Abstract
In this study, the effect of high hydrostatic pressure (HHP) on antigenicity, free sulfhydryl group (SH) content, hydrophobicity (Ho), fluorescence intensity and circular dichroism data of soybean β-conglycinin was studied. The antigenicity of soybean β-conglycinin was decreased significantly at pressures 200–400 MPa. The antigenicity inhibition rate of β-conglycinin declined from 92.72 to 55.15%, after being treated at 400 MPa for 15 min. Results indicated that free sulphydryl (SH) groups and surface Ho of β-conglycinin were significantly increased at pressures 200–400 MPa and 5–15 min, whereas these properties decreased at the treatments above 400 MPa and 15 min. The maximum fluorescence intensity was noticed at 400 MPa and 15 min. The circular dichroism data analysis revealed that the amount of β-turns and unordered structure significantly increased, while the content of α-helix1 and β-strand1 noticeably decreased. These results provide evidence that HHP-induced the structural modification of β-conglycinin and could alter the antigenicity of β-conglycinin.
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2972-2) contains supplementary material, which is available to authorized users.
Keywords: β-Conglycinin, Soybean, High hydrostatic pressure, Antigenicity, Structural properties
Introduction
Soybean proteins have high nutritional value and high functional properties among vegetable proteins and have been widely utilized in food and feed industries (He et al. 2015). The protein content of soybean seed is about 40% (Hancock et al. 2000). However, soybean contains many anti-nutritional factors, including lectin, trypsin inhibitors, α-amylase inhibiting factor, agglutinins, antigen proteins and others (Ma et al. 2010). Glycinin (11S globulin) and β-conglycinin (7S) are the two primary proteins in soybean, accounting for 70% of storage proteins (Wang et al. 2011).
The β-conglycinin, soybean 7S storage protein, a major soybean storage protein, accounting for 10.0–12.7% of soybean seed or about 30% of the total protein. β-Conglycinin, one of the most allergenic proteins that contain three subunits, which was named as α′ (76 kDa), α (72 kDa), and β (52 kDa) (Krishnan et al. 2009). And almost 25% of soybean-sensitive patients were identified as the α subunit of β-conglycinin (Ogawa et al. 1995). The nutrition of soybean proteins is reduced because of antigenicity of β-conglycinin. Several previous studies have reported that the IgE antibodies in soybean allergy patients can recognize the three subunits of β-conglycinin (Krishnan et al. 2009). Therefore, it is essential to develop a processing technique capable of eliminating or reducing the antigenicity of β-conglycinin.
Previous studies have shown that food processing, such as high pressure and thermal treatments can influence the IgE binding capacity of food proteins (Cabanillas et al. 2015). Although thermal treatment can reduce the immunoreactivity of most soybean allergens, some allergens are heat resistant (Wilson et al. 2004). As a new non-thermal processing technology, high hydrostatic pressure (HHP) can modify the second, tertiary, and quaternary structures of proteins and alter some of epitope structures, which can reduce the allergenicity and improve the functional properties of food proteins, and has become one of the most promising techniques for food preservations. Compared with the thermal processing, HHP does not affect vitamins, amino acids, flavor compounds and other small molecules (Puppo et al. 2011). During last decades, the industrial process of HHP to change the structure of food proteins has become more and more increasing interest.
Recent studies have shown that HHP can influence the allergenicity and functional properties of food protein. Hu et al. (2011) stated that high pressure induces conformational changes in peanut proteins, and according to their results, the binding of peanut allergens to IgE decreased with increasing pressure. Zhou et al. (2016) reported that HHP treatment with pressure ranges from 300 to 700 MPa can considerably reduce the allergenicity of ginkgo seeds protein (GSP), due to the structural changes which can either destroy or bury the conformational IgE-binding epitopes. Li et al. (2012) confirmed that at 300 MPa for 15 min, the allergenicity of HHP-treated soy protein isolate (SPI) decreased 48.6% compared with the native SPI for infant formula, owing to the inactivation of certain conformational epitopes. Furthermore, they investigated four physical approaches about allergenicity and structural properties of SPI for infant formula and indicated that in the aspect of reducing allergenicity of SPI for infant formula, the reducing efficiency of HHP treatment is much higher than those of other three treatments (Li et al. 2016). In studies conducted by Omi et al. (1996), 300 MPa (25 min, 20 °C) HHP treatment of soybean seed resulted in the release of 7S globulin. Peñas et al. (2011) pointed out that soybean seeds treated with HHP, the allergenicity of soybean sprouts was obviously decreased. HHP to the soybean seeds before could be used for the processing of hypoallergenic soybean sprouts in industrial (Peñas et al. 2011). However, It is still poorly understood about the influence of HHP on the antigenicity and structural properties of β-conglycinin, the main component of soybean antigen. Therefore, what we investigate in this study was the antigenicity and structural properties of native β-conglycinin after HHP treatment.
