Abstract
Objective
To estimate the proportion of potentially preventable stillbirths in the United States.
Methods
We conducted a secondary analysis of 512 stillbirths with complete evaluation enrolled in the Stillbirth Collaborative Research Network (SCRN) from 2006 to 2008. The SCRN was a multisite, geographically, racially, and ethnically diverse, population-based case-control study of stillbirth in the United States. Cases underwent standard evaluation that included maternal interview, medical record abstraction, biospecimen collection, postmortem examination, placental pathology and clinically recommended evaluation. Each case was assigned probable and possible causes of death using the Initial Causes of Fetal Death (INCODE) algorithm system. For this analysis, we defined potentially preventable stillbirths as those occurring in nonanomalous infants, ≥24 weeks of gestation and weighing ≥ 500g that were: 1) intrapartum; 2) due to medical complications; 3) due to placental insufficiency; 4) multiple gestation (excluding twin–twin transfusion); 5) due to spontaneous preterm birth; or 6) due to hypertensive disorders of pregnancy.
Results
Of the 512 stillbirths included in our cohort, causes of potentially preventable stillbirths included: Placental insufficiency (65; 12.7%); Medical complications of pregnancy (31; 6.1%); Hypertensive disorders of pregnancy (20; 3.9%); preterm labor (16; 3.1%); intrapartum (9; 1.8%); and multiple gestations (4; 0.8%). Twenty-seven cases fit 2 or more categories, leaving 114 (22.3%) potentially preventable stillbirths.
Conclusions
Based on our definition, almost a fourth of stillbirths are potentially preventable. Given the predominance of placental insufficiency among stillbirths, identification and management of placental insufficiency may have the most immediate effect on stillbirth reduction.
Introduction
The stillbirth rate in the U.S. in 2013 was 5.96 per 1,000 births.1 The rate declined from 6.61 to 6.05 per 1,000 births from 2000 through 2006 and has remained relatively stable for the past decade.2 While the rate of stillbirth worldwide has decreased as well, the global rate is much higher (18.4/1,000 births), with the majority of stillbirths occurring in middle and low-income countries.3 Furthermore, stillbirth rates are higher in the United States (U.S.) than in many other high-income countries, and rates continue to decrease in other high-income countries, suggesting there may be opportunities in the U.S. for improvement.3 Thus, the objective of our study was to determine the proportion of potentially preventable stillbirths in a diverse U.S. sample.
Materials and Methods
We conducted a secondary analysis of data from the Stillbirth Collaborative Research Network (SCRN). The SCRN conducted a multicenter, population-based case-control study that attempted to enroll all stillbirths and a representative sample of live births in five distinct geographically defined areas between March 2006 and September 2008. Details of the study design and methodology have been published.4,5 Stillbirths were defined as births at or after 20 weeks’ gestation with Apgar scores of 0 at 1 and 5 minutes and no signs of life on direct observation. Deliveries resulting from termination of a live fetus were excluded. Gestational age was determined using data from assisted reproductive technology, first day of last menstrual period, and ultrasound.5 All participants gave written informed consent and the study was approved by the institutional review boards of each clinical site and the Data-Coordinating and Analysis Center.
Cases of stillbirth were enrolled in the SCRN at the time of ascertainment of stillbirth. They had standardized maternal interview, medical record abstraction, biospecimen collection, postmortem examination and placental pathology, and attempted karyotype.4 The postmortem and placental examinations were performed by perinatal pathologists with a systematic protocol and using diagnostic criteria that had been determined prior to initiation of the study.6,7 Designated study pathologists underwent centralized training. Additional clinically indicated testing (suggested by SCRN investigators) was performed at the discretion of the primary clinician. Recommended testing included karyotype, antibody screen, syphilis serology, toxicology screen, assessment for fetal-maternal hemorrhage and antiphospholipid antibodies.4,5 Biospecimens including maternal blood, fetal umbilical blood, placental tissue and fetal tissue were collected and stored to complete clinically recommended and experimental testing that was not performed at the time of delivery. These tests included antibody screen, syphilis serology, parvovirus serology, anticardiolipin and anti-beta-2-glycoprotein-I antibodies, fructosamine, bile acids and chromosomal microarray.
