Abstract
Deodorised water extracts of aromatic plants are obtained as by-products of essential oil isolation and usually discarded as waste. However, phytochemical composition of these extracts encourages their further utilization as food additives or functional food ingredients. In this study we investigated phytochemical composition, antioxidant and in vivo antiproliferative activity of deodorised water extract of Thymus pannonicus All. (DWE). HPLC analysis revealed rosmarinic acid (RA) (71.11 ± 1.54 mg/g) as the most abundant constituent of the extract, followed by salvianolic acid H (14.83 ± 0.79 mg/g, calculated as RA). DWE exhibited pronounced antioxidant activity in vitro, in FRAP and DPPH tests (FRAP value: 7.41 mmol Fe/g and SC50: 3.80 μg/g, respectively). Using the model of Ehrlich carcinoma cells in mice that were treated with DWE prior, at the time, and after tumour cells implantation, the tumour growth suppression and redox status of malignant cells (i.e., activities of antioxidant enzymes, level of glutathione and intensity of lipid peroxidation) were followed. DWE applied as pretreatment caused disturbance of antioxidant equilibrium as well as apoptosis/necrosis of up to 90% EAC cells. Results obtained in the present study revealed chemopreventive potential and possibility of T. pannonicus DWE usage. High content of RA and other phenolic compounds explains, at least in part, the observed effects.
Keywords: Thyme, Water extract, Antioxidant, Prooxidant, Cytotoxicity, HPLC
Introduction
Plants belonging to family Lamiaceae, such as rosmary, lemonbalm, sage, or thyme, are commonly used as medicinal plants, spices, condiments, or as sources of essential oils. Additionally, these aromatic herbs may be used directly or in dosed preparations as food supplements in order to sustain or promote health. Regarding chemical composition, these herbs are abundant with essential oil, phenolic acids and flavonoids (Franz et al. 2011). Lemon-scented plants of thyme species Thymus pannonicus All. (Hungarian thyme, Eurasian thyme), are traditionally used for treatment of mild gastrointestinal and respiratory complaints, as well as a refreshing herbal drink, or as an aromatizing agent for homemade jams, cookies, etc. As it contains more than 70% of citral in the essential oil, this species could represent an attractive raw material for commercial exploitation as a source of highly valued essential oil (Maksimović et al. 2008).
Deodorised water extracts are obtained as by-products during essential oil isolation by hydrodistillation. Although usually considered as waste, they represent a rich source of phenolic compounds with favourable pharmacological properties, and potentially may be used as functional food ingredients, nutritional supplements or food additives (Dorman et al. 2003, 2004). Furthermore, plant extracts and phytoconstituents are considered as a good starting point for the ongoing pursue for the cancer chemopreventive and chemotherapeutic agents. For that issue, effects of plant phenolics on supression of malignant cells proliferation (by various mechanisms) as well as on attenuating consequences of excessive production of reactive oxygen species might be beneficial (Kozics et al. 2013; Link et al. 2010; Osakabe et al. 2004; Paluszczak et al. 2010). However, plant phenolic compounds might act as both antioxidants and prooxidants, and the final outcome in cancer cells is still hardly predictable (Trachootham et al. 2009).
In the present study, we investigated phytochemical composition and antiproliferative potential of deodorised water extract of Hungarian thyme (DWE) in the model of induced Ehrlich ascites carcinoma (EAC) in mice. In addition to assessing the effects of DWE on tumour growth parameters (i.e. ascites volume, tumour cell number and tumour cell viability), redox status of cancer cells was also followed. Ehrlich ascites carcinoma (EAC) cells were implanted into mice that were either pretreated, treated at the time of implantation, or posttreated with DWE.
Materials and methods
Chemicals
All solvents and reagents used were of analytical grade. For the HPLC analysis solvents and phosphoric acid were of HPLC gradient grade. The reference compounds N-acetyl-l-cysteine (NALC), apigenin, (-)-epicatechin, and luteolin 7-O-glucuronide were purchased from Sigma Aldrich, whereas rosmarinic acid (RA) and luteolin were obtained from Carl Roth. Salvianolic acid H (3′-O-(8′′-Z-caffeoyl)-rosmarinic acid), apigenin 7-O-glucuronide, and luteolin 7-O-diglucuronide were previously isolated from the aerial parts of Thymus pannonicus (Arsenijević et al., 2016).
Plant material and extraction procedure
Aerial parts of Thymus pannonicus All. were collected during flowering period on Mt. Vršačke planine (Serbia). Plant material was identified according to Flora Europaea (Jalas 1972). Voucher specimen was deposited at Herbarium of Department of Botany, Faculty of Agriculture, University of Belgrade. The aerial parts were spread out in a thin layer and air-dried (EMEA/HMPC/246816/2005 2006). Dried herb (60 g) was comminuted, 1200 mL of water was added, and subjected to hydrodistillation in a Clevenger-type apparatus (5 ml graduated trap, Šurlan, Pula) for 2 h. The residual plant material was separated from the liquid by filtration (Grade 1 filter paper, Whatman) and the filtrate was evaporated to dryness under reduced pressure (Rotavapor RII, Büchi), yielding 9.80 g of dry DWE.
