Abstract
The effect of the partial replacement of cocoa butter (CB) by cocoa butter equivalent (CBE) in the release of volatile compounds in dark chocolate was studied. The fatty acid profile, triacylglyceride composition, solid fat content (SFC) and melting point were determined in CB and CBE. Chocolate with CB (F1) and with different content of CBE (5 and 10%—F2 and F3, respectively) were prepared. Plastic viscosity and Casson flow limit, particle size distribution and release of volatile compounds using a solid phase microextraction with gas chromatography (SMPE-GC) were determined in the chocolate samples. The melting point was similar for the studied samples but SFC indicated different melting behavior. CBE showed a higher saturated fatty acid content when compared to CB. The samples showed similar SOS triglyceride content (21 and 23.7% for CB and CBE, respectively). Higher levels of POS and lower POP were observed for CB when compared to CBE (44.8 and 19.7 and 19 and 41.1%, respectively). The flow limit and plastic viscosity were similar for the studied chocolates samples, as well as the particle size distribution. Among the 27 volatile compounds identified in the samples studied, 12 were detected in significantly higher concentrations in sample F1 (phenylacetaldehyde, methylpyrazine, 2,6-dimethylpyrazine, 2-ethyl-5-methylpyrazine, 2-ethyl-3,5-dimethylpyrazine, tetramethylpyrazine, trimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, phenethyl alcohol, 2-acetylpyrrole, acetophenone and isovaleric acid). The highest changes were observed in the pyrazines group, which presented a decrease of more than half in the formulations where part of the CB was replaced by the CBE.
Keywords: Fat equivalent to cocoa butter, Cocoa butter, Volatile compounds, Chocolate
Introduction
Chocolate is a product obtained from the combination of different components of processed cocoa beans (cocoa mass or liquor, cocoa powder and cocoa butter) with other ingredients (Suzuki et al. 2011). It has a distinctive flavor with specific notes related to the cocoa bean genotype, the growth conditions and the processing factors (Afoakwa et al. 2008b). The typical sensory characteristics of chocolate are determined by the fermentation and drying of cocoa beans and also by the technological processes employed in chocolate making, such as refining, conching, tempering and crystallizing (Crafack et al. 2014).
Fermentation is a key processing stage that causes the death of the bean and facilitates removal of the pulp and subsequente drying. In addition, the formation of flavor precursors, such as amino acids and reducing sugars, is initiated along with the development of the cocoa color. The Maillard reaction occurs during the roasting process with the conversion of the amino acids and sugars into volatile compounds, such as pyrazines, aldehydes, alcohols, ethers, furans, thiazoles, pyrones, acids, esters, amines, oxazoles and pyrroles (Afoakwa et al. 2008b; Vítová et al. 2009). A complex mixture of up to 500 volatile compounds is responsible for the flavor of cocoa and chocolate. Pyrazines and heterocyclic nitrogen compounds with low molecular weight and high volatility represent 20% of volatile chocolate compounds in chocolate. High amounts of esters and hydrocarbons (13%), and acids (11%) are also reported (Perego et al. 2004).
The perception of the flavor of chocolate occurs when the cocoa butter (CB), which represents the continuous lipid phase, melts and the volatile compounds are released. It has been proposed that structural changes in the lipid phase can affect the release of volatiles, thus modifying the flavor perceived by the consumer (Nightingale et al. 2012). The volatility of aromatic compounds in lipids is dependent on the length of the chain and also on the degree of insaturation of fatty acids present in the triglycerides (Relkin et al. 2004). Roberts et al. (2003) and Miettinen et al. (2004) reported that the fat content of a food matrix and the degree of lipophilicity of volatile compounds can affect their release and, consequently the development of the flavor.
The CB is responsible for the dispersion of the other components of the formulation and the final quality of the chocolate, providing an exclusive melting profile. Its physico-chemical properties, including the rapid melting close to body temperature, result from the composition and symmetry of triacylglycerols (TAGs) comprised of around 42% of 1-palmitate-2-oleate-3-stearate triacylglycerol (POS); 24% of 1,3-distearate-2-oleate triacylglycerol (SOS), and 22% of 1,3-dipalmitate-2-oleate triacylglycerol (POP) (Naik and Kumarb 2014; Marty-Terrade and Marangoni 2015).
Considering the high cost of CB, vegetable fats known as cocoa butter equivalent (CBE) are commonly used in chocolate formulations. CBE are obtained by the fractioning and/or enzymatic interesterification of oils rich in TAGs such as POP (palm and palm kernel oils) and SOS (from exotic species such as karité, kokum or mango seed butters) (Jahurul et al. 2013; Rios et al. 2014).