Materials and methods
Materials
Soy protein isolate (SPI) was obtained from Ceres Biotechnology Group Co., Ltd (Shangdong Province, China). 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB) was procured from Sigma (St. Louis, MO, USA). Low molecular weight protein markers, 1,8-anilinonaphthalenesulfonate (ANS), and bovine serum albumin (BSA) were procured from Beijing Solarbio Science & Technology Co., Ltd (China).
Purification of soybean β-conglycinin
Soybean β-conglycinin was extracted by following Nagano et al. (1992). Firstly, the β-conglycinin was separated by salt precipitation and then further purified by passing through a Sepharose 6B gel filtration column (Beijing Solarbio Science & Technology Co., Ltd) in order to avoid contamination from glycinin. SDS-PAGE and Western Blot analysis showed that the purified β-conglycinin was not contaminated with glycinin, and the purified β-conglycinin could specially bind to β-conglycinin antiserum, and this confirmed the immunological reactivity of the purified β-conglycinin (data are shown in supplementary materials).
High hydrostatic pressure (HHP) treatment
HHP treatment was performed in a 5 L steel-vessel (model HHP-600 MPa-5L; KEFA Hitech Food Machine Company Co., Ltd., Baotou, China) equipped with pressure and temperature controls. The pressure build-up at 250 MPa/min, and when the duration time reached, released the pressure rapidly. Water was used as the medium for pressure transmitting, and during the whole procedure, temperature was controlled within 20 ± 2 °C. HHP treatment at 200, 300, 400, and 500 MPa were selected to perform for 5, 10, 15 and 20 min for 1% β-conglycinin solutions. The treatment of each sample was carried out in triplicate. The pressurized samples were stored at − 20 °C for further assays.
Assessment of β-conglycinin antigenicity by indirect competitive enzyme-immunoassay (icELISA)
Antigenicity of HHP-treated samples was determined by icELISA (Zhang et al. 2014). β-Conglycinin antigen was coated on microtiter plates (0.4 μg/mL in CBS, 4 °C, overnight). Solutions of HHP-treated and untreated β-conglycinin were incubated with rabbit anti-β-conglycinin serum (1:8000 in PBS, 4 °C, overnight). Next day, the plates were washed using PBS-T and blocked with 5% skim milk in 0.01 M pH 7.4 PBS. After washing, the reactive mixtures of HHP-treated, untreated samples, and rabbit anti-β-conglycinin serum were added and incubated (37 °C, 1 h). After the wells being washed, goat anti-rabbit IgG-HRP was added, and at the temperature of 37 °C incubated for 1 h. 3,3′,5,5′-tetramethyl benzidine (TMB, 100 μL/well) was added and 10 min later, terminating the reaction using 2 M sulphuric acid, and reading the plates at 450 nm.
The inhibition rate of HHP-treated β-conglycinin was calculated as follows:
where B is the optical density (OD) of HHP-treated samples and B0 is the OD of untreated β-conglycinin.
Measurement of free sulfhydryl (SH) contents
The sulfhydryl was measured based on Beveridge et al. (1974). One mL of the β-conglycinin solution (the β-conglycinin sample were adjusted to 1 mg/mL, using 0.086 M pH 8.0 Tris–HCl containing 0.004 M EDTA, 0.09 M Gly, and 8 M urea,) was mixed with Ellman’s reagent (40 μL). After being vortexed, the absorbance was detected at 412 nm using a UV–Vis spectrophotometer (Shanghai Precision & Scientific Instrument Co., Ltd (China).
Determination of surface hydrophobicity (Ho)
The Ho of β-conglycinin was measured following Wang et al. (2008). β-Conglycinin solutions were diluted (0.00625–0.2 mg/ml) with PBS (0.01 M, pH 7.0). The resultant β-conglycinin solution (4 mL) was then mixed with 20 μL ANS (8.0 mM). Under the wavelength of 280 nm (excitation) and 562 nm (emission) using a Cary Eclipse (EL06053220) to detect the fluorescence intensity of the solution. The indexes of Ho was defined as the initial slope of the fluorescence intensity plotted against protein concentration (mg/mL).