HPLC analysis
Prior to HPLC analysis DWE aqueous solution (5 mg/mL) was filtered through a 0.45 μm syringe filter (Captiva, Agilent Technologies). Chromatographic separation was performed on an Agilent 1100 HPLC system with diode–array detection under following conditions: Zorbax Eclipse XDB-C18 analytical column (250 × 4.6 mm; particle size 5 μm), flow rate 0.8 mL/min, temperature 25 °C, injection volume 20 μL. Gradient elution was applied, with binary mobile phase consisted of solvent A, 0.03% phosphoric acid, and solvent B, 10% of A in acetonitrile: initial 10% of B, rising to 25% in 5 min, kept constant till 15 min, 15–20 min rising to 30%, 20–25 min increased to 50%, 25–30 min increased to 70% of B, and returning to initial conditions till 35 min. The chromatograms were recorded at 210, 250, 320 and 350 nm.
Identification of compounds was performed by comparing their UV spectra and retention times with those obtained for available standards and isolated compounds (RA, salvianolic acid H, luteolin 7-O-glucuronide, luteolin 7-O-diglucuronide and apigenin 7-O-glucuronide). UV spectra were also compared with the literature data, having in mind that spectral features of a flavonoid molecule are tightly connected with its substitution pattern (Mabry et al. 1970).
External calibration was applied for the determination of phenolic acids, catechin derivatives, luteolin heterosides and apigenin heterosides, using RA (at 320 nm, y = 32000x-27, r2 = 0.9993, concentration range 0.100–1.000 mg/mL), (-)-epicatechin (210 nm, y = 272430x-20, r2 = 0.9811, 0.002–0.100 mg/mL), luteolin (350 nm, y = 59410x-51, r2 = 0.9999, 0.005–0.020 mg/mL), and apigenin (350 nm, y = 61304x-69, r2 = 0.9999, 0.005–0.020 mg/mL) as the reference standards, respectively.
Antioxidant activity in vitro: FRAP and DPPH tests
The antioxidant activity in vitro was assessed using FRAP (Ferric-Reducing Antioxidant Power) method, for the determination of reducing activity, and DPPH (2,2-diphenyl-1-picrylhydrazyl) test for the assessment of antiradical activity. The tests were carried out as proposed by Szőllősi and Szőllősi-Varga (2002) and Cuendet et al. (1997), respectively, with minor modifications, as performed in Kukić et al. (2006). For the determination of FRAP value, a calibration curve using 200–1000 µmol/L FeSO4 × 7H2O standard solutions (corresponding to 3.45–32.23 μmol/L of working standards) was made, and the result was expressed as mmol Fe2+/g of DWE. The concentration of the extract that caused 50% of DPPH scavenging, i.e. SC50DPPH, was calculated by regression analysis for samples containing different aliquots of DWE solution. As the reference antioxidants, RA and NALC were used.
Evaluation of cytotoxic and antioxidant/prooxidant activity in vivo
Animal care and treatment
Animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animal Resources edited by the Commission of Life Sciences, National Research Council, and the Directive 2010/63/EU of the European Parliament and of the Council of Europe. Experimental protocol was reviewed and approved by University of Novi Sad Ethics Committee (EK: I-2013-07). Male and female Hannover National Medical Institute (Hann:NMRI) mice were obtained from Biochemical Laboratory, Clinical Centre Vojvodina (Novi Sad, Serbia). 6–8 weeks old mice weighing 25 ± 2.5 g were used in the experiment. They were fed with standard mice chow (LM2, Veterinarski zavod, Subotica, Serbia) with free access to tap water, in a temperature (25 °C) and humidity controlled (30–50%) animal house, under 12 h light/day cycles. Animals were divided into groups of six, that were either untreated (CTRL, control group, i.e. mice with implanted EAC cells) or treated with DWE water solution (5%, m/V) 0.2, 0.5, 1, 1.5 or 2 mL/kg b.w. i.p. per day, under the following conditions: (a) group G1 (pretreated group)–mice treated with DWE during 7 days before the EAC implantation; (b) group G2 (treated group)–mice treated with DWE during 7 days starting from the day of the EAC implantation; and (c) group G3 (posttreated group)–mice treated with DWE during 7 days starting from the seventh day after EAC implantation. The implantation process was conducted by transferring the EAC ascites from one animal by intraperitoneal injection. These experiments were repeated with male and female mice separately. After treatment, mice were anesthetised and sacrificed, and the ascites of the carcinoma were collected for further analysis. The experiments were repeated in the same way using the antioxidant NALC instead of DWE.
Determination of tumour cell number and cell viability
The ascites from the abdomen was transferred to Krebs–Ringer phosphate buffer solution (0 °C, pH 7.4) and subjected to sequential centrifuging at 4500 rpm (MSE HIGH SPEED, 4 °C) and 12,000 rpm (Eppendorf 3200, 2.5 min) to obtain a dense cell suspension (1:1). The cell number was counted in a Neubauer chamber and expressed as number of cells/mm3. Cell viability was determined by the Trypan blue exclusion method and expressed as percentage of damaged cells.
