Abstract
The purposes of this study were to identify physicochemical properties and evaluate bioactive compound levels and antioxidant characteristics at 30 day intervals during the 90 days of fermentation of gochujang fortified with five different varieties of red pepper: Juktoma pepper (RP1), facing heaven pepper (RP2), Thai chili pepper (RP3), bird’s eye pepper (RP4), and red bell pepper (RP5). Physicochemicals properties, including reducing sugar, capsaicin, pH, β-carotene, and color parameters, of gochujang were evaluated. Antioxidant compounds of total polyphenols and total flavonoids were analyzed with antioxidant activities of DPPH and FRAP assays. The results showed that gochujangs (GRP1, GRP5) fortified with RP1, and RP5, had consistently higher values of reducing sugars, total polyphenols, and total flavonoids with antioxidant activities, but lower values of capsaicin, pH, β-carotene, and color parameters as compared to GRP2, GRP3, GRP4 during 90 days of fermentation. GRP5 especially had the highest reducing sugar, amino acid contents, total polyphenols, and total flavonoids with antioxidant activities and the lowest value of capsaicin during the 90 days of fermentation.
Keywords: Red pepper, Fermentation, Antioxidant activity, Physicochemical properties, Gochujang
Introduction
Gochujang is a fermented soybean-based red pepper paste that has been an integral part of Korean cuisine for more than 300 years. Gochujang is prepared by fermenting a mixed paste of red pepper (Capsicum annuum L.) powder, glutinous rice, salt, meju, and water. Various chemical components of gochujang combine during the fermentation process and affect the qualities of color and pungency from the red pepper: the sweet taste from the degraded carbohydrates, umami taste from the degraded proteins, and salty taste from the salts. Also, gochujang is known to have beneficial health effects, including anti-obesity (Shin et al. 2016), anti-diabetic (Kwon et al. 2009), and anti-cancer effects (Kim et al. 2005). The major bioactive effects among the various biological compounds in gochujang are related to both polyphenolic compounds and capsaicin derivatives in meju and red pepper. Meju is a fermented cooked soybean product that contains various types of metabolites and phytochemicals, with decreasing glycosides and increasing aglycones of isoflavones (Yun et al. 2012). Capsaicin, a key component of red pepper, has also been known to exert antioxidant, anti-inflammatory, anti-cancer, and anti-obesity effects (Anandakumar et al. 2008; Gannon et al. 2016; Kim and Lee 2014; Zheng et al. 2016; Clark and Lee 2016).
Many studies have shown the effects of additives on the taste of gochujang (Lee et al. 2011), aroma quality assessment of gochujang (Kang and Baek, 2014); and the effect of different microflora on the quality improvement of gochujang (Jang et al. 2011; Kim et al. 2008). However, there is limited information regarding the physicochemical characteristics and antioxidant properties of gochujang fortified with various types of red pepper varieties during the fermentation period. Therefore, the aim of this study was to identify the changes in physicochemical properties and bioactive compounds levels during the 90 days of fermentation of five gochujang samples, each fortified with a different variety of red peppers (Capsicum annuum L.): Juktoma pepper (RP1), Facing heaven pepper (RP2), Thai chili pepper (RP3), Bird's eye pepper (RP4), and Red bell pepper (RP5). The physicochemical properties and bioactive compound levels, including the total polyphenols, flavonoids contents, β-carotene, and capsaicin concentration; were analyzed at 30 days interval. Furthermore, the antioxidant activities of gochujang were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the ferric reducing/antioxidant power (FRAP) assays.
Materials and methods
Materials and reagents
“Juktoma” pepper (RP1) from Korea, “Facing Heaven” pepper (RP2) from China, “Thai Chili” pepper from Thailand (RP3), “Bird's Eye” pepper (RP4) from Vietnam, and “Red Bell” pepper (RP5) were used in this experiment were from 2015 harvest. RP1 was purchased from a local market in Jungeup agricultural market, Jeollabuk-do, South Korea. RP2 was obtained from a local farm in Kunming city, Yunnan Province, China. RP3 was purchased from Coman International Co. (Hwasung, Korea). RP4 was purchased from Hachannarae Co. (Hwaseong, Korea). RP5 was purchased from a local market, Hongseong-gun, Gangwon-do, Korea. Other materials including waxy rice powder, domestic solar sea salt, and meju powder were purchased at the Jungeup agricultural market. Folin-Ciocalteu’s reagent, gallic acid, rutin, DPPH, potassium persulfate, trichloroacetic acid (TCA), and potassium ferricyanide were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). All chemicals and reagents used in this study were of analytical grade.
Preparation and extraction
Gochujang samples were prepared based on the method described by Sunchang’s gochujang preparation (Jin et al. 2007) with a 2-step process of saccharification and fermentation (Table 1). In the first step, barley malt was soaked in deionized water overnight at a temperature of 20 °C, and then filtered. Glutinous rice powder was added to the filtered barley malt solution while stirring, and then heated at 60 °C for saccharification. In the second step, salt was added into the saccharified solution, and then boiled for 30 min. After cooling to 40 °C, red pepper powder and meju powder were added and mixed well into the rice paste. The mixed paste, gochujang, was then fermented at 25 °C for 90 days in an aerobic incubator (Hanbaek Scientific Co., Bucheon, Korea). Gochujang was mixed with deionized water into semi-liquid at the ratio of 1:3. The samples were extracted thrice with deionized water for 1 h at room temperature in a shaker (NB-101 M Shaker, N-Biotek, Korea). The extracts were centrifuged (4000 rpm, 10 min) and the supernatants were collected. The collected supernatants were stored at − 70 °C for 2 days and then dried in a freeze–dryer. Finally, the dried extracts were stored at − 20 °C before analysis.
Table 1.
