Abstract
Lotus (Nelumbo nucifera) root has been used as an edible vegetable in East Asia for thousands of years. The present research was aimed to explore the physicochemical, nutritional and microbiological safety of lotus root fermented sugar syrup as a fermented food supplement or condiment for human health benefits. In this study, the physicochemical, nutritional and microbiological safety properties of lotus root syrup fermented with 57° Brix brown sugar at different time periods until 6 months (180 days) was investigated. There was a significant improvement as compared to 57° Brix brown sugar broth (as a control) in the total acceptability and physicochemical properties of lotus root sugar syrup samples such as pH and color improvement. The red color values of 180 days lotus root fermented sugar syrup samples were significantly enhanced (6.85 ± 0.58) when compared with the control (0.20 ± 0.15). In addition, the total protein content was increased from 8.27 ± 0.86 to 392.33 ± 7.19 μg/mL, along with the increase in fermentation time reaching to the level of consumption acceptability. All the lotus root fermented sugar syrup samples were subjected to microbiological analysis. It was found that the coliform, Bacillus cereus, Escherichia coli, Salmonella and Staphylococcus aureus counts were not detected in majority of the samples, confirming the high degree of hygiene processing of lotus root fermented sugar syrup samples for its use as a food supplement or condiment.
Keywords: Lotus root, Fermentation, Physicochemical analysis, Nutritional analysis, Microbiological analysis
Introduction
Day-by-day increase in human population has resulted in an enormous pressure on natural resources. In order to meet the demanding level of various human needs, remarkable attention is being paid on diversification of the present agriculture. Accordingly, nowadays, herbal drugs have become the major subject of attention and global importance due to their ability to possess medicinal, therapeutic and economic implications (Dhanarasu and Al-Hazimi 2013). The current scenario of medicinal plants rich in bioactive components has confirmed that they have been used in folk medicine since ancient time for treating life-style related diseases throughout the world (Stoclet et al. 2004). Medicinal plants and herbs are of great importance to the health of individuals, and scientific investigations of traditional herbal remedies for metabolic disorders have proven to be natural remedies to provide valuable lead for the development of alternative food supplements and/or therapeutic agents (Kochhar et al. 2006). Hence, continuous development of new innovative natural functional foods has led to increased consumer awareness regarding health and the beneficial roles of foods for improving life quality (Verschuren 2002).
Although perfect storage/fermentation of foods provides consumers with several health beneficial effects, vegetables being a rich sources of vitamins and minerals are often used for storage in several countries due to the possibility to further improve the nutritional value of foods through storage/fermentation (Marica et al. 2007; Nazzaro et al. 2008). Proper storage with sugar or salt is a well-known process for improving the digestibility and flavor of fermented substrates including vegetables and plants along with enhanced nutritional and shelf life properties. Yoon et al. (2006) showed that stored/fermented cabbage juice served as a healthy beverage for vegetarians and lactose-allergic consumers. In addition, apple, plum, pear, beetroot and carrot juices fermented with the use of brewer’s yeast resulted in the enhanced nutritional value (Joshi and Devender 2005; Marica et al. 2007).
Nelumbo nucifera (lotus) is a medicinal plant in the monogeneric family Nelumbonaceae. Rhizome of lotus (lotus root) is recognized as one of the most delicious and nutritious vegetables in Asian countries along with its regular use in traditional medicine (Mukherjee et al. 2009; Huang et al. 2011). A number of studies have confirmed that lotus root contains high levels of polyphenolic compounds and possesses several beneficial health properties, such as hypoglycemic, anti-inflammatory and antioxidant potential (Yan et al. 2009).
To the best of our knowledge, use of fresh cut lotus in the food industry has been limited due to its short shelf-life as well as other factors such as its limited post-harvest status. A number of studies have shown that lotus root extracts increase the nutritional value of food due to their potential benefits for human health (Joshi and Devender 2005; Marica et al. 2007). Hence, addition of root extract in daily food diet can enhance nutritional quality and therapeutic value of food products. In the present study, by aiming the importance of storing strategy, nutritional quality and safety aspects, we developed a new lotus root fermented sugar syrup as a functional food and assessed its physicochemical characteristics, nutritional and microbiological quality during the overall storing period before human consumption.
Materials and methods
Collection of plant material
The lotus roots were collected in March 2012 from a lotus farm, located in Geumgang-dong, Dong-gu, Daegu, Republic of Korea, and no specific permissions were required for these locations/activities for their use in research purpose.
Processing for the development of lotus root fermented sugar syrup
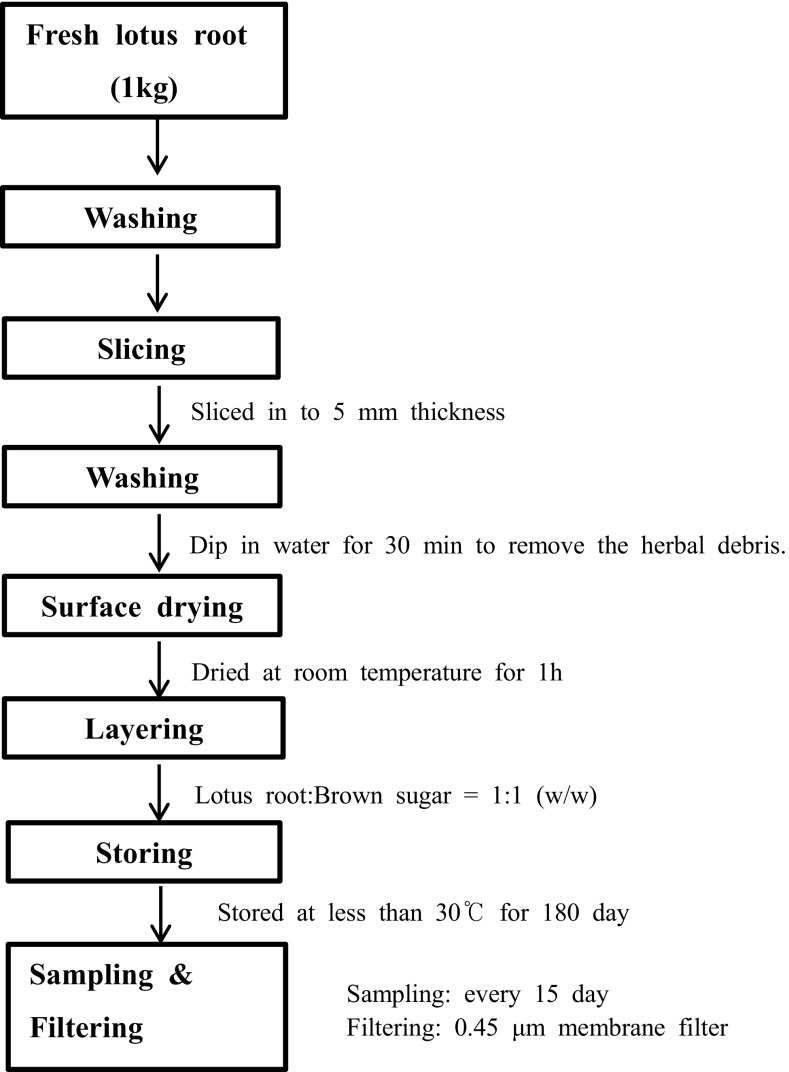
Lotus roots were washed with tap water and sliced into approximately 5 mm thickness. The lotus root slices (1 kg) were again carefully washed with water and surface dried at room temperature for about 1 h. The lotus root slices were placed inside a glass jar followed by layering of 1 kg brown sugar (1:1, w/w) to obtain 57° Brix brown sugar. Concerning hygiene point of view, the glass jar was capped and kept at room temperature (less than 30 °C). To maintain uniformity, manual agitation was conducted with a rotating steel rod (mechanically turned over the substrate) every other day. Detailed storing processing method is shown in Fig. 1. Every 15 days, fermented lotus root sugar syrup samples (15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180 days) were filtered through a 0.45 μm membrane filter to analyze the changes in physicochemical characteristics, microbial population and nutritional components. Experimental conditions were optimized, and experiment was carried out in 2–3 lots in order to achieve reproducibility of data. All experiments were conducted in comparison with 57° Brix brown sugar broth as a control.