Biochemical tests
Samples were diluted with Krebs–Ringer phosphate buffer and the activities of antioxidant enzymes were determined in EAC cells by standard laboratory protocols. The activity of xanthine oxidase (XOD) was evaluated following the procedure given by Bergmayer (1970), catalase (CAT) according to Beers and Sizer (1950), peroxidase (Px) according to Simon et al. (1974), glutathione peroxidase (GSHPx) according to Beuthler (1984) and glutathione reductase (GR) according to Goldberg and Spooner (1983). The quantity of reduced glutathione (GSH) was determined according to Beuthler et al. (1983). The intensity of lipid peroxidation (LPx) was assessed using the Buege and Aust (1978) protocol by determining the content of oxidative products of cell membrane lipids, and expressed in malonyldialdehyde (MDA) equivalents.
Statistical analysis
HPLC analysis and in vitro tests were run in triplicate and the results were expressed as mean ± standard deviation (SD). The results of in vivo experiment were obtained from six samples (animals) and expressed as mean ± SD. Mean values between the groups in biochemical analyses were considered significantly different at p < 0.05 confidence level, after the performance of the ANOVA followed by Bonferonni and Tuckey post hoc tests.
Results and Discussion
Phytochemical composition of DWE
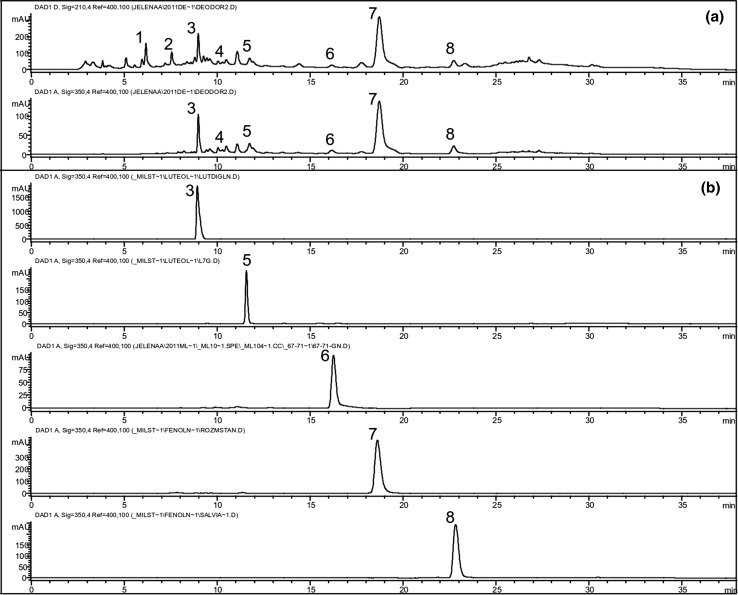
The deodorised water extract (DWE) of aerial parts of T. pannonicus was obtained as a by-product of essential oil isolation. Several phenolic compounds, namely phenolic acids, flavone heterosides, and catechin derivatives, were identified in DWE by HPLC (Fig. 1, Table 1). RA, phenolic acid, a derivative of caffeic acid, was the most abundant in the extract (71.11 ± 1.54 mg/g), followed by salvianolic acid H, i.e. caffeic acid trimer (14.83 ± 0.79 mg/g, expressed as RA). Among flavones, heterosides of luteolin and heterosides of apigenin were detected. The contents of luteolin 7-O-diglucuronide and luteolin 7-O-glucuronide were 5.54 ± 0.17 and 3.05 ± 0.16 mg/g, expressed as luteolin, respectively, whereas the total amount of apigenin heterosides was 3.35 ± 0.36 mg/g, expressed as apigenin. Total catechin derivatives constituted 4.37 ± 0.07 mg/g of extract, calculated as (-)-epicatechin.
Fig. 1.
Chromatograms of Thymus pannonicus deodorised water extract (DWE) recorded at 210 and 350 nm, respectively (a), and chromatograms of reference compounds recorded at 350 nm (b). 1, Catechin derivative; 2, Catechin derivative; 3, Luteolin 7-O-diglucuronide; 4, Apigenin 7-O-heteroside, 5, Luteolin 7-O-glucuronide; 6, Apigenin 7-O-glucuronide; 7, Rosmarinic acid; 8, Salvianolic acid H
Table 1.
Phytochemical composition of Thymus pannonicus deodorised water extract (DWE)
| # | R t (min) | Constituent | Content (mg/g DWE) |
|---|---|---|---|
| 1 | 6.18 | Catechin derivativea | 2.36 ± 0.04 |
| 2 | 7.56 | Catechin derivativea | 2.01 ± 0.03 |
| 3 | 8.99 | Luteolin 7-O-diglucuronideb | 5.54 ± 0.17 |
| 4 | 10.06 | Apigenin 7-O-heterosidec | 1.53 ± 0.06 |
| 5 | 11.75 | Luteolin 7-O-glucuronideb | 3.05 ± 0.16 |
| 6 | 16.19 | Apigenin 7-O-glucuronidec | 1.82 ± 0.30 |
| 7 | 18.76 | Rosmarinic acidd | 71.11 ± 1.54 |
| 8 | 22.76 | Salvianolic acid Hd | 14.83 ± 0.79 |
Results are expressed as mean ± SD (n = 3)
aCalculated as (-)-epicatechin
bCalculated as luteolin
cCalculated as apigenin
dCalculated as rosmarinic acid
The presence of phenolic constituents was confirmed previously in the polar extracts of Thymus species. RA was often the main constituent of previously analysed extracts of thyme plants, similar to the results obtained in this study. Furthermore, the presence of flavone glucuronides is also common for aqueous extracts of aerial parts of Thymus species (Boros et al. 2010; Dorman et al. 2004; Martins et al. 2015). On the other hand, salvianolic acid H (3′-O-(8′′-Z-caffeoyl)-rosmarinic acid), relatively abundant compound in the currently analysed extract of T. pannonicus, was only sporadically identified in the extracts of thyme species (Dapkevicius et al. 2002; Pereira et al. 2013).