Extrinsic emission fluorescence spectroscopy
Using the fluorescent probe ANS to determine the fluorescence spectra of HHP treated β-conglycinins. β-Conglycinin solutions were dissolved in 10 mM PBS at the concentration of 0.2 mg/ml. The resultant dispersions were excited at 280 nm, and in this study, emission spectra from 450 to 700 nm was measured.
Circular dichroism (CD) spectra
The CD spectroscopic was recorded at 25 °C on a MOS-450 spectropolarimeter (BioLogic Science Instrument, France). The parameters were set as follows: acquisition duration, 1 s; step resolution, 1 nm; bandwidth, 0.5 nm. Protein samples dissolved into final concentration 0.1 mg/mL with PBS, and scanned from 190 to 250 nm (far-UV region) with quartz cuvette (2 mm). The protein concentration of β-conglycinin was measured by Bradford method (1976). A mean value of 107.4 for soybean β-conglycinin was used to calculate the average residual weight of proteins (Koshiyama and Fukushima 1973). Using the DICHROWEB procedure (Whitmore and Wallace 2004) to analyze the compositions of secondary structure in each sample. The average of eight scans spectra was the result of recorded.
Statistical analysis
Three independent experiments were performed for each parameter. Data obtained from the experiments were analyzed of variance using the SPSS 16.0 (IBM Corporation, NY, USA), and the significance level of p ≤ 0.05 was established. Duncan’s multiple range tests were used to detect for discrepancies between means.
Results and discussion
Effect of HPP on antigenicity of β-conglycinin
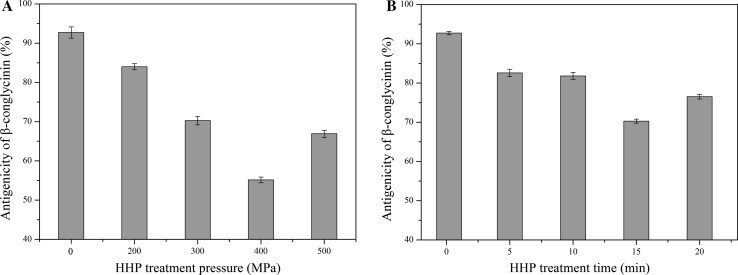
The antigenicity of β-conglycinin was found to be lower at HHP treated pressure 200, 300, 400 and 500 MPa compared to untreated sample (Fig. 1a). Compared to raw β-conglycinin, the antigenicity of HHP treated β-conglycinin decreased drastically (p < 0.05) between 200 and 400 MPa. After being treated by HHP at 400 MPa, the antigenicity inhibition rate of β-conglycinin decreased from 92.72 to 55.15%, with a reduction up to 37%. Pressurized protein at 400 MPa showed the greatest decline in antigenicity. Figure 1b indicated that HHP treatment at different times resulted in a decreased antigenicity of β-conglycinin compared to control sample, and the lowest antigenicity of β-conglycinin was observed at 15 min.
Fig. 1.
Effects of HHP treatment pressure and time on antigenicity of β-conglycinin (a HHP treatment time is fixed at 15 min; b HHP treatment pressure is fixed at 400 MPa)
There was similar phenomenon observed by Zhou et al. (2016), who revealed that HHP treatment at 300–700 MPa can reduce the allergenicity of ginkgo seed protein (GSP) significantly, and when the pressure further increased to 500, 600, and 700 MPa, the allergenicity of the protein samples was completely eliminated. The authors attributed this phenomenon to the seriously destroyed structures of the proteins. The higher the pressure was, the more conformational epitopes were eliminated. The current study results are in accordance with those obtained by Li et al. (2012) for SPI, who reported a decrease in the allergen content of SPI, after HHP treatment, because of aggregation and/or denaturation of soy protein.
Many similar studies have shown that HHP can decline the allergenicity of β-lactoglobulin, α-casein, whey proteins, and others (Meng et al. 2017; Hu et al. 2016; Ambrosi et al. 2016). However HHP treatment could not effectively change the immunoreactivity of hazelnut and walnut allergens (Prieto et al. 2014; Cabanillas et al. 2014), these difference may be connected with the different type of antigenic determinant in the protein.