Fig. 1.
Schematic presentation for the processing of lotus root fermented sugar syrup samples
Measurement of physicochemical quality of lotus root fermented sugar syrup
Determination of pH
For the pH measurement of the samples, 30 mL of lotus root fermented sugar syrup of various fermentation periods was filtered with Whatman paper No. 2 (Advantec, Tokyo, Japan). The pH was measured by using a pH meter (Orion 35 star pH Benchtop, Thermo electroncorporation; Beverly, MA, USA).
Color analysis
The color values (L*, a* and b*) of lotus root fermented sugar syrup samples were measured using a chromameter (CR-300, Minolta; Osaka, Japan). Lotus root sugar syrup samples of various storing periods were placed on the surface of a white standard plate and subjected to measure the Hunter color values (where L* = 96.43, a* = + 0.03 and b* = + 1.79). The L* value indicates the lightness on 0 to100 scale, representing dark to light. The a* value gives the degree of the red-green color with a highly positive a* value, indicating more red color. The b* value indicates the degree of yellow-blue color with highly positive b* value indicating more yellow color.
Measurement of nutritional quality of lotus root fermented sugar syrup
Analysis of total sugar and protein contents
The total contents of sugar and protein in lotus root fermented sugar syrup samples were determined using a phenol–sulfuric acid method (Masuko et al. 2005) and Bradford assay (Bradford 1976), respectively.
Quantitative analysis of amino acid content
For the analysis of amino acids, 3 mL of the lotus root fermented sugar syrup samples were diluted with 3 mL 20% trichloroacetic acid. They were then incubated at 4 °C for 4 h and centrifuged at 3000 rpm for 20 min. The supernatant was filtered through 0.45 µm filter. A 10 μL of each sample was analyzed using an amino acid analyzer (L-8800, Hitachi; Tokyo, Japan). Chromatography was performed using Hitachi ion exchange column (4.6 mm in width × 60 mm in length). Mobile phases were lithium citrate buffer (pump 1) and ninhydrin reagents (pump 2), and flow rate was 0.35 mL/min for pump 1 and 0.3 mL/min for pump 2, respectively. Detector used was ultraviolet ray detector (Juan Marcos et al. 2010).
Quantitative analysis of free sugar content
For high performance liquid chromatography (HPLC) analysis of free sugars such as sucrose, glucose and fructose in lotus root fermented sugar syrup samples, each sample (2.5 mL) was diluted using distilled water to make a total volume of 25 mL and then incubated at 30 °C for 30 min, followed by centrifugation at 10,000 rpm for 30 min (AOAC 2000). The supernatant was passed through the Sepak-C18 (Waters, Milford, Massachusetts, USA) and 0.45 µm filter and then analyzed using HPLC (Model 600E, Waters; Milford, MA, USA). Chromatography was performed using Sugar-Pak 1 column (60 mm in width × 300 mm in length) with mobile phase of Ca-EDTA buffer, and flow rate was maintained at 0.5 mL/min. Detector used was a refractive index detector.
Measurement of microbiological quality of lotus root fermented sugar syrup
Total coliform count
The total coliform counts in lotus root fermented sugar syrup samples and brown sugar broth (control) were determined according to Korean Food Standard Codex (2013). Every 15 days of interval, fermented lotus root sugar syrup samples were adequately diluted with 0.1% (w/v) peptone water using a serial dilution method. A 1 mL of each diluted sample was poured onto a deoxycholate lactose agar plate for the total coliform count using pour plate technique (KFDA 2009). The plates were incubated at 35 °C for 24 h, and then the number of colonies formed on the plates was counted as colony forming units (CFU) of the samples. Each test for each sample was carried out in triplicate.
Enumeration of Bacillus cereus (B. cereus) count
Enumeration of B. cereus count was performed by spreading 1 mL of each lotus root fermented sugar syrup sample onto the surface of mannitol-egg yolk-polymyxin agar (MYP) plates (Oxoid; Hampshire, England), and the plates were incubated at 30 °C for 24 h (MFDS 2013a). Confirmatory tests were performed by streaking the observed colonies on nutrient agar media, and then the grown colonies were further confirmed by using an API 50 CHB kit (bioMerieux; Marcy I’ Etoile, France).
Enumeration of Staphylococcus aureus (S. aureus), Escherichia coli (E. coli) and Salmonella count
According to the methods described by the Ministry of Food and Drug Safety (MFDS 2013a, 2013b), lotus root fermented sugar syrup samples were subjected to screening for the presence or absence of foodborne microorganisms, including S. aureus, E. coli O157:H7 and Salmonella species, by using selective culture dependent techniques. Colonies showing typical pathogen morphologies were subjected to microscopic examination. Isolates were obtained by streaking colonies onto nutrient agar plates and then identified using a microbial pathogen detection API kit (API kit (bioMerieux; Marcy I’ Etoile, France).
