might be lost continuously via the shell pores. CO2 in egg is formed when the balance of the carbonate-bicarbonate buffer system was shifted towards production of CO2 in egg (Akter et al. 2014). The loss in CO2 of stored hen and quail egg was reported by Akter et al. (2014); Jin et al. (2011); Silversides and Scott (2001); Itoh et al. (1981). Thus, storage temperature and time had the significant influence on pH of albumen from duck egg.
Change in trypsin inhibitor
With different storage temperatures and times, trypsin inhibitory activity of albumen was found to be varied (Table 1). Total trypsin inhibitory activity in freshly laid eggs was highest (10.47 kunits/mg solid). The decrease in trypsin inhibitory activity was found as storage time increased. After 15 days, activities of 7.66 and 6.79 kunits/mg solid were obtained in albumen from egg stored at 4 °C and room temperature, respectively. In general, higher activity was noticeable in albumen of egg stored at 4 °C, compared to room temperature (P < 0.05) at the same storage time. However, the storage time and temperature had no interaction effect on trypsin inhibitory activity (P > 0.05). Ovalbumin was transferred to ovalbumin isoform (S-ovalbumin) after storage, especially at high temperature (Qiu et al. 2012). Takenawa et al. (2015) reported that ovalbumin had inhibitory effect on trypsin activity. Therefore, the transformation or conformational conversion of a specific part of the folded structure of ovalbumin plausibly resulted in the loss of its biological activity, especially protease inhibitory activity (Rehault-Godbert et al. 2010). Decrease in antiprotease activity correlated with the deterioration of some pivotal proteins such as clusterin, lysozyme, ovotranferrin during storage at high temperature (Qiu et al. 2012). Rehault-Godbert et al. (2010) reported that long storage (after 30 days) at 37 °C was associated with reduced antitryptic and antichymotryptic activity of hen egg albumen, which was related with the degradation of ovalbumin and ovotransferrin. Moreover, Kopeć et al. (2005) also stated that after 2 weeks of storage at 15 °C, the activity of trypsin inhibitors in hen egg albumen decreased. Thus, trypsin inhibitor was still retained to a higher extent when duck egg was kept at low temperature.
Change in protein pattern
Protein is the dominant constituent of duck albumen with an average amount of 8.8%. Ovalbumin (40%), ovotransferrin (2%), ovomucoid (10%), lysozyme (1.2%), and ovomucin (3%) are considered as the main proteins found in duck albumen (Huang and Lin 2011). SDS–PAGE patterns of albumen from duck egg stored at 4 °C and room temperature under non-reducing and reducing condition are illustrated in Fig. 1. Ovalbumin with MW of 45 kDa was found as the most dominant protein. Under non-reducing condition, protein with MW of 81–84 and 70–72 kDa were also observed. Protein with MW of 81–84 kDa was more likely ovotransferrin. Under reducing condition, the thick band of ovotransferrin appeared as the thin band. Ovotransferrin has 15 disulfide bonds and folded into two lobes and four domains. Each lobe is composed of 2 distinct α- and β-domains (Abeyrathne et al. 2013). Reducing agent (β-ME) most likely broke down disulfide bonds of this protein and those domains were dissociated from each other. Additionally, the bands with MW of 13–16 kDa more likely belong to lysozyme, which is known as one of the major bacteriolytic proteins found in egg albumen (Abeyrathne et al. 2013). Three bands were observed under reducing condition. Those had MW of 181.48, 151.48 and 126.44 kDa.
Fig. 1.