For our analysis, only stillbirths with complete postmortem evaluation and placental histopathology were included. Demographic details of this population have been previously published.8 Results of karyotype and microarray were combined and considered collectively as “genetic testing results”.9 The presence of antiphospholipid antibodies was determined using anticardiolipin antibody and beta-2 glycoprotein antibody testing as previously described.10
The Initial Causes of Fetal Death (INCODE) algorithm developed by the SCRN11 was utilized in an attempt to assign causes of death for each case. INCODE distinguishes between probable causes, possible causes, or “condition present” for each potential cause of stillbirth. INCODE defines a “probable cause of death” as a condition or disease with a high likelihood of causing the stillbirth and a “possible cause of death” as one for which there is reasonable certainty it is involved in the pathophysiologic pathway leading to stillbirth. Conditions present, but not meeting criteria for probable or possible causes of death were considered “present”.11 Potential causes of stillbirth were further divided into broad categories including maternal medical conditions, obstetric complications, placental conditions, infection, fetal genetic or structural abnormalities, hypertensive disorders of pregnancy, umbilical cord abnormalities and other.11,5
There is no generally accepted definition of what constitutes a “preventable” cause of stillbirth. In theory, many stillbirths may be ultimately preventable and ideally most stillbirths would be eliminated. Nonetheless, there is some degree of subjectivity in what constitutes a “preventable” stillbirth. Accordingly, we developed a definition for potentially preventable stillbirths specific for this analysis considering a wide range of possibly preventable causes. (Figure 1)
Figure 1.
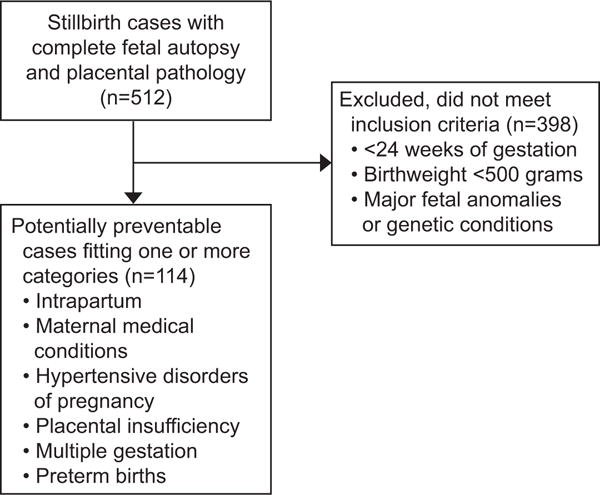
Flowchart of study design and analysis.
First, we categorized stillbirths that occurred prior to 24 weeks gestation and those weighing less than 500 grams as not preventable. Such infants would be considered previable or periviable and might not be candidates for operative delivery and resuscitation in many centers. We also categorized infants with major anomalies or genetic conditions that are not considered compatible with ex utero life or life of meaningful quality as not preventable.11 Although in utero death of some of these fetuses might be preventable, the overall perinatal mortality would not change since these would become neonatal deaths.
We then considered categories of potentially preventable stillbirths based on currently available knowledge and obstetric practices and using INCODE criteria for probable and possible causes of death (Table 1). The diagnostic criteria for these conditions were based on the definitions set forth in the INCODE classification tool.11 First, we considered intrapartum stillbirths since intervention with cesarean delivery likely could have prevented these losses. Second, we evaluated maternal medical complications such as diabetes, chronic hypertension and systemic lupus erythematosus. Perinatal mortality associated with these conditions is dramatically improved with contemporary medical care.12,13 The third category included hypertensive disorders of pregnancy. As with medical complications, optimal medical care substantially reduces fetal death in women with these conditions.14 Fourth, we considered stillbirths due to placental insufficiency as defined by INCODE.11 This included clinical evidence of placental insufficiency such as fetal growth restriction and oligohydramnios as well as rare and serious placental pathology such as decidual vasculopathy.11 Obstetric surveillance throughout the pregnancy and subsequent care is intended to screen for these conditions and prevent stillbirth, albeit imperfectly.15 Fifth, we assessed uncomplicated multiple gestations since appropriate obstetric care reduces the risk of stillbirth.16 We defined uncomplicated multiple gestations as those without twin-twin transfusion syndrome, twin reversed arterial perfusion and monoamnionicity. Finally, we analyzed stillbirths associated with preterm birth (and related pathways) since some proportion of these may be prevented by treatment with cerclage and, or progestins.17,18
Table 1.