Analysis for total bacteria, yeast and fungi in lotus root fermented sugar syrup samples
To analyze the changes for total bacteria, yeast and fungi during fermentation period, each lotus root fermented sugar syrup sample (0–180 day) was diluted with 0.1% (w/v) peptone water and spreaded on to the agar plates of following media: plate count agar (incubated at 37 °C for 24 h) for total bacteria, yeast-mould agar (incubated at 28 °C for 3–5 days) for yeast and potato dextrose agar (incubated at 25 °C for 3–5 days) for fungi, as described by Korean Food Standard Codex (2013). The population on each agar plate was then subjected for microbial count. Brown sugar broth was used for comparisons. All the experiments were performed in triplicates.
Statistical analysis
All experiments were performed in triplicate and presented as mean values ± standard deviation. Analysis of variance and Duncan’s multiple range test were performed using SPSS 20.0 (SPSS Inc.; Chicago, IL, USA.) with a level of statistical significance of p < 0.05.
Results and discussion
Physicochemical quality of lotus root fermented sugar syrup
pH
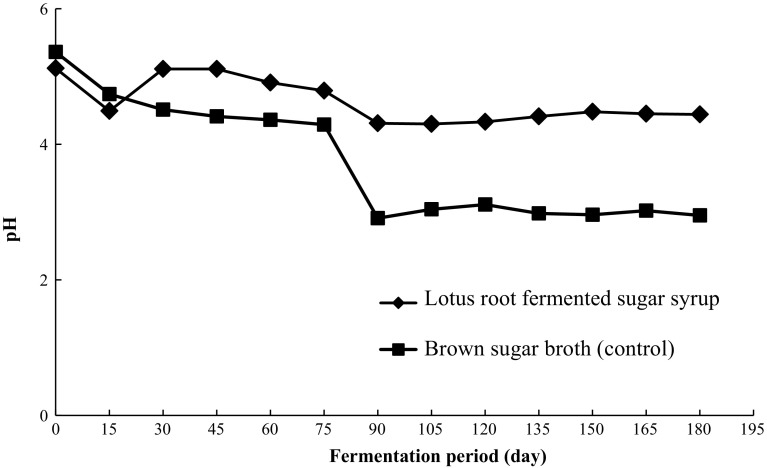
The pH and acidity changes were monitored during the fermentation of lotus root fermented sugar syrup samples on every 15 days of interval. It is well known that long time fermentation and storage lowers the pH of foods by increasing the level of acid present in them, which makes them safer for consumption. The changes in pH during fermentation of lotus root sugar syrup samples are shown in Fig. 2. The initial pH of the lotus root fermented sugar syrup samples on 0 day of storage was found to be 5.12 ± 0.01. Since then, the pH of lotus root fermented sugar syrup samples became more acidic with pH value of 4.49 ± 0.01 on day 15, 4.79 ± 0.01 on day 75 and 4.31 ± 0.01 on day 90, respectively.
Fig. 2.
Changes in pH during storage of lotus root fermented sugar syrup samples
In case of control (brown sugar broth), it was observed that on 0 day of storage, the pH of brown sugar samples was 5.36 ± 0.01, while it decreased to 4.74 ± 0.01 on day 15 with similar trends as observed for lotus root sugar syrup samples. After 90 days of storage, the pH of control became strongly acidic (2.9–3.1). Similar pH range has been considered optimal for vegetable-based products such as juices or condiments (Bau et al. 2014). The decrease of pH during storage or fermentation is common in fermented foods and can be attributed to the growth of bacteria and lactic acid production as also reported previously (Lucey 2004).
Color parameters
Color parameter in lotus root fermented sugar syrup samples and brown sugar broth (control) during different fermentation periods showed significant differences. The red color of the lotus root sugar syrup samples is preferred as a consumer acceptability level. As a result, lotus root sugar syrup samples showed an increase in red color (a*) according to the fermentation period (Table 1). There was a slight reduction in yellow color (b*) at the end of fermentation, and values decreased from 45.64 ± 0.70 to 39.38 ± 0.34 (Table 1), which might be due to the Maillard reaction occurring during the longer fermentation, since roots of lotus contain a number of amino acids, sugars and other components responsible for browning reaction (Mao et al. 2013). Gonçalves et al. (2007) reported that the loss of yellowness can also be attributed to the isomerization, oxidation and degradation of β-carotene, which can be promoted by acid formation. While in case of only brown sugar broth (control), there was a significant decrease in both red (from 7.60 ± 0.54 to 0.20 ± 0.15) and yellow (from 46.30 ± 0.35 to 30.63 ± 0.46) colors at the end of fermentation period. In addition, fermentation of the lotus root may also contain the enzymes to hydrolyze the proteins and starch by Maillard reaction, forming a certain amount of substances such as amino acids and sugars resulting in a unique color and flavor (Mao et al. 2013). In this study, the color parameters showed more pronounced modifications for 180 days of fermentation; and the L*, a* and b* parameters showed statistically significant differences (p < 0.05).
Table 1.
