Abstract
Roscoea procera Wall. is one of the important Himalayan medicinal plant used in traditional as well as in modern health care system. The present study aimed to find out the influence of different phenophases on the phenolic compounds and anti-oxidant properties by analysing after every week for over 4 months from shoot bud initiation to the preparation of senescence. Concentration of total phenolic content were found to be about 1.5 times higher in preparation of senescence phase (6.10 mg GAE/g dry weight or dw) as compared to vegetative growth phase. Similarly, total flavonoid concentration ranged from 4.36 to 5.65 mg querectin equivalents/g dw. The concentration of selected phenolic compounds, i.e., gallic acid, catechin and p-coumaric acid was quantified by reverse phase-high performance liquid chromatography and varied significantly among the different phenophases. While, anti-oxidant activity was found 2–3 times higher in preparation of senescence phase as compared to vegetative phase. Thus, these results concluded that in R. procera, November month (preparation of senescence phase) could be recommended for extracting optimum level of total phenolics, flavonoids and anti-oxidant activity. These results will be further helpful for obtaining maximum benefits from the species and to reduce pressure on reproductive phase while ensuring its conservation.
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2967-z) contains supplementary material, which is available to authorized users.
Keywords: Phenophases, Roscoea procera, Medicinal plants, Phenolic acids, Anti-oxidant activity, Seasonal variation
Introduction
The members of family Zingiberaceae (ginger family) are widely distributed throughout the tropics, particularly in South-East Asia, and are used in medicines, food, spices, dyes, perfume and aesthetics (Tushar et al. 2010). Among these, Roscoea procera is one of the important members of family Zingiberaceae, traditionally used in Indian system of medicines. R. procera is endemic to Himalaya distributed from Jammu and Kashmir to southwest China within an altitudinal range of 1700–3000 masl (Shah 2006). Species is used in preparation of many folk preparations such as tonics and polyherbal rejuvenating formulations, such as Chyawanprash (Rawat et al. 2014a). This species has also been reported to be use in fever, malaria, burning, phthisis; root powder mixed with black pepper is applied on boils for quick healing; decoction of root used in jaundice and boiled rhizome eaten with salt in Nepal (Shah 2006; Sahu et al. 2010; Kumari et al. 2011; Rawat et al. 2016).
Beneficial effects of medical plants are generally as a result of numerous compounds from diverse chemical groups acting together, including a range of phenolic compounds. Phenolics have an array of health-promoting benefits. These are of current interest of research due to their important biological and pharmacological properties, especially the anti-oxidant, anti-inflammatory, anti-allergic, anti-mutagenic and anti-carcinogenic activities (Bravo 1998; Zern and Fernandez 2005; Bhatt et al. 2012; Singh et al. 2016). Many degenerative diseases, such as aging, brain dysfunction, cancer, heart diseases and immune system decline, could be a result of cellular damage caused by free radicals, and that anti-oxidants present in human diet may play an important role in disease prevention (Hertog et al. 1993; Lu and Foo 2001). Anti-oxidants delay oxidative degradation of food products, maintain nutritional quality and prevent formation of toxic oxidation products (Moure et al. 2001). Phenolic compounds showed strong anti-oxidant activity due to their ability of hydrogen ion donation, metal chelation and scavenging property.
Phenolic compounds and/or anti-oxidant activity of plants are known to vary throughout the annual seasons due to genetic determinants and in response to various environmental conditions such as, herbivory, UV-radiation, day length, temperature and nutrient availability (Tegelberg et al. 2002). These seasonal changes may be particularly dramatic in high altitude environments with low temperature and short summer season (Grace 2005). A large variation in phenolic compounds and/or anti-oxidant activity has been reported in aerial and root part of different species during different ontogenetic stages and seasons (Pamplona et al. 2006; Policegoudra et al. 2007; Bruni and Sacchetti 2009; Rawat et al. 2014b).
When considering medicinal plants, it is important to account the seasonal change in quality of the produce that determine the optimal harvest time. The objectives of the present study are to explore the influence of phenophases during the growth of R. procera on phenolic content and anti-oxidants properties.
Materials and methods
Plant materials collection
The rhizomes of R. procera were collected from Majkhali forest of Almora District, India (altitude: 1710 masl; latitude: N29°40′10.0″; longitude: E79°31′54.0″). 20–25 plants were randomly harvested periodically with 7 days intervals from first week of July to first week of November in 2014. Each sampling was performed on Monday at 3–4 p.m. on weekly basis (indicated as week number). The botanical identity of the species was authenticated after consultation with Botanical Survey of India, Dehradun (Uttarakhand) and the voucher specimen deposited at GBPNIHESD herbarium. Immediate after collection, rhizomes/roots were cleaned, washed and dried in hot air oven at 50 °C for 15 days (NSW-142, Narang Scientific Works, New Delhi, India). Dried samples were grounded to fine powder using Wiley Grinder Mill (Macro Scientific, India).