Despite the ever-growing use of CBE in chocolate, the impact caused by their use to replace CB in terms of the release of the volatile compounds in chocolate is little known. The objective of this study was to investigate the effect of the partial replacement of CB by CBE on the release of volatile compounds in dark chocolate.
Materials and methods
Materials
Deodorized CB and cocoa mass were obtained from ADM Cocoa-Joanes Industrial (Ilheus, Brazil); CBE was obtained from Triângulo Alimentos (Itápolis, Brazil).; sugar from Raizen (Araçatuba, Brazil); soy lecithin from Imcopa (Araucaria, Brazil); PGPR (Polyglycerol Polyricinoleate) from Danisco Brazil (Pirapozinho, Brazil), and vanillin from Frey + Lau (Henstedt-Ulzburg, Germany).
Physico-chemical characterization of CB and CBE
CB and CBE were characterized for the following parameters according to the AOCS (2009): melting point (Cc 3.25); SFC (Cd 16b-93), using a nuclear magnetic resonance spectrometer mq20 (Bruker, Karlsruhe, Germany) and a dry bath TCON 2000 (Duratech, Indianapolis—USA) equipped with temperature control in the range of 0–70 °C. The fat samples were previously submitted to the following sequential heat treatment: heating at 100 °C (15 min); 5 min at 60 °C; 90 min at 0 °C; 40 h at 26 °C and 90 min at 0 °C. SFC was measured at the following temperatures: 10, 20, 25, 30, 35, 40 e 45 °C; fatty acid profile (Ce 1-62), using an Shimadzu CG 2010 gas chromatograph (Kyoto, Japan), with a capillary column RTX 2330, 100 m length, 0.25 mm internal diameter and 0.20 μm thick film. The chromatograph operating conditions were: split = 1:20; column flow = 1.16 mL/min.; detector temperature: 250 °C; injector temperature: 250 °C; oven temperature: 140 °C—6 min, 140–240 °C (4 °C/min), 240 °C—6 min; makeup gas: nitrogen; carrier gas: helium; injected volume: 1.0 μL; TAG composition: (Ce 5-86) was determined using an Agilent 6850 gas chromatograph Series CG System (California—USA) capillary column DB-17 HT Agilent Catalog: 122-1811 (50% phenyl-ethylpolysiloxane), 10 m length, 0.25 mm internal diameter and 0.15 µm thick film. Split = 1:100, column temperature program: initial temperature 250 °C raised at the rate of 5.0 °C/min to a final temperature of 350 °C. The detector temperature was 375 °C and the injector temperature was 360 °C. The carrier gas was helium, flow rate 1.0 mL/min, volume injected 1.0 μL and the sample concentration was 10 mg/mL diluted in tetrahydrofurane.
Chocolate production
The dark chocolate was formulated with 50% sugar; 34.5% cocoa mass; 0.3% soy lecithin; 0.2% of PGPR and 0.01% of vanillin. Formulation 1 (F1) was prepared with CB (15%); Formulation 2 (F2) was prepared with 10% of CB and 5% of CBE 5%, and the Formulation 3 (F3) with 5% of CB and 10% of CBE. The mixture of ingredients (sugar and cocoa mass) was performed in a pilot conch JAX Inox (Tambau—Brazil) with a capacity of 10 kg of product for a period of 15 min. After the mixture, the dry conching process (65 °C for 5 h) was carried out, only with sugar and cocoa mass. Then for the liquid conching, the CB and/or CBE were added. After 1 h of the liquid conching soy lecithin, PGPR and vanillin were added. The mass refining process was made in batch using a Netzsch mill balls PE 05 (Pomerode, Brazil) with a grinding chamber with a capacity of 3 L. A digital micrometer Mitutoyo 293, (Suzano, Brazil) was used to monitorate the reduction of the particle size in order to obtain particles with a maximum size of 25 µm. For the tempering process the chocolate (1 kg), was cooled from 45 to 28 °C using a cooling rate of 2.0 °C/min on a granite table. Then the chocolate was shaped into rectangular acrylic mold, (12.5 cm × 7 cm × 1.2 cm) crystallized in a refrigerator (8° C/45 min.), demolded manually, and packaged in metallised packaging.
Casson plastic viscosity and Casson yield value
The plastic viscosity and yield value of the samples were determined according to Liang and Hartel (2004) using a programmable rheometer Brookfield DV3T (Middleborough, EUA), equipped with an adapter for small samples and a cylinder spindle SC 4-27 coupled to a thermostatic bath Brookfield (Middleborough, EUA) kept at 40 °C. Before the measurement cycles, the chocolate samples were pre-sheared at 20 RPM for 10 min at 40 °C. Readings were performed in triplicate using ramp up (0.5–100 RPM) and ramp down (100–0.5 RPM) for each sample. The torque was recorded every 30 s reading at each point of the ramp. The data were analyzed according to the Casson model below modified to fluid rheology:
| 1 |
where τ is the shear stress (obtained from torque data) and and γ is the shear rate (obtained from the data RPM). The two parameters used to adjust the Casson model were τc (yield value) e ηc (plastic viscosity).
