Abstract
Objective
Accumulating evidence suggests a role of semaphorins in vascular homeostasis. Here we investigate the role of semaphorin 7A (Sema7A) in atherosclerosis and its underlying mechanism.
Approach and Results
Using genetically-engineered Sema7A−/−ApoE−/− mice, we showed that deletion of Sema7A attenuates atherosclerotic plaque formation primarily in the aorta of ApoE−/− mice on a high fat diet. A higher level of Sema7A in the atheroprone lesser curvature suggests a correlation of Sema7A with disturbed flow (d-flow). This notion is supported by elevated Sema7A expression in human umbilical venous endothelial cells (HUVECs) either subjected to oscillatory shear stress or treated with the PKA/CREB inhibitor H89. Further studies using the partial carotid artery ligation model showed that d-flow in the left carotid artery of Sema7A+/+ApoE−/− mice promoted the expression of endothelial Sema7A and cell adhesion molecules (CAMs), leukocyte adhesion, and plaque formation, while such changes were attenuated in Sema7A−/−ApoE−/− mice. Further studies showed that blockage of β1 integrin, a known Sema7A receptor, or inhibition of FAK, MEK1/2 or NFκB, significantly reduced the expression of CAMs and THP-1 monocyte adhesion in Sema7A overexpressing HUVECs. Studies using chimeric mice suggest that vascular, most likely endothelial Sema7A plays a major role in atherogenesis.
Conclusions
Our findings indicate a significant role of Sema7A in atherosclerosis by mediating endothelial dysfunction in a β1 integrin-dependent manner.
Keywords: Atherogenesis, Endothelial phenotypic changes, Disturbed blood flow, Leukocyte recruitment, Semaphorin 7A
Journal Subject Code: Vascular Disease
Introduction
Endothelial cells (ECs) sense and respond to various pathophysiological stimuli in maintaining vascular integrity and homeostasis.1–4 When exposed to disturbed blood flow (d-flow), these cells undergo major phenotypic changes that lead to increased vascular permeability, cytokine release and leukocyte adhesion.4–6 Such structural and functional changes in vessel wall increase the susceptibility to atherosclerosis.7–10 Previous studies looking into mechanisms underlying the hemodynamic regulation of vascular endothelium have unveiled an array of flow responsive candidates, including membrane receptors, adhesion molecules, glycocalyxes, and cytoskeleton proteins that are considered as promising therapeutic targets for atherosclerotic disease.8, 11, 12
Semaphorins are originally identified as regulators of neuronal growth, while individual family members have been shown to play roles in other physiological and pathological conditions, including immune responses, organogenesis, angiogenesis, vascular development and disorders13, 14. Semaphorin 7A (Sema7A) is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein15 with an N-terminal seven-bladed β-propeller Sema domain, a plexin-semaphorin-integrin domain, an immunoglobulin-like domain and a C-terminal GPI anchoring domain.16 The Sema domain contains an RGD motif commonly found in integrin-binding proteins.17 Expression of Sema7A and its known membrane receptors, integrin β1 and plexin C1, were found in neurons, endothelial cells, platelets, monocytes, T cells, dendritic cells (DCs) and cancer cells.18 Previous studies showed that Sema7A is involved in the regulation of inflammatory response, cancer progression, and neuron growth.17, 19, 20 Recently, lung EC Sema7A was reported to be upregulated by HIF-1α, an oxygen sensitive transcription factor, in the setting of acute inflammation induced by hypoxia, LPS or seawater.21–23 However, there has been no report whether Sema7A participates in the development of atherosclerosis.
We have previously shown that disruption of Sema4D, another member of semaphorin family, protects against the development of atherosclerosis.24 In this study, we utilized a partial carotid artery ligation (PCL) model in ApoE−/− mice fed with a high fat diet (HFD), and showed that endothelial Sema7A expression is increased by surgically induced d-flow, while genetic deletion of Sema7A ameliorated atherosclerosis in ApoE−/− mice. Sema7A appeared to cause endothelial cell dysfunction through endothelial β1 integrin/FAK/MEK1/2/NFκB pathway, acting as a proatherogenic molecule.
Materials and methods
Materials and methods are available in the online-only Data Supplement.