Effect of HPP on free sulfhydryl (SH) content of β-conglycinin
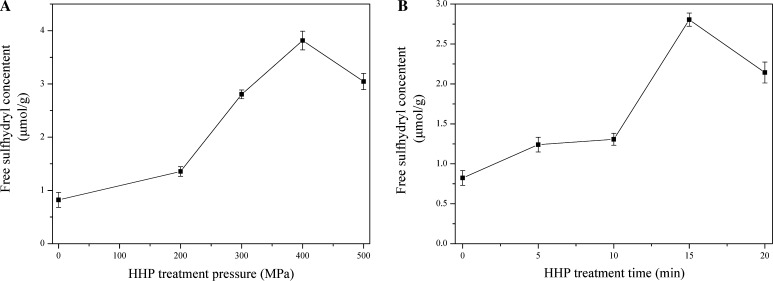
As demonstrated by Sen et al. (2002), a reduction of free SH indicated cross-linking effect on protein, whereas increasing usually indicates protein unfolding and backbone fragmentation. The free SH content in β-conglycinin changed after HHP treatment, which was mainly due to intramolecular non-covalent cleavage (Messens et al. 1997). The data showed that in the HHP treatment at 200–400 MPa, the SH content kept increasing. These results suggested that high pressure treatment may denature the β-conglycinin and trigger the SH–S–S interchange reaction and the variations in SH content may also cause variations in the properties of β-conglycinin. While, this value declined above 400 MP (Fig. 2a). A significant (p < 0.05) increase was obtained at 400 MPa for 5–15 min, which indicating that the conformation of β-conglycinin was significantly altered, whereas, from 15 to 20 min, a significant decrease (p < 0.05) was noticed at HPP treatment (Fig. 2b). Four hundred MPa for 15 min gave the maximum increase, at this pressure and time level, the free SH groups seems to be stable with a low degree of aggregation, whereas at high levels, the free SH content decreased. And unfolded proteins aggregation became outstanding due to hydrophobic interaction, which enhanced the reformation of S–S bonds, decreased the free SH content.
Fig. 2.
Effects of HHP treatment pressure and time on free sulfhydryl content of β-conglycinin (a HHP treatment time is fixed at 15 min; b HHP treatment pressure is fixed at 400 MPa)
These results indicated that HHP-induced structural modification can be quite complex, and may involve more than one mechanism of denaturation. The present study results were consistent with Li et al. (2012), who noticed similar trends in changes of free SH in SPI treated with HHP. In their study, the free SH content was highest at 300 MPa, and this was lower than our result (400 MPa). This difference could result from the various soybean cultivar and extration methods. In Zhang and others’ investigation (2003), free SH content of HHP-treated glycinin increased gradually in the range of 0–600 MPa, but the concentration of glycinin solution utilized for HHP processing was not given. These results confirmed the occurrence of HPP-induced protein unfolding and subsequent aggregation/re-association of the unfolded proteins.
However, there were also contrary observations to our results, who found that all the HHP treated sample showed lower SH content whatever the concentration used (Kajiyama et al. 1995). Some researchers suggested that the changes in free SH content was owing to the SH/S–S interchange reactions that induced the formation of S–S bonds (Galazka et al. 2000; Puppo et al. 2004). The contrary results and conclusion from these authors might due to pH and pressure, which played a major role on HHP treated samples’ aggregation.