Chemicals and reagents
All chemicals and reagents used were of analytical grade. 2,2-Diphenyl-2-picrylhydrazyl (DPPH) radical, gallic acid, ascorbic acid, chlorogenic acid, caffeic acid, ρ-coumaric acid, 3-hydroxy-benzoic acid, catechin and quercetin were procured from Sigma–Aldrich (Steinheim, Germany); sodium carbonate, 2-(N-morpholino)ethanesulfonic acid (MES buffer), potassium persulphate, ferric chloride, sodium acetate, potassium acetate, aluminum chloride, glacial acetic acid, hydrochloric acid from Qualigens (Mumbai, India); 2,2-azinobis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 2,4,6-tri-2-pyridyl-1,3,5-triazin (TPTZ), methanol and ethanol from Merck Co, (Darmstadt, Germany).
Extract preparation
Methanol was selected as a solvent due to its better solubility for hydrophobic and hydrophilic compounds in naturally occurring phytochemicals for analytical purpose. Sample (1.0 g) of each powdered rhizome was extracted in 100 ml of 80% (v/v) methanol in an orbital shaker at 22° ± 1 °C for 12 h followed by sonication at 50 Hz for 10 min. The extract was then filtered through a Whatman No. 1 filter paper and stored at 4 °C until determination of total phenolics, flavonoids and phenolic compounds, and anti-oxidant activities.
Phytochemicals analysis
Total phenolics were measured based on the method of Singleton et al. (1999) modified by Rawat et al. (2011). Briefly, a small portion (0.25 ml) of the methanolic extract described above was transferred into a test-tube containing 2.25 ml of distilled water, followed by addition of 0.25 ml of Folin–Ciocalteu’s reagent and allowed to stand for reaction for 5 min at 22° ± 1 °C. The mixture was neutralised by adding 2.50 ml of 7% (w/v) sodium carbonate and kept in the dark at 22° ± 1 °C for 90 min. The absorbance of the resulting blue-colour solution was measured at 765 nm using a spectrophotometer (Hitachi U-2001, Tokyo, Japan). Quantification was based on a standard curve of gallic acid prepared in 80% (v/v) methanol and the results were expressed in mg gallic acid equivalents (GAE)/g dry weight (dw).
Total flavonoid content in the methanolic extract of each sample was determined using aluminium chloride colorimetric method described by Bhatt et al. (2012). Briefly, the extract (0.50 ml) was diluted with 1.50 ml of distilled water, followed by the addition of 0.50 ml of 10% (w/v) aluminium chloride, 0.10 ml of 1.0 M potassium acetate and 2.80 ml of distilled water. This mixture was incubated at 22° ± 1 °C for 30 min and absorbance was measured at 415 nm. Quantification was carried out on the basis of standard curve of quercetin prepared in 80% (v/v) methanol and the results were expressed in mg quercetin equivalents (QE)/g dw.
HPLC analysis of phenolic compounds
High performance liquid chromatography (HPLC) was used to measure the composition and concentrations of phenolic compounds in each sample. The HPLC system (Merck-Hitachi, Tokyo, Japan) was equipped with an L-7100 series pump connected to an L-7400 series UV–Vis detector fitted with Winchrome 99 software (Infotech Instrument, Mumbai, India). Rhizome extract were separated in a LichroCart 100 RP-18e column (250 mm × 4.6 mm i.d, 5 µm pore size; Merck Pvt. Ltd, (Tokyo, Japan) using on 80:20:1 (v/v/v) water:methanol:acetic acid mix as the mobile phase at a flow rate of 0.8 ml/min in the isocratic mode and total run time was kept as 45 min for a single analysis. Spectra of gallic acid, catechin, 3-hydroxybenzoic acid, p-coumaric acid, and ellagic acid were recorded at 254 nm, and p-caffeic acid and chlorogenic acid were recorded at 370 nm. The identification of individual phenolic compounds was based on their retention times compared to external standards (Sigma-Aldrich, St. Louis, MO, USA). UV–Vis spectra of the pure standards at different concentrations were used to plot calibration curves for the quantification of each phenolic compound. The reproducibility of the standards during the quantitative analysis was < 3.0% (intra-day relative standard deviation) for each phenolic compound. The results were expressed in mg/100 g dw of rhizomes.