Results
Genetic deletion of Sema7A reduces plaque size in the aorta of ApoE−/− mice
To investigate the role of Sema7A in atherosclerosis, we generated Sema7A−/−ApoE−/− mice and fed them the HFD to induce atherosclerosis. After 12 w, the mice were sacrificed and their aortas were stained with Sudan IV. Atherosclerotic lesion size was 51.5% smaller in Sema7A−/−ApoE−/− mice than in Sema7A+/+ApoE−/− mice (5.0 ± 1.0% of the total aorta area vs 10.3 ± 1.1%, P< 0.01) and the reduction of the lesion size was most prominent in the aortic arch (59.2%) and less in the descending aorta (41.5%) (Figure 1A, B). Histological studies showed that areas of lipid accumulation in the aortic root were 51.8% less in Sema7A−/−ApoE−/− mice than in Sema7A+/+ApoE−/− mice (18.6 ± 3.1% vs 38.6 ± 3.8%, P< 0.001) (Figure 1C, D). Similar serum lipid profiles and body weights were found in Sema7A−/−ApoE−/− and Sema7A+/+ApoE−/− mice (Table II in the online-only Data Supplement). In addition, immunostaining also showed high levels of Sema7A protein in human atherosclerotic carotid tissues compared with human healthy abdominal aorta (Figure 1E). These results indicate that Sema7A participates in atherosclerosis development.
Figure 1.
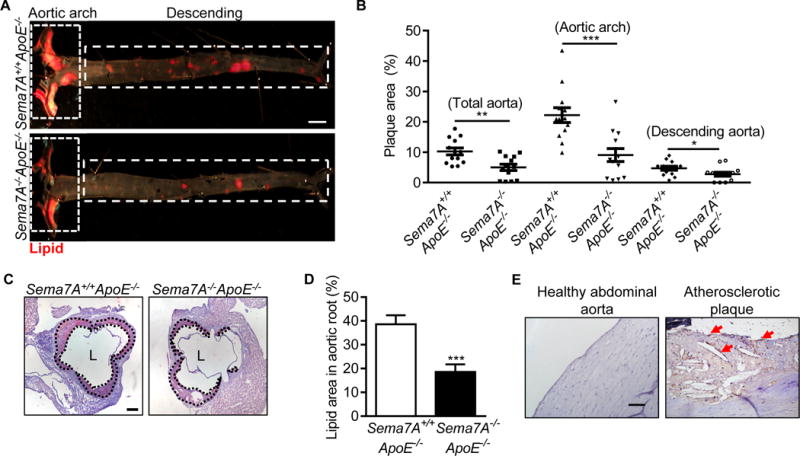
Sema7A deletion reduces lipid deposition in the aorta of ApoE−/− mice and vascular Sema7A is up-regulated during atherogenesis (A) Aortic plaque areas in Sema7A+/+ApoE−/− and Sema7A−/−ApoE−/− mice on HFD for 12 w were analyzed by en face Sudan IV-staining (Bar=2 mm). (B) Quantitative analysis for total aorta, aortic arch and descending aorta are shown. Data are mean ± SEM (n≥10 mice per group). *P<0.05; **P<0.01; ***P<0.001. (C, D) Aortic root sections from Sema7A+/+ApoE−/− and Sema7A−/−ApoE−/− mice on HFD for 12 w were stained with Oil Red O. Plaque area were demarcated by dotted lines (Bar=200 μm). (E) Sema7A expression in human atherosclerotic plaques was detected by immunostaining in brown (arrows). Nuclei were stained by hematoxylin (Bar=100 μm).
D-flow upregulates vascular endothelial Sema7A expression
Sema7A deletion primarily reduces plaque formation in aortic arch, suggesting a higher expression level of Sema7A in this region. Indeed, higher expression levels of Sema7A mRNA and protein in the aortic arch (AA) were observed compared with that in the descending aorta (DA) (Figure 2A, B). The lesser curvature (LC) of the aorta is an atheroprone region that encounters d-flow as compared to the greater curvature (GC) (Figure 2C). Further studies showed that Sema7A mRNA and protein levels were higher in LC than in GC (Figure 2D, E). To test if hemodynamic stress increases Sema7A expression in vivo, we induced d-flow by PCL in WT mice25 (Figure 2C and Figure I in the online-only Data Supplement). Compared with the untied right common carotid artery (RCA) with steady flow (s-flow), left common carotid artery (LCA) showed a 4-fold increase in Sema7A mRNA levels 48 h after the ligation (Figure 2F). To explore the cellular source for the increased Sema7A expression under d-flow, we examined Sema7A protein expression in carotid artery endothelium after the PCL by en face staining. Prominent endothelial Sema7A protein was detected in the ligated LCA, as shown by co-staining of Sema7A and CD31, whereas little Sema7A staining was found in the control RCA (Figure 2G). Consistently, Sema7A mRNA level in cultured human umbilical vein endothelial cells (HUVECs) exposed to oscillatory shear stress (OS) was significantly higher than that in the cells exposed to laminar shear stress (LS) (Figure 2H). These data indicate that d-flow upregulates vascular endothelial Sema7A expression.