Effect of HPP on surface hydrophobicity (Ho) of β-conglycinin
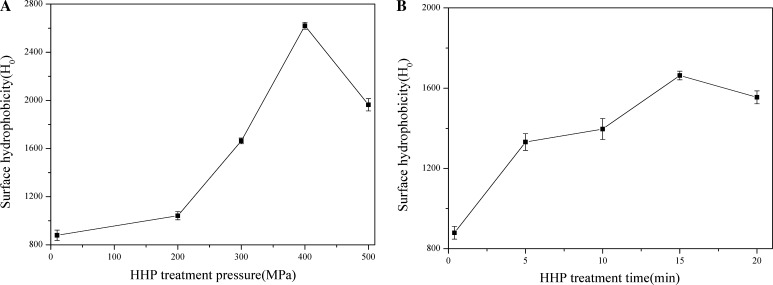
A significant (p < 0.05) increase in Ho of β-conglycinin was observed when the pressure were increased to 200–400 MPa and for 5–15 min, whereas this value declined at 500 MPa for 20 min (Fig. 3). These results indicated that HHP-induced the molecular unfolding and resulted in exposure of the hydrophobic domains of the protein molecules. Actually, HHP processing on Ho also reflects the difference in protein aggregation and unfolding/refolding extent at different pressure levels. Higher pressure (500 MPa) could not increase the hydrophobic region further, on the contrary, result in a decrease tendency of the hydrophobic regions, owing to the degree of binding increased among denatured proteins, leading to an increase in high molecular weight aggregates, and a decrease in Ho. Molina et al. (2001) reported an increase of Ho in SPI, 7S and 11S fractions at the pressure treatment above 200 MPa. In their investigation, 7S fraction showed the highest Ho at 400 MPa whereas 11S fraction performed the highest Ho at 200 MPa. The authors concluded that 400 MPa treatment broke the 7S into partially denatured monomers and thus more hydrophobic domains were exposed. Puppo et al. (2004) performed a HHP treatment on SPI (1%) and observed that Ho increased significantly at 400 MPa compared to 600 MPa. Therefore, it is reasonable to speculate that the unfolded β-conglycinin at higher pressure (≥ 400 MPa) could readily be participated in the rearrangement of hydrophobic domains. Furthermore, a previous report showed that a gradual increase to 450 MPa and ultimately reaching 500 MPa significantly reduced the hydrophobicity of patatin (Elahi and Mu 2017). However, in another study on SPI, the surface hydrophobicity was increased under the pressure they studied (200, 400 and 600 MPa), compared with the control (Wang et al. 2008). These authors attributed this behavior to the various soybean protein presented in SPI.
Fig. 3.
Effects of HHP treatment pressure and time on surface hydrophobicity of β-conglycinin (a HHP treatment time is fixed at 15 min; b HHP treatment pressure is fixed at 400 MPa)
Conformational changes of the protein can also be inferred based on alterations to the surface hydrophobicity of β-conglycinin. Because the increased Ho of the HHP-treated β-conglycinin indicated that the protein was unfolded, exposing more hydrophobic regions after HHP treatment. This finding illustrated that the tertiary and quaternary structure of β-conglycinin were changed by HHP.
Extrinsic emission fluorescence spectroscopy
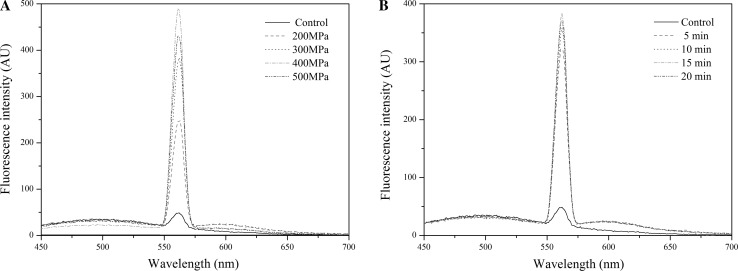
The fluorescence intensity increased significantly at 200–400 MPa, compared with the control (Fig. 4), partially attributing to the exposure of the Phe and Tyr residues. However, the fluorescence intensity decreased when the pressure reached at 500 MPa, which was due to the rearrangements hydrophobic domains in β-conglycinin. The Trp residues in β-conglycinin gradually migrated into a hydrophobic environment, and this might further support the existing structural rearrangements. At 400 MPa, the fluorescence intensity increased in the range of 5–15 min while decreased when the time up to 20 min, compared with the control. The trends of fluorescence intensity of HHP treated β-conglycinin revealed that the hydrophobic regions of β-conglycinin exposed at 200–400 MPa and 5–15 min. But, when processed at 500 MPa and 20 min, exposed hydrophobic groups might aggregate or re-associate to form a more stable structure. The present study results agreed with the results reported by Li et al. (2012), who demonstrated the fluorescence peak of SPI would be reduced at higher levels. Our results were not consistent with Zhang et al. (2003), in their study, the fluorescence peak of soybean glycinin was increased between 100 and 500 MPa. This difference may be due to the difference in soybean protein.
Fig. 4.
