Abstract
The purpose of investigation was to assess the phytochemical and nutraceutical of walnut in leaf extracts through diverse quantitative and qualitative phytochemical tests followed by array of assays. The screening of 50 elite walnut genotypes which exhibited wide range of discrepancy in terms of phytochemicals as well as their anti-oxidant potential was done. Walnut genotypes displayed maximum divergence in quercetin content (2.86–5.78 mg/100 g) as represented by cluster analysis. The phenolic rich genotypes exhibiting total phenols (37.61–46.47 mg/g GAE) having higher DPPH potential (IP of 32.82–73.50) where as genotypes that accumulate flavonoids/flavanols (5.52–28.48 mg/g QE and 4.11–21.76 mg/g QE showed immense FRAP activity (418.92–1067.94 µM Fe2+/g FW). There was positive correlation between the phenolics content and anti-oxidant potential. The results showed oil content of 50.1–85.08% and kernel percentage 25.21–81.92% of all walnut genotypes. To evaluate the anti-proliferative potential of walnut genotypes, Trypan blue exclusion test, MTT assay and Griess assay was used. Each assay was repeated with different positive controls against a panel of human cancer cell lines viz THP-1, U2OS, IMR-32 and HBL-100 and then compared with the walnut extracts for their efficiency in anti-proliferative activity. The SPS 1 walnut extract at concentration of 500 µg/ml exhibited 10% cell viability and with 1000 µg/ml walnut extract there was consequent decline towards (6.25%) viability. The results indicated that walnut leaf constitutes an excellent source of effective natural antioxidants and chemo-preventive agents that can act as anti cancer agents.
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2970-4) contains supplementary material, which is available to authorized users.
Keywords: Walnut, DPPH, FRAP, Phenolics, Flavanoids, Flavonols
Introduction
Juglans regia L. is conventionally cultivated as fruit having high nutritional value and for procurement of commercial wood. Kernel of walnut contains high protein and oil content which categorizes it as vital for human nutrition. Consequently, the walnut is categorized as a premeditated species for human diet and is integrated in the FAO inventory of precedence plants (Ramos 1985). Walnuts are nutritionally known for predominant concentrations of fats, proteins, vitamins and minerals. Besides, walnut possesses high content of phytochemicals like flavonoids, sterols, phenolics acids and associated polyphenols (Cerda et al. 2005). Walnut seeds are popularized as nutraceutical due to depressing effect on total and LDL-cholesterol and escalating effect on HDL-cholesterol which in turn declines the possibility of coronary disease (Davis et al. 2007). Walnut has been found to exhibit anti-cancer activities probably due to higher amounts of antioxidants in walnut oil (Miraliakbari and Shahidi 2008). Indeed, many components of the walnut tree display antioxidant prospective, counting with the shoot, kernel and bark. All the parts of walnut tree exhibit antioxidant and antimicrobial prospective, in addition to its anti-proliferative, anti-nociceptive, anti-asthmatic, hepato-protective, anti-diabetic, anti-fertility, anti-inflammatory, lipolytic and numerous other characteristics it positively influence human health (Vinson and Cai 2012). Walnuts have elevated levels of omega-6 and omega-3 PUFA, which are indispensable for dietary fatty acids (Kaur et al. 2014). Clinical research recommends that omega-3 PUFA have considerable function in anticipation of coronary heart disease (Hallahan et al. 2016). Walnuts are renowned as compared to other nuts due to higher concentration of polyunsaturated fat content prominently α-linolenic acid ALA levels together with antioxidants like tocopherol (Amarowicz et al. 2017).
Walnut fruits are rich in phenolic compounds (Slatnar et al. 2015). Phenols are significant phyto-constituents owing to their scavenging competence on free radicals owing to their hydroxyl groups. Consequently, plant phenolics may possibly add persistently to their antioxidant action (Kubola and Siriamornpun 2008). The data describe positive correlation between concentrations of phenolics in walnut leaves with respective antioxidant potential. Walnut leaves are extensively used as conventional medicines as antimicrobial, antihelmintic, astringent, keratolytic, antidiarrhoeal, hypoglycaemic, depurative, carminative, haemorrhoidal symptomatology, sinusitis and stomach pain (Wenzel et al. 2017). Moreover, the researches in pharmacology and therapeutics have shown that J. regia leaves have hypoglycaemic, antioxidative, antimicrobial, and antihypertensive effects.
Juglans regia L. leaves are good sources of flavonoids (Abuajah et al. 2015). In addition to their metabolic functions, flavonoids are premeditated to be significant compounds in the human nutrition, even though they are usually recognized as non-nutrients. Flavonoids have effectual antioxidant potential, which have been connected to variable immune role and increasing anticancer action. In addition, numerous pharmacological consequences have been attributed to flavonoids, for instance central vascular effects and anti-inflammatory, anti-hepatotoxic, anti-tumor, antimicrobial, antiviral and enzyme-inhibiting functions (Uysal et al. 2016).
Quercetin, the major component of the flavonol, subclass of flavonoids, is a universal nutritional constituent. It has been recurrently utilized as a representative component exhibiting the defensive potential of flavonoids. Quercetin has extensive assortment of natural potential, which comprise compelling antioxidant, anti-diabetic, anti tumour, antiviral efficacy (Hossen et al. 2015). Quercetin also exhibit anti-proliferative potential in vitro against ovarian, breast and stomach cancer cell lines (Filipa Brito et al. 2015). Keeping in view all these nutritional facts, our research study intends to evaluate the bioefficacy of walnut as fruit as well as its leaves as potential phytochemical.
Materials and method
Sample collection, preparation and experimental reagents
Fifty walnut genotypes were selected from experimental field genebank of Central Institute of Temperate Horticulture, Rangreth, Srinagar (Jammu and Kashmir), India representing local selections and exotic varieties (Table S1, Fig.SI). These genotypes of J. regia L., were evaluated and multiplied under different eco-geographical positions transversely the state of Jammu and Kashmir, India.
Chemicals and reagents
Methanol, Ethanol, Gallic acid, Folin–Ciocalteu, Sodium Carbonate, Aluminium chloride, potassium acetate, TPTZ (2,4,6-tripyridyl-s-triazine), Ferric chloride, Hydrogen chloride, Ferrous sulphate, Ascorbic acid, DPPH (1,1-diphenyl-2-picryl-hydrazyl). Quercetin was obtained from Sigma, Germany); 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) was obtained from Sigma Aldrich Co., St Louis, USA. All chemicals and reagents utilized in the experiments are of analytical grade.
Preparation of extracts
Finely powdered and air-dried leaf material was taken for experiments. Fine powder of 3 g was ground with a pestle and mortar in 50 ml of methanol stirred for 1 h on a magnetic stirrer and kept overnight. Centrifugation for 15 min at 4000 rpm was used to collect the supernatant and filtered through a Whatman filter paper. The solvent was vaporized by using a rotary evaporator to obtain a semisolid slurry sort of extract. Each extract was freshly prepared to avoid extra sample dilapidation.
Determination of total polyphenolic content (TPC)
Total phenol contents of different leaf extracts of walnut were calculated by the modified Folin–Ciocalteu method (Omoruyi et al. 2012). 1 ml of each extract (1 mg/ml), 5 ml of Folin–Ciocalteu reagent (previously diluted with distilled water 1:10 v/v) and 4 ml (75% w/v) of sodium carbonate (Na2CO3) were mixed together. The tubes were then vortexed and kept for 30 min at 40 °C for colour development. Absorbance was then measured at 765 nm using spectrophotometer. Gallic acid in the range of 0–400 mg/l was used for calibration of standard curve. The results were expressed as mg of gallic acid equivalents/g of dried leaf sample.
Determination of total flavonoid and flavonols content
Total flavonoids in the walnut leaf extract was determined using the method of Chang et al. (2002) which is based on the formation of flavonoid-aluminium complex. 1.5 ml of methanol (60%), 1 ml of 2% aluminium chloride, and 6 ml of 5% potassium acetate was mixed with 1 ml of the extract (1 mg/ml) or quercetin (standard flavonoid compound), and the mixtures were allowed to stand at room temperature for 40 min for yellow colour development which indicated the presence of flavonoid. The absorbance was then measured at 415 nm using spectrophotometer. Quercetin at concentrations of 0, 25, 50, 100 and 200 ppm in methanol was used for calibration of standard curve. Same method was employed for flavonol determination but the incubation period was 150 min instead of 40 min and the absorbance was measured at 440 nm. Total flavonols was also expressed in terms of quercetin equivalent (mg/g), which is the common reference compound.
Antioxidant activity
Ferric reducing antioxidant potential (FRAP) assay
The FRAP assay was done using the Benzie and Strains method (1996) with minor modification. The FRAP reagent was prepared by mixing sodium acetate buffer (300 mmol/l, pH 3.6), 10 mmol/l TPTZ solution in 40 mmol/l HCl and 20 mmol/l FeCl3 solution in ratio of 10:1:1 (v/v), respectively. The FRAP reagent was freshly prepared and was warmed to 37 °C in a water bath before use. 200 µl of extract was mixed with 1.8 ml of the FRAP reagent. The absorbance was then measured at 593 nm after 40 min. FeSO4 solution (0, 40, 80, 160, 320, 640 µmol/l) was used for calibration of standard curve.