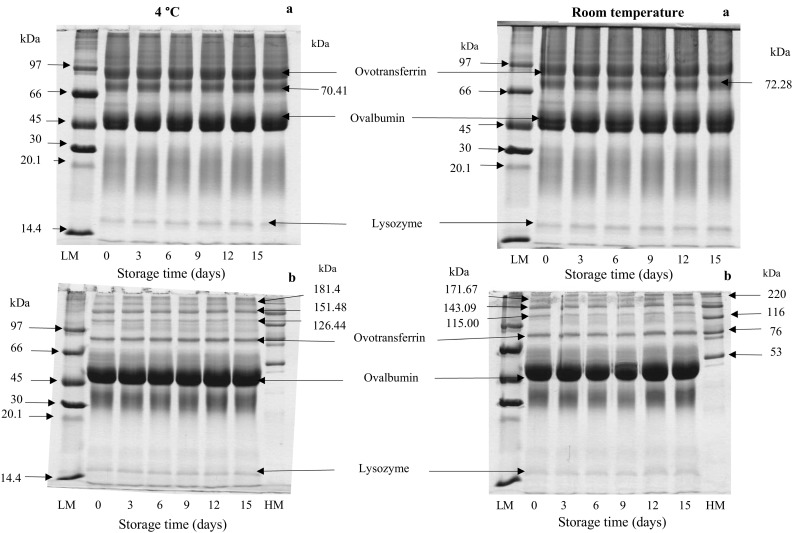
SDS–PAGE patterns of albumen from duck egg during 15 days of storage at different temperatures under non-reducing (a) and reducing (b) conditions
Overall, no remarkable change in protein patterns of albumin from duck egg stored at low temperature for 15 days. Although there was no drastic degradation of most proteins in egg albumen, some proteins such as trypsin inhibitors might undergo denaturation in which the bioactivity could be reduced. Ovalbumin was converted to S-ovalbumin in hen egg after 15 days of storage at 37 °C (Qiu et al. 2012). When eggs were stored under refrigerated condition, this transformation was negligible (Alleoni 2006). These results were supported by Rehault-Godbert et al. (2010) and Qiu et al. (2012) who found the differences in SDS–PAGE pattern of hen egg albumen stored at 20 and 4 °C. However, the intensity of the bands of ovalbumin and ovotransferrin kept at 37 °C seemed to increase after 14 days storage. Itoh et al. (1981) reported that SDS–PAGE pattern of thin quail egg white stored at 30 °C showed less clear bands of ovalbumin and ovostranferrin. Higher temperature generally caused the higher changes in proteins of stored egg. Nevertheless, storage temperature in the present study had no profound effect on protein pattern of duck egg kept for up to 15 days. This might be due to the differences in molecular property and stability of proteins between duck egg and hen egg. Duck eggs are more stable during storage at room temperature than chicken eggs. Duck egg albumen was not severely affected by long storage times as that of hen eggs reported by Huang and Lin (2011).
Effect of storage temperature and storage time on gelling properties of albumen during storage
Texture profile analysis
Hardness, adhesiveness, springiness, cohesiveness, resilience, chewiness and gumminess of albumen from duck egg stored at different temperatures and times are shown in Table 2. Hardness is correlated to the strength of the gel structure under the first compression cycle (Lau et al. 2000). A significant interaction between storage time and temperature was observed for hardness (P < 0.05). The hardness of albumen gel increased markedly after 3 days of storage for both storage temperatures (P < 0.05). However, higher hardness was found for egg kept at 4 °C (26.97 N) than that kept at room temperature (24.19 N). After 6 days of storage, hardness of both gels of duck eggs stored at room temperature and 4 °C decreased continuously up to the end of storage (15 days). However, these values were still higher than that of freshly laid eggs. pH generally affects turbidity and hardness of heat-induced ovalbumin gels. This was governed by alternation of the net charge of protein and the reactivity of sulfhydryl groups. When pH increases naturally in albumen during the storage of eggs, the gel elasticity and gel rigidity are increased (Phillips and Williams 2011). In general, pI of egg albumen is 5 (Croguennec et al. 2002). At alkaline pH found in duck egg albumen (8.6–9.2), the repulsion of negatively charged domains could be enhanced, leading to the unfolding of structure. This could favor the interaction between proteins and the inter-junction could be formed in gel network. Raikos et al. (2007) found that the highest hardness values for whole hen egg were obtained at pH 8 and the lowest value was found at pH 2. Additionally, Phillips and Williams (2011) reported that a maximum hardness of the egg white ovalbumin gel was found at pH of 9.0. With increasing storage time, hardness decreased. This might be caused by higher S-ovalbumin content, which could not undergo aggregation effectively on cooking. This led to the lower gel strengths, compared to that of N-ovalbumin (Hammershøj et al. 2002; Phillips and Williams 2011). Furthermore, heat is the driving force for protein denaturation and gelation. The temperature of heating is of major importance in forming gels. The optimum gelation of hen egg albumen was reported to occur at 80–85 °C with heating time of 30–60 min. The denaturation temperature of ovalbumin was shifted from 84.5 to 92.5 °C for S-ovalbumin, while an intermediate form was denatured at 88.5 °C (Phillips and Williams 2011; Woodward 1990).
Table 2.