Categories of potentially preventable stillbirth
| Category | Definition |
|---|---|
| Intrapartum | Fetal cardiac activity present on admission with stillborn infant |
| Maternal medical complications | Diabetes mellitus, gestational diabetes, chronic hypertension, antiphospholipid syndrome, systemic lupus erythematosus |
| Hypertensive disorders of pregnancy | Gestational hypertension, pre-eclampsia |
| Placental insufficiency | Abnormal placental pathology findings (small, fibrin, infarcts, thrombotic vasculopathy), fetal growth restriction |
| Multiple gestation | Otherwise uncomplicated multiple gestation (excluded TTTS*, TRAP†, monoamniotic pregnancies) |
| Preterm birth | PPROM‡, preterm labor, chorioamnionitis |
: Twin-twin transfusion syndrome
: Twin reversed arterial perfusion syndrome
: Preterm prelabor rupture of membranes
Descriptive statistics were used to characterize stillbirths by preventable category and cause. Preventable stillbirths were compared by race, ethnicity, and gestational age using contingency table analysis. Univariate regression analysis was used to assess associations between potentially preventable stillbirth and pregnancy characteristics. Analyses were performed with STATA, College Station, TX.
Results
Of the 663 women (676 stillbirths) enrolled in the SCRN, 500 women (75.4%) consented to their 512 stillbirths undergoing a complete postmortem examination and are included in this analysis. Of these, 114 cases (22.3%) were classified as potentially preventable, and were included in one or more of the potentially preventable categories. Table 2 depicts the number of stillbirths in each category of potential preventable etiologies. Twenty-seven cases were included in more than one category. Twenty-three fit two categories and four cases were included in three categories. Many of the cases that fit more than one category included placental insufficiency (21/27; 77.8%). For example, placental insufficiency often overlapped with antiphospholipid syndrome or hypertensive disorders of pregnancy; in fact, 32.3% (21 of 65 cases) of placental cases had another cause identified as well. Many of the intrapartum stillbirths were associated with abruption, and review indicated that clinical management, including the use of emergent cesarean,19 appeared to be appropriate in most cases. Nonetheless, we retained these intrapartum stillbirths as part of the potentially preventable cohort due to the fact that intrapartum stillbirths occurring at a viable gestational age represent a target for stillbirth prevention.
Table 2.
Cases of potentially preventable stillbirth by causes or category.
| Maternal Medical Conditions | Hypertensive Disorders of Pregnancy | Placental Insufficiency | Multiple Gestation | Preterm Birth | Intrapartum | |
|---|---|---|---|---|---|---|
| Total Cases | 31 | 20 | 65 | 4 | 16 | 9 |
| % of Total Stillbirths (n=512) (95% CI) |
6.1% (4.0–8.1) |
3.9% (2.2–5.6) |
12.7% (9.8–15.6) |
0.8% (0–1.5) |
3.1% (1.6–4.6) |
1.8% (0.6–2.9) |
| % of Potentially Preventable Stillbirths (n=114) (95% CI) |
27.2% (19.0–35.4) |
17.5% (10.6–24.5) |
57.0% (47.9–66.1) |
3.5% (0.1–6.9) |
14.0% (7.7–20.4) |
7.9% (2.9–12.8) |
Table 3 shows each category of potentially preventable stillbirth, stratified by gestational age at stillbirth. There was a nonsignificant increase in the proportion of preventable stillbirths due to placental insufficiency with increasing gestational age, with the most common gestational age window of stillbirth for these cases being after 37 weeks gestation. Other causes of stillbirth did not vary by gestational age. Figure 2 displays the distribution of potentially preventable stillbirth cases overall versus those that were not deemed potentially preventable. There was an increase in the proportion of potentially preventable cases with increasing gestational age (p = 0.023). Notably, 39 (34.2%) potentially preventable stillbirths occurred after 37 weeks’ gestation when there would have been minimal risk of complications of prematurity. Indeed, nearly half of all stillbirths after 37 weeks’ gestation were “potentially preventable” by our definitions. An additional 32 (28.1%) occurred between 32 – 37 weeks’ gestation; an epoch associated with meaningful risk of complications due to prematurity but a high probability of survival and relatively good long term outcomes.
Table 3.