Determination of particle size distribution
The particle size distribution of the chocolate samples was performed according to Afoakwa et al. (2009), using a MasterSizer® 2000 Laser Diffraction Particle Size Analyzer (Malvern Instrument Ltd., Worcestershire, England). Size distribution was quantified as relative volume of particles in size bands presented as size distribution curves (Malvern Master-Sizer Micro Software v 2.19). The parameters obtained were specific surface area, largest particle size (Dv90), mean particle volume (Dv50) and smallest particle size (Dv10).
Volatile compounds
Static headspace isolation of volatile compounds was performed using SMPE-GC for 30 min at 55 °C onto a divinylbenzene/carboxen/polydimethylsiloxane, 50/30 µm Supelco fiber (Bellefonte, EUA). The fiber was previously conditioned (300 °C for 45 min) according to the supplier’s instructions. The sample preparation and extraction was performed according to Afoakwa et al. (2009), with some modifications. Chocolate (4 g) was mixed with water (3 g) and NaCl (1 g), and heated to 55 °C and intermittently stirred for 60 min for headspace equilibration. Each experiment had a system control sample, made by stirring an empty vial under the same conditions. Volatile compounds were desorbed (1 min) into the splitless injector, 10:1 (230 °C) in an Agilent GC system 7890A and an Agilent quadrupole mass spectrometer 5975C (Agilent Technologies—Santa Clara, USA) using a capillary column (polyethylene glycol, 30 m × 0.25 mm × 0.25 µm) Agilent J&W VF-WAXms (Santa Clara, USA). The temperature programme was: 2 min at 50 °C; 4 °C/min to 240 °C and finally 15 min at 230 °C with a flow of 1.3 mL/s. Compounds were fragmented using electron-impact ionisation (70 eV), with a source temperature of 200 °C, a scan range of 30–300. Components were identified based on comparison of mass spectra with those of spectral libraries NIST MS search 2.0, 2011(distributed Agilent Technologies) and quantified from the total area of the peaks (abundance × 105).
Statistical analysis
One-way analysis of variance (ANOVA) was performed and the mean values were compared by Tukey’s test (5% level of significance—p < 0.05). All analyses were carried out in duplicate or triplicate and the results were expressed as mean ± standard deviation (SD).
Results and discussion
Physico-chemical characterization of CB and CBE
Table 1 shows the results for melting point, SFC and fatty acids and triacylglycerols profiles of CB and CBE. The melting points observed for CB and CBE were very similar (34.5 and 34.8 °C respectively). Temperatures of melting point from 27 to 35 °C and from 30 to 45 °C have been reported for CB and CBE, respectively, depending on the origin and the fat modification process (Naik and Kumarb 2014; Torbica et al. 2014; Ribeiro et al. 2012; Saldaña et al. 2002; Depoortere 2011).
Table 1.
Melting point, SFC, fatty acids and TAGs profiles of the CB and CBE
| CB | CBE | |
|---|---|---|
| Melting point (°C) | 34.5 | 34.8 |
| SFC (%) | ||
| 10 °C | 81.0 | 90.0 |
| 20 °C | 66.5 | 73.2 |
| 25 °C | 57.4 | 63.0 |
| 30 °C | 29.0 | 43.5 |
| 35 °C | 0.1 | 3.5 |
| 40 °C | 0.0 | 0.1 |
| Fatty acids profile (%) | ||
| C 14:0 myristic | 0.1 | 0.0 |
| C 16:0 palmitic | 24.9 | 39.3 |
| C 16:1 palmitoleic | 0.7 | 0.0 |
| C 18:0 stearic | 34.1 | 23.3 |
| C 18:1 oleic | 33.1 | 32.0 |
| C 18:2 linoleic | 2.8 | 2.8 |
| C 20:0 arachidic | 0.6 | 0.0 |
| Others | 3.7 | 2.6 |
| TAGs profile (%) | ||
| PPS | 0.30 | 0.53 |
| POP | 18.98 | 41.08 |
| PLP | 1.89 | 3.06 |
| PSS | 0.30 | 0.08 |
| POS | 44.82 | 19.69 |
| POO | 2.37 | 5.26 |
| PLS | 0.55 | 1.09 |
| PLO | 0.52 | 0.50 |
| SSS | 1.66 | 0.43 |
| SOS | 21.05 | 22.73 |
| SOO | 5.08 | 2.95 |
| OOO + SLS | 1.43 | 2.09 |
| SLO | 0.39 | 0.09 |
| SOA | 0.66 | 0.42 |
Based on technical specifications provided by the manufacturer, a CBE with similar physicochemical characteristics to the cocoa butter was used in this study. Although CB and CBE showed similar melting points, the SFC indicated that they have different melting behavior. The SFC at 35 °C obtained for CBE (3.5%) was higher when compared to CB (0.1%) indicating an inferior melting performance in the mouth for CBE.