Effect of storage temperature and time on textural properties of albumen gel from duck egg
| Hardness (N) | Cohesiveness | Adhesiveness (N s) | Springiness (cm) | Gumminess (N) | Chewiness (N cm) | Resilience | |
|---|---|---|---|---|---|---|---|
| Storage time (day) | |||||||
| 0 | 19.08d* | 0.72f | − 0.66c | 0.92a | 13.67a | 12.56c | 0.41f |
| 3 | 28.47a | 0.78e | − 0.62b | 0.94b | 22.11b | 20.72a | 0.52e |
| 6 | 28.32a | 0.80d | − 0.60a | 0.94b | 22.86a | 20.84a | 0.56d |
| 9 | 26.93b | 0.83b | − 0.60a | 0.94b | 22.16b | 20.88a | 0.59b |
| 12 | 25.75c | 0.84a | − 0.60a | 0.92a | 21.36c | 20.04b | 0.60a |
| 15 | 25.51c | 0.82c | − 0.61ab | 0.92a | 21.25c | 20.01b | 0.58c |
| Storage temperature | |||||||
| 28–30 °C | 24.19b | 0.82a | − 0.61a | 0.93a | 19.90b | 18.30a | 0.58a |
| 4 °C | 26.97a | 0.78b | − 0.63b | 0.93a | 21.16a | 19.78b | 0.51b |
| P value | |||||||
| Temperature | < 0.05 | < 0.05 | < 0.05 | NS | < 0.05 | < 0.05 | < 0.05 |
| Day | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Temperature × day | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| MSE | 0.49 | 0.00 | 2.14 | 0.00 | 0.37 | 0.53 | 0.00 |
MSE mean square error, NS not significant
* Means within storage time or temperature with different superscripts are different at P < 0.05
The gradual increase in cohesiveness of albumen gel was observed with increasing storage time at both storage temperatures up to day 12 (Table 2). At the same time of storage, higher values were found in sample kept at room temperature. At day 15, cohesiveness decreased slightly (P < 0.05), regardless of storage temperature. Cohesiveness is a parameter to measure of the difficult level in breaking down the internal structure of gel (Lau et al. 2000). Springiness of albumen gels is shown in the Table 2. Springiness of albumen gel increased slightly within the first 3 days (P < 0.05) and remained constant until day 12. In general, no marked difference in springiness was obtained between albumen from egg kept both temperatures during the first 9 days of storage. Springiness (%) is considered as “elasticity” or “rubberiness” of the gel in the mouth, and is a parameter to show how much the gel structure is broken down by the initial compression (Lau et al. 2000).
Adhesiveness values of albumen gels increased during storage (P < 0.05). There were marked differences in adhesiveness were noticeable between albumen from duck egg stored at the same storage time (P < 0.05). Adhesiveness (N s) is used to determine the textural properties of egg protein gels, which is defined as the work necessary to overcome the attractive forces between the product and a specific surface (Raikos et al. 2007).
Gumminess and chewiness of gels showed the increase within the first 6 days of storage. Thereafter, the values remained unchanged and decreased at day 12 or 15, respectively. In general, the values were lower in gels from egg stored at room temperature, compared to those kept at 4 °C (P < 0.05). The changes in gumminess and chewiness were in agreement with those of hardness. Gumminess and chewiness suggest the resistance to compression force (Yilmaz et al. 2012).
Resilience was increased throughout the storage at both temperatures. Nevertheless, the decrease was noticeable at day 15 of storage, regardless of storage temperatures. Resilience is considered as how well a product fights to regain its original position (Yilmaz et al. 2012). In addition, the interaction of storage time and temperature significantly affected almost all parameters of texture profile analysis. This study suggested that properties of albumen gels were improved when duck eggs were kept at least 3 days at room temperature and 6 days at 4 °C. Those changes could be plausibly due to the transformation of some particular proteins involved in gelation of duck egg albumen.