Anti-oxidant activity
Three different in vitro anti-oxidant assays namely, ABTS, DPPH, and FRAP assay were used in this study. Total anti-oxidant activity was measured by modified ABTS method described by Badhani et al. (2015). In brief, 10 ml 7.0 μM ABTS salt and 10 ml 2.45 μM potassium persulphate were combined to produce ABTS radical cations (ABTS·+) and kept in the dark at 22 ± 1 °C for 16 h. The ABTS·+ radical solution was diluted to an absorbance of 0.70 ± 0.05 at 734 nm using 80% (v/v) methanol. For each assay 3.90 ml of the diluted ABTS·+ solution was added to 0.10 ml of the 80% (v/v) methanolic extract of each and mixed thoroughly. The reaction mixture was allowed to stand for 6 min at 22º ± 1 °C in the dark. The absorbance was recorded at 734 nm as compared to a blank prepared with 0.10 ml of 80% (v/v) methanol alone.
The traditional DPPH assay as described by Bhatt et al. (2012) was followed in this study. Twenty-five ml of 0.4 mM DPPH reagent was prepared in 80% (v/v) ethanol added to 25 ml of 0.2 M MES buffer (adjusted to pH 6.0 with 1.0 M NaOH) and then 25 ml of 20% (v/v) ethanol was added. Subsequently, 2.7 ml of this DPPH radical cation preparation (DPPH· ) was mixed with 0.9 ml of 80% (v/v) methanolic extract of each sample and allowed to stand for 20 min at 22º ± 1 °C, at dark. The decline in absorbance at 520 nm was recorded in a UV–Vis spectrophotometer (Hitachi U-2001, Tokyo, Japan).
Ferric reducing anti-oxidant power (FRAP) assay was performed following the method described by Jugran et al. 2013. The FRAP reagent was prepared by adding ten volumes of 300 mM sodium acetate buffer (3.1 g of sodium acetate plus 16 ml glacial acetic acid/l) to one volume of 10 mM 2,4,6-tri-2-pyridyl-1,3,5-triazine (TPTZ) prepared in 40 mM HCl, and one volume of 20 mM ferric chloride. The mixture was pre-warmed to 37 °C and 3.0 ml of this mixture was added to 0.10 ml methanolic extract and placed at 37 °C for 8 min. The absorbance was recorded at 593 nm. In all the anti-oxidant assays, a standard curve of various concentrations of ascorbic acid was prepared for the equivalent quantification of anti-oxidant potential. Results of anti-oxidant activity were expressed in millimole (mM) ascorbic acid equivalent (AAE)/100 g dw plant material.
Statistical analyses
All the determinations of total phenolic and flavonoid content, and anti-oxidant activities, were performed in five replicates of each sample. Values for each sample were calculated as mean ± standard deviation (SD) and subjected to analysis of variance (ANOVA). Significant differences among mean values were tested using Duncan’s multiple range test (DMRT; p ≤ 0.05) by SPSS software Version 17.0 (SPSS Inc, Chicago, IL).
Results
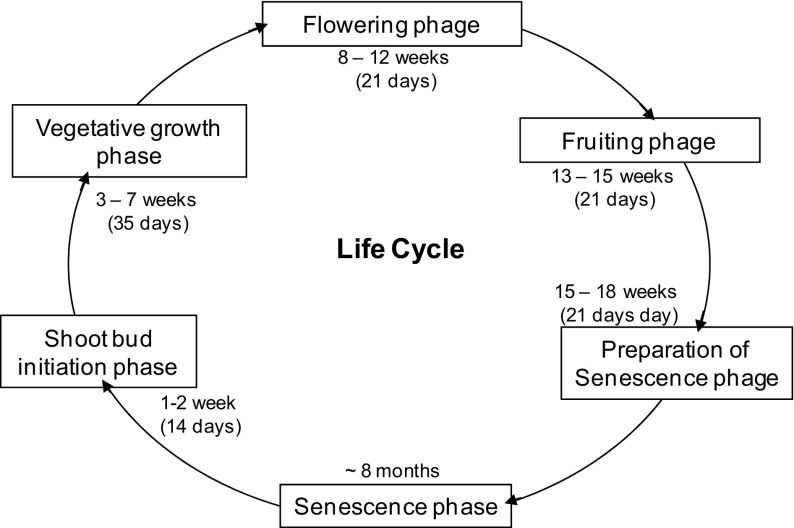
The active growth phases, of R. procera above the ground (about 18 weeks or 4 months) could be divided into 5 different phenophases. It was observed that shoot bud initiation takes place in first 2 weeks (shoot bud initiation phase) followed by vegetative growth for next 5 weeks (vegetative growth phase), flowering for 5 weeks (flowering phase), fruiting and seed maturation (fruiting phase). After that plant prepare itself for prolonged senescence in next 3 weeks (preparation of senescence phase). Finally plant goes for senescence during chilling cold for about 8 months till next year in dormant condition (Fig. 1).
Fig. 1.