Color parameters of lotus root fermented sugar syrup samples
| Fermentation period (day) | Lotus root sugar syrup samples | Brown sugar broth (control) | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| 0 | 60.96 ± 1.25a | 3.77 ± 0.53f | 45.64 ± 0.70a | 58.20 ± 1.14d | 7.60 ± 0.54a | 46.30 ± 0.35a |
| 15 | 56.21 ± 1.09cd | 4.13 ± 0.26f | 38.29 ± 0.65h | 58.83 ± 0.94cd | 6.19 ± 1.13bc | 44.43 ± 2.50b |
| 30 | 56.40 ± 1.43cd | 5.71 ± 0.78d | 44.50 ± 0.15bc | 60.26 ± 0.21c | 5.65 ± 0.20c | 44.60 ± 0.29b |
| 45 | 56.92 ± 1.02c | 5.97 ± 0.45d | 44.08 ± 0.51bc | 59.38 ± 0.55cd | 6.18 ± 0.39bc | 45.01 ± 0.46b |
| 60 | 55.75 ± 0.83cd | 6.39 ± 0.42cd | 43.81 ± 0.69c | 59.33 ± 0.94cd | 6.80 ± 0.58b | 46.39 ± 0.43d |
| 75 | 56.54 ± 0.40c | 6.55 ± 0.22bcd | 44.72 ± 0.30b | 59.27 ± 1.03cd | 6.37 ± 0.53bc | 47.15 ± 0.32a |
| 90 | 62.26 ± 0.12a | 3.53 ± 0.08f | 41.44 ± 0.17e | 66.88 ± 0.66a | 1.19 ± 0.28d | 33.31 ± 1.03d |
| 105 | 58.84 ± 0.77b | 5.11 ± 0.27e | 42.38 ± 0.29d | 64.54 ± 0.91b | 1.31 ± 0.13d | 32.97 ± 0.31d |
| 120 | 50.55 ± 0.68f | 7.82 ± 0.17a | 39.52 ± 0.33g | 64.45 ± 0.23b | 1.07 ± 0.20d | 35.41 ± 0.77c |
| 135 | 54.65 ± 0.84de | 6.65 ± 0.32bcd | 40.87 ± 0.15e | 66.52 ± 1.09a | 0.92 ± 0.20d | 31.86 ± 0.72de |
| 150 | 53.26 ± 1.12e | 6.92 ± 0.38bc | 40.87 ± 0.43ef | 66.55 ± 0.79a | 0.92 ± 0.24de | 31.86 ± 1.01de |
| 165 | 53.01 ± 0.97e | 7.15 ± 0.36b | 40.35 ± 0.34f | 67.01 ± 0.85a | 0.13 ± 0.19ef | 31.87 ± 0.94de |
| 180 | 53.10 ± 1.36e | 6.85 ± 0.58bc | 39.38 ± 0.34g | 67.80 ± 0.83a | 0.20 ± 0.15f | 30.63 ± 0.46e |
L*: lightness; a*: redness; b*: yellowness. All values were expressed as mean ± standard deviation of triplicate determinations
Superscripts sharing a common letter in the same column are not significantly different at p < 0.05 by Duncan’s multiple range test
Total sugar and protein content
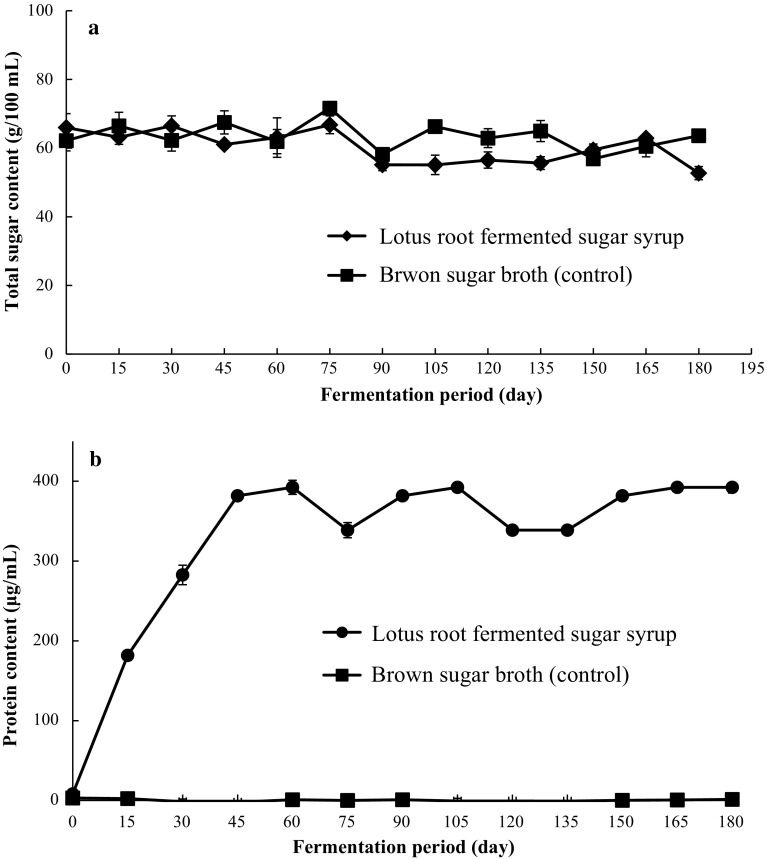
The total sugar and protein contents of lotus root fermented sugar syrup samples were determined from the standard calibration curve of glucose and bovine serum albumin, respectively. The obtained values of total sugar and protein content for 180 days fermented lotus root sugar syrup samples were found to be 52.71 ± 1.91 g/100 mL and 392.33 ± 7.19 μg/mL, respectively (Fig. 3a, b).
Fig. 3.
Changes in total sugar content (a) and total protein content (b) during storage of lotus root fermented sugar syrup samples
The sugar contents in 15 days lotus root fermented sugar syrup samples and control (brown sugar broth) were found to be 66.78 ± 2.58 g/100 mL for both the samples, and there was no significant difference in total sugar level at the end of fermentation period (180 days) in both the cases (Fig. 3a). There might be the possibility that total sugar content in fermented lotus root sugar syrup samples undergoes for breakdown in other sugar moieties, however, the total level of sugar content remained constant as observed during initial days of fermentation. Similar results were also observed with our previous finding in case of lotus leaf sugar syrup samples (Hwang et al. 2015).
On the other hand, the amount of protein content in the lotus root fermented sugar syrup samples reached about 381.80 ± 5.71 μg/mL (Fig. 3b). Interestingly, the protein content in the lotus root sugar syrup samples was the highest at day 60 (392.33 ± 8.84 μg/mL) and maintained until 180 days (392.33 ± 7.19 μg/mL) (Fig. 3b). However, there was almost no protein in the brown sugar broth (control).
Due to Maillard reaction, the lotus root fermented sugar syrup showed an increase in redness (a*) and slight reduction in yellowness (b*) during the fermentation period. Color changes corresponded to change in protein and amino acid contents during fermentation period. As shown in Table 2, fermentation period affected the contents of a few groups of amino acids. Aspartic acid, threonine, serine, glutamic acid, glycine and alanine increased on 90 and 180 days of fermentation while few amino acids such as proline showed increases in their content on 90 days following a slight reduction in their amount (Table 2). Similar findings on the relationship of Maillard reaction with amino acid contents were also observed in fresh and stored pear fruits (Coimbra et al. 2011). Amino acids are a class of important compounds present in foods for human nutrition and supply building-blocks required for protein biosynthesis (Pilipenko et al. 1999; Silva et al. 2004). Therefore, to have proper nutritional value, the contents of amino acids should not be less in food products. It has been also observed that during the fermentation period of lotus root fermented sugar syrup, the protein content was slightly increased on 60 days and 180 days of fermentation, giving positive aspects for its maintained nutritional quality. All these facts conclude that the mechanism of the Maillard reaction in this study doesn’t affect much of the protein and amino acid contents in their negative approach. Concerning these results, it can be assumed that lotus root sugar syrup samples could be a good source of protein content.
Table 2.