Color
Color values of albumen gel of duck eggs stored at 4 °C and room temperature are shown in Table 3. The highest lightness (L*) and whiteness of albumen gels were found at day 0 and decreased continuously as the storage time of duck egg increased (P < 0.05). The gels color became darker with increasing storage time. Nevertheless, the extent of decrease was higher when egg was kept at higher temperature. There was the decrease in b*-values of albumen gel throughout the storage. For a*-value, the value of both gels decreased up to day 15 (P < 0.05). ΔE* of gels from fresh egg was 5.54. The sharp increase was found in gel after the eggs were stored for 3–6 days. For gel from egg stored at room temperature, the higher value was observed, compared to that of gel from egg kept at 4 °C (P < 0.05). These data confirmed that color values of egg albumen gel were significantly affected by both storage time and temperature, in which the interaction between two factors was noticeable. Those changes might be related with the dissociation of ovomucin components, especially the soluble β component. Degradation of O-glycosidic bonds following the solubilization of β-ovomucin releases the carbohydrates (hexoses, hexosamine, sialic acid) especially at alkaline pH (Guyot et al. 2013). With increasing storage time, the pH became more increased, which could favor those reactions. As a result, the browning reaction (Maillard reaction) between reducing sugar and amino acid could occur to a higher extent. Refrigerated condition could retard the degradation of O-glycosidic bonds. Coincidentally, Maillard reaction could be retarded. On the other hand, the higher storage temperature could augment those changes as evidenced by the darker color of gel of stored duck egg.
Table 3.
Effect of storage temperature and time on color and whiteness of albumen gel from duck egg
| L* | a* | b* | ΔE* | Whiteness | |
|---|---|---|---|---|---|
| Storage time (day) | |||||
| 0 | 88.35a* | − 2.52a | − 2.45a | 5.54f | 87.79a |
| 3 | 81.84b | − 3.76b | − 3.47b | 11.88e | 81.21b |
| 6 | 80.98c | − 3.98c | − 3.50b | 12.75d | 80.24c |
| 9 | 73.24d | − 4.00c | − 5.38c | 22.54c | 72.83d |
| 12 | 71.81e | − 4.14d | − 6.02d | 23.13b | 69.75e |
| 15 | 71.44f | − 4.12d | − 6.48e | 24.03a | 69.09f |
| Storage temperature | |||||
| 28–30 °C | 73.28b | − 4.04b | − 4.65b | 20.52a | 72.53b |
| 4 °C | 82.26a | − 3.43a | − 4.46a | 11.85b | 81.43a |
| P value | |||||
| Temperature | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Day | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Temperature × day | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| MSE | 0.15 | 0.00 | 3.09 | 0.12 | 0.12 |
MSE mean square error
* Means within storage time or temperature with different superscripts are different at P < 0.05
Microstructure
Scanning electron micrographs of albumen gel from duck eggs stored at 4 °C and room temperature for 3 and 15 days were compared as illustrated in Fig. 2. Fresh duck egg gels were less compact with opened structure. Its network contained random aggregate with less connectivity. After 3 days of storage at 4 °C and room temperature, the denser networks with higher connection were observed. No large voids were observed. There was no difference in gel network of albumen from duck egg stored at temperatures between 4 °C and room temperature. The denser and compact structure of gel coincided with the increased hardness. With increasing storage time, pH became more alkaline. As a result, unfolding of protein was enhanced, more likely due to the increased repulsion mediated by the negatively charged domain, in which the subsequent aggregation induced by heat could be enhanced. Gel with better viscoelastic properties and high water-holding capacity was due to the high cross-linking (Croguennec et al. 2002). After 15 days of storage, the gel network became coarser, especially gel from egg kept at room temperature. The larger voids were obviously observed. The less compactness with the increase voids was related with the decrease in hardness of gel (Table 2). The result revealed that the storage temperature and time affected gel structure of albumen from duck egg in different fashions, where various networks were formed.
Fig. 2.