Potentially preventable cases of stillbirth by gestational age
| 24 0/7 – 27 6/7 weeks n cases (%)* |
28 0/7 – 31 6/7 weeks n cases (%)* |
32 0/7 – 36 6/7 weeks n cases (%)* |
37 0/7 – 41 6/7 weeks n cases (%)* |
P-value | |
|---|---|---|---|---|---|
| Total | 22 (19.3%) | 21 (18.4%) | 32 (28.1%) | 39 (34.2%) | |
|
| |||||
| Maternal medical complications | 6 (5.3%) | 7 (6.1%) | 10 (8.8%) | 8 (7.0%) | 0.67 |
| Chronic HTN† | 3 (2.6%) | 6 (5.3%) | 4 (3.5%) | 0 (0%) | |
| Diabetes Mellitus | 2 (1.8%) | 1 (0.9%) | 4 (3.5%) | 6 (5.3%) | |
| APS‡ | 2 (1.8%) | 0 (0%) | 2 (1.8%) | 2 (1.8%) | |
| Placental Insufficiency | 14 (12.3%) | 12 (10.5%) | 18 (15.8%) | 21 (18.4%) | 0.91 |
| Hypertensive disorders of pregnancy | 5 (4.4%) | 5 (4.4%) | 5 (4.4%) | 5 (4.4%) | 0.64 |
| Gestational HTN† | 3 (2.6%) | 0 (0%) | 2 (1.8%) | 2 (1.8%) | |
| Pre-eclampsia | 2 (1.8%) | 5 (4.4%) | 3 (2.6%) | 3 (2.6%) | |
| Multiple gestation | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) | 0.96 |
| Preterm birth | 5 (4.4%) | 1 (0.9%) | 3 (2.6%) | 7 (6.1%) | 0.27 |
| PPROM or PTL§ | 2 (1.8%) | 1 (0.9%) | 1 (0.9%) | 0 (0%) | |
| Infection | 3 (2.6%) | 0 (0%) | 2 (1.8%) | 7 (6.1%) | |
| Intrapartum | 3 (2.6%) | 0 (0%) | 4 (3.5%) | 2 (1.8%) | 0.92 |
: Percentage of potentially preventable stillbirths
: Hypertension
: Antiphospholipid antibody syndrome
: Preterm prelabor rupture of membranes or Preterm labor
: N = 114 potentially preventable stillbirth cases as denominator for percentage calculation
Figure 2.
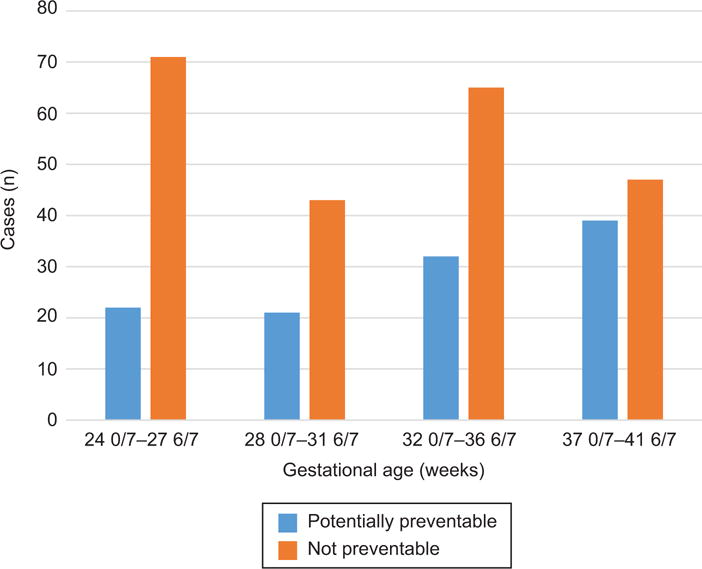
Potentially preventable stillbirth stratified by gestational age.
We also stratified cases of potentially preventable stillbirth by race and ethnicity. Potentially preventable stillbirths accounted for 25% of stillbirths among Hispanic (n = 42), 19% in non-Hispanic White (n = 35), 27% in non-Hispanic black women (n = 30) and 19% in other race, ethnicity categories (n = 7). These proportions were not significantly different (p = 0.474).