Gochujang ingredients used in the experiment
| Ingredients | Samples | ||||
|---|---|---|---|---|---|
| GRP1 | GRP2 | GRP3 | GRP4 | GRP5 | |
| Barley malt (g) | 275 | 275 | 275 | 275 | 275 |
| Glutinous rice (g) | 1265 | 1265 | 1265 | 1265 | 1265 |
| Meju powder (g) | 275 | 275 | 275 | 275 | 275 |
| Red pepper powder (g) | 1375 | 1375 | 1375 | 1375 | 1375 |
| Salt (g) | 660 | 660 | 660 | 660 | 660 |
| Water (g) | 3300 | 3300 | 3300 | 3300 | 3300 |
GRP1 Gochujang made with RP1 (red pepper 1, Juktoma), GRP2 Gochujang made with RP2 (red pepper 2, facing heaven), GRP3 Gochujang made with RP3 (red pepper 3, Thai chili); GRP4 Gochujang made with RP4 (red pepper 4, bird’s eye), GRP5 Gochujang made with RP5 (red pepper 5, red bell)
Physicochemical property
The reducing sugar content of gochujang was analyzed by the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959). A 0.5 mL sample of gochujang extract was mixed with 2 mL of DNS reagent, followed by incubation at 100 °C for 3 min. After cooling, the absorbance at 570 nm was measured. The reducing sugar content (%) was expressed as glucose equivalents. The pH of gochujang was measured by a calibrated pH meter (Orion 3-STAR BT pH meter, USA).
Capsaicin
Capsaicin was extracted from the samples of gochujang by applying the technique described by Collins et al. (1995). The capsaicin was extracted from a 2.4 g sample of gochujang in 6 mL acetonitrile by heating in a water bath at 80 °C for 4 h. The suspended solution was left to cool, added to 9 volumes of high performance liquid chromatography (HPLC) grade water, filtered into a 2 mL glass vial using 0.45 μm syringe filter (Sartorius Stedim Biotech, Germany), and then used for HPLC (Agilent Technologies 1100 series, Palo alto, CA, USA) on ZORBAX Eclipse XDB-C18 (4.6 × 250 mm, 5 μm, Agilent, Santa Clara, CA, USA), associated with UV–Visible detector VWD HP 1100 operating at 338 nm (excitation = 340 nm).
Color parameters
The color values of gochujang were analyzed using a spectrocolorimeter (JS-555, Color Techno System Co. Ltd. Japan) tristimulus color analyzer calibrated with a white porcelain reference plate. The color coordinates of the uniform color space CIE-LAB , , were determined by its reflectance and chromaticity. The value indicates brightness ranging from black ( = 0) to white ( = 100). The value indicates redness ranging from − 60 (green) to 60 (red) and the value ranges from − 60 (blue) to 60 (yellow). Chroma () was calculated as follows:
| 1 |
Amino acids
For the determination of free amino acids in gochujang, a 3.0 g sample was homogenized at 12,000 rpm for 3 min with 20 mL ice-cold 6.0% (v/v) perchloric acid using ACE homogenizer (Nissei AM-7, Nihonseiki Kaisha Ltd., Tokyo, Japan). The homogenized sample was incubated in an ice bath for 30 min before centrifugation at 3000 g for 10 min. The supernatant was filtered through a Whatman No. 41 filter paper and the pH of the filtrate was adjusted to pH 7.0 using a 33% (W/V) KOH solution. The sample solution centrifuged at 3000 g for 10 min to remove the precipitate of potassium perchlorate. The supernatant was acidified to pH 2.2 with 10 M HCl solution and then diluted to 50 mL with distilled water. Two milliliters of the extract solution was transferred into a clean tube and 1.0 mL of lithium citrate buffer (pH 2.2) was added. The amino acids within the extract solution were determined using HP 1100 liquid chromatograph (Hewlett Packard Wilmington, DE, USA) with a variable wavelength detector VWD HP 1100 operating at 338 nm (excitation = 340 nm). Separation was carried out with a Zorbax Eclipse AAA Rapid Resolution column (150 × 4.6 mm I.D., 5 μm particle size, Agilent Technologies, Palo Alto, CA, USA). A linear gradient profile of mobile phases, comprising 40 mM Na2HPO4, pH 7.8 (solvent A) and CAN: MeOH: water 45: 45: 10 (v/v) (solvent B), 0% B (0–1.9 min), 0–57% (1.9–18.1 min), 57–100% (18.1–18.8 min), 100% (18.8–22.3 min), 100–0% (22.3–23.2 min) and 0% (23.2–26 min), was applied at a flow rate of 2.0 mL min−1. The column was equilibrated for 5 min under initial conditions prior to injection of the next sample. The column temperature was 40 °C. In order to determine amino acids in gochujang, pre-column derivatization with o-phthalaldehyde (OPA) was used and 20 μL portions were injected into the HPLC system. Data analysis was performed using Chemstation software (Hewlett Packard, Wilmington, DE, USA).
Antioxidant contents
The total polyphenol content of gochujang was determined according to the method described by Arnous et al. (2001). Briefly, a 1.6 mL aliquot of diluted gochujang was mixed with 0.1 mL of Folin-Ciocalteu reagent, followed by the addition of 0.3 mL of 1 mol/L Na2CO3 solution. The reaction was then allowed to proceed for 1 min. The absorbance at 765 nm was measured after 20 min at room temperature in the dark. The total polyphenol content was expressed as milligrams of gallic acid equivalents (GAE).
The total flavonoid content was analyzed according to the method described by Shen et al. (2009). Two mL of deionized water and 0.15 mL of 0.5 mol/L NaNO2 solution were mixed with 0.5 mL of gochujang, and allowed to react at room temperature for 5 min. Then, 0.3 mL of 0.4 mol/L AlCl3·6H2O solution was added and the samples were incubated for 5 min before the addition of 0.3 mL of 1 mol/L NaOH solution. The absorbance was measured at 415 nm after 15 min incubation. The total flavonoid content was expressed as rutin equivalents (RE).
Antioxidant activity
The free radical scavenging activity of gochujang, based on the scavenging activity of the stable DPPH free radical, was determined using the method described by Brand-Williams et al. (1995) with slight modifications. Briefly, 0.2 mL of gochujang was added to 1.0 mL of 0.2 mmol/L DPPH dissolved in ethanol solution. After incubating the solution at room temperature in the dark for 30 min, the absorbance was measured at 517 nm, and the radical scavenging activity was expressed as percent inhibition:
| 2 |
where A C was the absorbance of the control (blank), and A S was the absorbance of the extract.