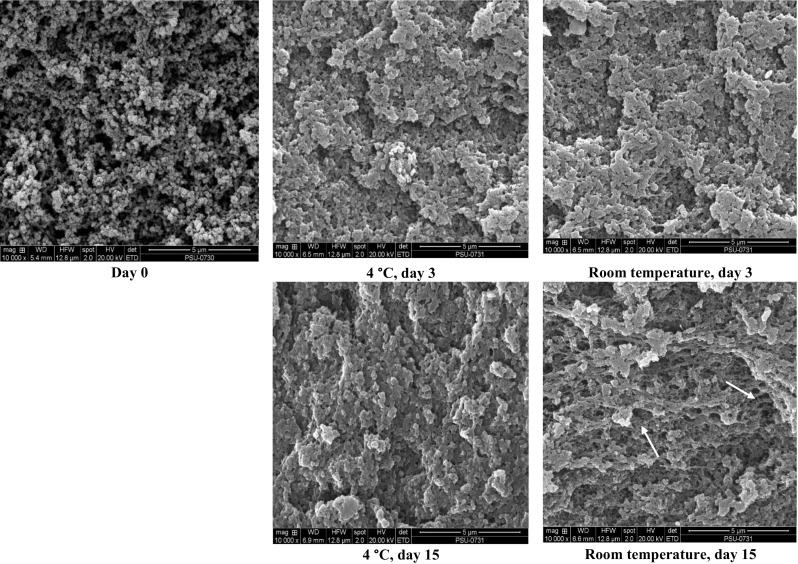
Scanning electron microscopic photograph of albumen gel from duck egg stored at 4 °C and room temperature for selected times. Magnification: ×10,000. Scale bar = 5 µm. Arrow sign indicates void
Conclusion
Duck egg albumen quality and functional property was affected by storage conditions. Eggs stored at refrigerated temperature could maintain the bioactive component, especially trypsin inhibitors during storage. Properties of albumen gel could be improved when eggs were kept at least 3 days. On the other hand, gel became weaker with the lowered whiteness with increasing storage time, particularly at room temperature. Thus, duck egg was suggested to be stored at 4 °C not longer than 6 days, in which albumen from duck egg could be used as the potential binder and gelling agent for food applications.
Acknowledgements
This work was supported by the Higher Education Research Promotion and the Thailand’s Education Hub for Southern Region of ASEAN Countries Project Office of the Higher Education Commission scholarship. The TRF Distinguished Research Professor Grant was also acknowledged.
Funding
Prince of Songkla University.
References
- Abeyrathne E, Lee H, Ahn D. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—a review. Poult Sci. 2013;92:3292–3299. doi: 10.3382/ps.2013-03391. [DOI] [PubMed] [Google Scholar]
- Akter Y, Kasim A, Omar H, Sazili AQ. Effect of storage time and temperature on the quality characteristics of chicken eggs. J Food Agric Environ. 2014;12:87–92. [Google Scholar]
- Alleoni ACC. Albumen protein and functional properties of gelation and foaming. Sci Agric. 2006;63:291–298. doi: 10.1590/S0103-90162006000300013. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersburg: Association of Analytical Communities; 2000. [Google Scholar]
- Bell D. Effects of temperature and storage time on egg weight loss. Poult Int. 1996;35:56–65. [Google Scholar]
- Benjakul S, Visessanguan W, Srivilai C. Porcine plasma protein as proteinase inhibitor in bigeye snapper (Priacanthus Tayenus) muscle and surimi. J Sci Food Agric. 2001;81:1039–1046. doi: 10.1002/jsfa.887. [DOI] [Google Scholar]
- Croguennec T, Nau F, Brule G. Influence of pH and salts on egg white gelation. J Food Sci. 2002;67:608–614. doi: 10.1111/j.1365-2621.2002.tb10646.x. [DOI] [Google Scholar]
- Department of Agriculture US . United States Standards, grades, and weight classes for shell eggs. Washington, DC: USDA; 2000. [Google Scholar]
- Ganesan P, Kaewmanee T, Benjakul S, Baharin BS. Comparative study on the nutritional value of pidan and salted duck egg. Korean J Food Sci. 2014;34:1–6. doi: 10.5851/kosfa.2014.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N, Jan S, Rehault-Godbert S, Nys Y, Gautier M, Baron F. Antibacterial activity of egg white: influence of physico-chemical conditions. World Poult Sci J. 2013;69:1–15. doi: 10.1017/S0043933913000160. [DOI] [Google Scholar]
- Hammershøj M, Larsen LB, Andersen A, Qvist K. Storage of shell eggs influences the albumen gelling properties. LWT Food Sci Technol. 2002;35:62–69. doi: 10.1006/fstl.2001.0811. [DOI] [Google Scholar]
- Huang JF, Lin CC. Production, composition, and quality of duck eggs. In: Nys YBM, Van Immerseel F, editors. Improving the safety and quality of eggs and egg products: volume 1: egg chemistry, production and consumption. 1. Cambridge: Woodhead Publishing Limited; 2011. pp. 487–504. [Google Scholar]
- Itoh T, Kobayashi S, Sugawara H, Adachi S. Some physicochemical changes in quail egg white during storage. Poult Sci. 1981;60:1245–1249. doi: 10.3382/ps.0601245. [DOI] [Google Scholar]
- Jin Y, Lee K, Lee W, Han Y. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian Australas J Anim Sci. 2011;24:279–284. doi: 10.5713/ajas.2011.10210. [DOI] [Google Scholar]