In the cohort of SCRN stillbirth cases with complete evaluation, there were many cases that did not meet our criteria of potentially preventable but represent groups of interest in stillbirth prevention. For example, 75 cases (14.6%) were extremely preterm births occurring prior to 24 weeks gestation. Most had obstetric causes of stillbirth based on INCODE including preterm labor, preterm prelabor rupture of membranes, cervical insufficiency and chorioamnionitis. In ideal circumstances, a proportion of these cases would be detected with cervical length screening and appropriate interventions taken when shortening is identified. This pathophysiologic pathway remains a major target for prevention of stillbirth, preterm birth and previable birth. Of term stillbirths (between 37 0/7 weeks’ and 41 6/7 weeks’ gestation), 38 (46.9%) were considered potentially preventable and 43 (53.1%) did not meet potentially preventable criteria based on our definition.
Characteristics associated with preventable stillbirth are shown in Table 4. Women with public insurance (publicly funded sources, excluded self-pay patients) were more likely to be in the potentially preventable group (60.8% versus 41.7%; p = 0.001). Tobacco use was also more common in the potentially preventable group (18.6% versus 10.5%; p = 0.022). Of interest, women without prenatal care were less likely to have preventable stillbirth (1.8% versus 7.2%; p = 0.039) than those with care. The remaining categories were similar among groups, although small numbers in many of these categories yielded little statistical power. Given the small number of variables associated with preventable stillbirth and the small numbers in some categories, we did not perform multivariable regression analysis.
Table 4.
Characteristics associated with preventable stillbirth (univariable regression analysis).
| Potentially preventable N=114 |
Not preventable N=398 |
p-value | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| Non-Hispanic Black | 30 (26.3) | 85 (21.4) | 0.269 | 1.31 (0.78–2.17) |
| Maternal age (years) | 29.0 (6.9) | 27.9 (6.5) | 0.118 | – |
| Maternal age <18 | 6 (5.3) | 12 (3.1) | 0.270 | 1.75 (0.52–5.17) |
| Maternal age >40 | 5 (4.4) | 17 (4.4) | 0.993 | 1.00 (0.28–2.92) |
| No prenatal care | 2 (1.8) | 27 (7.2) | 0.039 | 0.24 (0.03–0.99) |
| <12 years education | 24 (22.0) | 79 (21.0) | 0.821 | 1.06 (0.60–1.82) |
| Public Insurance | 62 (60.8) | 138 (41.7) | 0.001 | 2.17 (1.35–3.51) |
| Obese | 35 (36.5) | 100 (31.8) | 0.389 | 1.23 (0.74–2.04) |
| Parous | 51 (44.7) | 180 (45.2) | 0.926 | 0.98 (0.63–1.52) |
| Tobacco Use | 21 (18.6) | 41 (10.5) | 0.022 | 1.94 (1.04–3.55) |
| Alcohol Use | 2 (1.8) | 11 (2.8) | 0.742 | 0.62 (0.07–2.90) |
| Drug Use | 6 (5.4) | 9 (2.3) | 0.092 | 2.41 (0.69–7.77) |
| Abuse | 1 (1.1) | 6 (1.9) | 1.000 | 0.56 (0.01–4.70) |
| Assisted Reproductive Technology | 3 (2.7) | 15 (3.9) | 0.775 | 0.69 (0.13–2.50) |
Discussion
Using pre-specified and conservative criteria, about a quarter of stillbirths that occur in the United States are potentially preventable. The most common cause of potentially preventable stillbirths was placental insufficiency, followed by maternal medical disorders, hypertensive conditions and spontaneous preterm birth. These conditions can be targeted for stillbirth reduction.
The concept of preventable stillbirth is subjective and debatable. In theory, many stillbirths are potentially preventable and under ideal circumstances, many would be avoided. Even some lethal genetic conditions might be “prevented” by using pre-implantation genetic screening. Nonetheless, some stillbirths are more easily prevented than others, and when faced with limited resources and knowledge gaps, it makes sense to focus on these.
There is no question that a majority of stillbirths in low resource settings could be avoided. Half or more of stillbirths that occur in these locations are intrapartum at viable gestational ages,20–23 and many are associated with potentially preventable infections.3 In contrast, it is less clear how many stillbirths can be prevented in high resource settings. There is some thought that all stillbirths other than those due to major genetic abnormalities or fetal malformations (about 10%) are preventable.24,25 However, this may be unrealistic given our present knowledge base.