Figure 2.
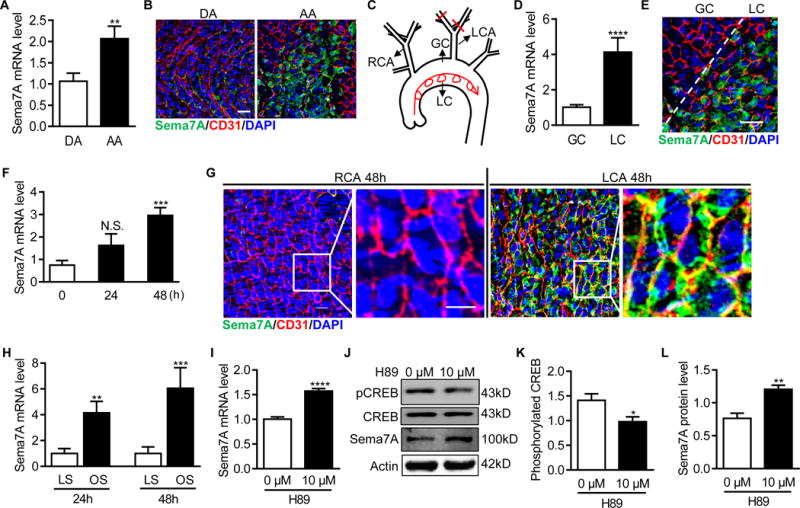
Disturbed blood flow up-regulates vascular endothelial Sema7A expression. (A, D) Total RNAs in the aortic arch (AA) or the descending aorta (DA) (A), and in the lesser (LC) or greater (GC) curvature regions of the aortic arch (D) from C57BL/6J mice were isolated. Sema7A mRNA expression was analyzed by qPCR normalized to GAPDH. Fold-changes are shown. Data are mean ± SEM. **P<0.01; ****P<0.0001. Results are representative of ≥3 independent experiments. (B, E) En face staining for CD31 (red) and Sema7A (green) on the aortic arch (AA) or the descending aorta (DA) (B) and the lesser (LC) or greater (GC) curvature (E) was shown. Nuclei were stained with DAPI (blue). Bar = 50 μm. The data are representative of ≥3 independent experiments. (C) The diagram of aortic arch and carotid arteries. (F) Total RNAs of LCA and the control RCA from C57BL/6J mice were isolated 24h and 48 h after PCL. The ratio of LCA to RCA was calculated (n≥5 mice per group). Data are mean ± SEM. ***P<0.001. The bar graphs represent the results from 3 independent experiments (one-way ANOVA with non-parametric multiple comparison test) (G) Carotid arteries from C57BL/6J 48 h after PCL were en face stained for CD31 (red) and Sema7A (green). Nuclei were stained with DAPI (blue). Bar = 10 μm. (H) Sema7A mRNA levels in HUVECs exposed to LS (15 dyn/cm2) or OS (±5 dyn/cm2, at 1 Hz) were analyzed by qPCR. Data are mean ± SEM, n=3, **P<0.01, ***P<0.001. (I-L) HUVECs treated with H89 for 24 h and Sema7A mRNA expression was analysed by qPCR (I) Sema7A protein expression (J, L) and CREB phosphorylation were analyzed by Western blotting (J, K). Data are mean ± SEM, n=3, *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. RCA: right common carotid artery; LCA: left common carotid artery; DA: descending aorta; AA: aortic arch; GC: greater curvature; LC: lesser curvature; LS: laminar shear stress; OS: oscillatory shear stress.
To understand how d-flow upregulates endothelial Sema7A expression, we performed in a silico promoter analysis and identified potential binding sites in human and mouse Sema7A promoter regions for cAMP response element-binding protein (CREB), a transcription factor known to respond to shear stress26, 27 (Figure II A, B in the online-only Data Supplement). As PKA is a canonical CREB upstream regulator in flow-mediated signaling, we examined Sema7A expression in HUVECs after inhibiting the PKA-CREB signaling using the PKA inhibitor H89. Results showed that H89 reduced CREB phosphorylation and increased Sema7A mRNA and protein expression (Figure 2I-L). These data suggest that suppression of PKA/CREB signaling may be an underlying mechanism in d-flow-induced endothelial Sema7A expression.