Schematic diagram of different phenophases in Roscoea procera life cycle
Total phenolic and flavonoid content
Total phenolic content varied from 3.97 to 6.10 mg GAE/g dw among different sampling times and phenophases (Table 1) and reached to its maximum level in preparation of senescence phase (week 18 = 6.10 mg GAE/g dw). However, it was lowest in later phase of flowering (week 12 = 3.97 mg GAE/g dw). With the growth of plant in vegetative growth phase and flowering phase, phenolic and flavonoid content declined and reached to its minimum level and plant started to develop new tuberous rhizomes. After attaining maximum biomass during flowering, these compounds again started to accumulate and stored in rhizomes. Similarly, minimum flavonoids content was recorded in vegetative growth phase (week 3 = 4.90 mg QE/g dw) and it was maximum in fruit maturation phase (week 14 = 5.82 mg QE/g dw).
Table 1.
Variation in total phenolic, flavonoid content and phenolic compounds in R. procera during different harvesting times
| Week no. | Phenophase | Total phenolic content1 | Total flavonoid content2 | Gallic acid3 | Catechin3 | p-Coumaric acid3 |
|---|---|---|---|---|---|---|
| 1 | Shoot bud initiation | 5.88 ± 0.12ij | 5.15 ± 0.10bcd | 37.33 ± 2.08fgh | 9.35 ± 0.79g | ND |
| 2 | Shoot bud initiation | 5.75 ± 0.07gh | 4.98 ± 0.12ab | 43.50 ± 1.50hi | 7.58 ± 0.63ef | ND |
| 3 | Vegetative growth | 5.50 ± 0.26fg | 4.90 ± 0.19a | 57.95 ± 3.56k | 11.31 ± 0.83h | 0.63 ± 0.13b |
| 4 | Vegetative growth | 5.95 ± 0.13ij | 5.04 ± 0.16abc | 47.33 ± 0.99i | 17.72 ± 0.88j | 0.29 ± 0.04a |
| 5 | Vegetative growth | 5.88 ± 0.16ij | 5.43 ± 0.18ef | 27.26 ± 1.42cde | 8.63 ± 0.32fg | ND |
| 6 | Vegetative growth | 5.58 ± 0.07fg | 5.69 ± 0.18hij | 14.11 ± 1.62ab | 0.84 ± 0.66a | ND |
| 7 | Vegetative growth | 5.76 ± 0.47gh | 5.33 ± 0.12de | 14.15 ± 1.81ab | 4.94 ± 0.41cd | 2.07 ± 0.08e |
| 8 | Flowering | 5.13 ± 0.15e | 5.77 ± 0.11ij | 9.40 ± 1.60a | 15.02 ± 1.55i | 1.59 ± 0.06d |
| 9 | Flowering | 4.37 ± 0.15bc | 5.52 ± 0.15fgh | 23.39 ± 4.45cde | 5.90 ± 0.71d | ND |
| 10 | Flowering | 4.40 ± 0.20bc | 5.62 ± 0.08ghi | 14.83 ± 2.30ab | 5.83 ± 0.43d | ND |
| 11 | Flowering | 4.58 ± 0.17c | 5.31 ± 0.07de | 33.24 ± 2.81efg | 22.76 ± 1.84k | ND |
| 12 | Flowering | 3.97 ± 0.10a | 5.16 ± 0.09cd | 49.22 ± 2.06j | 2.85 ± 0.47b | ND |
| 13 | Fruiting | 4.19 ± 0.09ab | 5.04 ± 0.11abc | 30.32 ± 1.45def | 3.86 ± 0.80bc | ND |
| 14 | Fruiting | 4.27 ± 0.25b | 5.82 ± 0.17j | 20.80 ± 1.59bc | 29.19 ± 0.93m | ND |
| 15 | Fruiting | 4.83 ± 0.15d | 5.17 ± 0.08cd | 27.64 ± 0.99cde | 16.81 ± 0.99j | ND |
| 16 | Preparation of senescence | 5.44 ± 0.25f | 5.65 ± 0.09hi | 38.16 ± 0.71gh | 6.10 ± 1.50de | 1.18 ± 0.07c |
| 17 | Preparation of senescence | 5.98 ± 0.13ij | 5.47 ± 0.14efg | 27.67 ± 3.12cde | 27.60 ± 1.09l | ND |
| 18 | Preparation of senescence | 6.10 ± 0.13j | 5.33 ± 0.10de | 60.72 ± 5.47k | 17.65 ± 0.71j | 0.57 ± 0.03b |
| Average | 5.20 | 5.35 | 30.13 | 11.88 | 0.35 |
For each column, values followed by the different superscript letters are statistically different at p < 0.05 as measured by the Duncan’s multiple range test
1Total phenolic content in mg gallic acid equivalent/g dw
2Total flavonoid content in mg quercetin equivalent/g dw; ND not detected
3mg/100 g dw
Phenolic composition
Individual phenolic compounds exhibited significant variations in different harvesting time (Table 1). Gallic acid content was varied significantly (p < 0.05) among different harvesting times and highest content was recorded in preparation of senescence and vegetative growth phase (week 18 = 60.72 mg and week 3 = 57.95 mg/100 g dw, respectively). However, lowest gallic acid was detected in flowering phase (week 8 = 9.40 mg/100 g dw). Catechin was detected highest in fruiting phase (week 14 = 29.19 mg/100 g dw) and lowest in vegetative growth phase (week 6 = 0.84 mg/100 g dw). p-Coumaric acid was only detected in few samples. Predominantly, it was detected in shoot bud initiation, vegetative growth and preparation of senescence phase; and not detected in flowering phase and fruiting phase. Maximum level of p-coumaric acid was detected in vegetative growth phase (week 7 = 2.07 mg/100 g dw).