Brazilian CB is considered relatively soft with a lower SFC at 20, 25 and 30 °C (around 68, 57 and 18% respectively). In order to obtain a higher SFC in the chocolate formulation, which is necessary in tropical regions, Brazilian CB needs to be mixed with CB from others countries such as Ghana, India, Nigeria, Ivory Coast and Malaysia, which have a higher SFC, around 80% at 25 °C (Ribeiro et al. 2012).
The fatty acids profiles observed for the samples studied were similar to reported in the literature for this type of fat (Saldaña et al. 2002; Ribeiro et al. 2012; Depoortere 2011; Lipp et al. 2001). Stearic and palmitic acids were the main saturated fatty acids determined for CB and CBE, respectively. The high content of palmitic acid in CBE indicate the use of palm oil or its fractions with other sources rich in oleic acid (Depoortere 2011; Lipp et al. 2001).
Although CB and CBE showed similar contents of TAGS such as SOS (21 and 23.7% respectively), the POP and POS content were different between the samples. This difference in the triglyceride composition influences the melting profile and performance of fats. Similar TAG compositions have been reported for CB from different regions of Brazil (Ribeiro et al. 2012). Depoortere (2011) reported a similar triacylglycerol composition for CBE. On the other hand, Undurraga et al. (2001) reported for a CBE obtained by enzymatic interesterification using fractions of palm oil a TAG composition similar with 23.4% of POP; 38.5% of POS, and 20.2% of SOS.
Rheological characterization
The substitution of CB with CBE did not significantly influence the rheological characteristics of the samples, which showed shear stress values of 6.48–6.52 Pa and plastic viscosity values of 2.59–2.62 Pa s.
The mass flow characteristics of the chocolate influence its flavor and texture and are thus directly related to the sensory characteristics of the final product (Nebesny and Zyżelewicz 2005; Afoakwa et al. 2009). Afoakwa et al. (2008a) reported that the rheological characteristics of the chocolate mass can be improved with the use of emulsifiers such as soy lecithin and PGPR. The fat content of the chocolate formulation also influences these characteristics and related with the release of volatile compounds. Afoakwa et al. (2009) reported for chocolate with fat content of 35%, and a lower plastic viscosity, exhibit greater release of components with cocoa-chocolate-praline notes (trimethypyrazine, 3-methylbutanal, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine, tetramethylpyrazine, linalool oxide and 2,3,5-triethyl-5-methylpyrazine) when compared with chocolates with lower fat contents (25% and 30%). In chocolates with higher fat content, the solid particles distributed in the lipid phase are more dispersed and have a more exposed surface area to release volatiles. On the other hand in chocolates with lower fat content, the particles are more packed, making it difficult to release volatiles.
Particle size distribution
The chocolate samples showed very similar particle size distributions (Table 2) since the process conditions and solid nonfat contents in the formulations were similar. The particle size distribution of chocolate is related to the rheological (Afoakwa et al. 2007) and sensory (Lenfant et al. 2013) characteristics. According Beckett (2011), dark chocolate tend to increase the flavor of cocoa with a smaller particle size. Afoakwa et al. (2009) reported a greater release of volatile compounds such as trimethypyrazine, 3-methylbutanal, 2,3-dimethylpyrazine, 2,5-dimethylpyrazine, tetramethylpyrazine, linalool oxide, 2,3,5-triethyl-5-methylpyrazine., 2-phenylethanol, furfuryl alcohol (furfurol), methylpyrazine, phenylacetaldehyde, 2,3,5-trimethyl-6-ethylpyrazine and 2-carboxaldehyde-1-H-pyrrole, characterized by the aromas of cocoa, chocolate, caramel, honey and sweetness in chocolate with smaller particle size (Dv90 between 18–25 µm), when compared to chocolate with larger particle size (Dv90 entre 35–50 µm). This increase in the release of volatiles is related to the increase of the surface area of the particles.
Table 2.