Sema7A-deficiency reduces d-flow induced endothelial ICAM-1 and VCAM-1 expression, leukocyte adhesion, and plaque formation
Endothelial adhesion molecules ICAM-1 and VCAM-1 are upregulated by d-flow and contribute to leukocyte recruitment, endothelial inflammation and dysfunction.28 To determine whether d-flow-induced Sema7A expression contributes to endothelial inflammation, we assessed ICAM-1 and VCAM-1 levels in carotid arteries in Sema7A+/+ and Sema7A−/− mice following PCL surgery. Compared to the control RCA, ICAM-1 and VCAM-1 mRNA levels were increased by 2.81- and 5.24-fold (P< 0.0001), respectively, in PCL-treated LCA from Sema7A+/+ mice. In contrast, such increases were abrogated in Sema7A−/− mice (Figure 3A, D). Carotid P-selectin mRNA levels were also reduced in LCA of Sema7A−/− mice compared to Sema7A+/+ mice, whereas E-selectin mRNA levels in PCL-treated LCA were similar in Sema7A+/+ and Sema7A−/− mice (Figure III in the online-only Data Supplement), indicating selective regulation by Sema7A on d-flow induced expression of adhesion molecules. Consistently, en face immunostaining showed higher endothelial ICAM-1 and VCAM-1 expression in LCA from Sema7A+/+ mice compared with that from Sema7A−/− mice (Figure 3B, C, E, F).
Figure 3.
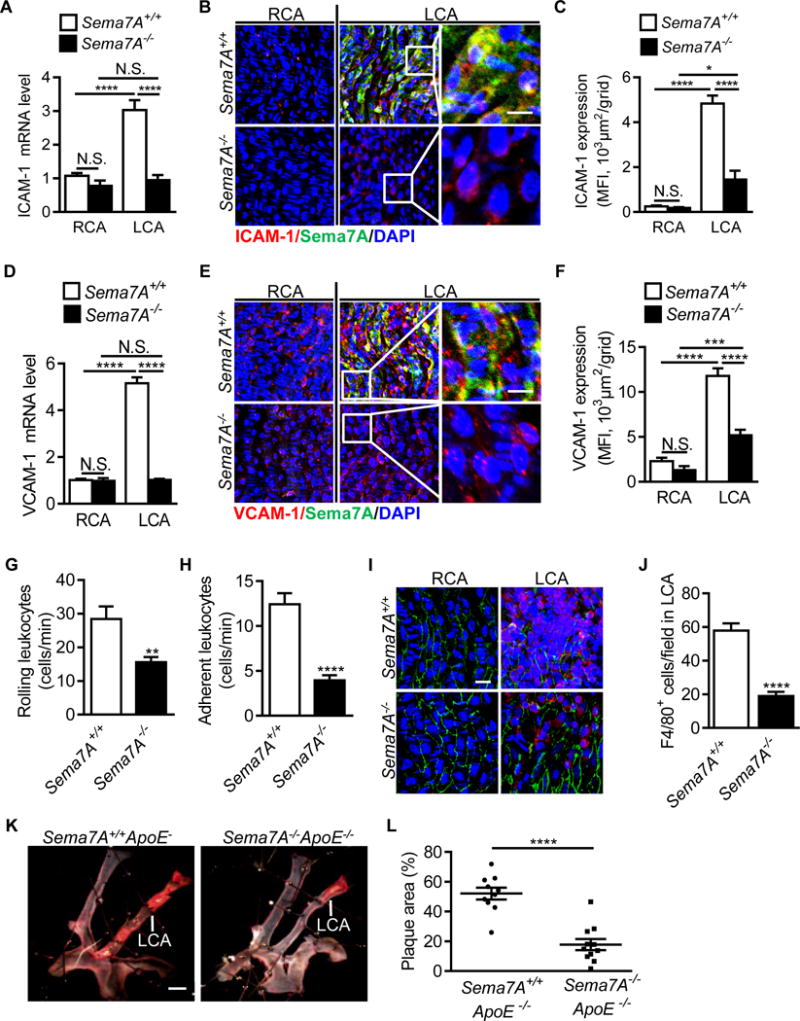
Sema7A deficiency reduces d-flow induced endothelial expression of ICAM-1 and VCAM-1, leukocyte adhesion and plaque formation. ICAM-1 and VCAM-1 mRNA (A, D) and protein (B, E) level in LCA and RCA in Sema7A+/+ and Sema7A−/− mice after PCL were analyzed by qPCR (n=9 mice per group) and en face immunostaining of aortic luminal surface (red: ICAM-1 or VCAM-1; green: Sema7A; blue: nuclei. Bar = 10 μm). Data are representative of ≥3 independent experiments. Mean fluorescent intensity for ICAM-1 (C) and VCAM-1 (F) was used for the statistical analysis of panels B and E, respectively. Each result includes 15-30 fields from 3 mice. Data are mean ± SEM. *P<0.05; ***P<0.001; ****P<0.0001. (G, H) Mice were injected with TNF-α around the cremaster muscle. Rolling and adhesion of leukocytes on cremaster venules (4-5 vessels per mouse) were analyzed under a real-time intravital microscope and counted visually as numbers of rolling (G) or adherent cells (H) on the vascular surface per min (n=6 mice per group). Data are mean ± SEM. **P<0.01; ****P<0.0001. Results are representative of ≥3 independent experiments. (I, J) Adhesion of F4/80+ leukocytes in partially ligated LCA was observed under a confocal microscope and recorded as numbers of adherent cells on the vascular surface per field (Bar = 20 μm). Data are mean ± SEM (n≥9 per group). ****P<0.0001. Results are representative of ≥3 independent experiments. (K) Sema7A+/+ApoE−/− and Sema7A−/−ApoE−/− mice were subjected to PCL. After 2 w on HFD, lipid deposition (in red) was analyzed by en face Sudan IV-staining. Bar=1 mm. The lesion surface areas were quantified and displayed as the percentage area of the LCA (L). Data are mean ± SEM (n≥9 mice per group). ****P<0.0001. RCA: right common carotid artery; LCA: left common carotid artery.