Anti-oxidant activity
Anti-oxidant activity measured by all the three anti-oxidant assays showed a similar trend as shown by total phenolic content during the different harvesting times (Fig. 2). Anti-oxidant activity measured by ABTS assay exhibited significantly higher value in preparation of senescence phase followed by vegetative growth phase (week 17 = 2.78 mM and week 18 = 2.71 mM AAE/100 g dw); however lower value was detected in flowering phase (week 11 = 2.31 mM; week 9 = 2.33 mM; week 10 = 2.34 mM AAE/100 g dw). Similarly, DPPH and FRAP assay revealed maximum values in preparation of senescence phase (week 18 = 1.79 mM and 2.41 mM AAE/100 g dw by DPPH and FRAP assay, respectively) and minimum in flowering phase (week 12 = 1.17 mM and 0.85 mM AAE/100 g dw by DPPH and FRAP assay, respectively).
Fig. 2.
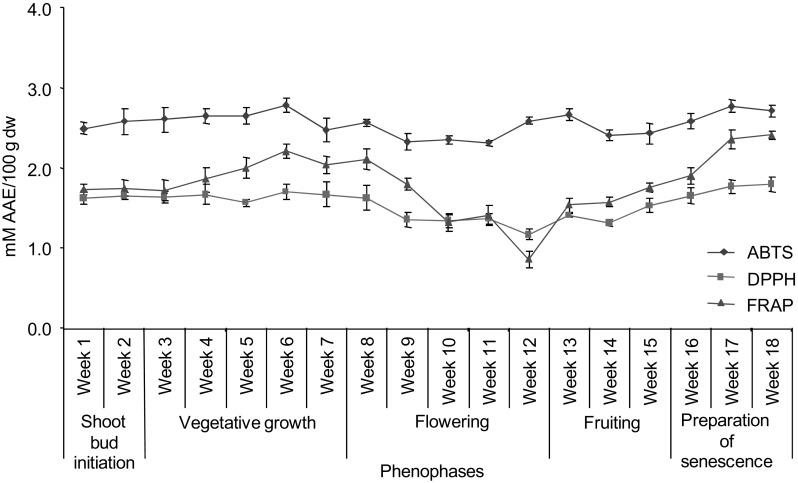
Variation in anti-oxidant activity in R. procera during different harvesting times and phenophases
Relationship between phenolic constituents and anti-oxidant activity
In order to find out possible relationship between phenolic constituents and anti-oxidant activity, correlation and linear regression analysis were preformed (Table 2). Results revealed that total phenolic content were positively correlated with anti-oxidant activities by all the assays. Total phenolic content showed comparatively closer relationship with DPPH assay and FRAP assay (p < 0.001) as compared to ABTS assay (p < 0.05). Whereas, total flavonoid content and different phenolic compounds did not reveal any significant relationship with anti-oxidant activity. Among the different anti-oxidant assays DPPH and FRAP assay exhibited similar type of response (p < 0.001). Individual phenolic compounds did not show any relationship with anti-oxidant activity due to high variation in different sampling weeks and indicated that anti-oxidant activity is a response of synergistic effect of many such compounds.
Table 2.