Antioxidant capacity of DWE in vitro
High content of phenolic compounds led to presumption that T. pannonicus DWE should exhibit antioxidant properties. The total reducing power of DWE assayed by FRAP method was substantial (4.09 mmol Fe/g), as well as its DPPH scavenging ability (SC50DPPH 19.89 μg/g), in comparison to the antioxidant activity assessed with NALC (FRAP value 10.3 mmol Fe/g; SC50DPPH 7.1 μg/g) as the reference compound. Determined by the same methods, RA accounted for around 25% of the reducing activity (FRAP value 16.5 mmol Fe/g) and 36% of the scavenging ability of DWE (SC50DPPH 3.9 μg/g). Bearing in mind the composition of DWE and previously established antioxidant properties of salvianolic acid H and flavonoids, especially glucuronides of luteolin (Dapkevicius et al. 2002; Kozics et al. 2013; Lu and Foo 2001), it can be concluded that these compounds significantly contributed to the reducing and anti-DPPH activity of analysed DWE, also.
Effects of DWE on Ehrlich ascites carcinoma cells in vivo
Cytotoxic and antiproliferative activity
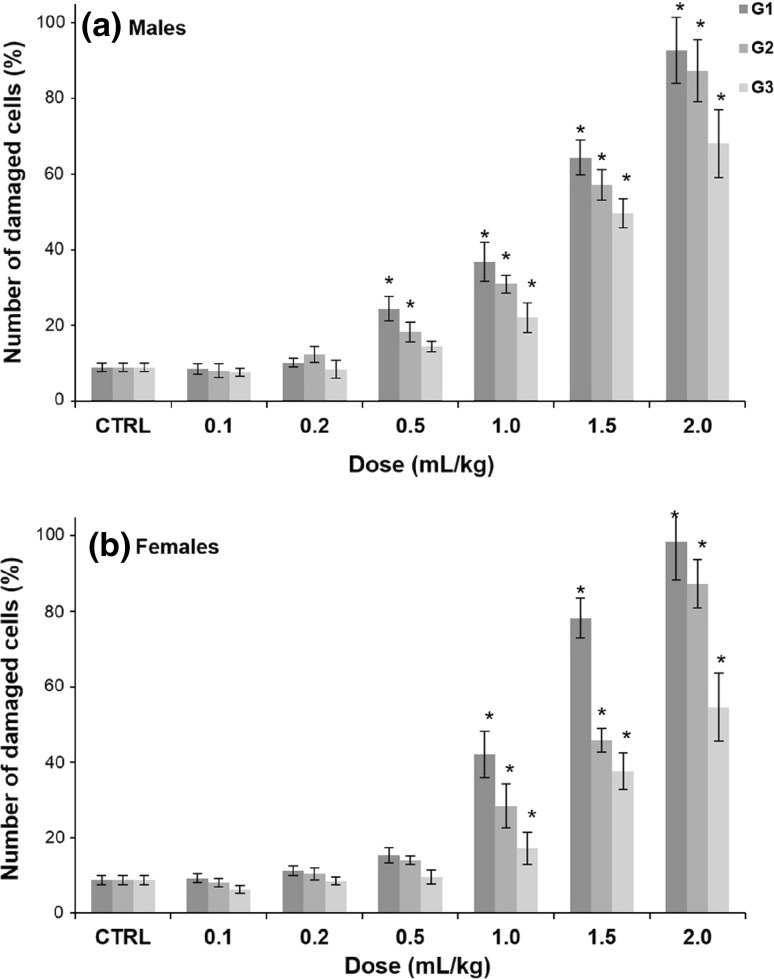
At first, the possible dose dependency of the induction of apoptosis/necrosis of EAC cells by DWE was investigated. Results presented in Fig. 2 showed that the extract inhibited the tumour growth in a dose dependent manner. Significantly higher percentage of damaged cells was noticed at higher doses of extracts (1.0, 1.5 and 2.0 mL/kg b.w.) in comparison to the untreated group (CTRL). DWE caused strong tumour growth inhibition, which was followed by a high percentage of apoptotic/necrotic cells, ranging from 49.7 and 39.6% for 1.5 mL/kg b.w. posttreated males and females (G3), to 92.7 and 98.4% for 2 mL/kg b.w. pretreated males and females (G1), respectively. In addition, certain differences were noticed between male and female groups. These preliminary results suggested that in the upcoming experiments, the highest dose (2 mL/kg b.w.) of DWE should be selected for the evaluation of other antitumour activity parameters.
Fig. 2.