Leukocyte rolling and adhesion, mediated by selectins and adhesion molecules, are crucial for monocyte recruitment and accumulation in atherogenesis.29 Given the role of Sema7A in upregulating the expression of ICAM-1 and VCAM-1, as well as P-selectin, we examined the functional relevance of Sema7A in endothelial inflammation. Using a real-time intravital microscopy, we monitored leukocyte rolling and adhesion on mouse cremaster venule endothelium primed by TNF-α.30 Leukocyte rolling and adhesion were decreased by 45.2% (28.5 ± 3.7 cells/min vs 15.6 ± 1.6 cells/min, P< 0.01) and 68.2% (12.4 ± 1.2 cells/min vs 4.0 ± 0.6 cells/min, P< 0.0001), respectively, in Sema7A−/− mice compared to Sema7A+/+ mice (Figure 3G, H, Supplemental Video I in the online-only Data Supplement). Using a modified PCL model, in which F4/80+ leukocytes adhered on the endothelium of ligated carotid artery were quantified, we showed that leukocyte adhesion was decreased by 67.2% (19.0 ± 2.5 cells/field vs 57.9 ± 4.3 cells/field, P< 0.0001) in Sema7A−/− mice compared to Sema7A+/+ mice (Figure 3I, J). These results indicate that Sema7A deficiency reduces d-flow induced upregulation of adhesion molecules and the consequent leukocyte adhesion in mice.
Reduced leukocyte recruitment is expected to inhibit atherogenesis. To evaluate the effect of Sema7A deletion on d-flow induced plaque formation in vivo we established an extended PCL model, in which Sema7A−/−ApoE−/− and Sema7A+/+ApoE−/− mice were subjected to PCL and fed HFD for 2 w, followed by in situ measurements of the plaque area. Although PCL induced atherosclerotic lesions developed in the LCA of both groups, the average lesion size in LCA from Sema7A−/−ApoE−/− mice was significantly reduced compared with that from Sema7A+/+ApoE−/− mice (17.8 ± 3.7% vs 52.1 ± 4.0%, P< 0.0001) (Figure 3K, L). These findings suggest that Sema7A promotes d-flow induced atherosclerotic plaque formation.
Sema7A overexpression enhances ICAM-1 and VCAM-1 expression in HUVECs and monocyte-endothelial cell interaction via β1 integrin pathway
To investigate the mechanism by which Sema7A regulates ICAM-1 and VCAM-1 expression and leukocyte adhesion in atherosclerosis, we generated a Sema7A-overexpressing endothelial cell line by transducing lentiviral vectors expressing human Sema7A with a GFP tag (Lenti-hSema7A-GFP) into HUVECs (Figure IVA in the online-only Data Supplement). Quantitative PCR, Western blotting and immunostaining were used to determine Sema7A expression in the cell line (Figure 4A, B and Figure IVB in the online-only Data Supplement). Compared to HUVECs transduced with the control virus (Lenti-pCDH-GFP), HUVECs transduced with Lenti-hSema7A-GFP expressed significantly higher levels of ICAM-1 and VCAM-1 (Figure 4C, D and Figure VC, D in the online-only Data Supplement).