DPPH scavenging activity
DPPH free radical scavenging assay was measured using the procedure with slight modifications (Xu et al. 2012). The diluted working solutions of the test extracts were prepared in methanol at a conc. of 15 µg/ml. Ascorbic acid in the conc. range of 1, 2, 4, 6, 8, 10 & 15 µg/ml was used as a standard. 1 ml of the extract was mixed with 1 ml of 0.002% DPPH solution dissolved in methanol. The mixture was shaken vigorously and incubated in the dark at room temperature for 30 min and the absorbance was measured at 517 nm using spectrophotometer. The decrease in absorbance of DPPH on addition of test samples in relation to the control was used to calculate the antioxidant activity, as percentage inhibition (%IP) of DPPH radical. Percentage Inhibition (%IP) = [(At=0 − At=15)]/(A t=0) * 100 where At=15: absorbance of the test sample after 15 min; A t=0: absorbance of the control after 15 min. Furthermore, the scavenging activity percentage (AA%) was determined (Mensor et al. 2001). AA% = 100 − [(Abssample − Abscontrol)/Absblank * 100] where mixture of methanol and DPPH in the ratio of 1:1 served as blank and mixture of standard (Ascorbic acid) and DPPH in the ratio of 1:1 was used as control. Here, the concentration of test sample as well as standard used was 15 µg/ml.
Oil content
The oil content of nuts was determined using the extraction method with Soxhlet apparatus. The oil contents of 5 g of walnut kernel were extracted by petroleum ether (b.p. 40–60 °C) in a Soxhlet apparatus, the remaining solvent was removed by vacuum distillation. Weight difference of tubes before and after the experiment was considered as oil content.
Morphological analysis
5–10 nuts from each tree were randomly collected during the harvest season and evaluated for nut characteristics such as nut weight, nut length, nut diameter, nut shape, kernel weight, kernel percentage and shell strength according to the walnut international descriptor (Eriksson 1998).
Extraction and isolation of quercetin
Sample and standard preparation for HPLC
Extracted leaf samples were dissolved in HPLC grade methanol at final concentration of 100 ppm. The standard was prepared as 100–500 ppm quercetins in HPLC grade methanol as per calibration. Samples were filtered through 0.2 µm syringe filters and then 10 µl filtered sample was injected into an HPLC coupled to a PDA detector.
HPLC analysis
The analysis was carried out in a Shimadzu HPLC (Kyoto, Japan) equipped with quaternary pumps, degasser coupled to a photo-diode-array detector and injection valve with a 20 µl loop. Separation was carried out with an injection volume of 10 μl, a flow rate of 1.1 ml/min with 10 min of run time. The analysis was carried out thrice for each sample. Quercetin was detected at 262 nm and the retention time was 1.915 min. Quercetin standard were obtained from Sigma Aldrich. Chromatographic separations were performed on C18 (250 mm × 4.6 mm), 5 µm column using a solvent system consisting of 60% methanol, 20% acetonitrile and 20% water in an isocratic mode. The mobile phase was filtered through a 0.45 μm membrane filter (Millipore, Bedford, MA, USA) and subjected to ultrasonication for 40 min. Class WP software (version 6.1) from Shimadzu was used for instrument control, data acquisition and data processing. Quantitative determinations were made by taking into account the respective peak areas of standards at particular retention time versus concentration and expressed in mg/g of walnut leaves.
Preparation of extracts for cell culture
The six samples showing the maximum phenolics content were further used for cell culture experiments. Extracts were weighed and stocks were prepared at a concentration of 100 mg/ml in dimethyl sulfoxide (DMSO).
Cell culture
The human monocyte THP-1 cells were cultured as suspension culture in RPMI-1640 medium. Other cell lines (IMR-32, HBL-100 and U2OS) were grown as monolayer in DMEM. Both the media were supplemented with fetal bovine serum (10%), penicillin–streptomycin (1%) in a humidified atmosphere of 5% CO2 at 37 °C. The medium was changed every 2 days or until the cells attained 80–90% confluency. The cells were counted by haemocytometer and seeded at a concentration of 1 × 105 cells/ml.
Trypan blue cell viability assay
The cell viability of THP-1 cells was assayed by trypan blue exclusion test. This assay enables us to accurately determine the cell viability. Cell viability is calculated as the number of viable cells divided by the total number of cells within the grids on the hemacytometer. The THP-1 cells (1 × 105 cells/ml) were plated in a 24-well plate and treated with 0, 0.5 and 1 mg/ml of the methanolic walnut extract for 24 h. Then 0.1 ml of trypan blue was added to 1 ml of cells in the ratio of 1:10. This was followed by loading of cell-dye mixture on a hemacytometer. The cells were counted and the numbers of viable cells were calculated by the given formula. The experiment was repeated in triplicate.
MTT assay
Anti proliferative activity of walnut extracts on human cancer cells (neuroblastoma cells IMR-32; human mammary epithelial cells HBL-100; human osteosarcoma U2OS) was determined by MTT assay as described previously by van Meerloo et al. (2011) with slight modification. MTT is a yellow water-soluble dye that is reduced to an insoluble coloured (dark purple) product, MTT-formazan in viable cells by the action of mitochondrial enzyme succinate dehydrogenase. This is a colorimetric assay that measures the reduction of yellow 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to MTT-formazan (3-[4,5-dimethylthiazol-2-yl]-3,5-diphenylformazan) by mitochondrial succinate dehydrogenase. Since formazan is an insoluble dye, so an organic solvent like Isopropanol is added to the cells in order to solubilise it and the released solubilized formazan is measured spectro-photometrically. Since reduction of MTT can only occur in metabolically active cells the level of activity is a measure of the viability of the cells.
In this assay, IMR-32, HBL-100 and U2OS cancer cells were seeded in 12-well plates (1 × 105 cells/ml) and treated with 0, 1 and 2 mg/ml of the methanolic extract. Cells treated with 0.5% DMSO served as the solvent control. Ethyl Pyruvate (0.6 µl) was used as a positive control and untreated cells were treated as negative control. The cells were incubated for 24 h after drug treatment. Then 200 µl (5 mg/ml) of MTT solution was added to each well and allowed to incubate at 37 °C for 2–3 h in a CO2 incubator. The media was aspirated out and 200 µl of acidified isopropanol was added. The plate was placed in dark on shaking rotor. Finally 50 μl of solution was harvested from 12-well plate and transferred to 96 well plate and absorbance was measured at 570 nm using 495 nm as reference in plate reader spectrophotometer. The experiment was repeated in triplicate. Cell viability was measured as the percentage of absorbance compared to control.
Griess cytotoxicity assay
Additionally, in order to understand the anti-proliferative activity of walnut extracts, Nitric Oxide (NO) production of IMR-32, HBL-100 and U2OS cancer cell lines was determined as described previously by van Meerloo et al. (2011). This assay is based on two-step diazotization reaction in which nitrite is first treated with sulfanilamide (diazotizing reagent) in acidic media to form a transient diazonium salt. Then this intermediate is treated with N-naphthyl-ethylenediamine (coupling reagent) to form a stable azo compound (purple in colour). In this assay, the culture supernatant (100 μl) from various treated groups of previous MTT experimental set up and 100 μl of Griess reagent were mixed and incubated for 30 min in dark for colour development. Then the absorbance of the reaction mixture was measured at 540 nm using an ELISA plate reader. The experiment was repeated in triplicate.
Statistical analysis
In this study, the data was subjected to various statistical tests like cluster analysis and correlation was carried out to conclude the data so as to ascertain the superlative genotypes exhibiting with high antioxidant potential estimated via DPPH and FRAP assay. All the experiments were carried out thrice. The results were represented as mean values and standard error of the mean. The existence of significant differences among the results for total phenols, flavonoids, flavonols, quercetin, oil content and the antioxidant properties of the extracts was analysed. The results obtained were subjected to one-way analysis of variance (ANOVA) and Duncan’s test were used. All statistical tests were done using SAS Enterprise Guide 4.2, SPSS 13 (SPSS Inc., USA) and OP-STAT software at a 5% significance level. Correlation analysis was carried out using Pearson’s test.
Results
Morphological characterization of walnut
Morphological analysis was carried out on the basis of some main qualitative and quantitative traits. The nut weight ranged from 4.82 g (BB-2) to 24.27 g (NDPB-1) and kernel weight ranged from 2.44 g (CITH W24) to 10.76 g (NDPB-1). Nut length ranged from 27.86 cm (SKUA 0020) to 56.83 cm (CWS 7) and nut diameter ranged from 22.10 cm (CWS 9) to 39.13 cm (NDPB-1). In terms of the morphological characteristics, kernel and nut weight are the significant characteristics for evaluation of kernel fraction which ranged from 25.21% (CithW24) to 81.92% (BB-2).
Nut shape varied greatly among genotypes. Majority of the nuts were round or elliptical in shape where as few were found to be oblate in shape. Shell strength also varied greatly among genotypes. Most nuts were found to possess weak shell strength whereas few nuts were found of intermediate to strong shell strength. Detailed description of morphological characters of selected 50 walnut genotypes is given in Table 1.
Table 1.