Specific surface area and particle size distribution of the dark chocolate samples
| Formulation | Specific surface area (m2/g) | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) |
|---|---|---|---|---|
| F1 | 0.88 ± 0.03 | 3.00 ± 0.02 | 9.38 ± 0.02 | 28.2 ± 0.03 |
| F2 | 0.90 ± 0.02 | 2.95 ± 0.03 | 9.41 ± 0.02 | 27.9 ± 0.04 |
| F3 | 0.89 ± 0.03 | 2.96 ± 0.04 | 9.47 ± 0.03 | 27.7 ± 0.03 |
Analysis of volatile compounds
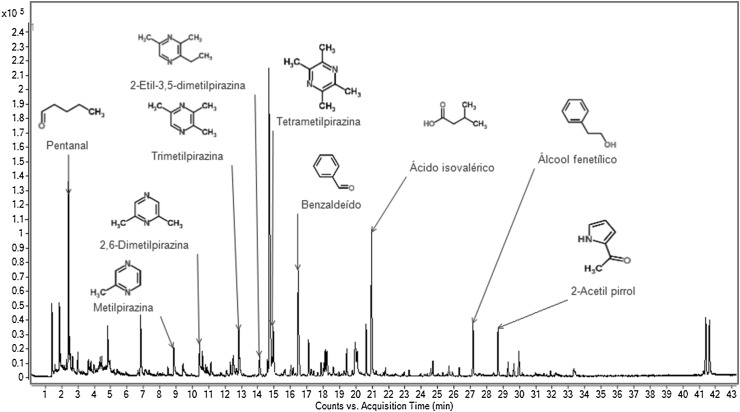
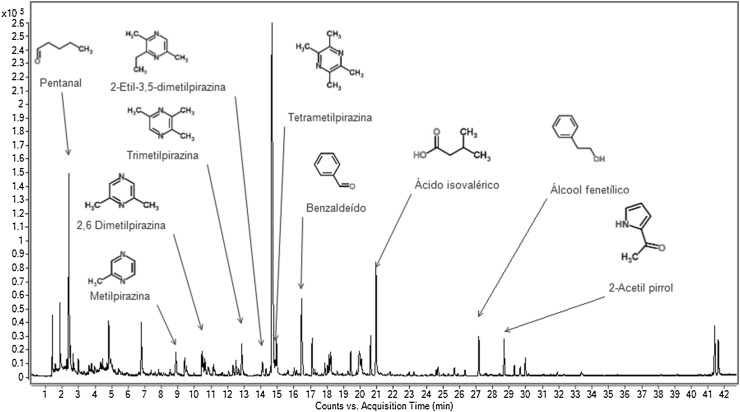
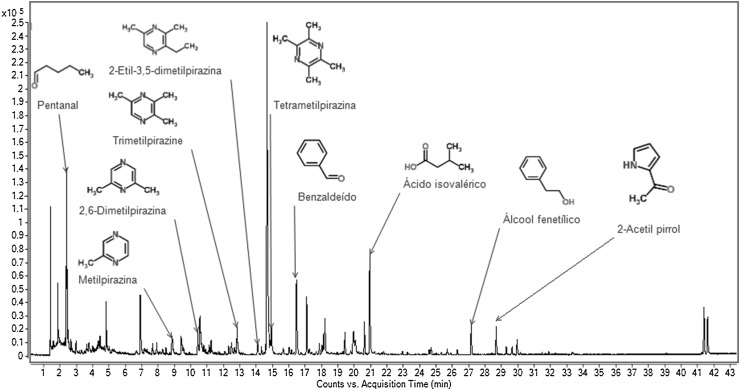
Table 3 and Figs. 1, 2 and 3 show that 27 volatile compounds were identified in the studied samples. The phenylacetaldehyde, methylpyrazine, 2,6-dimethylpyrazine, 2-ethyl-5-methylpyrazine, 2-ethyl-3,5-dimethylpyrazine, tetramethylpyrazine, trimethylpyrazine, 3-ethyl-2,5-dimethylpyrazine, phenethyl alcohol, 2-acetylpyrrole acetophenone and isovaleric acid sample were detected in significantly higher concentrations (p ≤ 0.05) in sample F1.
Table 3.