Figure 4.
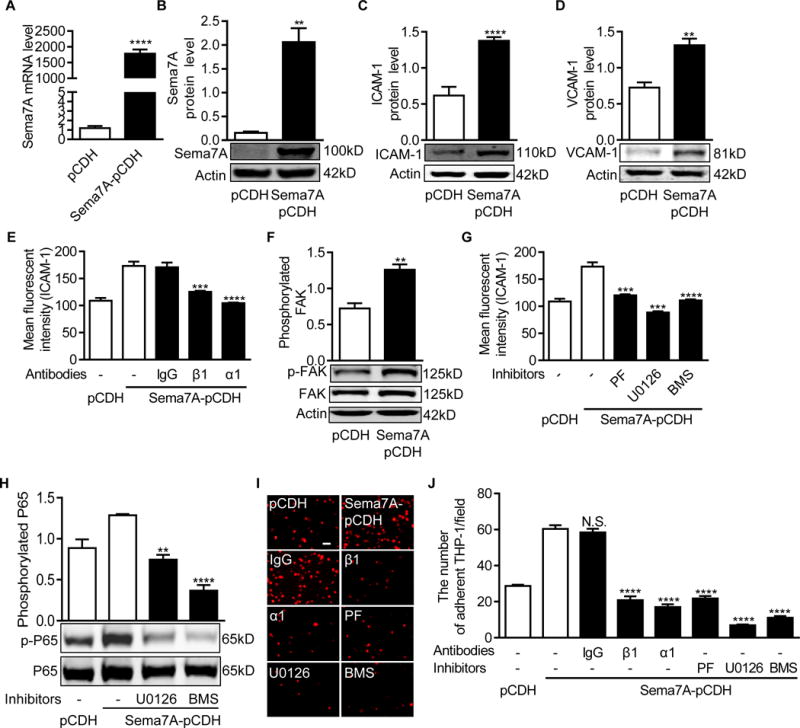
Sema7A overexpression enhances ICAM-1 and VCAM-1 expression in HUVECs and monocyte-endothelial cell interaction via β1 integrin and the downstream FAK/MEK1/2/NFκB pathway (A) Sema7A mRNA expression in the Lenti-pCDH-hSema7A-GFP-transduced HUVECs was analyzed by qPCR (n≥5 per group). Data are mean ± SEM. ***P<0.001. (B~D) Sema7A (B), ICAM-1 (C) and VCAM-1 (D) proteins in Lenti-pCDH-hSema7A-GFP-transduced HUVECs were analyzed by Western blotting normalized to β-actin and displayed as fold-changes relative to Lenti-pCDH-GFP-transduced control HUVECs. Data are mean ± SEM. Results are representative of ≥3 independent experiments. **P<0.01; ***P<0.001; ****P<0.0001. (E) Sema7A-overexpressing and control HUVECs were pre-treated with blocking antibodies against β1 (P5D2) or α1 (5E8D9) integrin subunits for 24 h and analyzed for ICAM-1 expression by flow cytometry. Data are mean ± SEM. ****P<0.0001. Results are representative of ≥3 independent experiments. (F) FAK phosphorylation in Sema7A-overexpressing HUVECs was analyzed by Western blotting normalized to total FAK, and displayed as fold-changes relative to the control HUVECs. Data are mean ± SEM. **P<0.01. Results are representative of ≥3 independent experiments. (G) Sema7A-overexpressing and control HUVECs were pre-treated with PF573228 (10μM), U0126 (10 μM) and BMS-345541 (20 μM) for 24 h. The cells were analyzed by flow cytometry for ICAM-1 expression. (H) Cells were treated with U0126 (10 μM) and BMS-345541 (20 μM), and the lysates were analyzed by Western blotting for NFκB (p65) phosphorylation. Data are mean ± SEM. **P<0.01; ****P<0.0001. Results are representative of ≥3 independent experiments. (I, J) THP-1 cells were stained by DiL and incubated with HUVECs for 30 min at 37°C. HUVECs were also pretreated with blocking antibodies against β1 or α1 integrin subunits or inhibitors for FAK, MAPK/MEK1/2 or NFκB for 24 h. Adherent THP-1 cells (red) on the monolayer of Sema7A-overexpressing or control HUVECs were visualized under a microscope (I) and statistically analyzed (J). Bar=20 μm. Data are mean ± SEM. ****P<0.0001. Results are representative of ≥3 independent experiments. PF: PF573228, FAK inhibitor; U0126: MAPK/MEK1/2 inhibitor; BMS: BMS-345541, NFκB inhibitor.