Nut and kernel characteristics in 50 walnut genotypes
| S. No. | Genotype name | Nut diameter (cm) | Nut length (cm) | Nut weight (g) | Kernel weight (g) | Kernel (%) |
|---|---|---|---|---|---|---|
| 1 | SHS-11 | ± 0.48 | ± 0.62 | ± 0.82 | ± 0.00 | 72.43q ± 4.94 |
| 2 | SHUA 004 | ± 0.45 | ± 0.90 | ± 0.45 | ± 0.28 | 45.64bcdefghijk ± 0.61 |
| 3 | GLS-6 | ± 0.13 | ± 0.15 | ± 0.34 | ± 0.24 | 57.37lmno ± 2.26 |
| 4 | S-289(09) | ± 0.47 | ± 1.38 | ± 0.25 | ± 0.45 | 53.91ijklmn ± 2.76 |
| 5 | CWS-7 | ± 0.69 | ± 16.48 | ± 0.32 | ± 0.13 | 57.50mnop ± 2.22 |
| 6 | BB-3 | ± 0.95 | ± 0.89 | ± 0.37 | ± 0.2 | 66.70pq ± 3.35 |
| 7 | WGB-13 | ± 0.09 | ± 0.87 | ± 0.51 | ± 0.13 | 50.00efghijklmn ± 3.09 |
| 8 | AB-1 | ± 0.55 | ± 1.06 | ± 0.39 | ± 0.41 | 38.98bcde ± 2.19 |
| 9 | SPS-1 | ± 0.55 | ± 1.05 | ± 0.31 | ± 0.28 | 49.33defghijklmn ± 1.32 |
| 10 | SULAIMAN | ± 0.66 | ± 1.46 | ± 0.65 | ± 0.54 | 44.38bcdefghij ± 3.19 |
| 11 | BB-1 | ± 0.36 | ± 5.45 | ± 0.17 | ± 0.34 | 44.98bcdefghijk ± 2.34 |
| 12 | GGS-1 | ± 0.38 | ± 1.67 | ± 0.42 | ± 0.1 | 46.14cdefghijklm ± 1.53 |
| 13 | CITH W24 | ± 0.67 | ± 0.27 | ± 0.45 | ± 0.22 | 25.21a ± 1.36 |
| 14 | NB-3 | ± 0.37 | ± 0.47 | ± 0.46 | ± 0.19 | 53.36hijklmn ± 2.58 |
| 15 | AMC-5 | ± 0.60 | ± 0.37 | ± 0.61 | ± 0.1 | 43.77bcdefghij ± 3.22 |
| 16 | KB-2 | ± 0.78 | ± 1.36 | ± 0.13 | ± 0.4 | 46.11cdefghijklm ± 3.16 |
| 17 | BB-2 | ± 0.50 | ± 0.37 | ± 0.29 | ± 0.09 | 81.93r ± 6.35 |
| 18 | VL-2 | ± 0.68 | ± 0.75 | ± 0.62 | ± 0.48 | 48.66cdefghijklmn ± 1.75 |
| 19 | GB-2 | ± 0.80 | ± 0.43 | ± 0.31 | ± 0.12 | 41.49bcdefg ± 1.29 |
| 20 | CITH W4 | ± 0.41 | ± 0.20 | ± 0.34 | ± 0.34 | 48.78cdefghijklmn ± 1.33 |
| 21 | CITH W15 | ± 0.38 | ± 1.09 | ± 0.64 | ± 0.33 | 42.02bcdefgh ± 0.86 |
| 22 | CITH W5 | ± 0.63 | ± 0.37 | ± 0.77 | ± 0.4 | 51.23fghijklmn ± 1.50 |
| 23 | SKUA0023 | ± 0.63 | ± 0.56 | ± 0.08 | ± 0.3 | 45.02bcdefghijk ± 1.48 |
| 24 | NDPB-1 | ± 0.59 | ± 0.68 | ± 0.51 | ± 0.54 | 44.36bcdefghij ± 2.14 |
| 25 | CITH W13 | ± 0.35 | ± 3.20 | ± 0.2 | ± 0.27 | 45.12bcdefghijk ± 1.05 |
| 26 | BRTS-4 | ± 0.91 | ± 0.93 | ± 0.74 | ± 0.37 | 42.88bcdefghi ± 0.63 |
| 27 | PTS-21 | ± 0.34 | ± 0.69 | ± 0.37 | ± 0.53 | 56.18klmno ± 4.20 |
| 28 | CITH W3 | ± 1.14 | ± 0.47 | ± 0.23 | ± 0.14 | 42.66bcdefghi ± 0.66 |
| 29 | S-285(09) | ± 1.48 | 1.49 | ± 0.32 | ± 0.36 | 38.14bcd ± 3.15 |
| 30 | GKS-1 | ± 0.40 | ± 0.62 | ± 0.3 | ± 0.07 | 43.33bcdefghij ± 0.57 |
| 31 | NUGGET | ± 1.37 | ± 1.11 | ± 0.37 | ± 0.45 | 41.00bcdef ± 3.92 |
| 32 | CITH W11 | ± 0.74 | ± 1.37 | ± 0.28 | ± 0.27 | 52.89ghijklmn ± 3.03 |
| 33 | KB-1 | ± 0.67 | ± 0.32 | ± 0.56 | ± 0.37 | 34.22ab ± 1.62 |
| 34 | SKUA 0024 | ± 1.21 | ± 0.7 | ± 0.46 | ± 0.25 | 59.44nop ± 0.98 |
| 35 | CHINOVA | ± 0.75 | ± 0.52 | ± 0.4 | ± 0.33 | 41.58bcdefg ± 3.06 |
| 36 | CPB-4 | ± 0.75 | ± 0.63 | ± 0.3 | ± 0.17 | 54.47jklmn ± 1.45 |
| 37 | HS-4 | ± 0.54 | ± 0.24 | ± 0.23 | ± 0.21 | 45.11bcdefghijk ± 1.38 |
| 38 | CWS-9 | ± 0.53 | ± 0.65 | ± 059 | ± 1.29 | 69.00q ± 14.93 |
| 39 | APS-13 | ± 0.80 | ± 0.67 | ± 0.6 | ± 0.29 | 53.39hijklmn ± 1.46 |
| 40 | SKUA 0020 | ± 0.14 | ± 0.37 | ± 0.55 | ± 0.43 | 47.00cdefghijklmn ± 3.15 |
| 41 | LG-10 | ± 0.47 | ± 1.79 | ± 0.58 | ± 0.9 | 50.33efghijklmn ± 5.95 |
| 42 | KD-1 | ± 0.71 | ± 0.50 | ± 0.59 | ± 0.33 | 37.56bc ± 2.07 |
| 43 | CITH W27 | ± 0.97 | ± 0.32 | ± 0.5 | ± 0.67 | 38.85bcde ± 3.78 |
| 44 | SKUA 003 | ± 1.03 | ± 0.86 | ± 0.46 | ± 0.14 | 49.60defghijklmn ± 3.09 |
| 45 | Wussan-8 | ± 0.32 | ± 0.30 | ± 0.17 | ± 0.12 | 53.28hijklmn ± 1.02 |
| 46 | BRUS-10 | ± 0.93 | ± 0.21 | ± 0.42 | ± 0.23 | 49.66defghijklmn ± 1.14 |
| 47 | AMC-4 | ± 1.84 | ± 0.58 | ± 0.32 | ± 0.28 | 59.50opq ± 1.56 |
| 48 | PBS-3 | ± 1.54 | ± 0.66 | ± 0.60 | ± 0.42 | 45.98cdefghijkl ± 1.63 |
| 49 | CITH W23 | ± 0.48 | ± 0.37 | ± 0.42 | ± 0.4 | 49.53defghijklmn ± 1.70 |
| 50 | NB-4 | ± 0.86 | ± 0.80 | ± 0.75 | ± 0.42 | 48.24cdefghijklmn ± 0.98 |
| Max value | 39.12 | 56.83 | 24.27 | 10.76 | 81.92 | |
| Min value | 22.10 | 27.86 | 4.82 | 2.44 | 25.21 | |
| Avg value | 31.01 | 36.65 | 13.06 | 6.24 | 48.96 |
Oil content
The oil content of the investigated nuts ranged from 50.1 (Cith W2) to 85.08% (GLS-6). Data are present in Table 2. On the basis of the estimated oil content, walnut genotypes were classified into three categories; 12 superlative genotypes exhibiting 70–85% oil, 8 good genotypes exhibiting 60–70% oil and 35 average genotypes exhibiting 50–60% oil.
Table 2.