The antioxidant capacity of gochujang was determined using the FRAP (ferric reducing/antioxidant power) assay described by Benzie and Strain (1996) with some modifications. The stock solutions included 300 mmol/L acetate buffers (pH 3.6), 10 mmol/L TPTZ (2,4,6-tripyridyl-s-triazine) solution dissolved in 40 mmol/L HCl, and 20 mmol/L FeCl3·6H2O solution. The working solution was prepared by mixing 25 mL of acetate buffer, 2.5 mL of TPTZ solution, and 2.5 mL of 20 mmol/L FeCl3·6H2O solution. Then 0.1 mL of gochujang was added to 0.3 mL of the FRAP solution with 2.7 mL distilled water and then incubated at room temperature in the dark for 30 min. Color changes were then measured at 593 nm and the standard curve was linear between 0 and 200 μmol/L of Trolox. The result was expressed as mmol/L Trolox equivalent per gram of gochujang.
Statistical analysis
All experiments were carried out in triplicate and data were expressed as mean ± standard deviation (SD) using SPSS version 17.0 (IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) and Duncan’s multiple comparison test were used to determine the significance of the difference among samples with a significance level of 0.05.
Results and discussion
Characteristics of red peppers
The weight, length, soluble solids, pH, capsaicin, total polyphenol, and total flavonoid contents of 5 varieties of red peppers are given in Table 2. The capsaicin content of RP2 (318.85 mg/100 g), RP3 (121.19 mg/100 g), and RP4 (140.88 mg/100 g) were higher than that of RP1 (17.50 mg/100 g), whereas RP5 (0.42 mg/100 g) was the lowest. The total polyphenol content of RP5 (639.38 mg GAE/100 g) was the highest, followed by RP1 (392.89 mg GAE/100 g), RP2 (371.79 mg GAE/100 g), RP3 (339.65 mg GAE/100 g), and RP4 (275.41 mg GAE/100 g). The total flavonoid content also showed similar patterns as those of the total polyphenol content. Based on this analysis, RP5 had not only the highest sugar content, total polyphenol and total flavonoid contents, but also the lowest capsaicin content.
Table 2.
Characteristics of dried red peppers
| Sample | Varieties | Weight (g) | Length (cm) | Soluble solids (°Brix) | pH | Capsaicin (mg/100 g) | Total polyphenol (mg GAE/100 g) | Total flavonoid (mg RE/100 g) |
|---|---|---|---|---|---|---|---|---|
| RP1 | Juktoma | 3.58 ± 0.26b | 11.38 ± 0.27e | 5.00 ± 0.00c | 4.51 ± 0.00b | 17.50 ± 0.08b | 392.89 ± 0.03d | 8.20 ± 0.08d |
| RP2 | Facing heaven | 0.42 ± 0.06a | 3.46 ± 0.11a | 4.53 ± 0.12b | 3.97 ± 0.01a | 318.85 ± 0.71e | 371.79 ± 0.01c | 7.24 ± 0.02c |
| RP3 | Thai chili | 0.61 ± 0.03a | 5.10 ± 0.37c | 4.60 ± 0.00b | 4.75 ± 0.01d | 121.19 ± 0.09c | 339.65 ± 0.04b | 6.06 ± 0.05b |
| RP4 | Bird’s eye | 0.51 ± 0.05a | 4.56 ± 0.22b | 4.20 ± 0.00a | 4.84 ± 0.01e | 140.88 ± 0.18d | 275.41 ± 0.02a | 4.43 ± 0.01a |
| RP5 | Red bell | 6.81 ± 0.34c | 8.68 ± 0.70d | 7.00 ± 0.00d | 4.53 ± 0.01c | 0.42 ± 0.05a | 639.38 ± 0.05e | 9.40 ± 0.03e |
Values are mean ± standard deviation; values followed by different letters in the same column are significantly different by Duncan’s multiple range test (p < 0.05)
RP1 Red pepper 1, RP2 Red pepper 2, RP3 Red pepper 3, RP4 Red pepper 4, RP5 Red pepper 5
Physicochemical properties of gochujang
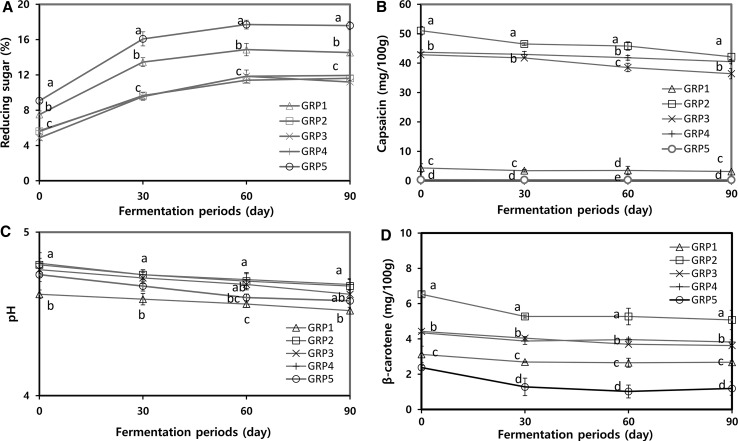
The reducing sugar content (4.87–9.06%) of gochujang after the preparation speedily increased until 30 days of the fermentation, and then slightly increased up to 60 days of the fermentation (11.40–17.71%) (Fig. 1a). For the fermentation period, the reducing sugar content of GRP5 was the highest and followed by GRP1; whereas GRP2, GRP3, and GRP4 were the lowest. The reducing sugars are produced from the degradation of starch, which is continuously converted to sugar, alcohol, and acids by an amylase from a natural microflora of meju such as Aspergillus oryzae and Bacillus sp. (Jang et al. 2011). Cho et al. (2013) have shown that β-glucosidase was produced during the manufacture of gochujang, and that the β-glucosidase activity of gochujang increased during the early period of fermentation and then decreased gradually because it contains salt and pungent compounds like hot pepper capsaicinoids, which may provide an inadequate environment for the growth of microorganisms. The capsaicin contents of gochujang for the fermentation period are shown in Fig. 1b. The capsaicin contents of gochujang after the preparation decreased until 90 days of the fermentation and the capsaicin contents of GRP2, GRP3, and GRP4 were much higher than those of GRP1 and GRP5 during the 90 days of the fermentation. Lee et al. (2015) have shown that the capsaicin content from gochujang decreased during the incubation period by using Aspegillus oryzae as the starter, but new metabolites including N-vanillylcarbamoylbutyric acid, N-vanillyl-9-hydroxy-8-methyloctanamide, ω-hydroxycapsaicin, 8-methyl-N-vanillylcarbamoyl-6(E)-octenoic acid, and 2-methyl-N-vanillylcarbamoyl-6(Z)-octenoic acid increased. The pH values of all gochujang samples decreased during the 90 days of the fermentation. On 90th day of the fermentation, the pH values ranged from 4.52 to 4.68 (Fig. 1c). The salt contents (9.95–10.29%) were almost constant during the 90 d of the fermentation (data not shown). It is well known that β-carotene is not only an effective quencher of singlet oxygen like a protective effect from UV and infra-red radiation, but also a color pigment of yellow-orange like carrots, sweet potatoes, apricots, and red pepper (Freitas et al. 2015). The β-carotene content of GRP2 was the highest after the preparation of gochujang, and flattened until 90th day of the fermentation after decreasing till the 30th day, whereas the content of GRP5 was the lowest and the patterns were similar when compared to other gochujangs during the fermentation (Fig. 1d). Panda et al. (2007) showed that the β-carotene content of sweet potato decreased depending on the lactic acid fermentation period. Degradation of β-carotene content in gochujang could also be due in part to the metabolism of bacteria and the environmental conditions such as temperature, pH, and sugar content.