Many high-resource settings have considerably lower stillbirth rates than the U.S.24 Currently, the U.S. ranks 25th in the world in third trimester stillbirths and many countries have over a 33% lower rate.25 In addition, other high-resource countries have recently reported dramatic decreases in stillbirth. For example, the stillbirth rate in the Netherlands decreased 6.8% (1.8 per 1,000 births) from 2000 to 2015 while the U.S. rate declined only 0.4% (3.0 per 1,000 births) during the same period.25
One could make a compelling argument that many of the cases we did not include as potentially preventable, were in fact, preventable. For example, there were many cases of obstetric complications such as preterm labor, preterm prelabor rupture of membranes and cervical insufficiency resulting in delivery at pre- or peri-viable gestational ages (the vast majority). In theory, some of those cases could have been identified through obstetric history or cervical length screening and prevented with progestational agents and, or cerclage.17,18 In addition, one is tempted to consider all cases with fetal growth restriction at viable gestational ages or all cases after 34 or 37 weeks gestation to be preventable. However, given the relatively poor ability of risk factors to predict stillbirth, the potential for causing harm, for example through iatrogenic prematurity, must be weighed against the potential for stillbirth prevention. Improved prediction of stillbirth risk in the third trimester warrants further research and we look forward to data from the NICHD sponsored Human Placenta Project. Another area of uncertainty is the effect of risk factor modification. Smoking, obesity, advanced maternal age and multiple gestation are all associated with stillbirth. Although one can estimate attributable risks,26 it is unclear how many stillbirths could be prevented by reduction in these risk factors, since they are risk factors for rather than causes of stillbirth.
There was no association with race, ethnicity and preventable stillbirth using our definitions. This may have been due to small sample size after stratification. Also, non-Hispanic black race is associated with stillbirth due to preterm labor pathways, many of which result in stillbirths occurring between 20 – 24 weeks gestation.8 In contrast, a higher percentage of stillbirths in non-Hispanic white women are due to placental causes, which were considered preventable in this study. Regardless, reduction in stillbirth disparity warrants focus and resources. Preventable stillbirth was more common in those women with public insurance, highlighting socioeconomic disparity in health outcomes and warranting further research. We were surprised that lack of prenatal care was not associated with potentially preventable stillbirth, although very small numbers in the study preclude meaningful conclusions. As expected, smoking and low socioeconomic status were associated with preventable stillbirth.
Our study had several weaknesses. The concept of “preventable stillbirth” is subjective and open to debate. Accordingly, we used several definitions in an attempt to acknowledge this subjectivity. Also, our definitions were conservative and most likely underestimate the percentage of preventable stillbirths based on rates in other countries. We also may have had too few stillbirths in each preventable category to identify maternal risk factors for preventable stillbirth. The study also had numerous strengths. The study included a large number of well-characterized stillbirths. The study was population-based and participants reflected racial, ethnic, and geographic diversity. Also, causes of death were ascertained using the evidence-based INCODE algorithm after extensive evaluation of cases.
In summary, we speculate that almost 25% of stillbirths in a large U.S. cohort are potentially preventable using conservative definitions. The most common causes of preventable stillbirths were placental abnormalities or insufficiency as well as maternal medical disorders, hypertensive diseases of pregnancy and preterm labor. These findings underscore the importance of obstetric surveillance to identify potential placental insufficiency as well as other maternal conditions that can be targeted in efforts to reduce stillbirth in the U.S. and other high resource settings.
Acknowledgments
This work, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review and approval of the manuscript, was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HD045953 Brown University, Rhode Island; U10-HD045925 Emory University, Georgia; U10-HD045952 University of Texas Medical Branch at Galveston, Texas; U10-HDO45955 University of Texas Health Sciences Center at San Antonio, Texas; U10-HD045944 University of Utah Health Sciences Center, Utah; and U01-HD045954 RTI International, RTP.
Footnotes
Comments and views of the author(s) do not necessarily represent the views of the NICHD.
Presented as a poster at the SMFM 36th Annual Pregnancy Meeting, Atlanta, GA, February 1-6 2016.