Total phenol, flavanoid, oil, quercetin content and antioxidative potential of walnut genotypes
| S. No. | Genotype name | % Inhibition (Arc sine Transformed values in °) | Oil content (%) | AA% | µM Fe2+/g FW | Phenols (mg/g GAE) | Total flavonoids (mg/g quercetin equivalent) | Total flavanols (mg/g quercetin equivalent) | Total quercetin content (mg/100 g FW) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SHS-11 | 60.26 ± 5.4 (34.51 ± 3.1) | 57 ± 1.2 | 65.38 | 908.14 ± 8 | 40.3 ± 0.24 | 20.66 ± 0.48 | 15.47 ± 0.27 | 3.6 ± 0.01 |
| 2 | SKUA-4 | 44.27 ± 0.9 (25.36 ± 0.5) | 59.02 ± 2.1 | 49.40 | 686.24 ± 13 | 39.4 ± 0.14 | 9.26 ± 0.52 | 7.07 ± 0.38 | 4.54 ± 0.01 |
| 3 | GLS-6 | 57.69 ± 0.9 (33.04 ± 0.5) | 85.08 ± 1.09 | 62.82 | 940.82 ± 3 | 42.83 ± 0.38 | 10.24 ± 0.03 | 8.40 ± 0.13 | 4.87 ± 0.01 |
| 4 | S-289 | 44.70 ± 0.1 (25.60 ± 0) | 79.82 ± 0.99 | 49.83 | 545.39 ± 16 | 38.65 ± 0.12 | 15.31 ± 1.61 | 11.51 ± 1.33 | 4.62 ± 0.02 |
| 5 | CWS-7 | 32.82 ± 5.7 (18.80 ± 3.3) | 53.5 ± 0.87 | 37.95 | 554.22 ± 10 | 38.89 ± 0.05 | 16.62 ± 0.41 | 12.55 ± 0.30 | 3.8 ± 0.01 |
| 6 | BB-3 | 54.96 ± 0.5 (31.48 ± 0.3) | 61.44 ± 1.23 | 60.09 | 717.29 ± 6 | 43.22 ± 0.32 | 7.75 ± 0.78 | 5.94 ± 0.62 | 4.35 ± 0.01 |
| 7 | WGB-13 | 54.62 ± 4.4 (31.28 ± 2.5) | 55.54 ± 0.09 | 59.74 | 835.92 ± 11 | 46.47 ± 0.89 | 14.69 ± 0.85 | 11.45 ± 0.59 | 4.73 ± 0.01 |
| 8 | AB-1 | 37.52 ± 10.2 (21.49 ± 5.8) | 64.94 ± 0.87 | 42.65 | 755.20 ± 6 | 38 ± 0.21 | 21.41 ± 0.16 | 16.41 ± 0.17 | 3.11 ± 0.01 |
| 9 | SPS-1 | 52.39 ± 0.2 (30.01 ± 0.1) | 58.54 ± 1.27 | 57.52 | 764.35 ± 1 | 44.14 ± 0.54 | 13.46 ± 0.75 | 11.01 ± 0.71 | 4.82 ± 0.01 |
| 10 | SULAIMAN | 48.72 ± 2.7 (27.90 ± 1.6) | 54.86 ± 1.66 | 53.85 | 492.12 ± 7 | 45.02 ± 0.17 | 8.54 ± 0.25 | 6.42 ± 0.15 | 5.67 ± 0.01 |
| 11 | BB-1 | 45.81 ± 7.1 (26.24 ± 4.1) | 54.18 ± 1.54 | 50.94 | 934.28 ± 3 | 43.02 ± 0.90 | 20.44 ± 0.21 | 15.54 ± 0.38 | 4.38 ± 0.01 |
| 12 | GGS-1 | 41.62 ± 4.6 (23.84 ± 2.6) | 64.2 ± 0.88 | 46.75 | 890.16 ± 11 | 39.94 ± 0.01 | 22.49 ± 0.78 | 18.08 ± 0.42 | 3.22 ± 0.01 |
| 13 | CITH W24 | 46.58 ± 2.7 (26.68 ± 1.5) | 51.9 ± 1.11 | 51.71 | 822.52 ± 3 | 40.47 ± 0.07 | 13.68 ± 0.03 | 10.47 ± 0.10 | 3.94 ± 0.01 |
| 14 | NB-2 | 44.27 ± 9.8 (25.36 ± 5.6) | 72.62 ± 1.21 | 49.40 | 735.26 ± 17 | 42.21 ± 0.66 | 26.78 ± 0.44 | 21.44 ± 0.30 | 3.82 ± 0.01 |
| 15 | AMC-5 | 63.50 ± 4.2 (36.37 ± 2.4) | 54.12 ± 0.69 | 68.63 | 1067.94 ± 1 | 44.47 ± 0.42 | 25.19 ± 0.18 | 20.20 ± 0.07 | 3.02 ± 0.01 |
| 16 | KB-2 | 47.09 ± 1.1 (26.97 ± 0.6) | 61.46 ± 0.95 | 52.22 | 699.64 ± 41 | 39.01 ± 0.08 | 15.24 ± 0.45 | 12.25 ± 0.64 | 4.32 ± 0.02 |
| 17 | BB-2 | 58.03 ± 4.8 (33.24 ± 2.8) | 62.24 ± 1.02 | 63.16 | 895.72 ± 12 | 42 ± 0.32 | 16.84 ± 0.44 | 13.66 ± 0.40 | 5.11 ± 0.01 |
| 18 | VL-2 | 46.92 ± 1 (26.87 ± 0.6) | 52.52 ± 1.09 | 52.05 | 565.98 ± 3 | 40.12 ± 0.48 | 13.69 ± 0.02 | 10.42 ± 0.01 | 3.45 ± 0.02 |
| 19 | GB-2 | 46.41 ± 0.4 (26.58 ± 0.2) | 73.8 ± 2.11 | 51.54 | 578.07 ± 9 | 41.3 ± 0.03 | 11.43 ± 0.52 | 8.67 ± 0.45 | 4.41 ± 0.01 |
| 20 | CITH W16 | 60.09 ± 1.1 (34.41 ± 0.6) | 50.1 ± 1.08 | 65.21 | 779.05 ± 5 | 44.9 ± 1.8 | 11.48 ± 0.21 | 8.90 ± 0.16 | 2.86 ± 0.01 |
| 21 | CITH W15 | 63.33 ± 0.1 (36.27 ± 0.1) | 50.74 ± 1.45 | 68.46 | 1078.40 ± 3 | 43.95 ± 0.62 | 16.82 ± 0.53 | 13.05 ± 0.54 | 3.54 ± 0.05 |
| 22 | CITH W5 | 52.91 ± 4.6 (30.30 ± 2.6) | 54.68 ± 1.61 | 58.03 | 523.50 ± 2 | 37.85 ± 0.42 | 13.56 ± 0.29 | 10.73 ± 0.19 | 4.54 ± 0.01 |
| 23 | SKUA-23 | 46.75 ± 1.1 (26.78 ± 0.6) | 51.08 ± 0.91 | 51.88 | 704.54 ± 14 | 38.48 ± 0.20 | 5.52 ± 0.03 | 4.11 ± 0.02 | 4.08 ± 0.02 |
| 24 | NDPB-1 | 73.50 ± 1.8 (42.10 ± 1) | 53.12 ± 0.84 | 78.63 | 979.38 ± 8 | 42.08 ± 0.03 | 19.94 ± 0.21 | 15.41 ± 0.97 | 3.2 ± 0.01 |
| 25 | CITH W13 | 55.56 ± 1.2 (31.82 ± 0.7) | 55.84 ± 0.88 | 60.68 | 764.02 ± 1 | 43.4 ± 0.45 | 8.22 ± 0.30 | 6.13 ± 0.26 | 3.05 ± 0.01 |
| 26 | BRTS-4 | 33.93 ± 2.1 (19.43 ± 1.2) | 52.2 ± 0.97 | 39.06 | 418.92 ± 1 | 40.29 ± 0.07 | 13.15 ± 0.48 | 10.58 ± 0.44 | 3.42 ± 0.02 |
| 27 | PTS-21 | 54.10 ± 0.5 (30.99 ± 0.3) | 59.8 ± 1.11 | 59.23 | 682.65 ± 2 | 42.12 ± 0.17 | 8.00 ± 0.66 | 5.74 ± 0.61 | 4.46 ± 0.01 |
| 28 | CITH W3 | 60.85 ± 2.8 (34.85 ± 1.6) | 77.98 ± 2.24 | 65.98 | 846.05 ± 19 | 44.32 ± 0.12 | 11.02 ± 0.30 | 8.47 ± 0.30 | 4.31 ± 0.11 |
| 29 | S-285 | 51.88 ± 1.2 (29.71 ± 0.7) | 59.02 ± 1.33 | 57.01 | 694.74 ± 7 | 38.58 ± 0.19 | 10.16 ± 1.04 | 7.83 ± 0.78 | 4.66 ± 0.01 |
| 30 | GKS-1 | 59.66 ± 4.5 (34.17 ± 2.6) | 57.1 ± 0.21 | 64.79 | 1022.75 ± 31 | 44.8 ± 0.15 | 23.64 ± 0.83 | 19.26 ± 0.60 | 2.98 ± 0.01 |
| 31 | NUGGET | 63.59 ± 0.1 (36.42 ± 0.1) | 53.12 ± 1.64 | 68.72 | 902.25 ± 8 | 43.7 ± 0.09 | 16.61 ± 0.55 | 12.93 ± 0.43 | 3.85 ± 0.04 |
| 32 | CITH W11 | 44.79 ± 5.5 (25.65 ± 3.1) | 51.74 ± 1.28 | 49.91 | 1057.48 ± 13 | 42.19 ± 0.34 | 21.17 ± 0.19 | 15.84 ± 0.12 | 3.55 ± 0.02 |
| 33 | KB-1 | 39.15 ± 3.1 (22.42 ± 1.8) | 69.92 ± 2.12 | 44.27 | 818.92 ± 109 | 39.63 ± 0.13 | 28.48 ± 0.12 | 21.76 ± 0.25 | 4.71 ± 0.01 |
| 34 | SKUA-24 | 37.18 ± 1.4 (21.29 ± 0.8) | 56.84 ± 1.11 | 42.31 | 538.53 ± 5 | 43.03 ± 0.58 | 14.04 ± 1.53 | 10.52 ± 1.22 | 5.67 ± 0.01 |
| 35 | CHENOVO | 51.45 ± 5.8 (29.47 ± 3.3) | 73.1 ± 0.08 | 56.58 | 891.47 ± 7 | 44.12 ± 0.42 | 15.62 ± 0.49 | 12.11 ± 0.38 | 3.5 ± 0.01 |
| 36 | CPB-4 | 54.53 ± 2.1 (31.23 ± 1.2) | 58.36 ± 0.64 | 59.66 | 831.99 ± 31 | 41.39 ± 0.53 | 18.78 ± 0.66 | 14.49 ± 0.54 | 2.91 ± 0.02 |
| 37 | HS-4 | 41.03 ± 10.6 (23.50 ± 6.1) | 57.88 ± 0.33 | 46.15 | 880.36 ± 37 | 39.39 ± 0.20 | 23.25 ± 0.26 | 17.19 ± 0.23 | 4.8 ± 0.03 |