Fig. 1.
Changes in physicochemical properties of five types of gochujang for 90 days of fermentation. Deferent letters above the bar for the fermentation period indicate the significance difference at p < 0.05, by Duncan’s multiple range test
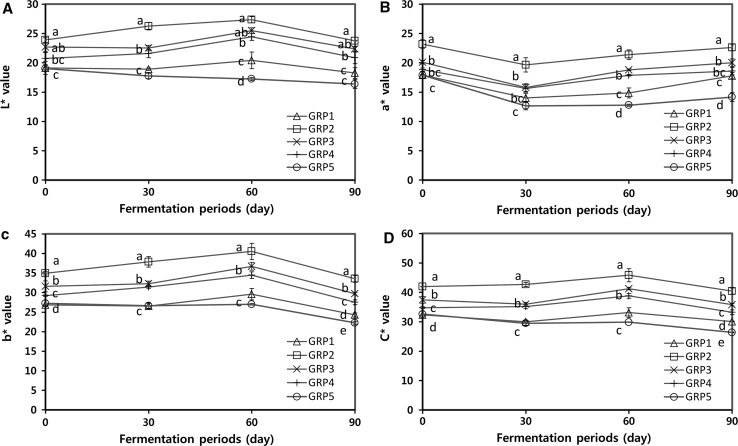
Color in gochujang is an important quality factor in consumers’ purchase intention. Lightness (), redness (), yellowness (), and chroma () of gochujang were determined during the fermentation period of 90 days (Fig. 2). GRP2 had the highest values in all color parameters, followed by GRP3 > GRP4 > GRP1 > GRP5. On 90th day of the fermentation, lightness ( value) and yellowness ( value) of gochujang samples overall decreased when compared to gochujangs samples from the day of preparation, while the slightly increasing trend was observed until the 60th day of fermentation (Fig. 2a, c). The values of gochujang samples showed decreasing trend up to the 30th of the fermentation, and then increased during the fermentation (Fig. 2b). Chacón-Ordóñez et al. (2016) have shown the main carotenoids of red bell pepper were β-carotene and capsanthin with various capsanthin esters. Lee et al. (2005) have shown that Korean traditional fermented radish kimchi made with red pepper powder decreased the capsaicin content whereas capsanthin level increased during the fermentation period. In the present results, the decreased values of gochujang until the 30th day of the fermentation might be positively related with the β-carotene (Fig. 1d) and the increased values of gochujang might be positively related to capsanthin (data not present in this result). Chroma () reflects color brilliance or purity with intensity of color saturation. The values of GRP2, GRP3, and GRP4 were the highest, indicating an increase of saturation until the 60th day of the fermentation, whereas GRP5 progressively decreased until 90th day of the fermentation (Fig. 2d).
Fig. 2.
Changes in color parameters of five types of gochujang for 90 days of fermentation. Deferent letters above the bar for the fermentation period indicate the significance difference p < 0.05, by Duncan’s multiple range test
Amino acid changes of gochujang
Changes in the free amino acids of gochujang during the fermentation period are shown in Table 3. A variety of amino acids were dynamically altered for the fermentation periods. Total amino acids of GRP5 was the highest followed by: GRP4 > GRP2 > GRP1 > GRP3 on 90th day of the fermentation. Contents of sweet taste amino acids (alanine, glycine, serine, threonine) increased in all gochujang samples during the fermentation period. Particularly, alanine content (38.57 mg/100 g to 55.34 mg/100 g) of gochujang were the highest among other sweet taste amino acids, followed by threonine (17.44 mg/100 g to 49.57 mg/100 g), glycine (7.17 mg/100 g to 17.11 mg/100 g), and serine (3.94 mg/100 g to 10.00 mg/100 g). Schiffman et al. (1981) showed that alanine (1.62 × 10−2 M) had the lowest threshold than those of other sweet amino acids such as glycine (3.09 × 10−2 M), threonine (2.57 × 10−2 M), and serine (2.09 × 10−2 M). Glutamate content and aspartate, known as umami taste amino acid, increased from 60 to 80 and 15 to 50%, respectively, during the fermentation period. Particularly, GRP5 had the highest concentrations of aspartate (116.09 mg/100 g), glycine contents (17.11 mg/100 g), and higher concentration of glutamate content (73.15 mg/100 g) when compared to GRP1, GRP2, GRP3 on 90th day of the fermentation. Branched-chain amino acids (leucine, isoleucine, valine) and sulfur-containing amino acids (methionine and cysteine) are related with bitter and sulfurous perception (Garbriel and Uneyama 2013). GRP4 had the highest content of branched chain amino acids, and relatively high content sulfur-containing amino acids. The results may be related to the microorganisms and their environmental conditions, such as pungent compounds like capsaicinoids and sugar content.