Contents of amino acid (µg/100 mL) in lotus root fermented sugar syrup samples
| Amino acid | Brown sugar broth (control) | Lotus root fermented sugar syrup | ||||
|---|---|---|---|---|---|---|
| 0 day | 180 day | 0 day | 15 day | 90 day | 180 day | |
| Aspartic acid | 0.60 ± 0.13a | nd | nd | nd | nd | nd |
| Threonine | nd | nd | 0.12 ± 0.00d | 22.87 ± 0.14c | 26.50 ± 0.01b | 27.82 ± 0.04a |
| Serine | nd | nd | 0.17 ± 0.02d | 23.53 ± 0.14c | 27.41 ± 0.04b | 28.72 ± 0.04a |
| Glutamic acid | nd | nd | 0.41 ± 0.02d | 13.44 ± 0.17c | 16.86 ± 0.10b | 16.98 ± 0.14a |
| Glycine | nd | nd | 0.59 ± 0.05d | 4.09 ± 0.03c | 5.44 ± 0.01b | 5.61 ± 0.12a |
| Alanine | nd | nd | 1.15 ± 0.02d | 28.31 ± 0.12c | 37.06 ± 0.04a | 34.71 ± 0.19b |
| Valine | 0.41 ± 0.015e | 0.34 ± 0.017e | 2.64 ± 0.95d | 23.31 ± 0.20c | 26.96 ± 0.32b | 28.50 ± 0.63a |
| Isoleucine | nd | nd | 0.12 ± 0.01d | 11.97 ± 0.74c | 13.39 ± 1.02b | 15.80 ± 1.84a |
| Leucine | nd | nd | 0.41 ± 0.03d | 9.21 ± 0.92c | 10.39 ± 0.69b | 12.21 ± 1.28a |
| Tyrosine | nd | nd | nd | 27.39 ± 2.47c | 31.83 ± 0.82b | 33.82 ± 2.05a |
| Phenylalanine | nd | nd | nd | 6.77 ± 2.00b | 7.73 ± 0.41ab | 8.12 ± 1.03a |
| β-Alanine | nd | nd | nd | 4.20 ± 0.57b | 4.84 ± 1.08b | 8.64 ± 0.39a |
| Ornithine | nd | nd | nd | 2.14 ± 0.02c | 2.99 ± 0.00a | 2.27 ± 0.02b |
| Lysine | nd | nd | 0.13 ± 0.00d | 1.74 ± 0.02c | 6.24 ± 0.01b | 9.98 ± 0.01a |
| 1-Mehyl histidine | nd | nd | nd | 1.72 ± 0.00b | 1.81 ± 0.04a | 1.71 ± 0.02b |
| Histidine | nd | nd | nd | 8.72 ± 0.02b | 9.51 ± 0.04a | 9.49 ± 0.06a |
| Arginine | nd | nd | nd | 66.58 ± 0.22c | 77.49 ± 0.23b | 98.09 ± 0.13a |
| Proline | nd | nd | nd | 6.96 ± 0.35c | 10.65 ± 0.21a | 8.97 ± 0.45b |
| Total | 1.01 | 0.34 | 5.74 | 262.95 | 317.1 | 351.44 |
All values were expressed as mean ± standard deviation of triplicate determinations
nd not detected
Superscripts sharing a common letter in the same row are not significantly different at p < 0.05 by Duncan’s multiple range test
Amino acid composition
To measure the content of amino acids in lotus root fermented sugar syrup samples, a modified procedure developed by Juan Marcos et al. (2010) was adopted. The profile of amino acids of lotus root sugar syrup samples is given in Table 2. A total of 18 kinds of amino acids and their derivatives were analyzed. The total yield of free amino acids in lotus root sugar syrup samples was 5.74 μg/mL at 0 day, 262.95 μg/mL at 15 day, 317.1 μg/mL at 90 day and 351.44 μg/mL at 180 day of storage (Table 2). While yield of total free amino acids at 0 day and 180 days old brown sugar broth (control) was found to be 1.01 µg/100 mL and 0.34 μg/100 mL, respectively. Arginine was found to be the most abundant amino acid among all the tested lotus root fermented sugar syrup samples (180 day), representing 98.09 ± 0.13 μg/100 mL at day 180 (Table 2). However, no changes in free amino acid content of brown sugar broth (control) were observed during storage time.
With respect to the lotus root fermented sugar syrup samples, almost all amino acids were increased over the fermentation period. Yu et al. (1990) also found that the amount of the amino acids in the final product of lotus root fermented sugar syrup was 4-times higher than before storage. Combining with the results of this study, it can be hypothesized that some of the amino acids were released by metabolism pathways and thus enriched the lotus root sugar syrup samples with health benefits and nutritional values. Besides, changes caused by fermentation were not only the increase of certain amino acids, but also a balance of all kinds of amino acids (Table 2).
In addition, results from other studies indicated that the fermentation process led to an overall increase in free amino acids and ammonia by 60-fold and 40-fold, respectively (Collar and Martinez 1993). Moreover, the net decrease in the level of some individual amino acids after fermentation suggests that they were metabolized to a greater extent than they were replaced by proteolytic activity. These findings are consistent with the nutritional requirements for amino acids described for a bacterium and yeast during wheat sour dough storage/fermentation (Collar and Martinez 1993). Previously it has been reported that amino acid assimilation by yeast fermentation was indicative of smaller increase of some individual amino acid components such as glycine, leucine, glutamine, lysine, histidine, phenylalanine and proline (Sarkar et al. 1993).
Sugar content
Free sugars are key taste components and significantly contribute to the sweet factor of fermented foods (Kim et al. 1998). In the present study, HPLC technique was used to determine the free sugar contents of lotus root sugar syrup samples. Standard solutions of the individual sugars were chromatographed separately to determine the retention time of each sugar compound. The individual free sugar contents were qualitatively determined by comparison with standard chromatograms and by doping the samples with reference compound. Table 3 shows the amount of total free sugar contents in lotus root fermented sugar syrup samples obtained from different storage periods. It was observed that the amount of sucrose was found to be decreased as the fermentation time increased. At 0, 15, 90 and 180 days of fermentation, the sucrose content was found to be 25.6 ± 0.06, 3.85 ± 0.07, 0.25 ± 0.07 and 0.30 ± 0.00 g/100 g, respectively, while glucose and fructose were found to be increased as the fermentation period increased (Table 3).
Table 3.