Correlation matrix in different parameters of phytochemicals and anti-oxidant activity in different seasons in R. procera (n = 18)
| TP | TF | Gallic acid | Catechin | p-Coumaric acid | ABTS | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|
| TP | 1 | |||||||
| TF | − 0.167 | 1 | ||||||
| GA | 0.251 | − 0.682** | 1 | |||||
| Cat | 0.094 | 0.154 | 0.069 | 1 | ||||
| pCA | 0.289 | 0.185 | − 0.176 | − 0.128 | 1 | |||
| ABTS | 0.563* | − 0.142 | 0.287 | 0.163 | − 0.113 | 1 | ||
| DPPH | 0.929*** | − 0.088 | − 0.091 | 0.103 | 0.211 | 0.618** | 1 | |
| FRAP | 0.770*** | 0.249 | 0.028 | 0.389 | 0.351 | 0.525* | 0.879*** | 1 |
TP total phenolic content, TF total flavonoid content
Level of Significance: * p < 0.05;** p < 0.01; *** p < 0.001
Discussion
Investigation on chemical constituents of medicinal plants used in folk preparation is important because of its potential biological performance. Recently, investigations in hydrophilic extractions are gaining much attention for biological activity as compared to essential oils (Rawat et al. 2011; Kocabey et al. 2016; Singh et al. 2016). Variation in phenolic content and anti-oxidant activity was observed in rhizome of R. procera during different phases of growth. Phenolic content and anti-oxidant activity showed a similar type of trend along with plant growth. It initially increased with shoot bud initiation phase but suddenly decreased with plant growth till flowering phase and again increased up to maximum level after flowering phase.
Total phenolic content in the rhizomes of R. procera was observed between 3.97 and 6.10 mg GAE/g dw which was higher than reported values in previous literature (2.92 mg GAE/g dw) on the same species (Rawat et al. 2014a). Also, level of total flavonoids was found comparatively lower than our previous study (7.64 mg QE/g dw) from west Himalaya (Rawat et al. 2014a) in same extraction solvent and experimental condition. These assays showed non-specificity with phenolics and related compounds, such as amino acids, tertiary aliphatic amines, certain purines, etc. It is reported that plant phenolics are surprisingly very high sometime in different parts and growth phase (Harborne 1993). Total phenolic content were observed 1.5 times higher in preparation of senescence phase (week 18) as compared to flowering phase (week 12). Flavonoid content was observed 1.2 fold higher in fruiting phase as compared to vegetative growth phase. Slight variation in chemical constituents in different studies may be due to different collection sites (genotype) with altered conditions of extraction. Similar trend of variation in phenolic anti-oxidant among different seasons along with developmental phases has been reported in other plants of family Zingiberaceae such as, Hedychium spicatum and Curcuma amada and suitable harvesting time has been optimised on the basis of their physiological maturity (Policegoudra et al. 2007; Rawat et al. 2014a, b).
Free radical-scavenging assays provide an information on the capability of anti-oxidant or anti-oxidant mixtures (extract), in preventing reactive radical species from reaching lipoproteins, polyunsaturated fatty acids, DNA, amino acids, proteins and sugars in biological and food systems (Katalinic et al. 2010). In different seasons, anti-oxidant activity was found higher in preparation of senescence phase (1.2 fold high with ABTS assay; 1.5 fold high with DPPH assay; and 2.8 fold high with FRAP assay) as compared to flowering phase. However, oxidative burst takes place during preparation of senescence phase in which a large quantity of reactive oxygen species like superoxide, hydrogen peroxide radicals, peroxy radicals, alkoxy radicals, singlet oxygen, etc., are generated. This process is earliest response of plant cells in natural course of senescence (Bhattacharjee 2005).
Besides, nutrient remobilization occurs during the preparation of senescence process in perennial plants, in which translocation of nutrients transpire from senescing plant parts such as stem, leaves and spike to surviving parts like rhizomes, bulbs and roots (Fischer 2007). In rhizomatous species, high content of phenolics may be an essential component for defence against various pathogens that are constantly challenging the underground tuberous roots, as evidence are available for antimicrobial activity of some of phenolics (King et al. 1972; Dorman and Dean 2000; Merkl et al. 2010). Decrease in level of poly-phenolics during growth may be attributed to strengthening the plant cell by polymerization of free phenolics into lignin and lignans (Randhir and Shetty 2005). In R. procera, there was an interesting pattern in development of roots and tuberous rhizome as initially along with the plant growth, new tuberous rhizome emerge. At flowering time it starts developing new tuberous rhizome and degenerating older rhizomes, thus maximum secondary metabolite content stored in newly developed tuberous rhizome on their maturity in preparation of senescence phase (Fig. 3). Thus, high level of phenolics and anti-oxidant in preparation of senescence phase may be selected as a marker for physiological maturity of plant and can be utilized for harnessing maximum potential of the species.
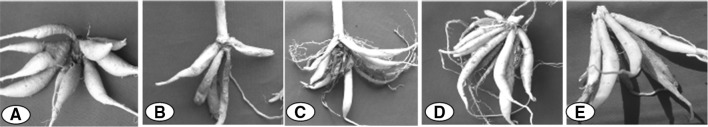
Fig. 3.