| 38 | CWS-9 | 43.25 ± 2.3 (24.77 ± 1.3) | 64.6 ± 0.14 | 48.38 | 766.63 ± 4 | 41.97 ± 0.24 | 16.90 ± 0.20 | 13.61 ± 0.61 | 4.87 ± 0.01 |
| 39 | APS-13 | 61.88 ± 6.7 (35.44 ± 3.8) | 57.7 ± 0.54 | 67.01 | 970.56 ± 40 | 43.9 ± 0.65 | 20.91 ± 0.57 | 16.80 ± 0.48 | 3.79 ± 0.02 |
| 40 | SKUA-20 | 43.85 ± 1 (25.11 ± 0.6) | 51.22 ± 0.62 | 48.97 | 786.90 ± 16 | 40.69 ± 0.20 | 15.91 ± 0.08 | 11.70 ± 0.44 | 5.78 ± 0.01 |
| 41 | LG-10 | 44.36 ± 2.2 (25.41 ± 1.3) | 71.36 ± 0.88 | 49.49 | 749.97 ± 15 | 42.13 ± 0.27 | 14.94 ± 0.18 | 11.93 ± 0.23 | 3.82 ± 0.01 |
| 42 | KD-1 | 41.88 ± 7.9 (23.99 ± 4.5) | 58.56 ± 1.34 | 47.01 | 975.78 ± 14 | 40.5 ± 0.24 | 23.36 ± 0.33 | 17.48 ± 0.28 | 4.57 ± 0.01 |
| 43 | CITH W7 | 43.68 ± 4.9 (25.01 ± 2.8) | 51.72 ± 0.76 | 48.80 | 754.87 ± 31 | 41.71 ± 0.24 | 15.76 ± 0.22 | 11.87 ± 0.11 | 3.92 ± 0.15 |
| 44 | SKUA-3 | 42.05 ± 0.4 (24.08 ± 0.3) | 60.58 ± 0.56 | 47.18 | 574.15 ± 13 | 37.61 ± 0.23 | 8.01 ± 0.68 | 5.81 ± 0.52 | 4.3 ± 0.01 |
| 45 | WUSSAN- 8 | 63.50 ± 3.4 (36.37 ± 2) | 54.64 ± 0.75 | 68.63 | 831.67 ± 9 | 42.96 ± 0.35 | 9.00 ± 0.18 | 6.80 ± 0.16 | 4.65 ± 0.01 |
| 46 | BRUS-10 | 61.03 ± 1.9 (34.95 ± 1.1) | 58.78 ± 0.89 | 66.15 | 774.48 ± 17 | 42.52 ± 0.26 | 12.73 ± 0.02 | 9.59 ± 0.05 | 5.23 ± 0.01 |
| 47 | CITH W14 | 70.85 ± 8.6 (40.58 ± 4.9) | 69.96 ± 0.97 | 75.98 | 887.88 ± 47 | 42.48 ± 0.42 | 9.60 ± 0.46 | 7.56 ± 0.46 | 3.62 ± 0.01 |
| 48 | PBS-3 | 38.89 ± 0.7 (22.27 ± 0.4) | 51.16 ± 1.23 | 44.02 | 527.09 ± 1 | 40.32 ± 0.40 | 11.96 ± 0.49 | 8.68 ± 0.30 | 4.47 ± 0.01 |
| 49 | CITH W23 | 44.02 ± 2.4 (25.21 ± 1.4) | 57.48 ± 1.45 | 49.15 | 526.11 ± 2 | 41.37 ± 0.15 | 12.14 ± 0.46 | 9.19 ± 0.32 | 3.07 ± 0.01 |
| 50 | NB-4 | 53.42 ± 0.7 (30.59 ± 0.4) | 57.8 ± 0.76 | 58.55 | 853.56 ± 2 | 43.49 ± 0.13 | 17.40 ± 0.63 | 13.15 ± 0.54 | 4.62 ± 0.02 |
| CD | 12.027 | 46.759 | 1.260 | 1.567 | 1.337 | 0.062 | |||
| SE(d) | 6.053 | 23.532 | 0.634 | 0.789 | 0.673 | 0.031 | |||
| SE(m) | 4.280 | 16.640 | 0.448 | 0.558 | 0.476 | 0.022 | |||
| CV | 14.845 | 3.758 | 1.864 | 6.230 | 6.870 | 0.910 |
Phytochemical determinations
Total phenols, flavonoids, and flavonols
The phenolic content of the walnut (J. regia) leaf extract ranged from 37.61 mg/g gallic acid equivalent (SKUA 3) to 46.47 mg/g GAE (WGB 13). On the basis of the estimated phenolic content, walnut genotypes were classified into three categories; 8 superlative genotypes exhibiting 43–47 mg/g GAE, 23 good genotypes exhibiting 40–43 mg/g GAE and 19 average genotypes exhibiting 37–40 mg/g GAE of walnut leaf extract (Table 2). The complete phenolic composition of varied extracts were determined as milligram gallic acid equivalent (mg GAE/g dry weight of plant extract via the subsequent formulae from the calibration curve: Y = 0.00050x + 0.00262; R2 = 0.99637, where Y is the absorbance and x is the gallic acid equivalent in mg/g.
Total flavonoids and flavonols of the extract ranged from 5.52 (SKUA0023) to 28.48 (KB1) mg/g quercetin equivalent and 4.11 (SKUA23) to 21.76 (KB1) mg/g QE respectively. On the basis of flavonoid content, walnut genotypes were classified into three categories; 9 superlative genotypes displaying 20–30 mg/g quercetin equivalent, 30 good genotypes exhibiting 10–20 mg/g QE and 11 average genotypes exhibiting 5–10 mg/g QE. Following the data on flavonol content, walnut genotypes were classified into three categories; 10 superlative genotypes showing 15–22 mg/g quercetin equivalent, 18 good genotypes exhibiting 10–15 mg/g QE and 22 average genotypes exhibiting 4–10 mg/g QE. Total flavonoid concentration was determined as quercetin equivalent (mg/g) via the subsequent formulae established on calibration curve: Y = 0.00618x − 0.01452; R2 = 0.99669, where Y is the absorbance and x is the Quercetin equivalent in mg/g. Total flavonols was determined as quercetin equivalent (mg/g) functioning as standard compound.
Quantification of quercetin in walnut leaves by RP-HPLC
Quercetin
The total quercetin content of the leaf extract of 50 walnut genotypes ranged from 2.86 mg/100 g (Cith W 16) to 5.78 mg/100 g (SKUA20). The minimum quercetin content of 2.86 and 2.91 mg/100 g was displayed by genotypes Cith W16 and CPB4 respectively. Similarly the maximum quercetin content of 5.11, 5.23, 5.67, 5.67 and 5.78 mg/100 g was displayed by genotypes BB2, Brus10, Sulaiman, SKUA24 and SKUA20 respectively.
Antioxidant potential of walnut
Antioxidant activity (DPPH free radical scavenging activity)
Higher and lower percentage of scavenging potential about 73.50 (NDPB1) and 32.82 (CWS7) respectively was recorded in walnut leaf extract which was appreciably higher than reference compound ascorbic acid. On the basis of the estimated percent inhibition, walnut genotypes were classified into three categories; 8 superlative genotypes exhibiting 60–75 percent inhibition (PI), 22 good genotypes exhibiting 45–60 PI and 20 average genotypes exhibiting 30–45 PI. Furthermore, the scavenging activity percentage (AA%) also followed the same trend as % Inhibition.
Ferric reducing antioxidant potential (FRAP)
FRAP observations ranged from 418.92 µM Fe2+/g FW (BRTS4) to 1067.94 µM Fe2+/g FW (AMC5). On the basis of the estimated FRAP values, walnut genotypes were classified into three categories; 23 superlative genotypes exhibiting 800–1100 µM Fe2+/g FW, 16 good genotypes exhibiting 600–800 µM Fe2+/g FW and 11 average genotypes exhibiting 400–600 µM Fe2+/g FW. The observations were e determined as µmol Fe (2)/g dry weight of plant extract via subsequent formulae determined on calibration curve: Y = 0.0017x − 0.00073; R2 = 0.9986 in which Y stands for absorbance and x is the µM Fe2+/g FW.