Table 3.
Amino acids content of five types of Gochujang (mg/100 g)
| Amino Acid | Fermentation day | GRP1 | GRP2 | GRP3 | GRP4 | GRP5 |
|---|---|---|---|---|---|---|
| Alanine | 0 | 47.95 ± 0.19 | 47.39 ± 0.13 | 44.43 ± 0.44 | 46.75 ± 0.10 | 38.57 ± 0.18 |
| 30 | 51.92 ± 0.51 | 53.29 ± 0.36 | 47.93 ± 0.07 | 48.79 ± 0.30 | 43.62 ± 0.31 | |
| 60 | 54.50 ± 1.48 | 55.65 ± 0.21 | 48.27 ± 27.95 | 50.76 ± 0.57 | 47.03 ± 0.22 | |
| 90 | 54.99 ± 1.20 | 55.34 ± 0.40 | 49.10 ± 0.14 | 52.99 ± 0.21 | 49.61 ± 0.32 | |
| Asparagine | 0 | 16.14 ± 0.51 | 19.7 ± 0.76 | 15.35 ± 0.43 | 21.21 ± 0.30 | 36.23 ± 0.35 |
| 30 | 19.06 ± 0.34 | 23.81 ± 0.58 | 18.82 ± 0.12 | 23.32 ± 0.12 | 40.45 ± 0.12 | |
| 60 | 21.13 ± 0.16 | 25.81 ± 0.44 | 19.84 ± 0.11 | 25.35 ± 0.26 | 43.95 ± 0.72 | |
| 90 | 22.12 ± 0.25 | 26.47 ± 0.60 | 20.87 ± 0.32 | 26.79 ± 0.36 | 45.71 ± 0.56 | |
| Aspartate | 0 | 64.64 ± 1.62 | 56.20 ± 0.48 | 34.52 ± 0.79 | 48.94 ± 0.40 | 77.62 ± 0.91 |
| 30 | 68.28 ± 1.58 | 65.94 ± 0.60 | 40.76 ± 0.03 | 54.55 ± 0.16 | 93.05 ± 1.72 | |
| 60 | 73.25 ± 0.58 | 70.65 ± 1.35 | 42.95 ± 0.27 | 60.10 ± 0.31 | 104.50 ± 2.55 | |
| 90 | 74.13 ± 0.21 | 74.98 ± 0.08 | 46.15 ± 0.60 | 64.51 ± 0.30 | 116.09 ± 1.14 | |
| Cystine | 0 | nd | nd | nd | 9.71 ± 0.16 | 7.13 ± 0.05 |
| 30 | nd | 9.95 ± 0.16 | nd | 9.85 ± 0.18 | 8.36 ± 0.06 | |
| 60 | 2.68 ± 3.79 | 10.31 ± 0.43 | 3.01 ± 4.26 | 9.90 ± 0.03 | 8.5 5 ± 0.10 | |
| 90 | 7.60 ± 0.31 | 10.06 ± 0.25 | 6.24 ± 0.09 | 9.98 ± 0.11 | 9.42 ± 6.25 | |
| Glutamate | 0 | 34.85 ± 0.49 | 38.85 ± 0.26 | 34.22 ± 0.28 | 50.20 ± 0.17 | 44.03 ± 0.67 |
| 30 | 48.08 ± 0.22 | 56.87 ± 0.05 | 49.59 ± 0.08 | 64.23 ± 0.80 | 57.46 ± 4.07 | |
| 60 | 57.42 ± 1.66 | 66.90 ± 0.20 | 55.40 ± 0.03 | 72.44 ± 1.70 | 64.41 ± 4.23 | |
| 90 | 59.06 ± 1.01 | 70.78 ± 0.59 | 58.22 ± 0.31 | 79.94 ± 0.56 | 73.15 ± 1.97 | |
| Glycine | 0 | 7.17 ± 0.47 | 10.48 ± 0.22 | 8.78 ± 0.37 | 10.75 ± 0.18 | 12.32 ± 0.25 |
| 30 | 9.64 ± 0.23 | 13.31 ± 0.16 | 10.50 ± 0.15 | 11.88 ± 0.25 | 14.91 ± 0.27 | |
| 60 | 10.81 ± 0.46 | 14.34 ± 0.29 | 10.90 ± 0.08 | 13.07 ± 0.13 | 16.11 ± 0.25 | |
| 90 | 11.13 ± 0.47 | 14.64 ± 0.08 | 11.48 ± 0.03 | 13.79 ± 0.05 | 17.11 ± 0.04 | |
| Histidine | 0 | 12.66 ± 0.31 | 13.26 ± 0.49 | 10.71 ± 0.12 | 15.80 ± 0.03 | 13.30 ± 0.45 |
| 30 | 16.45 ± 0.21 | 18.47 ± 0.12 | 14.24 ± 0.11 | 19.23 ± 0.13 | 18.24 ± 0.20 | |
| 60 | 18.83 ± 0.02 | 21.34 ± 0.29 | 16.08 ± 0.34 | 21.42 ± 0.26 | 20.96 ± 0.09 | |
| 90 | 19.72 ± 0.12 | 22.66 ± 0.17 | 17.63 ± 0.27 | 22.93 ± 0.25 | 23.45 ± 0.29 | |
| Isoleucine | 0 | 10.68 ± 0.17 | 10.68 ± 0.17 | 10.00 ± 0.14 | 14.68 ± 0.20 | 10.18 ± 0.42 |
| 30 | 14.60 ± 0.02 | 14.60 ± 0.02 | 12.48 ± 0.11 | 16.97 ± 0.18 | 13.99 ± 0.07 | |
| 60 | 16.46 ± 0.03 | 16.46 ± 0.03 | 13.46 ± 0.20 | 18.73 ± 0.04 | 15.22 ± 0.17 | |
| 90 | 17.17 ± 0.04 | 17.17 ± 0.04 | 14.05 ± 0.16 | 19.62 ± 0.11 | 17.37 ± 0.09 | |
| Leucine | 0 | 12.13 ± 0.05 | 12.13 ± 0.05 | 11.75 ± 0.11 | 20.84 ± 0.06 | 15.23 ± 0.61 |
| 30 | 19.79 ± 0.27 | 19.79 ± 0.27 | 17.16 ± 0.03 | 25.57 ± 0.18 | 22.09 ± 0.55 | |
| 60 | 22.82 ± 0.26 | 22.82 ± 0.26 | 18.78 ± 0.20 | 27.80 ± 0.25 | 25.99 ± 0.46 | |
| 90 | 24.34 ± 0.14 | 24.34 ± 0.14 | 20.40 ± 0.43 | 30.06 ± 0.31 | 29.08 ± 0.65 | |
| Lysine | 0 | 16.13 ± 0.13 | 16.13 ± 0.13 | 12.68 ± 0.03 | 17.78 ± 0.05 | 12.98 ± 0.37 |
| 30 | 23.45 ± 1.15 | 23.45 ± 1.15 | 18.20 ± 0.06 | 22.83 ± 0.35 | 17.97 ± 0.04 | |
| 60 | 25.49 ± 0.10 | 25.49 ± 0.10 | 19.87 ± 0.25 | 25.21 ± 0.16 | 20.51 ± 0.07 | |