Emission fluorescence spectra of β-conglycinin treated by HHP (a HHP treatment time is fixed at 15 min; b HHP treatment pressure is fixed at 400 MPa) (using ANS as a fluorescence probe)
Circular dichroism (CD) analysis
The changes of secondary folding level of β-conglycinin were analyzed by Far-UV CD. The results (Table 1) showed that the secondary structure of native β-conglycinin consisted of four structure, which were consistent with the previous reports by Li et al. (2007). The data indicated that the unordered and β-turns content of HHP-treated β-conglycinin increased, and helix1 and strand1 content decreased, while the content of the strand2 and helix2 did not change obviously. Zhang et al. (2003) tested the HHP treated soybean glycinin using circular dichroism spectra and found that α-helix and β-sheet content decreased significantly, but the content of random coil increased at HHP pressure of 500 MPa or higher. The current study findings agreed with those of the early studies, in which the random coil content increased, and the α-helix content decreased with the increase of pressure (Puppo et al. 2004). HHP treatment could lead to unfolding of the helices structure and compression of strands structure in SPI (Li et al. 2012). These differences with our results might derive from the different parameters of HHP treatment, such as concentration and pH.
Table 1.
Effects of HHP on secondary structure composition of β-conglycinin
| Treatment | Helix1 | Helix2 | Strand1 | Strand2 | Turns | Unordered | Total |
|---|---|---|---|---|---|---|---|
| Control | 0.190 ± 0.004a | 0.147 ± 0.002c | 0.295 ± 0.003d | 0.076 ± 0.004g | 0.189 ± 0.003i | 0.251 ± 0.003l | 1.148 ± 0.003o |
| 400 MPa, 15 min | 0.145 ± 0.003b | 0.144 ± 0.003c | 0.109 ± 0.002e | 0.084 ± 0.002h | 0.216 ± 0.002j | 0.316 ± 0.004m | 1.014 ± 0.003p |
| 500 MPa, 15 min | 0.152 ± 0.003b | 0.146 ± 0.003c | 0.127 ± 0.003f | 0.074 ± 0.003g | 0.226 ± 0.004k | 0.274 ± 0.003n | 0.999 ± 0.003q |
All values were mean ± standard deviation of three values
Different superscripts (a–q) in the same column indicate the significantly different at the p < 0.05 level
After HHP treatment, the secondary structure of β-conglycinin changed and helix1 and strand1 could be destroyed; however, the extent of these alterations depended on the pressure value. At the same time, the content of random coil increased considerably, which corresponded with a significant decrease in antigenicity. The helix1 and strand1 content decreased with the reduction in antigenicity, indicating that antigenicity epitopes are probably localized within the helix1 and strand1 structures of β-conglycinin.
Conclusion
This study has demonstrated that the antigenicity and structures of β-conglycinin could be strongly affected by HHP treatment. The antigenicity of β-conglycinin significantly decreased after HHP treated at 400 MPa. The values of free SH content and Ho increased significantly after HPP treatments at 200–400 MPa and in the range of 5–15 min. The maximum fluorescence intensity peak was observed at 400 MPa and 15 min, and higher HHP treatment pressure and processing time did not cause a longer maximum emission wavelength. The unordered and β-turns content of β-conglycinin increased significantly after HHP treatments. Thus, HHP treated at 400 MPa can be employed for antigenicity reduction of β-conglycinin, which can improve the security of soybean products.
Electronic supplementary material
Below is the link to the electronic supplementary material.
SDS-PAGE and Western Blot of purified β-conglycinin (A: SDS-PAGE of purified β-conglycinin; B: Western Blot of purified β-conglycinin) (M: Marker; 1: purified β-conglycinin) (TIFF 1272 kb)
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC, 31671778) and Key Scientific Research Projects of Henan Colleges and Universities under Grant No. (16A550001).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-017-2972-2) contains supplementary material, which is available to authorized users.