Volatile compounds identified in samples of dark chocolate and its description of odor
| Volatile compounds | (Abundance × 105) | Description of odor* | ||
|---|---|---|---|---|
| F1 | F2 | F3 | ||
| Aldehydes | ||||
| 2-Methylpropanal | 6.63a | 7.24a | 7.17a | Malt and chocolate |
| 2-Methylbutanal | 32.8a | 36.6a | 34.8a | Malt and chocolate |
| Pentanal | 3.36a | 2.60a | 1.90a | Pungent and bitter almond |
| Benzaldehyde | 27.6a | 22.5a | 21.2a | Walnut, bitter almond and grass |
| Phenylacetaldehyde | 13.3a | 10.7b | 10.1b | Honey and floral |
| 2-Furaldehyde | 3.74a | 3.72a | 3.24a | Caramel and sweet |
| Pyrazines | ||||
| Methylpyrazine | 8.53a | 7.05b | 5.51c | Cocoa and hazelnuts |
| 2.6-Dimethylpyrazine | 7.23a | 5.29c | 5.83b | Roasted, baked, walnut and coffee |
| 2.5-Dimethylpyrazine | 4.08a | 4.86a | 4.61a | Baked, boiled and popcorn |
| 2.3-Dimethylpyrazine | 2.21a | 2.37a | 2.23a | Cooked, nut, caramel, cocoa and sweet |
| 2-Ethyl-6-methylpyrazine | 2.78a | 2.28a | 3.18a | Cocoa and roasted |
| 2-Ethyl-5-methylpyrazine | 5.25a | 3.16b | 2.83c | Cocoa and roasted |
| Trimethylpyrazine | 13.4a | 8.89b | 7.49b | Cocoa. roasted and baked |
| 2-Ethyl-3.5-dimethylpyrazine | 6.18a | 4.27b | 3.23c | Roasted, smoke, cocoa and chocolate |
| 3-Ethyl-2.5-dimethylpyrazine | 3.89a | 2.78b | 2.08b | Roasted, smoke, cocoa and chocolate |
| Tetramethylpyrazine | 13.4a | 10.2b | 8.12c | Coffee with milk, roasted and chocolate |
| Furans | ||||
| 5-Methylfurfural | 2.84a | 2.11a | 1.88a | Caramel and sweet |
| Furan-3-methanol | 9.54a | 7.97a | 6.73a | – |
| Alcohols | ||||
| 2.3-Butanediol | 7.81a | 7.99a | 7.96a | Sweet and floral |
| (2R.3R)-(−)-2.3-Butanediol | 7.43a | 7.64a | 7.48a | Sweet and floral |
| 2-Phenylethanol | 10.7a | 8.81b | 7.36c | Floral |
| Ketones | ||||
| Acetophenone | 6.90a | 4.81b | 3.84c | Floral and sweet |
| Pyrroles | ||||
| 2-Acetyl-pyrrole | 9.05a | 7.34b | 6.13c | Caramel |
| Acids | ||||
| acetic acid | 102.0a | 122.0a | 99.9a | Astringent and vinegar |
| Butyric acid | 5.41a | 4.50a | 4.09a | Rancid butter |
| Isovaleric acid | 34.1a | 28.0b | 25.4c | Rancidity |
| Others | ||||
| Vanillin | 13.0a | 12.96a | 12.98a | Vanilla |
*Afoakwa et al. (2009) and Crafack et al. (2014)
**Same letters in the same line indicate that the values are not statistically different from each other (Tukey test p ≤ 0.05)
***Different letters in the same line indicate that the values are statistically different from each other (p ≤ 0.05 Tukey test)
Fig. 1.
Volatile compounds in dark chocolate formulated with CB
Fig. 2.
Volatile compounds in dark chocolate formulated with 5% of CBE
Fig. 3.
Volatile compounds in dark chocolate formulated with 10% of CBE
The aldehydes and pyrazines are the mainly volatile compounds responsible for the aroma in both cocoa mass and chocolate. When the CB was replaced by CBE, the greatest changes were observed in the pyrazine group. The release of volatile compounds in foods is not well understood yet. Several studies indicate that the volatility of aromatic compounds in lipids longer fatty acids chains and saturated fatty acids absorb greater quantities of volatile compounds (Roberts et al. 2003; Relkin et al. 2004). This may explain the decrease in the release of volatile compounds in the samples formulated with CBE. The saturated fatty acids observed in CBE were 2.9% higher than in the CB. The effect of lipids on aromatic compounds is dependent mainly on the lipophilicity of aromatic compounds (Miettinen et al. 2004). Fat has a significant effect on the partitioning of volatile compounds between the product and the gas phase, with lipophilic aromatic compounds being the most affected (Van Ruth et al. 2002; Shiota et al. 2011). Table 3 shows that seven volatile compounds of the pyrazine group reduced significantly the release, out of a total of ten compounds. Heterocyclic aromatic compounds are the most volatile compounds for the cocoa aroma, and among them are alkylpyrazines which contribute to the desirable aroma of chocolate. These pyrazines are considered to be more complex and more alkyl-substituted, and thus have higher lipophilicity (Welty et al. 2001; Afoakwa et al. 2009). Therefore the greater degree of saturation and higher hydrophobicity of the CBE may be associated with the decrease in the release of the pyrazines. The volatility of the aromatic compounds is greatly dependent on the temperature, molecular interactions and the partition coefficient of the particular compound, which will determine the affinity of the volatile molecules for each phase. Since chocolate is a continuous lipid phase, the highest flavor perception occurs due to the retronasal action of the volatile compounds released during the melting. Structural changes of the lipid phase may alter the release of volatiles affecting as a result the chocolate flavor profile (Afoakwa et al. 2009; Welty et al. 2001; Nightingale et al. 2012).