| 90 | 26.62 ± 0.06 | 26.62 ± 0.06 | 20.77 ± 0.41 | 27.28 ± 0.17 | 22.59 ± 0.01 | |
| Methionine | 0 | nd | nd | nd | 5.13 ± 0.36 | 4.23 ± 0.27 |
| 30 | 5.12 ± 0.60 | 4.99 ± 0.16 | nd | 4.41 ± 0.12 | 5.77 ± 0.04 | |
| 60 | 5.30 ± 0.54 | 4.93 ± 0.41 | 3.72 ± 0.16 | 4.65 ± 0.05 | 5.90 ± 0.08 | |
| 90 | 7.61 ± 0.43 | 5.38 ± 0.35 | 3.38 ± 0.53 | 5.11 ± 0.07 | 6.18 ± 0.05 | |
| Phenylalanine | 0 | 10.50 ± 0.63 | 14.22 ± 0.03 | 14.80 ± 0.06 | 16.38 ± 0.40 | 11.77 ± 0.04 |
| 30 | 14.78 ± 0.36 | 19.24 ± 0.54 | 18.77 ± 0.10 | 19.34 ± 0.19 | 16.67 ± 0.33 | |
| 60 | 16.68 ± 0.31 | 21.31 ± 0.32 | 19.85 ± 0.29 | 21.08 ± 0.25 | 18.63 ± 0.16 | |
| 90 | 17.18 ± 0.22 | 21.87 ± 0.04 | 20.51 ± 0.25 | 22.67 ± 0.25 | 20.57 ± 0.16 | |
| Serine | 0 | 3.94 ± 0.12 | 5.76 ± 0.19 | 5.22 ± 0.11 | 5.80 ± 0.06 | 4.64 ± 0.03 |
| 30 | 5.51 ± 0.16 | 7.69 ± 0.30 | 6.82 ± 0.35 | 10.00 ± 4.28 | 5.71 ± 0.01 | |
| 60 | 6.58 ± 0.43 | 7.99 ± 0.07 | 7.17 ± 0.09 | 7.94 ± 0.03 | 6.60 ± 0.03 | |
| 90 | 6.74 ± 0.59 | 8.20 ± 0.09 | 7.54 ± 0.09 | 8.18 ± 0.14 | 7.01 ± 0.16 | |
| Threonine | 0 | 17.44 ± 0.40 | 41.82 ± 0.34 | 23.83 ± 0.55 | 37.39 ± 0.47 | 17.44 ± 0.48 |
| 30 | 23.89 ± 0.18 | 47.86 ± 0.14 | 29.48 ± 0.20 | 39.85 ± 0.15 | 24.09 ± 0.58 | |
| 60 | 28.25 ± 0.28 | 49.57 ± 0.08 | 31.21 ± 0.18 | 42.14 ± 0.50 | 27.77 ± 0.55 | |
| 90 | 29.58 ± 0.73 | 49.56 ± 0.34 | 32.56 ± 0.14 | 43.57 ± 1.52 | 30.14 ± 0.11 | |
| Tyrosine | 0 | 7.57 ± 0.36 | 9.36 ± 0.06 | 8.61 ± 0.04 | 11.25 ± 0.12 | 8.41 ± 0.02 |
| 30 | 10.94 ± 0.45 | 14.96 ± 0.31 | 12.63 ± 0.04 | 15.77 ± 0.19 | 13.35 ± 0.27 | |
| 60 | 13.04 ± 0.51 | 16.84 ± 0.37 | 14.22 ± 0.08 | 16.45 ± 0.21 | 14.80 ± 0.17 | |
| 90 | 13.33 ± 0.38 | 17.77 ± 0.39 | 15.05 ± 0.26 | 18.74 ± 0.40 | 17.29 ± 0.64 | |
| Valine | 0 | 14.42 ± 0.26 | 19.63 ± 0.18 | 17.63 ± 0.18 | 23.15 ± 0.16 | 18.91 ± 0.16 |
| 30 | 17.96 ± 0.35 | 24.99 ± 0.04 | 20.97 ± 0.04 | 25.52 ± 0.65 | 23.91 ± 0.21 | |
| 60 | 19.95 ± 0.10 | 27.18 ± 0.12 | 21.98 ± 0.34 | 27.83 ± 0.22 | 26.47 ± 0.04 | |
| 90 | 20.19 ± 0.05 | 27.90 ± 0.26 | 22.74 ± 0.26 | 29.27 ± 0.25 | 27.88 ± 0.23 | |
| Total | 0 | 276.22 ± 17.54 | 315.61 ± 17.01 | 252.53 ± 12.59 | 340.92 ± 15.50 | 321.63 ± 19.37 |
| 30 | 344.35 ± 18.63 | 404.27 ± 19.04 | 318.35 ± 15.05 | 397.85 ± 17.45 | 405.51 ± 22.80 | |
| 60 | 385.21 ± 20.09 | 442.35 ± 20.61 | 339.98 ± 15.40 | 430.32 ± 19.23 | 452.95 ± 25.53 | |
| 90 | 396.30 ± 21.48 | 458.30 ± 21.48 | 357.07 ± 15.88 | 460.34 ± 20.84 | 497.05 ± 28.33 |
GRP1 Gochujang made with RP1 (Juktoma), GRP2 Gochujang made with RP2 (facing heaven), GRP3 Gochujang made with RP3 (Thai chili), GRP4 Gochujang made with RP4 (bird’s eye), GRP5 Gochujang made with RP5 (red bell)
Antioxidant properties of gochujang
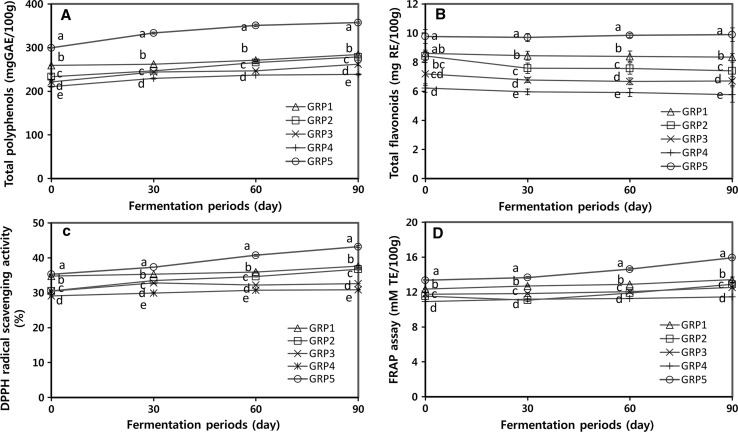
There is increasing evidence that consumption of phenolics and polyphenolics in foods may lower the risk of diseases because of their antioxidant properties (Gharras 2009; Shahidi and Ambigaipalan 2015). To clarify the antioxidant properties of gochujang during the fermentation period, the total polyphenols and total flavonoids contents were evaluated (Fig. 3a, b). The total polyphenol contents of gochujangs increased during the fermentation period (Fig. 3a). The total polyphenol content of GRP5 (357.38 mg/100 g) was significantly highest on 90th day of the fermentation than those of other gochujangs in the following order: GRP1 (284.27 mg/100 g) > GRP2 (278.79 mg/100 g) > GRP3 (261.77 mg/100 g) > GRP4 (238.57 mg/100 g). The total flavonoid contents of gochujangs very slightly decreased and flattened during the fermentation period and did not significantly change (Fig. 3b). The total flavonoid content of GRP5 (9.89 mg/100 g) was the highest on 90th day of the fermentation, followed by: GRP1 (8.35 mg/100 g) > GRP2 (7.41 mg/100 g) > GRP3 (6.70 mg/100 g) > GRP4 (5.77 mg/100 g).