Contents of free sugars in lotus root fermented sugar syrup samples (g/100 g)
| Free sugars | Lotus root fermented sugar syrup | Brown sugar broth (control) | ||||
|---|---|---|---|---|---|---|
| 0 day | 15 day | 90 day | 180 day | 0 day | 180 day | |
| Sucrose | 25.6 ± 0.06 | 3.85 ± 0.07 | 0.25 ± 0.07 | 0.30 ± 0.00 | 25.6 ± 0.06 | 21.50 ± 0.27 |
| Glucose | 3.10 ± 0.03 | 11.15 ± 0.64 | 7.90 ± 0.14 | 9.05 ± 0.07 | 3.10 ± 0.03 | 4.40 ± 0.06 |
| Fructose | 1.90 ± 0.01 | 7.40 ± 0.85 | 11.45 ± 0.21 | 11.25 ± 0.07 | 1.90 ± 0.01 | 2.70 ± 0.04 |
| Total | 30.60 ± 1.06 | 22.40 ± 1.56 | 19.60 ± 0.42 | 20.60 ± 0.14 | 30.60 ± 1.06 | 28.50 ± 3.75 |
All values were expressed as mean ± standard deviation of triplicate determinations
Fructose level was the lowest at 0 day of fermentation, while it significantly increased at 180 days of fermentation to the high amount (11.25 ± 0.07 g/100 g). In contrast to glucose and fructose, the concentration of sucrose was much lower (0.30 ± 0.00 g/100 g) for 180 days lotus root fermented sugar syrup samples (Table 3). Jing et al. (2015) also observed similar results with storage/fermentation of pumpkin juice. These results confirmed the metabolism of sugar during fermentation period by the microbial population produced in the lotus root samples. Tang and Zhong (2002) revealed that sucrose as a carbon source was suitable for the bioactive free sugars production.
In addition to this, the nutritional quality of lotus root fermented sugar syrup in aspects of amino acids, proteins and sugar contents revealed that the lotus root fermented sugar syrup provides a positive aspect for containing various nutritive components. In our earlier studies, we have reported that the lotus root fermented sugar syrup samples containing phenolic and flavonoid compounds exhibited remarkable antioxidant and tyrosinase inhibitory activities as compared to brown sugar broth (control) (Shukla et al. 2016). The high scavenging properties of lotus root fermented sugar syrup samples might be due to phenolic compounds containing hydroxyl groups acting as radical scavengers. The total phenolic and flavonoid contents in lotus root fermented sugar syrup samples were also found to be increased by fermentation period. Overall, these findings suggest that the lotus root fermented sugar syrup could be a natural food supplement with potent functional activities and nutritional values.
Total coliforms
Overall, all the tested lotus root fermented sugar syrup samples showed microbiological quality to the consumer acceptable level. In the present research, total coliform counts in lotus root fermented sugar syrup samples were determined in order to evaluate the distribution of microorganisms during fermentation period. Coliforms were not found in any of the lotus root fermented sugar syrup samples, implying that the lotus root fermented sugar syrup samples were relatively hygenic as the fermentation proceeded (Table 4). Gilbert et al. (2000) reported the detection limit range of > 2, 2–3 and < 3 log CFU/g for coliform bacteria to be satisfactory, acceptable and unsatisfactory, respectively. The presence of higher levels of coliforms in food samples indicates the possibility of poor personal hygiene, insufficient production conditions or cross-contamination in the manufacturing process of food, as well as the potential presence of other enteric pathogens (Rompre et al. 2002).
Table 4.
Counts of total coliforms and Bacillus cereus various microorganisms in lotus root sugar syrup samples
| Fermentation period (day) | Sample | Total coliforms | Bacillus cereus | Salmonella species | Escherichia coli O157:H7 | Staphylococcus aureus |
|---|---|---|---|---|---|---|
| 15 | Lotus root fermented sugar syrup | nd | nd | nd | nd | nd |
| Brown sugar broth | nd | nd | nd | nd | nd | |
| 60 | Lotus root fermented sugar syrup | nd | nd | nd | nd | nd |
| Brown sugar broth | nd | nd | nd | nd | nd | |
| 90 | Lotus root fermented sugar syrup | nd | nd | nd | nd | nd |
| Brown sugar broth | nd | nd | nd | nd | nd | |
| 180 | Lotus root fermented sugar syrup | nd | nd | nd | nd | nd |
| Brown sugar broth | nd | nd | nd | nd | nd |
nd not detected
B. cereus
B. cereus is known to cause distinctly different types of foodborne illnesses in human beings, including gastrointestinal illness (Lund et al. 2000). In the present study, the presumptive test (culture dependent test on MYP plates) confirmed the presence of B. cereus colonies on MYP agar plates in all lotus root fermented sugar syrup samples collected during different fermentation time intervals, and no colonies of B. cereus were found throughout the fermentation period (Table 4). This meant the consumer acceptance level to the detection limit (> 104 CFU/g) as defined by the Ministry of Food and Drug Safety (MFDS 2013a, b). Based on the findings of microbial analysis, it was confirmed that samples of lotus root fermented sugar syrup can be considered safe for consuming purposes.
S. aureus, E. coli O157:H7 and Salmonella
In the present study, all lotus root fermented sugar syrup samples were examined for hazardous microbial counts. S. aureus, E. coli O157:H7 and Salmonella species were not detected in any of the tested samples (Table 4). The United States Food and Drug Administration established that hazardous doses of Staphylococcus toxin can be achieved in bacterial populations greater than 105 cells/g in contaminated foods (USFDA 1999). A single colony of E. coli O157:H7, Salmonella species and/or S. aureus is hazardous and may contribute to food poisoning (MFDS 2013b).