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.MacDorman MF, Gregory EC. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep. 2015;64:1–24. [PubMed] [Google Scholar]
- 2.Gregory EC, MacDorman MF, Martin JA. Trends in fetal and perinatal mortality in the United States, 2006–2012. NCHS Data Brief. 2014 Nov;(169):1–8. [PubMed] [Google Scholar]
- 3.Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. doi: 10.1016/S0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 4.Parker CB, Hogue CJR, Koch MA, Willinger M, Reddy U, Thorsten VR, et al. for the Stillbirth Collaborative Research Network Stillbirth Collaborative Research Network: Design, methods and recruitment experience. Paediatric and Perinatal Epidemiology. 2011;25:425–35. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA. 2011;306:2469–79. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination. Am J Perinatol. 2011;28:781–92. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) Postmortem Examination Protocol. Am J Perinatol. 2012;29:187–202. doi: 10.1055/s-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA. 2011;306(22):2459–2468. doi: 10.1001/jama.2011.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367:2185–93. doi: 10.1056/NEJMoa1201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silver RM, Parker CB, Reddy UM, et al. Antiphospholipid antibodies and stillbirth. Obstet Gynecol. 2013;122:641–57. doi: 10.1097/AOG.0b013e3182a1060e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley DJ, Goldenberg R, Conway D, Silver RM, Saade G, Varner MV, et al. Stillbirth Collaborative Research Network: Initial Causes of Fetal Death (INCODE) Obstet Gynecol. 2010;116:254–60. [Google Scholar]
- 12.Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi: 10.1007/s11892-015-0580-y. [DOI] [PubMed] [Google Scholar]
- 13.Johnstone FD, Lindsay RS, Steel J. Type 1 diabetes and pregnancy: trends in birth weight over 40 years at a single clinic. Obstet Gynecol. 2006 Jun;107(6):1297–302. doi: 10.1097/01.AOG.0000218706.38886.10. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad AS, Samuelsen SO. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies. BJOG. 2012 Nov;119(12):1521–8. doi: 10.1111/j.1471-0528.2012.03460.x. Epub 2012 Aug. [DOI] [PubMed] [Google Scholar]
- 15.Silver RM, Page JM. Interventions to prevent stillbirth. Sem Fet Neonat Med. 2017;22:135–145. doi: 10.1016/j.siny.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Saccone G, Berghella V. Planned delivery at 37 weeks in twins: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Med. 2016;29:685–9. doi: 10.3109/14767058.2015.1016423. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Nicolaides K, Conde-Aguedo A, et al. vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–71. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 19.Boyle A, Preslar JP, Hogue CJ, Silver RM, Reddy UM, Goldenberg RL, et al. Route of Delivery in Women With Stillbirth: Results From the Stillbirth Collaborative Research Network. Obstet Gynecol. 2017;129:693–698. doi: 10.1097/AOG.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhutta ZA, Das JK, Bahl R, et al. What will it take to avert preventable newborn deaths and stillbirths and at what cost? Lancet. 2014;384:347–70. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 21.McClure EM, Saleem S, Goudar SS, Moore JL, Esamai F, Garces A, et al. Stillbirth trends in low-middle income countries 2010 – 2013: A population-based, multi-country cohort study from the Global Network. Reproductive Health. 2015;12(Suppl 2):S7. doi: 10.1186/1742-4755-12-S2-S7. 8 June 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg RL, Saleem S, Pasha O, Harrison M, McClure EM. Reducing Stillbirths in Low-Income Countries. 2015 ACTAOBGYN Scandanavia. Acta Obstet Gynecol Scand. 2016;95:135–43. doi: 10.1111/aogs.12817. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Griffin J, Harrison M, Rouse DJ, Moran K, Jones BJ, McClure EM. An analysis of the impact of clinical interventions to reduce stillbirths in sub-Saharan Africa. BJOG. 2016 Oct 5; doi: 10.1111/1471-0528.14304. [DOI] [PubMed] [Google Scholar]
- 24.de Bernis L, Kinney MV, Stones W, et al. Stillbirths: ending preventable deaths by 2030. Lancet. 2016;387:703–16. doi: 10.1016/S0140-6736(15)00954-X. [DOI] [PubMed] [Google Scholar]
- 25.Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: recall to action in high-income countries. Lancet. 2016;387:691–702. doi: 10.1016/S0140-6736(15)01020-X. [DOI] [PubMed] [Google Scholar]
- 26.Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]