Fig. 3.
Changes in antioxidant properties of five types of gochujang for 90 days of fermentation. Deferent letters above the bar for the fermentation period indicate the significance difference p < 0.05, by Duncan’s multiple range test
To evaluate the antioxidant activities of gochujang, we performed antioxidant activity measurements such as DPPH radical scavenging activity and FRAP assay. DPPH radical scavenging activity is described by the ability to undergo single electron transfer by two components in the reaction mixture of antioxidants with oxidant, such as DPPH radicals. The assay is also operationally simple and easy to use (MacDonald-Wicks et al. 2006). The DPPH free radical scavenging activities of all gochujang samples increased during the fermentation period (Fig. 3c). The DPPH activity of GRP5 (43.19%) was the highest, followed by GRP1 (38.28%), GRP2 (37.59%), GRP3 (34.59%), and GRP4 (31.88%) on 90th day of the fermentation (p < 0.05). These results were similar to the increase of total polyphenol concentrations and might be related to the characteristics of GRP5, which contains the lowest capsaicin and the highest total polyphenol with total flavonoids (Table 2). The FRAP values of GRP5 was the highest among the gochujang samples during the fermentation period, whereas the value of GRP4 was the lowest (Fig. 3d). These results were similar to those obtained in the DPPH radical scavenging activity and the increase in the antioxidant activities of gochujang samples may be due to the increase in total polyphenols, even though β-carotene and total flavonoid contents decreased until the 30th day of the fermentation period.
Conclusion
In this study, GRP1 and GRP5 had higher values of reducing sugar and antioxidant compounds including total polyphenols and total flavonoids, with antioxidant activities when compared to GRP2, GRP3, and GRP4. The concentrations of capsaicin, β-carotene, and pH of GRP1 and GRP5 were lower compared to those of GRP2, GRP3, and GRP4 during the 90 days of the fermentation period. The high capsaicin content may be related to hostile environment for the growth of bacteria in gochujang, resulting in difference of physicochemical and antioxidant properties. Particularly, GRP5, shown the lowest capsaicin content, had the highest reducing sugar content and antioxidant activities. GRP5 may be positively related to not only health benefits with high total polyphenols and total flavonoids values, but also savory sensation with high reducing sugar and umami amino acids contents. These results can be utilized in gochujang development with increased antioxidant properties.
Acknowledgements
This research was supported by WonKwang University under research Grant 2015. There is no potential conflict of interest reported by the authors.
Contributor Information
Hyun Jung Yang, Email: hjwhite15@khu.ac.kr.
Young Soon Lee, Email: yysllee@hanmail.net.
Il Sook Choi, Phone: 82-63-850-6657, Email: choiis@wku.ac.kr.