Rhizome of Roscoea procera in different phenophases. a Rhizome in shoot bud initiation phase, b new tuberous rhizome emerged during the vegetative growth phase, c in flowering phase, new tubers started developed and older rhizome degenerating, d in fruiting phase, new tuberous rhizome attained its full size and secondary metabolite content started to stored in it, e during preparation of senescence phase, translocation of nutrients occurs from degrading aerial parts to newly developed rhizomes and it attained maximum level of secondary metabolites
Generally, phenolic compounds are synthesised in inner membrane of vacuole and gradually diffused to vacuole of vascular parenchyma and sub-epidermal cells and stored. Commonly, the parenchyma cells of plants actively synthesize, store and modify phenolic substances, and release the stored phenolic compounds to perform their protective effect when plant gets encounter attacks (Franceschi et al. 1998, 2000). It proved for them a self protective effect, especially inhibit herbivores utilization rate of protein, enzyme activity, integrity maintaining of the cell membrane, etc. Phenolic compounds are utilised in the cell wall formation and intercellular spaces of the host and considered that the intercellular phenolic compounds would interact with the cell membrane of the fungi and inhibit the growth of fungal filament and degrade them (Lindroth and Batzli 1984; Li et al. 2012).
Thus, rhizomes of R. procera may be considered a source of powerful anti-oxidants which can be used as substitute for synthetic additives in food production process, in order to prevent/delay oxidative deterioration of food products. Determined differences in phenolic composition and biological activity of rhizomes extracts from different phenophases showed greatest phenolic potential and best anti-oxidant properties in extracts of October, suggesting it the best harvesting time for further use as functional food ingredient.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Director of GBPNIHESD, for the facilities and encouragement. Colleagues of Biodiversity Conservation & Management and Environmental Physiology & Biotechnological application groups are thankful for the cooperation and help during the study. Financial support from Young Scientist Scheme of Science and Engineering Research Board, Department of Science and Technology (DST No. SB/YS/LS-162/262), New Delhi is acknowledged.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2967-z) contains supplementary material, which is available to authorized users.
Contributor Information
Sandeep Rawat, Phone: 05962-241041, Email: sandeep_rawat15@rediffmail.com.
Arun K. Jugran, Phone: 01346-252603, Email: arunjugran@gmail.com
References
- Badhani A, Rawat S, Bhatt ID, Rawal RS. Variation in chemical constituents and antioxidant activity in Yellow Himalayan (Rubus ellipticus Smith) and hill raspberry (Rubus niveus Thunb.) J Food Biochem. 2015;39:663–672. doi: 10.1111/jfbc.12172. [DOI] [Google Scholar]
- Bhatt ID, Dauthal P, Rawat S, Gaira K, Jugran A, Rawal RS, Dhar U. Characterization of essential oil composition, phenolic content and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci Hortic. 2012;136:61–68. doi: 10.1016/j.scienta.2011.12.032. [DOI] [Google Scholar]
- Bhattacharjee S. Reactive oxygen species and oxidative burst: role in stress, senescence and signal transduction in plants. Curr Sci. 2005;89:1113–1121. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutri Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Bruni R, Sacchetti G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wart (Hypercum perforatum L. Hyperiaceae/Guttiferare) Molecules. 2009;14:680–725. doi: 10.3390/molecules14020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJD, Dean SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Fischer AM. Nutrient remobilization during leaf senescence. In: Gum S, editor. Senescence processes in plants. Oxford: Blackwell Publishing Ltd; 2007. pp. 87–107. [Google Scholar]
- Franceschi VR, Krekling T, Berryman AA, Christiansen E. Specialized phloem parenchyma cells in Norway spruce (Pinaceae) bark are an important site of defence reactions. Am J Bot. 1998;85:601–615. doi: 10.2307/2446529. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Krekling T, Christiansen E. Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae) Amer J Bot. 2000;87:314–326. doi: 10.2307/2656627. [DOI] [PubMed] [Google Scholar]
- Grace S. Phenolics as antioxidant. In: Msernof N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell; 2005. pp. 141–168. [Google Scholar]
- Harborne JB. New naturally occurring plant polyphenols. In: Scalbert A, editor. Polyphenolic phenomena. Paris: INRA; 1993. pp. 19–22. [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- Jugran A, Rawat S, Dauthal P, Mandal S, Bhatt ID, Rawal RS. Association of ISSR markers with biochemical traits of Valeriana jatamansi Jones. Ind Crop Prod. 2013;44:671–676. doi: 10.1016/j.indcrop.2012.09.004. [DOI] [Google Scholar]