Total bacteria, yeast and fungi
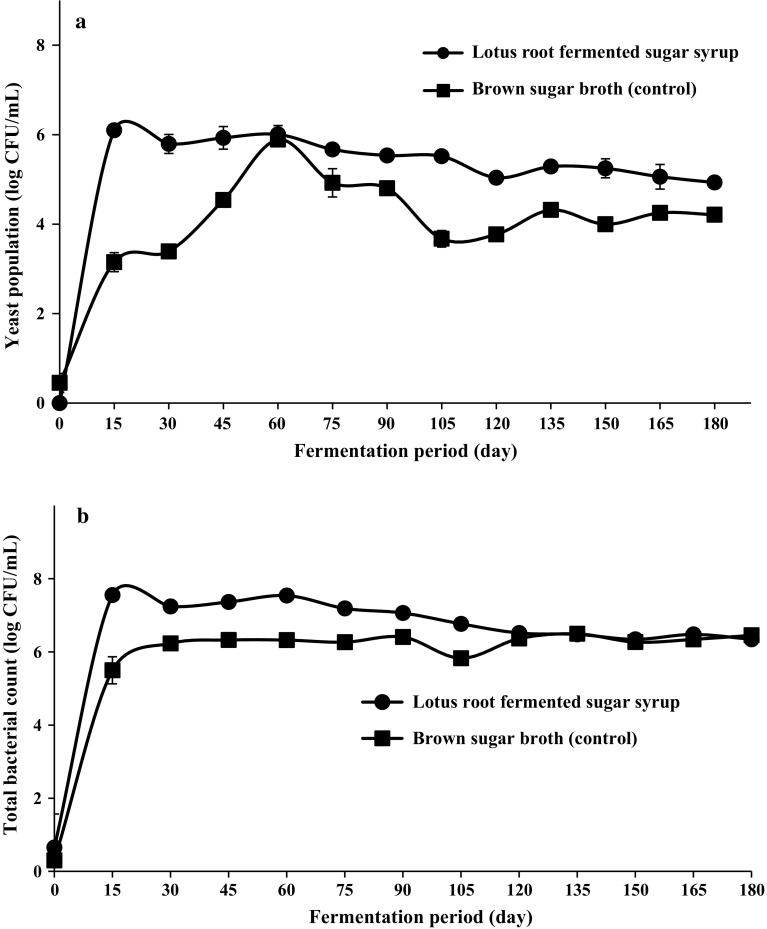
The total microbial counts were obtained by analyzing the bacterial, yeast and fungal growth in lotus root fermented sugar syrup samples. Yeast population in lotus root fermented sugar syrup samples showed very slow decrease after 15 days of fermentation, which was continuously slow and steady until the end of the fermentation day (180 day). Yeast count in lotus root fermented sugar syrup sample at 15 days and 180 days of fermentation was observed at 6.0 log CFU/mL and 4.9 log CFU/mL respectively, confirming the decreased counts as the fermentation period increased. While in case of brown sugar broth (control), at 15 days and 180 days, yeast count was observed as 3.1 log CFU/mL and 4.2 log CFU/mL, respectively (Fig. 4a). It was also confirmed in our earlier studies that during fermentation of lotus leaf sugar syrup samples (lotus leaf fermented broth), the dominant microflora included several species of yeast, including Saccharomyces cerevisiae (Hwang et al. 2015), although in the present work, we just performed the total yeast population count rather than their identification.
Fig. 4.
Microbial population in lotus root fermented sugar syrup samples as storing days. a Total yeast population (Log CFU/mL), b total bacterial count (Log CFU/mL)
Total bacterial population in lotus root fermented sugar syrup sample at day 15 and day 180 of fermentation was 7.5 log CFU/mL and 6.3 log CFU/mL respectively, confirming the decreased counts as the fermentation period increased. While in case of brown sugar broth (control), at day 15 and 180, yeast count was observed as 5.4 log CFU/mL and 6.4 log CFU/mL, respectively (Fig. 4a, b). These results confirm that the bacterial population showed slight reduction as the fermentation period increased. However, these bacterial counts could not prove the presence of hazardous bacterial populations, as we already confirmed the safety aspects by enumerating the hazardous bacterial species and found no growth. Therefore, it might be possible that the total bacterial count includes beneficial bacterial species, such as Bacillus and Lactobacillus.
Further, in case of fungal colony counts, it was observed that there was no fungal growth observed in lotus root fermented sugar syrup samples after 15 day, of fermentation, while in case of brown sugar broth (control), various black and blue fungal colonies were observed after day 15 (data not shown). With this observation, it can be hypothesized that the lotus root fermented sugar syrup samples might have antifungal effects against various fungal colonies.
Conclusion
The present study was undertaken to produce a sugar-based new lotus root fermented sugar syrup samples with the increased amount of nutritive quality due to higher consumer demand of lotus root. This study provided a scientific approach for development of the functional syrup supplement through the fermentation of lotus root using brown sugar, which enhanced its physicochemical, nutritional and microbiological characteristics. The contents of major sugars (sucrose, glucose and fructose) in lotus root fermented sugar syrup samples were found to be reduced after fermentation for 15 days along with a marked increase in essential amino acids. The significant (p < 0.05) increase in redness and loss of yellowness of the lotus root fermented sugar syrup samples during fermentation was observed. The fermentation period reflected the changes of lotus root fermented sugar syrup samples in both microbiological and physicochemical characteristics during the process of fermentation. By this way, lotus root fermented with sugar syrup gives good flavor (tested with a small group of laboratory personnels) as well as enhance its shelf life that can be stored up to 180 days at room temperature. The results of our study suggest that new acceptable storage strategies applied on the medicinal plants or any of their parts may lead to the development of a new product of choice which could potentially improve the nutritional value of lotus root fermented sugar syrup samples with additional health benefits. In conclusion, production of naturally fermented beverage from lotus root could be considered as a promising method of utilization of vegetable during the seasonal glut by making the vegetable available in the form of syrup or beverage throughout the year with health beneficial effects.
Acknowledgements
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A3011096).
Footnotes
Shruti Shukla and Juyeon Park have contributed equally to this research.