Beta1 integrin is a Sema7A receptor that mediates cell-cell interaction in axon outgrowth and T cell response.17, 20 Given the presence of β1 integrin in endothelial cells, we hypothesized that Sema7A may mediate endothelial dysfunction through β1 integrin. Flow cytometry showed that β1 integrin blockage by a monoclonal antibody (P5D2) significantly reduced ICAM-1 expression compared to an isotypic control (IgG) (Figure 4E). Similarly, pre-incubation with a blocking antibody against integrin α1 (5E8D9) subunit reduced ICAM-1 expression in Sema7A overexpressing HUVECs (Figure 4E), suggesting that Sema7A mediates endothelial phenotypic changes, at least in part, through the α1β1 heterodimer.
Previous studies showed that activation of focal adhesion kinase (FAK) by Sema7A binding to β1 integrin activates downstream mitogen-activated protein kinase (MAPK).17 NFκB, the downstream molecule to MAPK, is a canonical switch for the expression of pro-inflammatory molecules including ICAM-1 and VCAM-1.31 We analyzed FAK/MAPK/NFκB signaling in Sema7A-overexpressing HUVECs. As expected, FAK phosphorylation was increased by Sema7A overexpression (Figure 4F). Treatment of PF573228 (10 μM), a specific inhibitor for FAK, U0126 (10 μM), a potent inhibitor for both MEK1 and MEK2, or BMS-345541 (20 μM), a highly selective inhibitor for the catalytic subunits of IKK-2 and IKK-1, attenuated ICAM-1 expression in Sema7A-overexpressing HUVECs (Figure 4G). Downstream NFκB P65 phosphorylation was also inhibited by U0126 (10 μM) and BMS-345541 (20 μM) (Figure 4H). These findings indicate that FAK/MEK1/2/NFκB may act downstream of the Sema7A-integrin β1 axis to mediate vascular endothelial dysfunction.
To determine the involvement of β1 integrin and the downstream FAK/MEK1/2/NFκB signaling in leukocyte-endothelial interaction, an in vitro monocyte-endothelial cell adhesion assay was performed. Sema7A-overexpressing HUVECs had a 1.1-fold increase in THP-1 cell adhesion compared with control HUVECs (60.4 ± 1.8 cells/field vs 28.7 ± 0.9 cells/field, P< 0.0001). However, the increased monocyte adhesion was attenuated by antibody blockage of integrin β1 or α1 subunits, or by inhibition of FAK, MEK1/2 or NFκB with small molecule inhibitors (Figure 4I, J). These results indicate that Sema7A enhances the expression of adhesion molecules and leukocyte adhesion on endothelial cells in a β1 integrin-dependent manner that involves downstream FAK/MEK1/2/NFκB signaling.
Endothelial Sema7A plays a major role in promoting leukocyte adhesion and plaque formation in ApoE−/− mice
Sema7A is expressed in many cell types.18 To distinguish the role of vascular vs blood cell-derived Sema7A in leukocyte adhesion, we performed an ex vivo leukocyte adhesion assay, in which aortic endothelium from Sema7A+/+ApoE−/− or Sema7A−/−ApoE−/− mice were treated with LPS and incubated with leukocytes from these mice. As shown in Figure 5A, although adhered monocytes on LPS-treated Sema7A+/+ aortic endothelium were ~30% less when incubated with Sema7A−/− leukocytes compared with Sema7A+/+ leukocytes (P< 0.05), monocyte adhesion was virtually blocked when LPS-treated Sema7A−/− endothelium was incubated with either Sema7A+/+ or Sema7A−/− leukocytes, indicating that vascular, most likely endothelial Sema7A plays a major role in monocyte adhesion in the aorta upon inflammatory stimulation.
Figure 5.