Some studies have suggested that the SFC of lipid matrixes can influence the release of volatile compounds in emulsions (Rabe et al. 2003; Relkin et al. 2004). However, according to Roberts et al. (2003), the effect of the SFC on the release of volatiles can be only observed in the presence of some percentage of solid in the lipid phase.
Table 1 shows that CBE has a higher solid fat content at all temperatures, when compared with CB. In addition, considering that the SPME-GC assay was carried out at 55 °C, the SFC of the fat was not a significant factor in terms of the changes in the release of volatiles in the chocolate samples, since at 40 °C the solids content in fats is almost zero.
Conclusion
The volatile compounds profile of dark chocolate is modified by the addition of CBE and the effect is more pronounced in the pyrazine group. The composition of fatty acids as well as the level of saturation was a determinant factor for the release of volatile compounds. The results showed that the selection of the CBE in the process of formulation and production of the dark chocolate is an important step and it is directly related to the overall final quality of the product.
Acknowledgements
The authors would like to thank Rossana Podestá from Department of Food Science and Technology (UFSC) and Rosane Kolling from Department of R&D of Duas Rodas company for their assistance with CG analysis.
Contributor Information
Cristiano de Silva Souza, Email: cristiano.qui@hotmail.com.
Jane Mara Block, Email: janeblock@gmail.com.
References
- Afoakwa EO, Paterson A, Fowler M. Factors influencing rheological and textural qualities in chocolate—a review. Trends Food Sci Technol. 2007;18(6):290–298. doi: 10.1016/j.tifs.2007.02.002. [DOI] [Google Scholar]
- Afoakwa EO, Paterson A, Fowler M, et al. Comparison of rheological models for determining dark chocolate viscosity. J Food Sci Technol. 2008;44(1):162–167. doi: 10.1111/j.1365-2621.2008.01710.x. [DOI] [Google Scholar]
- Afoakwa EO, Paterson A, Fowler M, et al. Flavor formation and character in cocoa and chocolate: a critical review. Crit Rev Food Sci Nutr. 2008;48(9):840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- Afoakwa EO, Paterson A, Fowler M, et al. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC–mass spectrometry and GC–olfactometry. Food Chem. 2009;113:208–215. doi: 10.1016/j.foodchem.2008.07.088. [DOI] [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. Champaign: AOCS Press; 2009. [Google Scholar]
- Beckett ST. Industrial chocolate manufacture and use. 4. Hoboken: Wiley-Blackwell; 2011. [Google Scholar]
- Crafack M, Keul H, Eskildsen CE, et al. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res Int. 2014;63:306–316. doi: 10.1016/j.foodres.2014.04.032. [DOI] [Google Scholar]
- Depoortere L (2011) The use and applicability of cocoa butter equivalents (CBEs) in chocolate products. Master thesis, Ghent University, Belgium
- Jahurul MHA, Sarker MZI, Norulaini NAN, et al. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J Food Eng. 2013;117(4):467–476. doi: 10.1016/j.jfoodeng.2012.09.024. [DOI] [Google Scholar]
- Lenfant F, Hartmann C, Watzke B, et al. Impact of the shape on sensory properties of individual dark chocolate pieces. LWT-Food Sci Technol. 2013;51(2):545–552. doi: 10.1016/j.lwt.2012.11.001. [DOI] [Google Scholar]
- Liang B, Hartel RW. Effects of milk powders in milk chocolate. J Dairy Sci. 2004;87(1):20–31. doi: 10.3168/jds.S0022-0302(04)73137-9. [DOI] [PubMed] [Google Scholar]
- Lipp M, Simoneau C, Ulberth F, et al. Composition of genuine cocoa butter and cocoa butter equivalents. J Food Compos Anal. 2001;14(4):399–408. doi: 10.1006/jfca.2000.0984. [DOI] [Google Scholar]
- Marty-Terrade S, Marangoni AG (2015) Impact of cocoa butter origin on crystal behavior. In: Garti N, Widlak NR (eds) Cocoa butter and related compounds: challenges in food systems. AOCS Press, Urbana, Illinois, United States