References
- Ambrosi V, Polenta G, Gonzalez C, Ferrari G, Maresca P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov Food Sci Emerg. 2016;38:294–301. doi: 10.1016/j.ifset.2016.05.009. [DOI] [Google Scholar]
- Beveridge T, Toma SJ, Nakai S. Determination of sh- and ss-groups in some food proteins using ellman’s reagent. J Food Sci. 1974;39(1):49–51. doi: 10.1111/j.1365-2621.1974.tb00984.x. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabanillas B, Maleki SJ, Rodríguez J, Cheng H, Teuber SS, Wallowitz ML, Crespo JF. Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem. 2014;157(1):141–147. doi: 10.1016/j.foodchem.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Cabanillas B, Cuadrado C, Rodriguez J, Hart J, Burbano C, Crespo JF, Novak N. Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem. 2015;183:18–25. doi: 10.1016/j.foodchem.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Elahi R, Mu TH. High hydrostatic pressure (HHP)-induced structural modification of patatin and its antioxidant activities. Molecules. 2017;22(3):438. doi: 10.3390/molecules22030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka VB, Dickinson E, Ledward DA. Influence of high pressure processing on protein solutions and emulsions. Curr Opin Colloid In. 2000;5(3–4):182–187. doi: 10.1016/S1359-0294(00)00055-8. [DOI] [Google Scholar]
- Hancock JD, Cao H, Kim IH, Li DF, Aumaitre A, Lee BD. Effects of processing technologies and genetic modifications on nutritional value of full-fat soybeans in pigs. Asian Austral J Anim. 2000;13(4):356–375. [Google Scholar]
- He L, Han M, Qiao S, He P, Li D, Li N, Ma X. Soybean antigen proteins and their intestinal sensitization activities. Curr Protein Pept Sc. 2015;16(7):613–621. doi: 10.2174/1389203716666150630134602. [DOI] [PubMed] [Google Scholar]
- Hu C, Chen H, Gao J, Luo C, Ma X, Tong P. High-pressure microfluidisation-induced changes in the antigenicity and conformation of allergen ara h 2 purified from chinese peanut. J Sci Food Agric. 2011;91(7):1304–1309. doi: 10.1002/jsfa.4318. [DOI] [PubMed] [Google Scholar]
- Hu G, Zheng Y, Liu Z, Deng Y, Zhao Y. Structure and ige-binding properties of α-casein treated by high hydrostatic pressure, UV-c, and far-IR radiations. Food Chem. 2016;204:46–55. doi: 10.1016/j.foodchem.2016.02.113. [DOI] [PubMed] [Google Scholar]
- Kajiyama N, Isobe S, Uemura K, Noguchi A. Changes of soy protein under ultra-high hydraulic pressure. Int J Food Sci Tech. 1995;30(2):147–158. doi: 10.1111/j.1365-2621.1995.tb01366.x. [DOI] [Google Scholar]
- Koshiyama I, Fukushima D. Comparison of conformations of 7s and 11s soybean globulins by optical rotatory dispersion and circular dichroism studies. Cereal Chem. 1973;50(1):114–121. [Google Scholar]
- Krishnan HB, Kim WS, Jang S, Kerley MS. All three subunits of soybean beta conglycinin are potential food allergens. J Agric Food Chem. 2009;57:938–943. doi: 10.1021/jf802451g. [DOI] [PubMed] [Google Scholar]
- Li YQ, Chen ZX, Mo HZ. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT Food Sci Technol. 2007;40(7):1167–1175. doi: 10.1016/j.lwt.2006.08.015. [DOI] [Google Scholar]
- Li H, Zhu K, Zhou H, Peng W. Effects of high hydrostatic pressure treatment on allergenicity and structural properties of soybean protein isolate for infant formula. Food Chem. 2012;132(2):808–814. doi: 10.1016/j.foodchem.2011.11.040. [DOI] [Google Scholar]
- Li H, Zhu K, Zhou H, Peng W, Guo X. Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric Immunol. 2016;27(5):1–20. doi: 10.1080/09540105.2015.1129602. [DOI] [Google Scholar]
- Ma X, He P, Sun P, Han P. Lipoic acid: an immunomodulator that attenuates glycinin-induced anaphylactic reactions in a rat model. J Agric Food Chem. 2010;58(8):5086–5092. doi: 10.1021/jf904403u. [DOI] [PubMed] [Google Scholar]
- Meng X, Bai Y, Gao J, Xin L, Chen H. Effects of high hydrostatic pressure on the structure and potential allergenicity of the major allergen bovine β-lactoglobulin. Food Chem. 2017;219:290–296. doi: 10.1016/j.foodchem.2016.09.153. [DOI] [PubMed] [Google Scholar]