Dose dependency of the induction of apoptosis and/or necrosis of EAC cells by Thymus pannonicus deodorised water extract (DWE) in males (a) and females (b). CTRL, untreated control group; G1, G2 and G3, animals pretreated, treated at the time of implantation or posttreated with DWE, respectively. *Significantly different from the CTRL group at p < 0.05
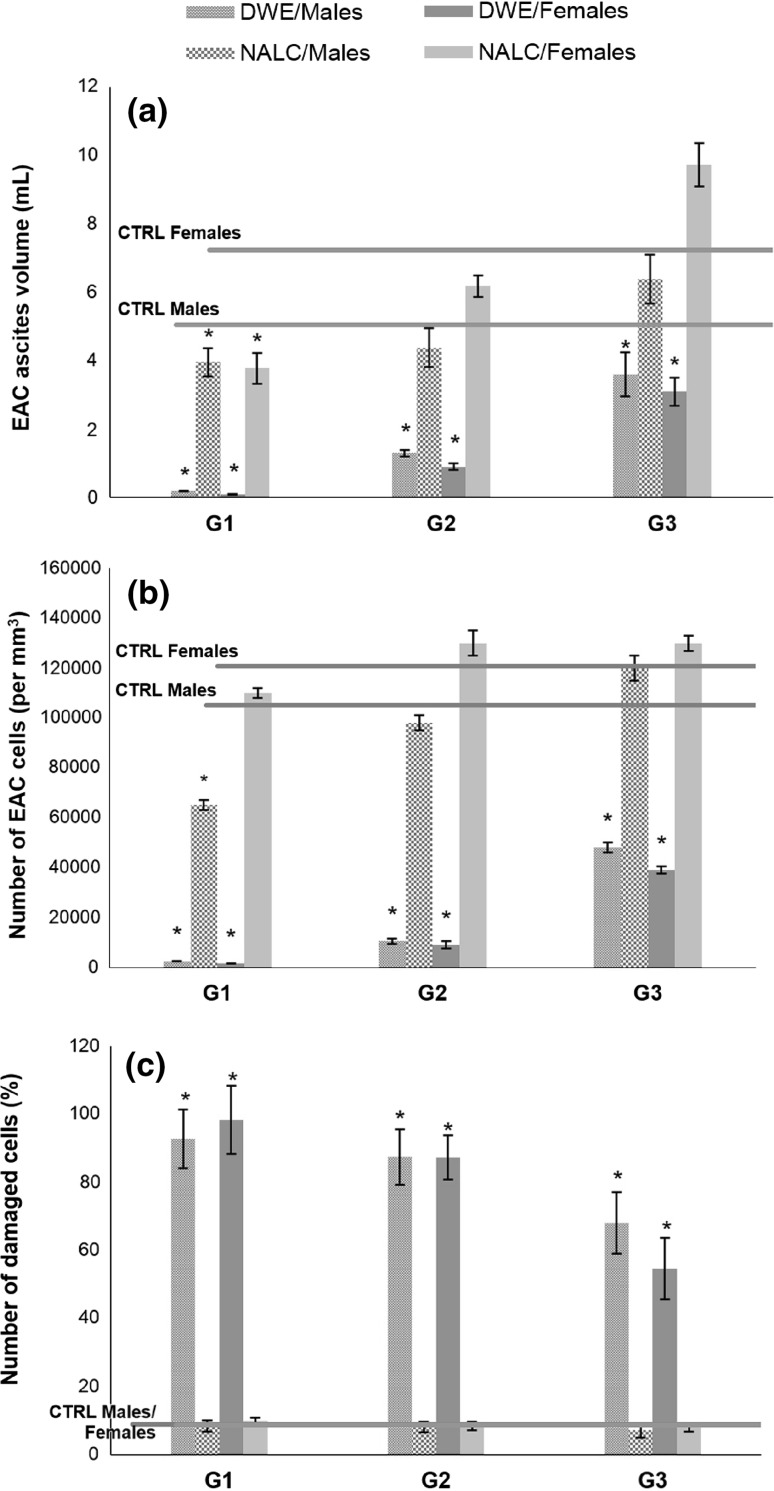
The ability of the extract to inhibit the tumour growth was analysed by following the tumour ascites volume (Fig. 3a), tumour cell number (Fig. 3b) and tumour cell viability (Fig. 3c). A significant decrease (96–98%) in the EAC ascites volume was observed in mice of both sexes pretreated with DWE (G1) (Fig. 3a). The same tendency was observed for both treated (G2) and posttreated animals (G3), but the efficiency of the extract was lower. Besides, it could be noticed that slightly better effects were obtained in female mice than in males. In contrast to the results obtained with DWE, NALC, the reference antioxidant, significantly inhibited the EAC ascites volume growth only in the pretreated animals. Moreover, applied as a posttreatment, it even promoted the ascites growth in female mice.
Fig. 3.
Effects of Thymus pannonicus deodorised water extract (DWE) and N-acetyl-l-cysteine (NALC) on EAC ascites volume (a), EAC cells number (b) and EAC cells viabilty (c). CTRL, untreated control group; G1, G2 and G3, animals pretreated, treated at the time of implantation of EAC cells, or posttreated with DWE or NALC, respectively. *Significantly different from the CTRL group at p < 0.05
The EAC cell numbers were decreased in all DWE-treated groups in comparison to the untreated animals (Fig. 3b). The highest decrease in EAC cells number was obtained in groups pretreated with DWE (G1): 1500/mm3 for females and 2500/mm3 for males, while the number of cells in untreated animals (CTRL) was 120,000/mm3. It can be noticed that the extract was less efficient when applied after the tumour implantation. Treatment with NALC resulted just in a small insignificant decrease in pretreated mice, while in posttreated animals NALC administration even promoted the increase in the number of tumour cells. Application of DWE significantly increased the percentage of damaged cells in all groups (G1–G3) (Fig. 3c). The best results were obtained in the pretreated animals (G2). In the groups treated with NALC, the percentage of damaged cells remained in the range of control group (CTRL).