- Miettinen SM, Hyvonen L, Linforth RST, et al. Temporal aroma delivery from milk systems containing 0–5% added fat, observed by free choice profiling, time intensity, and atmospheric pressure chemical ionization-mass spectrometry techniques. J Agric Food Chem. 2004;52(26):8111–8118. doi: 10.1021/jf040218v. [DOI] [PubMed] [Google Scholar]
- Naik B, Kumarb V. Cocoa butter and its alternatives: a reveiw. J Bioresour Eng Technol. 2014;1:07–17. [Google Scholar]
- Nebesny E, Zyżelewicz D. Effect of lecithin concentration on properties of sucrose-free chocolate masses sweetened with isomalt. Eur Food Res Technol. 2005;220(2):131–135. doi: 10.1007/s00217-004-1009-z. [DOI] [Google Scholar]
- Nightingale LM, Cadwallader KR, Engeseth NJ. Changes in dark chocolate volatiles during storage. J Agric Food Chem. 2012;60(18):4500–4507. doi: 10.1021/jf204718z. [DOI] [PubMed] [Google Scholar]
- Perego P, Fabiano B, Cavicchioli M, et al. Cocoa quality and processing: a study by solid-phase microextraction and gas chromatography analysis of methylpyrazines. Food Bioprod Process. 2004;82(4):291–297. doi: 10.1205/fbio.82.4.291.56402. [DOI] [Google Scholar]
- Rabe S, Krings U, Berger RG. Influence of oil-in-water emulsion characteristics on initial dynamic flavour release. J Sci Food Agric. 2003;83(11):1124–1133. doi: 10.1002/jsfa.1513. [DOI] [Google Scholar]
- Relkin P, Fabre M, Guichard E. Effect of fat nature and aroma compound hydrophobicity on flavor release from complex food emulsions. J Agric Food Chem. 2004;52(20):6257–6263. doi: 10.1021/jf049477a. [DOI] [PubMed] [Google Scholar]
- Ribeiro APB, Silva RC, Gioielli LA, et al. Physico-chemical properties of Brazilian cocoa butter and industrial blends. Part I chemical composition, solid fat content and consistency. Grasas Aceites. 2012;63(1):79–88. doi: 10.3989/gya.069011. [DOI] [Google Scholar]
- Rios RVR, Pessanha M, Almeida PF, et al. Application of fats in some food products. J Food Sci Technol. 2014;34(1):3–15. doi: 10.1590/S0101-20612014000100001. [DOI] [Google Scholar]
- Roberts DR, Pollien P, Watzke B. Experimental and modeling studies showing the effect of lipid type and level on flavor release from milk-based liquid emulsions. J Agric Food Chem. 2003;51(1):189–195. doi: 10.1021/jf025646k. [DOI] [PubMed] [Google Scholar]
- Saldaña M, Mohamed RS, Mazzafera P, et al. Extraction of ocoa butter from Brazilian cocoa beans using supercritical CO2 and ethane. Ind Eng Chem Res. 2002;41(26):6751–6758. doi: 10.1021/ie0203936. [DOI] [Google Scholar]
- Shiota M, Isogai T, Iwasawa A, et al. Model studies on volatile release from different semisolid fat blends correlated with changes in sensory perception. J Agric Food Chem. 2011;59(9):4904–4912. doi: 10.1021/jf104649y. [DOI] [PubMed] [Google Scholar]
- Suzuki RM, Montanher PF, Visentainer JV, et al. Proximate composition and quantification of fatty acids in five major Brazilian chocolate brands. J Food Sci Technol. 2011;31(2):541–546. doi: 10.1590/S0101-20612011000200040. [DOI] [Google Scholar]
- Torbica AM, Pajin B, Omorjan R, et al. Physical properties of chocolate with addition of cocoa butter equivalent of moderate hardness. J Am Oil Chem Soc. 2014;91(1):39–48. doi: 10.1007/s11746-013-2357-2. [DOI] [Google Scholar]
- Undurraga D, Markovits A, Erazo S. Cocoa butter equivalent through enzymic interesterification of palm oil midfraction. Process Biochem. 2001;36(10):933–939. doi: 10.1016/S0032-9592(00)00260-0. [DOI] [Google Scholar]
- Van Ruth S, King C, Giannouli P. Influence of lipid fraction, emulsifier fraction, and mean particle diameter of oil-in-water emulsions on the release of 20 aroma compounds. J Agric Food Chem. 2002;50(8):2365–2371. doi: 10.1021/jf011072s. [DOI] [PubMed] [Google Scholar]
- Vítová E, Loupancova B, Štoudková H, et al. Effect of fat composition on some physico-chemical parameters and sensorial evaluation of dark chocolate. J Food Nutr Res. 2009;48(2):72–79. [Google Scholar]
- Welty WM, Marshall RT, Grün IU, et al. Effects of milk fat, cocoa butter, or selected fat replacers on flavor volatiles of chocolate ice cream. J Dairy Sci. 2001;84(1):21–30. doi: 10.3168/jds.S0022-0302(01)74447-5. [DOI] [PubMed] [Google Scholar]