References
- AOAC . Official methods of analysis. 17. Washington DC, USA: Association of Official Analytical Chemists; 2000. p. 942. [Google Scholar]
- Bau TR, Garcia S, Ida EI. Evaluation of a functional soy product with addition of soy fiber and fermented with probiotic kefir culture. Braz Arch Biol Technol. 2014;57:402–409. doi: 10.1590/S1516-89132014005000005. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coimbra MA, Nunes C, Cunha PR, Guine R. Amino acid profile and Maillard compounds of sun-dried pears. Relation with the reddish brown colour of the dried fruits . Eur Food Res Technol. 2011;233:637–646. doi: 10.1007/s00217-011-1563-0. [DOI] [Google Scholar]
- Collar C, Martinez CS. Amino acid profiles of fermenting wheat sour doughs. J Food Sci. 1993;58:1324–1328. doi: 10.1111/j.1365-2621.1993.tb06175.x. [DOI] [Google Scholar]
- Dhanarasu S, Al-Hazimi A. Phytochemistry, pharmacological and therapeutic applications of Nelumbo nucifera. Asian J Phytomed Clin Res. 2013;1:123–136. [Google Scholar]
- Gilbert RJ, Louvois J, Donovan T, Little C, Nye K, Riberiro CD, Richard J, Roberts D, Bolton FJ. Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of scale. Commun Dis Public Health. 2000;3:163–176. [PubMed] [Google Scholar]
- Gonçalves EM, Pinheiro J, Abreu M, Brandão TRS, Silva CLM. Modelling the kinetics of peroxidase inactivation, colour and texture changes of pumpkin (Cucurbita maxima L.) during blanching. J Food Eng. 2007;81:693–701. doi: 10.1016/j.jfoodeng.2007.01.011. [DOI] [Google Scholar]
- Huang B, He J, Ban X, Zeng H, Yao X, Wang Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011;87:46–53. doi: 10.1016/j.meatsci.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Hwang D, Charchoghlyan H, Lee JS, Kim M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: volatile and nonvolatile metabolite profiling during fermentation. Int J Food Sci Technol. 2015;50:1988–1995. doi: 10.1111/ijfs.12882. [DOI] [Google Scholar]
- Jing Z, Liu W, Chen D, Zhou D, Song Y, Zhang Y, Ni Y, Li Q. Microbiological and physicochemical analysis of pumpkin juice fermentation by the basidiomycetous fungus Ganoderma lucidum. J Food Sci. 2015;80:241–251. doi: 10.1111/1750-3841.12741. [DOI] [PubMed] [Google Scholar]
- Joshi VK, Devender A. Panorama of research and development of wines in India. J Sci Ind Res. 2005;64:9–18. [Google Scholar]
- Juan Marcos AA, Purevdorj NO, Kayoko T, Shimada KI, Michihiro F, Mitsuo S. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010;199:279–285. [Google Scholar]
- KFDA (2009) Outbreak food poisoning. Korea Food and Drug Administration. http://e-start.kfda.go.kr. Accessed 21 sep 2016
- Kim JS, Yoo SM, Choi JS, Park HJ, Hong SP, Chang CM. Physicochemical properties of traditional Cheonggukjang produced in different regions. Agric Chem Biotechnol. 1998;41:377–383. [Google Scholar]
- Kochhar A, Nagi M, Sachdeva R. Proximate composition, available carbohydrates, dietary fiber and anti-nutritional factors of selected traditional medicinal plants. J Hum Ecol. 2006;19:195–199. doi: 10.1080/09709274.2006.11905878. [DOI] [Google Scholar]
- Korean Food Standards Codex (2013) vol 2. Korea Food and Drug Administration, Korea, pp 10–23
- Lucey JA. Cultured dairy products: an overview of their gelation and texture properties. Int J Dairy Technol. 2004;57:77–84. doi: 10.1111/j.1471-0307.2004.00142.x. [DOI] [Google Scholar]
- Lund BM, Baird-Parker TC, Gould GW. The microbiological safety and quality of food. Gaithersburg, Maryland, USA: Aspen Publishers Inc; 2000. p. 15. [Google Scholar]
- Mao C, He G, Du X, Cui M, Gao S. Biochemical changes in the fermentation of the soy sauce prepared with bittern. Adv J Food Sci Technol. 2013;2:144–177. doi: 10.19026/ajfst.5.3234. [DOI] [Google Scholar]
- Marica R, Maja V, Slavica SM, Milan M. Contribution of lactic acid fermentation to improved nutritive quality vegetable juices enriched with brewer’s yeast autolysate. Food Chem. 2007;100:599–602. doi: 10.1016/j.foodchem.2005.09.077. [DOI] [Google Scholar]
- Masuko T, Minam A, Iwaskin N, Majima T, Nishimura S, Lee YC. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- MFDS (2013a) Ministry of Food and Drug Safety (korea): food-borne pathogen test methods. http://www.mfds.go.kr. Accessed 21 sep 2016
- MFDS (2013b) Ministry of Food and Drug Safety: food-borne pathogen standard limits and specifications. korea. http://fse.foodnara.go.kr/residue. Accessed 21 sep 2016
- Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) phytochemical and therapeutic profile. J Pharm Pharmacol. 2009;61:407–422. doi: 10.1211/jpp.61.04.0001. [DOI] [PubMed] [Google Scholar]
- Nazzaro F, Fratianni F, Sada A, Orlando P. Symbiotic potential of carrot juice supplemented with Lactobacillus spp. and inulin or fructooligosaccharides. J Sci. 2008;88:2271–2276. [Google Scholar]
- Pilipenko LN, Kalinkov AY, Spektor AV. Amino acid composition of fruit in the manufacture of sedimentation-stabilized dispersed products. Chem Nat Compd. 1999;35:208–211. doi: 10.1007/BF02234937. [DOI] [Google Scholar]
- Rompre A, Servais P, Baudart J, De-Roubin MR, Laurent P. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods. 2002;49:31–54. doi: 10.1016/S0167-7012(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Cook PE, Owens JD. Bacillus fermentation of soybeans. World J Microbiol Biotechnol. 1993;9:295–299. doi: 10.1007/BF00383066. [DOI] [PubMed] [Google Scholar]
- Shukla S, Lee JS, Park J, Hwang DJ, Park JH, Kim M. Quantitative analysis of functional components from Nelumbo nucifera root fermented broth with antioxidant and tyrosinase inhibitory effects. J Food Biochem. 2016;40:248–259. doi: 10.1111/jfbc.12242. [DOI] [Google Scholar]
- Silva BM, Andrade PM, Valentao P, Ferreres F, Seabra RM, Ferreira MA. Free amino acid composition of quince (Cydonia oblonga Miller) fruit (pulp and peel) and jam. J Agric Food Chem. 2004;52:1201–1206. doi: 10.1021/jf030564x. [DOI] [PubMed] [Google Scholar]
- Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, Bedoui J, Chataigmeau M, Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Tang YJ, Zhong JJ. Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acid. Enzyme Microb Technol. 2002;31:20–28. doi: 10.1016/S0141-0229(02)00066-2. [DOI] [Google Scholar]
- USFDA (1999) United States Food and Drug Analysis: guidelines for food pathogens. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/#y1999. Accessed 21 sep 2016
- Verschuren PM. Summary report—functional foods: scientific and global perspectives. Br J Nutr. 2002;88:125–130. doi: 10.1079/BJN2002609. [DOI] [PubMed] [Google Scholar]
- Yan SL, Wang QZ, Peng GH. Determination of catechin in lotus rhizomes by high-performance liquid chromatography. Int J Food Sci Nutr. 2009;60:432–438. doi: 10.1080/09637480701780062. [DOI] [PubMed] [Google Scholar]
- Yoon KY, Woodams EE, Hang YD. Production of probiotic cabbage juice by lactic acid bacteria. Biores Technol. 2006;97:1427–1430. doi: 10.1016/j.biortech.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Yu DS, Yi JP, Kong XR. A comparative analysis of amino acid in the fermentation broth, the mycelia and the fruiting body of Ganoderma lucidum. J Amino Acid. 1990;2:44–46. [Google Scholar]