The obtained data on the EAC ascites volume, EAC cell number, and cell viability suggested that pretreatment with DWE interfered with the establishment of tumour growth more significantly in comparison with the effects observed in case of simultaneous administration with the tumour cells. Additionally, certain gender-dependent differences in response to DWE treatment were noticed, which could be related to the oestrogen-dependent nature of Ehrlich tumour cells. Furthermore, pronounced differences between the effects of DWE and NALC were observed, indicating that the mechanism of DWE action goes beyond its activity as an antioxidative agent.
Antioxidant/prooxidant activity: activities of antioxidant enzymes, glutathione content and intensity of lipid peroxidation
Activities of the antioxidant enzymes (XOD, CAT, Px, GSHPx, GR), as well as the amount of GSH and intensity of LPx in the EAC cells are shown in Table 2. Some differences in the antioxidant enzymes activities were noticed between the animals that received DWE treatment and the control group (CTRL). Significant increase in the activity of XOD (i.e. marker of prooxidant activity in cells) was noticed in all groups, being the most pronounced in the pretreated group (G1). The CAT activity was not changed significantly upon the treatment with DWE, except in the pretreated females. The Px activity was increased in the pretreated (G1) and treated groups (G2), and without significant changes in the posttreated group (G3).
Table 2.
Effects of Thymus pannonicus deodorised water extract (DWE) (5% m/V, 2 mL/kg b.w.) on the activities of antioxidant enzymes, reduced glutathione (GSH) level and intensity of lipid peroxidation (LPx) in EAC cells
| Group | XOD (µmol mL−1) | CAT (µmol mL−1) | Px (µmol mL−1) | GR (µmol mL−1) | GSHPx (µmol mL−1) | GSH (nmol mg−1) | LPx (nmol MDA mg−1) |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| CTRL | 0.123 ± 0.011 | 0.445 ± 0.012 | 0.279 ± 0.001 | 1.38 ± 0.01 | 0.772 ± 0.009 | 1.50 ± 0.02 | 0.029 ± 0.001 |
| G1 | 3.22 ± 0.14* | 0.458 ± 0.029 | 0.922 ± 0.11* | 3.45 ± 0.21* | 4.82 ± 0.36* | 0.312 ± 0.11* | 0.91 ± 0.11* |
| G2 | 1.56 ± 0.18* | 0.311 ± 0.021 | 0.627 ± 0.065 | 2.78 ± 0.17 | 5.11 ± 0.34 | 0.587 ± 0.12* | 0.078 ± 0.053 |
| G3 | 0.538 ± 0.12 | 0.284 ± 0.10 | 0.302 ± 0.078 | 2.07 ± 0.26 | 3.67 ± 0.25 | 0.945 ± 0.11* | 0.074 ± 0.061 |
| Females | |||||||
| CTRL | 0.152 ± 0.013 | 0.512 ± 0.023 | 0.328 ± 0.001 | 2.24 ± 0.05 | 0.783 ± 0.011 | 1.63 ± 0.01 | 0.031 ± 0.001 |
| G1 | 4.15 ± 0.15** | 0.247 ± 0.11** | 0.912 ± 0.13** | 3.78 ± 0.34 | 3.87 ± 0.64** | 0.248 ± 0.12** | 0.24 ± 0.09** |
| G2 | 1.69 ± 0.17** | 0.287 ± 0.12 | 0.547 ± 0.078 | 2.92 ± 0.18 | 3.12 ± 0.57 | 0.694 ± 0.09** | 0.064 ± 0.037 |
| G3 | 0.684 ± 0.13 | 0.413 ± 0.069 | 0.327 ± 0.091 | 2.07 ± 0.31 | 2.08 ± 0.34 | 1.26 ± 0.05 | 0.040 ± 0.012 |
Values are expressed as mean ± SD of six mice at p < 0.05. Activities of xanthine oxidase (XOD), catalase (CAT), peroxidase (Px), glutathione reductase (GR) and glutathione peroxidase (GSHPx) are expressed in µmol/mL EAC cells. The content of glutathione (GSH) and intensity of lipid peroxidation (LPx) are expressed in nmol/mg of protein content in EAC cells
CTRL, untreated control group; G1, G2 and G3, animals pretreated, treated at the time of implantation of EAC cells, or posttreated with DWE, respectively
* Significantly different from the male control group at p < 0.05. ** Significantly different from the female control group at p < 0.05
The activity of enzyme complex GR and GSHPx, involved in the regeneration of GSH, was increased in all DWE-treated mice (GSHPx activity was affected more, approx. sixfold in groups G1 and G2) (Table 2), indicating that the induction of oxidative stress may play an important role in antitumour properties of the applied extract. The observed changes were brought to a lesser extent in female groups. These observations were also in accordance with the results obtained for the measured amount of GSH (Table 2).