Screening of walnut extract for immunostimulation of monocytes
Among the diverse phytochemicals procured from plants, polyphenols are known to be most active candidates against cancer. Quercetin also exhibited anti-proliferative potential in vitro against ovarian, breast and stomach cancer cell lines. Walnut possess anti-cancerous activity may be due to the presence of various chemical constituents such as omega 3 fatty acids, vitamin E (mainly the c-tocopherol form), phytosterols, juglone, ellagic acid, gallic acid and flavonoids (quercetin, carotenoids and melatonin). So in order to evaluate the anti-proliferative potential of walnut genotypes, Trypan blue exclusion test, MTT anti-proliferative assay and Griess assay was performed. Each assay was repeated with different positive controls against a panel of human cancer cell lines viz THP-1, U2OS, IMR-32 and HBL-100 and then compared with the walnut extracts for their efficiency in anti-proliferative activity.
Anti-proliferative activity of walnut extracts on human cancer cell lines
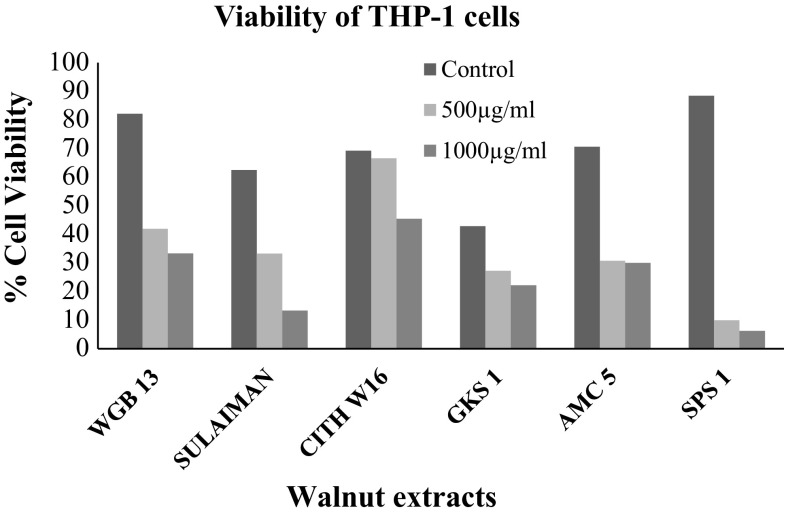
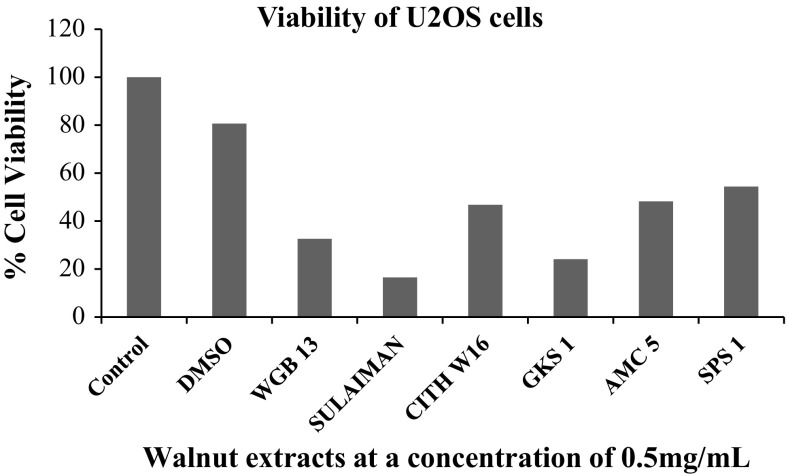
GAE of walnut leaf extract as represented in Table 2. The six genotypes represented by superlative category (WGB 13, SULAIMAN, CITH W16, GKS 1, AMC 5 and SPS 1) were further used for determining anti-proliferative activity of methanolic extracts of walnut leaves against a panel of human cancer cell lines. The cell viability of human monocytic THP-1 cells was assayed by means of trypan blue exclusion test (Fig. 1). It was observed that the extracts resulted in loss of cell viability in a concentration-dependent manner. As the concentration of walnut extracts increased from 0 to 1000 µg/ml, the cell viability decreased progressively and the maximum decrease in cell viability was obtained with extract SPS 1. The SPS 1 walnut extract at concentration of 500 µg/ml exhibited 10% cell viability and with 1000 µg/ml walnut extract there was consequent decline towards (6.25%) viability.
Fig. 1.
Cell viability of THP-1 cells determined by cell counting in Trypan blue exclusion assay
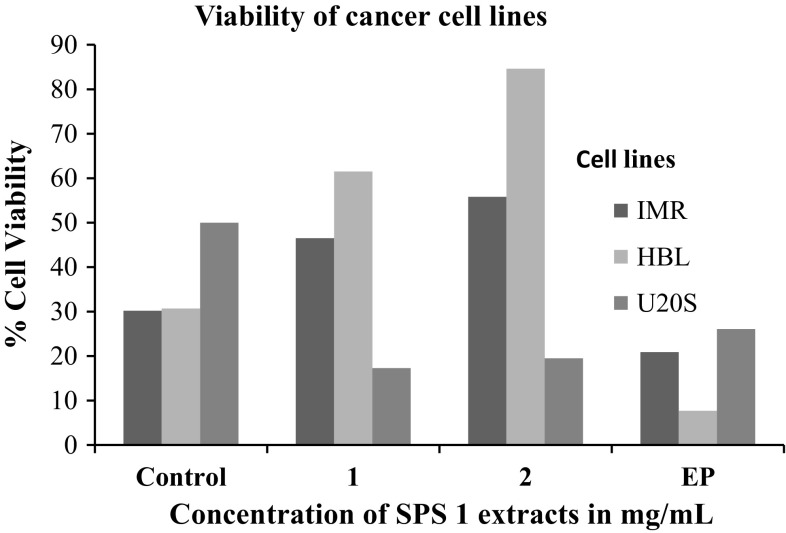
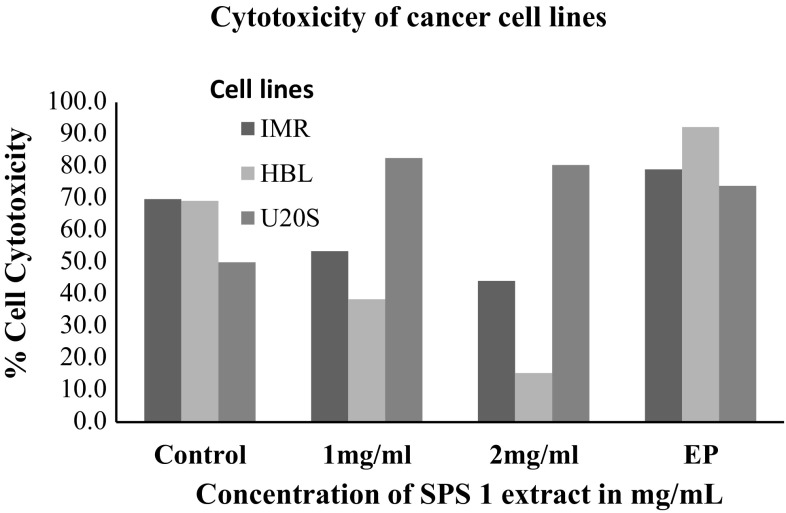
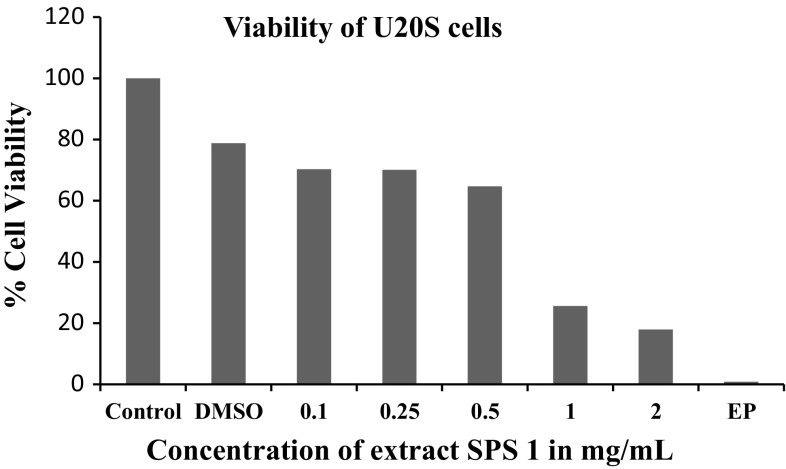
Keeping in view the potential anti-proliferative activity of extract SPS 1, it was further used to treat other cancer cell lines viz. human neuroblastoma cells IMR-32; human mammary epithelial cells HBL-100 and human osteosarcoma U2OS in order to check its anti proliferative action on these cell lines using MTT assay. A considerable difference was found in the sensitivity of the three cell lines to walnut extract SPS-1 as is clearly depicted in Fig. 2. The results indicated that U2OS cells were sensitive to this extract exhibiting 17.3% cell viability as compared to IMR-32 and HBL-100 cells which showed 46.5 and 61.5% cell viability which was insignificant. Interestingly, it was found that walnut extract SPS 1 at a concentration of 1–2 mg/ml was much potent antiproliferative agent than the positive control (Ethyl pyruvate) itself. Additionally, antiproliferative activity of SPS 1 extract was also determined by estimating the production of nitric oxide (NO) in IMR-32, HBL-100 and U2OS cells using Griess assay (Fig. 3). The results obtained in all the three cell lines were in agreement with the percentage of viability obtained by MTT assay. Furthermore, in order to calculate IC50 of SPS1 in U2OS cells, a concentration gradient (0, 0.1, 0.25, 0.5, 1 and 2 mg/ml) of methanolic walnut extract (SPS 1) was set up. IC50 value is defined as the amount of extract that inhibits 50% of cell growth (Fig. 4). From our experiments it was determined that SPS 1 extract showed potent anti-proliferative effect with IC50 at 0.5 mg/ml against U2OS cells. Finally, optimized IC50 value i.e., 0.5 mg/ml of all methanolic extract walnut samples (WGB 13, SULAIMAN, CITH W16, GKS 1, AMC 5 and SPS 1) was used to evaluate their anti-proliferative activity against U2OS cancer cell line (Fig. 5). All the six walnut extracts showed significant anti-proliferative activity on U2OS cells. Among all the extracts, SULAIMAN showed strongest antiproliferative effect with 16.5% cell viability followed by GKS (24.1%), WGB 13 (32.6%), CITH W16 (46.8%), AMC 5 (48.2%) and SPS 1 (54.4%). The results obtained herein strongly indicate that walnut tree constitutes an excellent source of effective natural antioxidants and chemo-preventive agents that can act as anti cancer agents.