References
- Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Vinodhkumar R, Devaki T. Capsaicin modulates pulmonary antioxidant defense system during benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother Res. 2008;22(4):529–533. doi: 10.1002/ptr.2393. [DOI] [PubMed] [Google Scholar]
- Arnous A, Makris DP, Kefalas P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem. 2001;49(12):5736–5742. doi: 10.1021/jf010827s. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chacón-Ordóñez T, Esquivel P, Jiménez VM, Carle R, Schweiggert RM. Deposition form and bioaccessibility of keto-carotenoids from mamey sapote (Pouteria sapota), red bell pepper (Capsicum annuum), and sockeye salmon (Oncorhynchus nerka) filet. J Agric Food Chem. 2016;64(9):1989–1998. doi: 10.1021/acs.jafc.5b06039. [DOI] [PubMed] [Google Scholar]
- Cho JY, Lee HJ, Shin HC, Lee JM, Park KH, Moon JH. Behavior of flavonoid glycosides contained in Korean red pepper paste (Gochujang) during fermentation: participation of a β-glucosidase inhibitor. Food Sci Biotechnol. 2013;22(5):1–8. [Google Scholar]
- Clark R, Lee SH. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016;36(3):837–843. [PubMed] [Google Scholar]
- Collins MD, Wasmund LM, Bosland PW. Improved method for quantifying capsaicinoids in Capsicum using high-performance liquid chromatography. HortScience. 1995;30(1):137–139. [Google Scholar]
- Freitas JV, Praҫa FS, Bentley MV, Gaspar LR. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int J Pharm. 2015;484(1–2):131–137. doi: 10.1016/j.ijpharm.2015.02.062. [DOI] [PubMed] [Google Scholar]
- Gannon NP, Lambalot EL, Vaughan RA. The effects of capsaicin and capsaicinoid analogs on metabolic molecular targets in highly energetic tissues and cell types. BioFactors. 2016;42(3):229–246. doi: 10.1002/biof.1273. [DOI] [PubMed] [Google Scholar]
- Gharras HE. Polyphenols: food sources, properties and applications—a review. Int J Food Sci Technol. 2009;44(12):2512–2518. doi: 10.1111/j.1365-2621.2009.02077.x. [DOI] [Google Scholar]
- Jang SJ, Kim YJ, Park JM, Park YS. Analysis of microflora in gochujang, Korean traditional fermented food. Food Sci Biotechnol. 2011;20(5):1435–1440. doi: 10.1007/s10068-011-0197-0. [DOI] [Google Scholar]
- Jin HS, Kim JB, Lee KJ. Major microbial composition and its correlation to the taste of Sunchang traditional kochujang. Korean J Food Nutr. 2007;20(4):363–386. [Google Scholar]
- Kang KM, Baek HH. Aroma quality assessment of Korean fermented red pepper paste (gochujang) by aroma extract dilution analysis and headspace solid-phase microextraction-gas chromatography-olfactometry. Food Chem. 2014;145:488–495. doi: 10.1016/j.foodchem.2013.08.087. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee JS. Anti-inflammatory activity of capsaicin and dihydrocapsaicin through heme oxygenase-1 induction in raw264.7 macrophages. J Food Biochem. 2014;38(4):381–387. [Google Scholar]
- Kim SO, Kong CS, Kil JH, Kim JY, Han MS, Park KY. Fermented wheat grain products and kochujang inhibit the growth of AGS human gastric adenocarcinoma cells. J Food Sci Nutr. 2005;10(4):349–352. [Google Scholar]
- Kim YS, Oh BH, Shin DH. Quality characteristics of Kochujang prepared with different Meju fermented with Aspergillus sp. and Bacillus subtilis. Food Sci Biotechnol. 2008;17(3):527–533. [Google Scholar]
- Kwon DY, Hong SM, Ahn IS, Kim YS, Shin DW, Park S. Kochujang, a Korean fermented red pepper plus soybean paste, improves glucose homeostasis in 90% pancreatectomized diabetic rats. Nutrition. 2009;25(7–8):790–799. doi: 10.1016/j.nut.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee KT, Kim MR. Effect of gamma-irradiated red pepper powder on the chemical and volatile characteristics of kakdugi, a Korean traditional fermented radish kimchi. J Food Sci. 2005;70(7):C441–C447. doi: 10.1111/j.1365-2621.2005.tb11466.x. [DOI] [Google Scholar]
- Lee SY, Park SL, Yi SH, Nam YD, Lim SI. Quality characteristics of low-salt gochujang added with Glycyrrhiza uralensis and Brassica juncea. J Food Sci Nutri. 2011;16(4):348–356. [Google Scholar]
- Lee M, Cho JY, Lee YG, Lee HJ, Lim SI, Park SL, Moon JH. Bioconversion of capsaicin by Aspergillus oryzae. J Agric Food Chem. 2015;63(26):6102–6108. doi: 10.1021/acs.jafc.5b01730. [DOI] [PubMed] [Google Scholar]
- MacDonald-Wicks LK, Wood LG, Garg ML. Methodology for the determination of biological antioxidant capacity in vitro: a review. J Sci Food Agric. 2006;86(13):2046–2056. doi: 10.1002/jsfa.2603. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Panda SH, Parmanick M, Ray RC. Lactic acid fermentation of sweet potato (Ipomoea batatas L.) into pickles. J Food Process Preserv. 2007;31(1):83–101. doi: 10.1111/j.1745-4549.2007.00110.x. [DOI] [Google Scholar]
- San Gabriel A, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids. 2013;45(3):451–461. doi: 10.1007/s00726-012-1371-2. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Sennewald K, Gagnon J. Comparison of taste qualities and thresholds of d- and l-amino acids. Physiol Behav. 1981;27(1):51–59. doi: 10.1016/0031-9384(81)90298-5. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages, and spices: antioxidant activity and health effects—a review. J Funct Foods. 2015;18(B):820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size, and weight. J Cereal Sci. 2009;49(1):106–111. doi: 10.1016/j.jcs.2008.07.010. [DOI] [Google Scholar]
- Shin HW, Jang ES, Moon BS, Lee JJ, Lee DE, Lee CH, Shin CS. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. J Food Sci Technol. 2016;53(2):1004–1013. doi: 10.1007/s13197-015-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun LS, Kim HY, Lee S, Lee JM, Muthaiya MJ, Kim BS, Oh JY, Song CK, Jeon EJ, Ryu HS, Lee CH. Mass spectrometry-based metabolite profiling and bacterial diversity characterization of Korean traditional meju during fermentation. J Microb Biotechnol. 2012;22(11):1523–1531. doi: 10.4014/jmb.1207.07003. [DOI] [PubMed] [Google Scholar]
- Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients. 2016;8(8):E495. doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed] [Google Scholar]