The hypothesis, that the oxidative stress was involved in tumour cells apoptosis/necrosis, was also tested by measuring the levels of cell membrane oxidative damage (LPx). The intensity of LPx, expressed as the content of MDA, in the DWE pretreated mice was increased significantly in comparison to the untreated group (CTRL), while no significant changes were detected in the treated (G2) and posttreated animals (G3). The changes of MDA content were in certain correlation with determined viability of EAC cells in these groups (Table 2, Fig. 3c).
Low values determined for the XOD activity and LPx intensity in EAC cells of the untreated animals (CTRL) indicated that the production of oxidants in cancer cells was suppressed. On the other hand, application of DWE caused increase of these parameters, statistically significant in the pretreated groups (G1). Furthermore, notably higher activity of the GSH regulating system (i.e. considerably increased GSHPx activity, moderate increase of GR activity, and resulting decreased content of GSH) was observed in animals that received DWE treatment. These results altogether implied that DWE application caused EAC cells to undergo a condition of oxidative stress, i.e. that DWE acted as a prooxidant in cancer cells. In other words, DWE, an extract characterized by high content of RA and other phenolic compounds, acted as a cytotoxic agent by provoking oxidative stress in EAC cells.
Antioxidative and prooxidative properties of RA, the dominant constituent in the investigated extract, were confirmed previously, as well as its other numerous biological activities (Muñoz-Muñoz et al. 2013; Petersen 2013). Furthermore, this compound is considered as a promising food-functional ingredient and dietary cancer chemopreventive agent (Fernández-Ginés et al. 2005; Link et al. 2010). In vitro cytotoxic activity of several different plant extracts abundant with RA, luteolin 7-O-glucuronide and other phenolics was observed previously, in addition to their ascertained in vitro cytotoxic effects as single compounds (Berdowska et al. 2013; Encalada et al. 2011; Stanojković et al. 2013). Moreover, the inhibition of cancer development by extracts of Prunella vulgaris (Feng et al. 2010) and Perilla frutescens (Osakabe et al. 2004) in preclinical in vivo models was attributed to RA and related compounds. However, their mechanisms of tumour growth inhibition are still speculative (Berdowska et al. 2013; Paluszczak et al. 2010; Stanojković et al. 2013). Similar to some previous reports on the inhibition of malignant transformation caused by plant extracts (Vulić et al. 2013), the results obtained in the present study imply that DWE elicited production of reactive oxygen species in malignant cells. However, the exact role of RA and other present phenolic compounds in this mechanism is not clarified and represents a topic for further investigation.
Conclusion
Based on the results obtained, prooxidative effect of deodorised water extract of T. pannonicus, obtained as a residual product in the process of essential oil production, might be involved in the cytotoxic and antiproliferative activities against cancer cells in vivo. The effects were more pronounced when the extract was applied prior to tumour cells implantation. The determined composition of the extract, with rosmarinic acid as the most abundant, followed by its derivative salvianolic acid H, and luteolin glucuronides, affirms its revealed chemopreventive potential. Further investigations on the exact mechanism of activity of individual constituents, as well as on the pharmacokinetic properties and toxicological profile of the extract are required to evaluate whether the studied deodorised water extract of T. pannonicus might be used as a chemopreventive agent.
Acknowledegement
This research was supported by the Ministry of Education, Science and Technological Development of Republic of Serbia (Grants Nos. TR 31089 and ON 173021).
References
- Arsenijević J, Drobac M, Šoštarić I, Ražić S, Milenković M, Couladis M, Maksimović Z. Bioactivity of herbal tea of Hungarian thyme based on the composition of volatiles and polyphenolic. Ind Crop Prod. 2016;89:14–20. doi: 10.1016/j.indcrop.2016.04.046. [DOI] [Google Scholar]
- Beers RFJ, Sizer JW. Spectrophotometric method for measuring of breakdown of hydrogen peroxide by catalase. J Biol Chem. 1950;195:133–140. [PubMed] [Google Scholar]
- Berdowska I, Zieliński B, Fecka I, Kulbacka J, Saczko J, Gamian A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013;141:1313–1321. doi: 10.1016/j.foodchem.2013.03.090. [DOI] [PubMed] [Google Scholar]
- Bergmayer UH. Methoden der enzymatischen Analyse. Weinheim: Chemie; 1970. pp. 483–484. [Google Scholar]
- Beuthler E. Glutathione reductase, glutathione peroxidase, catalase, glutathione. In: Beutler E, editor. Red cell metabolism: a manual of biochemical methods. New York: Grune and Stratton; 1984. [Google Scholar]
- Beuthler E, Duron O, Kelly B. Improved methods for the determination of blood glutathione. J Lab Clin Med. 1983;61:882–889. [PubMed] [Google Scholar]