- Katalinic V, Smole-Mozina S, Skroza D, Generalic I, Abramovic H, Miloš M, et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) Food Chem. 2010;119:715–723. doi: 10.1016/j.foodchem.2009.07.019. [DOI] [Google Scholar]
- King AD, Bayne HG, Jurd L, Case C. Antimicrobial properties of natural phenols and related compounds: obtusastyrene and dihydro-obtusastyrene. Antimicrob Agents Chemother. 1972;1:263–267. doi: 10.1128/AAC.1.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabey N, Yilmaztekin M, Hayaloglu AA. Effect of maceration duration on physicochemical characteristics, organic acid, phenolic compounds and antioxidant activity of red wine from Vitis vinifera L., Karaoglan. J Food Sci Technol. 2016;53:3557–3565. doi: 10.1007/s13197-016-2335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P, Joshi GC, Tiwari LM. Diversity and status of ethano-medicinal plants of Almora district in Uttarakhand, India. Int J Biodiv Conserv. 2011;3:298–326. [Google Scholar]
- Li Z, Tang T, Ning X, Bai M, Wu H. The synthesis and storage sites of phenolic compounds in the roots and rhizomes of Echinacea purpuria. Am J Plant Sci. 2012;3:551–558. doi: 10.4236/ajps.2012.34066. [DOI] [Google Scholar]
- Lindroth RL, Batzli GO. Plant phenolics as chemical defences: effects of natural phenolics on survival and growth of Prairie Voles (Microtus ochrogaster) J Chem Ecol. 1984;10:229–244. doi: 10.1007/BF00987851. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. doi: 10.1016/S0308-8146(01)00198-4. [DOI] [Google Scholar]
- Merkl M, Hrádková H, Filip V, Šmidrkal JS. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci. 2010;28:275–279. [Google Scholar]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, et al. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Pamplona CR, De Souza MM, Machado MS, FelhoVC Navarro D, Yunes RA, Monache FD, Niero R. Seasonal variation and analgesic properties of different parts from Curcuma zedoaria Roscoe (Zingiberaceae) grown in Brazil. Zietschirift fur Naturforschung Tubingen. 2006;61:6–10. doi: 10.1515/znc-2006-1-202. [DOI] [PubMed] [Google Scholar]
- Policegoudra RS, Kumar MHS, Aradhya MS. Accumulation of bioactive compounds during growth and development of mango ginger (Curcuma amada Roxb.) rhizomes. J Agric Food Chem. 2007;55:8105–8111. doi: 10.1021/jf0715469. [DOI] [PubMed] [Google Scholar]
- Randhir R, Shetty K. Development stimulation of total phenolic and related antioxidant in light and dark germinated corn by natural elicitors. Process Biochem. 2005;40:1721–1732. doi: 10.1016/j.procbio.2004.06.064. [DOI] [Google Scholar]
- Rawat S, Bhatt ID, Rawal RS. Variation in total phenolic compounds and antioxidant potential of Hedychium spicatum Buch. Ham. ex D. Don in west Himalaya, India. J Food Comp Anal. 2011;24:574–579. doi: 10.1016/j.jfca.2010.12.005. [DOI] [Google Scholar]
- Rawat S, Andola H, Bhatt ID, Giri L, Dhyani P, Jugran A, Rawal RS. Assessment of nutritional and antioxidant potential of selected vitality strengthening Himalayan medicinal Plants. Int J Food Prop. 2014;17:703–712. doi: 10.1080/10942912.2012.654563. [DOI] [Google Scholar]
- Rawat S, Bhatt ID, Rawal RS, Nandi SK. Effect of developmental stages on total phenolic composition and anti-oxidant activities in Hedychium spicatum Buch. Ham. ex D. Don (Van haldi) J Hortic Sci Biotechnol. 2014;89:557–563. doi: 10.1080/14620316.2014.11513120. [DOI] [Google Scholar]
- Rawat S, Jugran A, Bhatt ID, Rawal RS, Nandi SK. Genetic diversity analysis in natural populations of Roscoea procera Wall. From West Himalaya, India. Braz J Bot. 2016;29:621–630. doi: 10.1007/s40415-016-0260-4. [DOI] [Google Scholar]
- Sahu MS, Mali PY, Waikar SB, Rangri VD. Evaluation of immunomodulatory potential of ethanolic extract of Roscoea procera rhizomes in mice. J Pharm Bioallied Sci. 2010;2:346–349. doi: 10.4103/0975-7406.72138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. Nature’s medicinal plants of Uttaranchal. Nainital: Gyanodaya Prakashan; 2006. [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53:4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenol and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tegelberg R, Aphalo PJ, Julkunen-Tiitto R. Effects of long-term, elevated ultraviolet-B radiation on phytochemicals in the bark of silver birch (Betula pendula) Tree Physiol. 2002;22:1257–1263. doi: 10.1093/treephys/22.17.1257. [DOI] [PubMed] [Google Scholar]
- Tushar R, Basak S, Sarma GC, Lngan N. Ethnomedical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol. 2010;132:286–296. doi: 10.1016/j.jep.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr. 2005;135:2291–2294. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.