- Messens W, Camp JV, Huyghebaert A. The use of high pressure to modify the functionality of food proteins. Trends Food Sci Technol. 1997;8(4):107–112. doi: 10.1016/S0924-2244(97)01015-7. [DOI] [Google Scholar]
- Molina E, Papadopoulou A, Ledward DA. Emulsifying properties of high pressure treated soy protein isolate and 7s and 11s globulins. Food Hydrocolloid. 2001;15(3):263–269. doi: 10.1016/S0268-005X(01)00023-6. [DOI] [Google Scholar]
- Nagano T, Hirotsuka M, Mori H, Kohyama K, Nishinari K. Dynamic viscoelastic study on the gelation of 7s globulin from soybeans. J Agric Food Chem. 1992;40(6):941–944. doi: 10.1021/jf00018a004. [DOI] [Google Scholar]
- Ogawa T, Bando N, Tsuji H, Nishikawa K, Kitamura K. Alpha-subunit of beta-conglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis. Biosci Biotechnol Biochem. 1995;59(5):831–833. doi: 10.1271/bbb.59.831. [DOI] [PubMed] [Google Scholar]
- Omi Y, Kato T, Ishida K, Kato H, Matsuda T. Pressure-induced release of basic 7s globulin from cotyledon dermal tissue of soybean seeds. J Agric Food Chem. 1996;44(12):3763–3767. doi: 10.1021/jf960231i. [DOI] [Google Scholar]
- Peñas E, Gomez R, Frias J, Baeza ML, Vidal-Valverde C. High hydrostatic pressure effects on immunoreactivity and nutritional quality of soybean products. Food Chem. 2011;125(2):423–429. doi: 10.1016/j.foodchem.2010.09.023. [DOI] [Google Scholar]
- Prieto N, Burbano C, Iniesto E, Rodríguez J, Cabanillas B, Crespo JF, Cuadrado C. A novel proteomic analysis of the modifications induced by high hydrostatic pressure on hazelnut water-soluble proteins. Foods. 2014;3(2):279–289. doi: 10.3390/foods3020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo C, Chapleau N, Speroni F, De Lamballerie-Anton M, Michel F, Añón C, Anton M. Physicochemical modifications of high-pressure-treated soybean protein isolates. J Agric Food Chem. 2004;52(6):1564–1571. doi: 10.1021/jf034813t. [DOI] [PubMed] [Google Scholar]
- Puppo MC, Beaumal V, Speroni F, Lamballerie MD, Añón MC, Anton M. β-conglycinin and glycinin soybean protein emulsions treated by combined temperature-high-pressure treatment. Food Hydrocolloid. 2011;25(3):389–397. doi: 10.1016/j.foodhyd.2010.07.005. [DOI] [Google Scholar]
- Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol. 2002;169(2):882–887. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- Wang XS, Tang CH, Li BS, Yang XQ, Li L, Ma CY. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloid. 2008;22(4):560–567. doi: 10.1016/j.foodhyd.2007.01.027. [DOI] [Google Scholar]
- Wang JM, Yang XQ, Yin SW, Zhang Y, Tang CH, Li BS, Yuan DB, Guo J. Structural rearrangement of ethanol-denatured soy proteins by high hydrostatic pressure treatment. J Agric Food Chem. 2011;59(13):7324. doi: 10.1021/jf201957r. [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. Dichroweb, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Blaschek K, Mejia E. Allergenic proteins in soybean: processing and reduction of p34 allergenicity. Nutr Rev. 2004;63(2):47–58. doi: 10.1111/j.1753-4887.2005.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li L, Tatsumi E, Kotwal S. Influence of high pressure on Conformational changes of soybean glycinin. Innov Food Sci Emerg. 2003;4(3):269–275. doi: 10.1016/S1466-8564(03)00043-2. [DOI] [Google Scholar]
- Zhang M, Zheng J, Ge K, Zhang H, Fang B, Jiang L, Guo H, Ding Q, Ren F. Glycation of α-lactalbumin with different size saccharides: effect on protein structure and antigenicity. Int Dairy J. 2014;34(2):220–228. doi: 10.1016/j.idairyj.2013.09.003. [DOI] [Google Scholar]
- Zhou H, Wang C, Ye J, Chen H, Tao R, Cao F. Effects of high hydrostatic pressure treatment on structural, allergenicity, and functional properties of proteins from ginkgo seeds. Innov Food Sci Emerg. 2016;34:187–195. doi: 10.1016/j.ifset.2016.02.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE and Western Blot of purified β-conglycinin (A: SDS-PAGE of purified β-conglycinin; B: Western Blot of purified β-conglycinin) (M: Marker; 1: purified β-conglycinin) (TIFF 1272 kb)