- Boros B, Jakabová S, Dörnyei A, Horváth G, Pluhár Z, Kilár F, Felinger A. Determination of polyphenolic compounds by liquid chromatography-mass spectrometry in Thymus species. J Chromatogr A. 2010;1217:7972–7980. doi: 10.1016/j.chroma.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Buege AL, Aust DS. Microsomal lipid peroxidation. In: Fleisher S, Parker L, editors. Methods in enzymology. New York: Academic Press; 1978. [DOI] [PubMed] [Google Scholar]
- Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 1997;80:1144–1152. doi: 10.1002/hlca.19970800411. [DOI] [Google Scholar]
- Dapkevicius A, Van Beek TA, Lelyveld GP, Van Veldhuizen A, De Groot A, Linssen JPH, Venskutonis R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J Nat Prod. 2002;65:892–896. doi: 10.1021/np010636j. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. doi: 10.1016/S0308-8146(03)00088-8. [DOI] [Google Scholar]
- Dorman HJD, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- EMEA/HMPC/246816/2005 (2006) Guideline on Good Agricultural and Collection Practice GACP for starting materials of herbal origin. European Medicines Agency—Committee on Herbal Medicinal Products, London
- Encalada MA, Hoyos KM, Rehecho S, Berasategi I, De Ciriano MG-Í, Ansorena D, Astiasarán I, Navarro-Blasco I, Cavero RJ, Calvo MI. Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum Nutr. 2011;66:328–334. doi: 10.1007/s11130-011-0256-y. [DOI] [PubMed] [Google Scholar]
- Feng L, Jia X, Zhu MM, Chen Y, Shi F. Antioxidant activities of total phenols of Prunella vulgaris L. in vitro and in tumor-bearing mice. Molecules. 2010;15:145–9156. doi: 10.3390/molecules15129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ginés JM, Fernández-López J, Sayas-Barberá E, Pérez-Alvarez JA. Meat products as functional foods: a review. J Food Sci. 2005;70:37–43. doi: 10.1111/j.1365-2621.2005.tb07110.x. [DOI] [Google Scholar]
- Franz C, Chizzola R, Novak J, Sponza S. Botanical species being used for manufacturing plant food supplements (PFS) and related products in the EU member states and selected third countries. Food Funct. 2011;2:720–730. doi: 10.1039/c1fo10130g. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Spooner RJ. Glutathione reductase. In: Bergmayer HU, editor. Methods of enzymatic analysis. Weinheim-Basel: Chemie; 1983. [Google Scholar]
- Jalas J, et al. Thymus L. In: Tutin T, Heywood V, Burges N, Moore D, Valentine D, Walters S, et al., editors. Flora Europea. Cambridge: Cambridge University Press; 1972. pp. 172–182. [Google Scholar]
- Kozics K, Klusová V, Srančíková A, Mučaji P, Slameňová D, Hunáková L, Kusznierewicz B, Horváthová E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013;141:2198–2206. doi: 10.1016/j.foodchem.2013.04.089. [DOI] [PubMed] [Google Scholar]
- Kukić J, Petrović S, Niketić M. Antioxidant activity of four endemic Stachys taxa. Biol Pharm Bull. 2006;29:725–729. doi: 10.1248/bpb.29.725. [DOI] [PubMed] [Google Scholar]
- Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Foo YL. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. doi: 10.1016/S0308-8146(01)00198-4. [DOI] [Google Scholar]
- Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York: Springer; 1970. [Google Scholar]
- Maksimović Z, Milenković M, Vučićević D, Ristić M. Chemical composition and antimicrobial activity of Thymus pannonicus All. (Lamiaceae) essential oil. Cent Eur J Biol. 2008;3:149–154. [Google Scholar]
- Martins N, Barros L, Santos-Buelga C, Silva S, Henriques M, Ferreira ICFR. Decoction, infusion and hydroalcoholic extract of cultivated thyme: antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015;167:131–137. doi: 10.1016/j.foodchem.2014.06.094. [DOI] [PubMed] [Google Scholar]
- Muñoz-Muñoz JL, Garcia-Molina F, Ros E, Tudela J, García-Canovas F, Rodriguez-Lopez JN. Prooxidant and antioxidant activities of rosmarinic acid. J Food Biochem. 2013;37:396–408. doi: 10.1111/j.1745-4514.2011.00639.x. [DOI] [Google Scholar]
- Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- Paluszczak J, Krajka-Kuźniak V, Baer-Dubowska W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett. 2010;192:119–125. doi: 10.1016/j.toxlet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Pereira OR, Peres AM, Silva AMS, Domingues MRM, Cardoso SM. Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC–UV and ESI–MS combined method. Food Res Int. 2013;54:1773–1780. doi: 10.1016/j.foodres.2013.09.016. [DOI] [Google Scholar]
- Petersen M. Rosmarinic acid: new aspects. Phytochem Rev. 2013;12:207–227. doi: 10.1007/s11101-013-9282-8. [DOI] [Google Scholar]
- Simon LM, Fatrai Z, Jonas DJ, Matkovics B. Study of metabolism enzymes during the development of Phaseolus vulgaris. Biochem Physiol Pflanz. 1974;166:389–393. doi: 10.1016/S0015-3796(17)30073-2. [DOI] [Google Scholar]
- Stanojković T, Kolundžija B, Ćirić A, Soković M, Nikolić D, Kundaković T. Cytotoxicity and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. (Lamiaceae) Dig J Nanomater Biostruct. 2013;8:845–854. [Google Scholar]
- Szôllôsi R, Szôllôsi Varga I. Total antioxidant power in some species of Labiatae (adaptation of FRAP method) Acta Biol Szeged. 2002;46:125–127. [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Vulić J, Ćebović T, Čanadanović V, Ćetković G, Djilas S, Čanadanović-Brunet J, Velićanski A, Cvetković D, Tumbas V. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013;4:713–721. doi: 10.1039/c3fo30315b. [DOI] [PubMed] [Google Scholar]