Fig. 2.
Anti-proliferative activity of methanolic walnut extract (SPS 1) against cancer cell lines (IMR-32, HBL-100 and U2OS)
Fig. 3.
Cytotoxicity of methanolic walnut extract (SPS 1) against cancer cell lines (IMR-32, HBL-100 and U2OS) determined by nitric oxide levels
Fig. 4.
Antiproliferative activity of methanolic walnut extract (SPS 1) against U2OS cell line
Fig. 5.
Anti-proliferative activity of different walnut extracts against U2OS cancer cells
Discussion
Walnuts possess diverse exclusive and dominant antioxidants that are obtainable in scanty eatables. This comprises plant phenolics, flavonoids, and flavonols with antioxidants and powerful free-radical scavenging and investigation has revealed that walnut polyphenols might assist in averting of drug-induced liver damage. Several research reports potential antioxidant properties of walnut leaves which is rich source of phytochemicals utilized in conventional medicines for prevention of venous paucity, hemorrhoids, hypoglycemia, diarrhoea, and other allied pathogenic infections (Pereira et al. 2008; Oliveira et al. 2008). Infusions of walnut leaves are also used in conventional medicine system. The shell is employed as filtration media to decant crude oil from water and the walnut green husk is the fundamental substance for the conventional walnut fluid. The results obtained by Oliveira et al. (2008) exhibited the prospective of this economical natural resource of phenolic components having antiradical and antimicrobial potential. However, it has been established that there exists significant extent of disparity amongst the cultivars evaluated which was quite relevant with concentration of phenolics in each walnut cultivar investigated (Oliveira et al. 2008) and in many other horticultural crops, including blueberry, apple and plum. Further it was established there is strong association between phenolics composition in walnut leaves and ecophysiology of environment and ontogeny of walnut cultivar. The observations has confirmed that walnut leaves harvested in July and late September exhibit higher antioxidant potential due to higher accumulation of phenolics at this stage. Data procured from total phenols in walnut leaves might be utilized in execution of harvesting time to procure best walnut products.
Walnut extracts of 0.5 mg/ml exhibit 90.2–92.6% DPPH activity (Pereira et al. 2008). Total phenolic contents in walnut green husks varying from 15.15 to 108.11 mg GAE of extract and the flavonoid concentration ranging from 3.59 to 22.91 mg QE per gram of leaf extract (Ghasemi et al. 2011) whereas the total phenolic content of the extracted walnut oils from all selected cultivars ranged from 0.18 GAE/g to 0.31 GAE/g (Gharibzahedi et al. 2014).
Quercetin is chemically the principal compound of flavonoid class and important component of human diet. Quercetin is established reference compound having extensive defense features of flavonoids class. Quercetin exhibit higher in vitro anti-proliferative activity aligned with ovarian, breast and stomach cancer cell lines. Quercetin has validated its potential in clinical research investigations. Our data anticipated that walnut extract has incredible potential to contribute electron to ROS and convert them to relatively higher stable reactive species and conclude free radical reaction activity (Pereira et al. 2008; Qamar and Sultana 2011).
Walnuts (J. regia L.) contain kernels that have high content of oil. It varies widely (52–75%), depending on the variety, cultivation, place of growing and irrigation of walnut trees. The observations regarding oil yield are in concordance with earlier research investigations in walnut kernel. These studies confirm about 60% oil content however certain walnut cultivars exhibit range from 52 to 70% depending on ecophysiology of habitat (Li et al. 2014). Pereira et al. (2008) recorded oil yield in the range of 78.83–82.4% which was more than earlier reports. However our study confirmed more oil yield in selected genotypes than earlier reported. Conversely, due to viable relevance of kernel oil, walnut can be used as prospective for nutrition supplements. Our results are at par with the findings of other researchers. As reported by Kazankaya et al. (2001) in Turkey, nut as well as kernel weight and kernel ratio of superior walnut genotypes were 11–12 g, 6–7 g and more than 45%, respectively. As per Ebrahimi et al. (2011), the nut weight varied from 7.52 to 17.73 g, kernel weight from 4.00 to 9.83 g, and kernel percentage ratio from 38.78 to 67.05% amongst calculated genotypes. The possible mechanism for effective antioxidant potential of walnut may be due to presence of anti-oxidant compounds like quercetin and other phenolics which quench pool of free radicals and inactivate them thereby conferring protection of cell membrane and various compounds against deleterious effects. Further the walnut selections with comparatively high anti-oxidant capacity can possibly offer higher market value owing to consumer preference for nutraceutical rich walnuts (Delaviz et al. 2017).
Our study confirms that investigated walnut genotypes exhibit higher antioxidant potential measured as percent inhibition. The major mechanism employed by natural antioxidants is their potential to scavenge free radicals prior to initiation of free radical chain reactions across cell membranes. DPPH assay owing to its simplicity has been extensively employed for evaluating antioxidant potential of diverse natural compounds. This assay is renowned for its accurate data acquisition on antioxidant activity of evaluated compounds. The rule underlying this assay in the color alteration of DPPH solution from purple to yellow as the radical is satiated by the antioxidant. The color transition was determined quantitatively via spectrophotometer absorbance at 517 nm and as free radicals are quenched by the extract, there is a decline in absorbance.
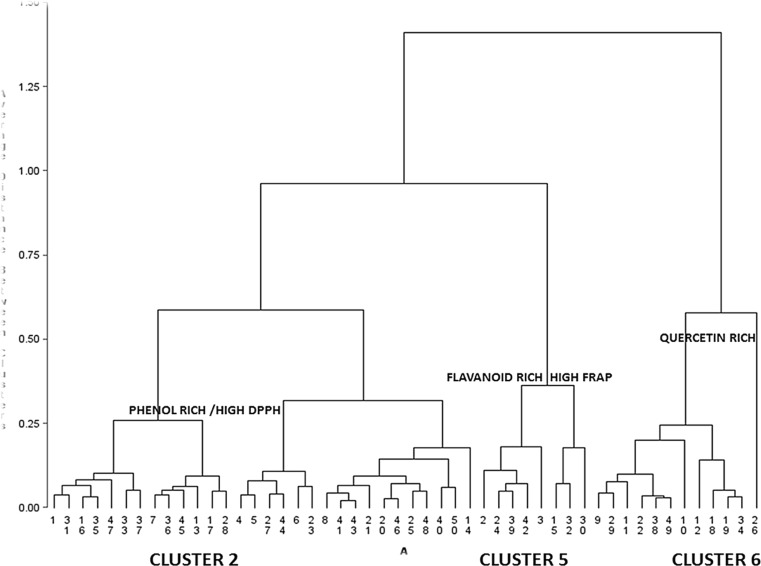
Methanolic extract of walnut leaf extract exhibited exceptionally higher anti-radical potential in quenching DPPH radical (analogous to the standard, Ascorbic acid). The study carried out by Carvalho et al. (2010) investigated phenolic fraction and antioxidant characteristics of methanolic and petroleum ether extracts resulting walnut (J. regia L.) seed, husk and leaf. This research illustrated highest phenolics fraction as well as correspondingly elevated levels of anti-oxidant potential in seeds, leaves and green husk in relevant order. On the contrary, petroleum ether display no or lower anti-oxidant potential. Rationally, methanolic extracts resulting from various walnut parts exhibited phenolic fraction than petroleum ether extracts. This information associates with the significant disparity in the solvent polarity and solubility of phenolic components in them. Actually, methanol (polar solvent) is believed as one of the best solvents for phenolics extraction. Cluster analysis pertaining to superlative genotypes exhibiting high DPPH and FRAP potential has been marked as CLUSTER 2, CLUSTER 5 and CLUSTER 6 having high accumulation of phenolics, flavonoids and quercetin respectively are represented in Fig. 6 The cluster 6 demonstrates higher number of quercetin rich genotypes than other metabolites. The concomitant increase in phenolics has been manifested in terms of antioxidant potential as described through the positive correlation (r = 0.53) between phenolics rich genotypes and their antioxidative potential. While as flavanoid/flavanol rich genotypes exhibited positive correlation (r = 0.37) with FRAP.