- Jones D, Musgrove M. Effects of extended storage on egg quality factors. Poult Sci. 2005;84:1774–1777. doi: 10.1093/ps/84.11.1774. [DOI] [PubMed] [Google Scholar]
- Kaewmanee T, Benjakul S, Visessanguan W. Effects of salting processes and time on the chemical composition, textural properties, and microstructure of cooked duck egg. J Food Sci. 2011;76:139–147. doi: 10.1111/j.1750-3841.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- Kopeć W, Skiba T, Korzeniowska M, Bobak Ł, Trziszka T. Activity of protease inhibitors and lysozyme of hen’s egg white depending on feed modification and egg storage. Pol J Food Nutr Sci. 2005;14:79–83. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau M, Tang J, Paulson A. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res Int. 2000;33:665–671. doi: 10.1016/S0963-9969(00)00111-3. [DOI] [Google Scholar]
- Lomakina K, Mikova K. A study of the factors affecting the foaming properties of egg white—a review. Czech J Food Sci. 2006;24:110–118. [Google Scholar]
- Machleidt W, et al. Mechanism of inhibition of papain by chicken egg white cystatin. FEBS Lett. 1989;243:234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Mmadi M, Amza T, Wang YC, Zhang M. Effect of desalination on physicochemical and functional properties of duck (Anas Plotyrhyncus) egg whites. Adv J Food Sci Technol. 2014;6:784–791. doi: 10.19026/ajfst.6.111. [DOI] [Google Scholar]
- Phillips GO, Williams PA. Egg proteins. In: Strixner T, Kulozik U, editors. Handbook of food proteins. Oxford: Woodhead Publishing Limited; 2011. pp. 150–209. [Google Scholar]
- Qiu N, Ma M, Zhao L, Liu W, Li Y, Mine Y. Comparative proteomic analysis of egg white proteins under various storage temperatures. J Agric Food Chem. 2012;60:7746–7753. doi: 10.1021/jf302100m. [DOI] [PubMed] [Google Scholar]
- Raikos V, Campbell L, Euston SR. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocoll. 2007;21:237–244. doi: 10.1016/j.foodhyd.2006.03.015. [DOI] [Google Scholar]
- Rehault-Godbert S, Baron F, Mignon-Grasteau S, Labas V, Gautier M, Hincke MT, Nys Y. Effect of temperature and time of storage on protein stability and anti-Salmonella activity of egg white. J Food Prot. 2010;73:1604–1612. doi: 10.4315/0362-028X-73.9.1604. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wu J, Renema R. Nutritional and health attributes of eggs. In: Guerrero-Legarreta I, editor. Handbook of poultry science and technology. Hoboken: Wiley; 2010. pp. 533–578. [Google Scholar]
- Robinson HW, Hogden CG. The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J Biol Chem. 1940;135:707–725. [Google Scholar]
- Samli H, Agma A, Senkoylu N. Effects of storage time and temperature on egg quality in old laying hens. J Appl Poult Res. 2005;14:548–553. doi: 10.1093/japr/14.3.548. [DOI] [Google Scholar]
- Silversides F, Scott T. Effect of storage and layer age on quality of eggs from two lines of hens. Poult Sci. 2001;80:1240–1245. doi: 10.1093/ps/80.8.1240. [DOI] [PubMed] [Google Scholar]
- Siyar S, Aliarabi H, Ahamdi H, Anshori H. Effect of different storage conditions and hen egg on egg quality parameters. Aust Poult Sci Symp. 2007;19:106–109. [Google Scholar]
- Takenawa T, Takahashi K, Le-Chang S, Okazaki E, Osako K (2015) The effect of ovalbumin on the protease activity. In: International symposium on aquatic products processing, Bogor, Indonesia, pp 39–41
- Woodward SA. Egg protein gels. In: Harris P, editor. Food gels. Dordrecht: Springer; 1990. pp. 175–199. [Google Scholar]
- Yilmaz MT, Karaman S, Dogan M, Yetim H, Kayacier A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis (TPA) parameters. J Food Eng. 2012;108:327–336. doi: 10.1016/j.jfoodeng.2011.08.005. [DOI] [Google Scholar]
- Zayas JF. Functionality of proteins in food. Berlin: Springer; 1997. [Google Scholar]