Fig. 6.
Schematic representation of 50 walnut genotypes displaying metabolically rich cluster CLUSTER 2 {Phenol rich/high DPPH}, CLUSTER 5 {Flavanoid rich/High FRAP}, Cluster 6 {Quercetin rich} with respective antioxidant potential
During recent years, oncologists are working extensively on natural anti-cancer plant based drugs. Walnut has been observed as an important folklore medicine for treatment of cancer. Numerous research reports has documented remarkable antiproliferative potential of walnut against HepG2 liver and Caco-2 colon cancer cells (Yang et al. 2009). Anti-cancer potential of walnut can be correlated with available phytoconstituents in walnut. In current study, we evaluated the inhibitory effects of various leaf extracts in three cancer cell lines IMR-32, HBL-100 and U2OS by MTT assay. Leaf extracts were screened on basis of maximum phenolics fraction and then evaluated against different cell lines. Since plant phenolics constitute one of the major groups of compounds and have a protective role in carcinogenesis, it was reasonable to determine their total amounts in various leaf extracts to discover if there is any correlation between anticancer properties and the amount of phenolic compounds. Following screening SPS-1 exhibited maximum response against three cell lines. Among cell lines, U2OS cells were most sensitive to this extract exhibiting 17.3% cell viability as compared to IMR-32 and HBL-100 cells. Previous research reports have also documented antiproliferative activity of methanolic walnut leaf extracts against A-498, 769-P renal, and Caco-2 colon cancer cells with IC50 ranging from 0.226 to > 0.5 mg/ml (Carvalho et al. 2010). Salimi et al. (2012) has also demonstrated chloroform fraction of walnut extract exhibit lowest IC50 values (0.36–0.81 mg/ml) against human oral squamous carcinoma (BHY), adenocarcinoma (HT-29) and breast adenocarcinoma (MCF7) cell lines and can induce cell cycle arrest following 24 h treatment. In contrast to our findings in which SPS-1 walnut extracts displayed IC50 value of 0.5 mg/ml against U2OS cells. Numerous other research reports confirmed antiproliferative effects of walnut extracts which modifies multiple gene targets within MCF-7 human breast cancer cells and reduce proliferation of these cells (Heuvel et al., 2012).
Conclusion
Walnuts and methanolic extracts prepared from walnut leaves are known as resources of phytochemicals. These compounds can enhance the effect of other antioxidants, such as fat-soluble vitamins and low molecular water soluble substances. Moreover, the high content of antioxidant components in plants, decide on their significant role in the prevention of lifestyle diseases. The results indicated J. regia as bioactive source having antioxidant potential. Walnut fruit in the early stages of maturity contained significantly more biologically active compounds than in the later stages of fruit maturity. Particularly noteworthy is the activity of the compounds contained in the leaves of J. regia, which may be easily accessible source of valuable substances.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to Central Institute of Temperate Horticulture (Indian Council of Agricultural Research, ICAR) and University Grants Commission (UGC) for financial support of this work.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2970-4) contains supplementary material, which is available to authorized users.
Contributor Information
Uzma Noor Shah, Email: uzmadanishnahvi@gmail.com.
Javid Iqbal Mir, Email: javidiqbal1234@gmail.com.
Nazeer Ahmed, Email: dnak59@rediffmail.com.
Sumira Jan, Email: sumira.sam@gmail.com.
Khalid Majid Fazili, Email: fazili@kashmiruniversity.ac.in.
References
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52(5):2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarowicz R, Gong Y, Pegg RB (2017) Recent advances in our knowledge of the biological properties of nuts. In: Wild plants, mushrooms and nuts: functional food properties and applications, pp 377–409
- Benzie IF, Strains JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jeronimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48(1):441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Cerda B, Tomas-Barberan FA, Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2005;53(2):227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Davis L, Stonehouse W, Loots DT, Mukuddem-Petersen J, van der Westhuizen F, Hanekom SJ, Jerling JC. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr. 2007;46:155–164. doi: 10.1007/s00394-007-0647-x. [DOI] [PubMed] [Google Scholar]
- Delaviz H, Mohammadi J, Ghalamfarsa G, Mohammadi B, Farhadi N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacog Rev. 2017;11(22):145. doi: 10.4103/phrev.phrev_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi A, Fatahi R, Zamani Z. Analysis of genetic diversity among some Persian walnut genotypes (Juglans regia L.) using morphological traits and SSRs markers. Sci Hort. 2011;130:146–151. doi: 10.1016/j.scienta.2011.06.028. [DOI] [Google Scholar]
- Eriksson G (1998) Sampling for genetic resources population in the absence of genetic knowledge. In: Noble hardwoods network, report of the second meeting, EUFORGEN, IPRG, pp 61–75
- Filipa Brito A, Ribeiro M, Margarida Abrantes A, Salome Pires A, Jorge Teixo R, Guilherme Tralhao J, Filomena Botelho M. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. 2015;22(26):3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Mousavi SM, Hamedi M, Khodaiyan F. Determination and characterization of kernel biochemical composition and functional compounds of Persian walnut oil. J Food Sci Technol. 2014;51(1):34–42. doi: 10.1007/s13197-011-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi K, Ghasemi Y, Ehteshamnia A, Nabavi SM, Nabavi SF, Ebrahimzadeh MA, Pourmorad F. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J Med Plants Res. 2011;5(7):1128–1133. [Google Scholar]
- Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, Davis JM. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry bjp-bp. 2016;209:192–201. doi: 10.1192/bjp.bp.114.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuvel JPV, Belda BJ, Hannon DB, Kris-Etherton PM, Grieger JA, Zhang J, Thompson JT. Mechanistic examination of walnuts in prevention of breast cancer. Nutr Cancer. 2012;64(7):1078–1086. doi: 10.1080/01635581.2012.717679. [DOI] [PubMed] [Google Scholar]
- Hossen MJ, Cho JY, Rahman MRT. Quercetin: promising flavonoid for drug development. EC Vet Sci. 2015;2:90–91. [Google Scholar]
- Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods—a review. J Food Sci Technol. 2014;51(10):2289–2303. doi: 10.1007/s13197-012-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazankaya A, Koyuncu F, Koyuncu MA, Yarilgaric T, Sen SM. Some nut properties of walnuts (Juglans regia L.) of Edremit country. Acta Hort. 2001;544:97–100. doi: 10.17660/ActaHortic.2001.544.11. [DOI] [Google Scholar]
- Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Y, Gong X, Zhao C. quality evaluation of walnut oil through HPLC and in vitro antioxidant activity. J Food Nutr Res. 2014;2:244–249. doi: 10.12691/jfnr-2-5-6. [DOI] [Google Scholar]
- Mensor LI, Menezes FS, Leitao GG, Reis AS, Dos Santos T, Coube CS, Leitao SG. Screening of Brazilian plants extracts for antioxidants activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–427. doi: 10.1016/j.foodchem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46(7):2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Omoruyi BE, Bradley G, Afolayan AJ. Antioxidant and phytochemical properties of Carpobrotus edulis (L.) bolus leaf used for the management of common infections in HIV/AIDS patients in Eastern Cape Province. Complement Altern Med. 2012;12(215):2–9. doi: 10.1186/1472-6882-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Qamar W, Sultana S. Polyphenols from Juglans regia L. (Walnut) kernel modulate cigarette smoke extract induced acute inflammation, oxidative stress and lung injury in Wistar rats. Hum Exp. 2011;30:499–506. doi: 10.1177/0960327110374204. [DOI] [PubMed] [Google Scholar]
- Ramos DE (1985) Walnut orchard management. Cooperative Extension, University of California, Division of Agriculture and Natural Resources
- Salimi M, Majd A, Sepahdar Z, Azadmanesh K, Irian S, Ardestaniyan MH, Rastkari N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm Biol. 2012;50(11):1416–1422. doi: 10.3109/13880209.2012.682118. [DOI] [PubMed] [Google Scholar]
- Slatnar A, Mikulic-Petkovsek M, Stampar F, Veberic R, Solar A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.) Food Res Int. 2015;67:255–263. doi: 10.1016/j.foodres.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Uysal S, Zengin G, Aktumsek A, Karatas S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J Funct Foods. 2016;22:518–532. doi: 10.1016/j.jff.2016.02.006. [DOI] [Google Scholar]
- van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Cancer cell culture: methods and protocols, pp 237–245 [DOI] [PubMed]
- Vinson JA, Cai YX. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012;3:134–140. doi: 10.1039/C2FO10152A. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Storer Samaniego C, Wang L, Burrows L, Tucker E, Dwarshuis N, Zand A. Antioxidant potential of Juglans nigra, black walnut, husks extracted using supercritical carbon dioxide with an ethanol modifier. Food Sci Nutr. 2017;5(2):223–232. doi: 10.1002/fsn3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JG, Hu QP, Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J Agric Food Chem. 2012;60:11625–11630. doi: 10.1021/jf303771s. [DOI] [PubMed] [Google Scholar]
- Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.