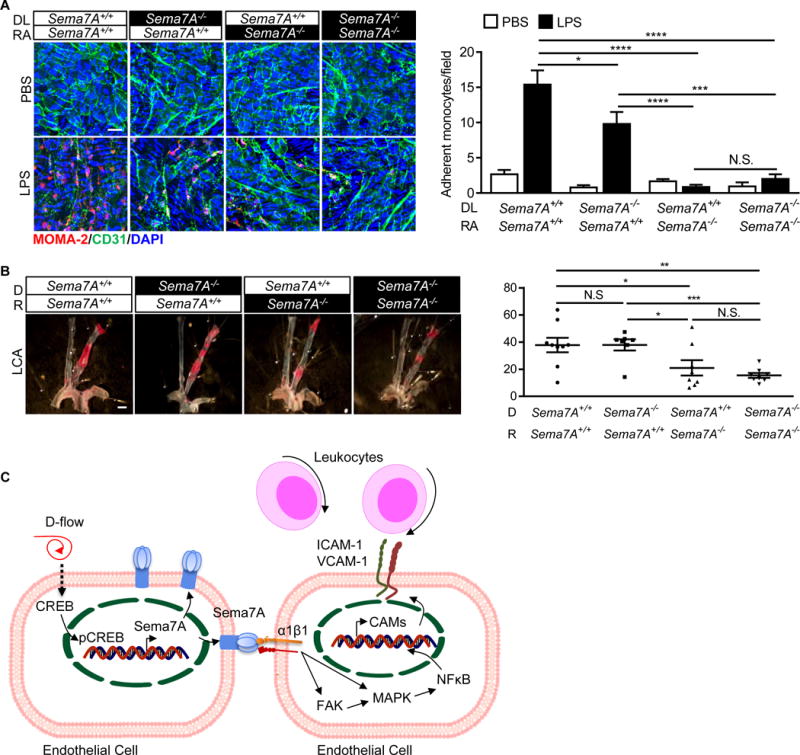
Endothelial Sema7A plays a major role in promoting leukocyte adhesion and plaque formation in ApoE−/− mice (A) Mouse thoracic aortas were isolated from Sema7A−/−ApoE−/− and Sema7A+/+ApoE−/− mice (8-w-old). The exposed endothelium was treated with LPS (5 μg in 2 mL medium per well) for 4 h and cultured with leukocytes from Sema7A−/−ApoE−/− or Sema7A+/+ApoE−/− mice for 30 min at 37°C. Adhered monocytes on the aortic endothelium were stained with a fluorescence-labeled anti-MOMA-2 antibody (red). Bar = 20 μm. Data are mean ± SEM. Results are representative of ≥3 independent experiments. *P<0.05; ***P<0.001; ****P<0.0001. (B) Sema7A+/+ApoE−/− and Sema7A−/−ApoE−/− mice were transplanted with Sema7A+/+ApoE−/− and Sema7A−/−ApoE−/− bone marrow cells and recovered for the following 4 w before subjected to PCL. After additional 2 w on HFD, atherosclerotic lipid deposition (in red) was analyzed by en face Sudan IV-staining. Bar=1 mm. The lesion surface areas were quantified and displayed as the percentage area of the LCA. Data are mean ± SEM (n≥7 mice per group). *P<0.05; **P<0.01; ***P<0.001. DL: donor leukocyte; RA: recipient aorta; D: donor; R: recipient; LCA: left common carotid artery. (C). A proposed model for the role of Sema7A in d-flow-induced endothelial phenotypic changes and leukocyte recruitment in atherogenesis. When exposed to d-flow, endothelial Sema7A expression is upregulated, potentially by the cAMP/CREB pathway. Endothelial Sema7A activates the transcription of ICAM-1/VCAM-1 genes through binding to its receptor integrin α1β1, resulting in FAK/MAPK/NFκB signaling pathway activation and the recruitment of leukocytes into atherosclerotic lesions. D-flow: disturbed flow.
To distinguish the role of vascular vs blood cell-derived Sema7A in d-flow induced atherogenesis in vivo, we generated chimeric mice of which blood cells of Sema7A+/+ApoE−/− or Sema7A−/−ApoE−/− mice were replaced with bone marrow-derived cells from these mice through bone marrow transplantation (BMT). A modified PCL was performed 4 w after BMT following with HFD for another 2 w. As shown in Figure 5B, atherosclerotic lesion size was significantly reduced in Sema7A−/−ApoE−/− mice compared with that in Sema7A+/+ApoE−/− mice when transplanted with either Sema7A−/−ApoE−/− or Sema7A+/+ApoE−/− blood cells. These results support the notions that Sema7A-deficiency reduces atherosclerotic lesion size, particularly in the aortic arch exposed to d-flow and that most likely endothelial, but not blood cell-derived, Sema7A plays a major role in the atherogenesis.
Discussion
In this study, we examined the role of Sema7A, an EC expressed semaphorin family member, in d-flow-induced vascular inflammatory changes, leukocyte adhesion, and atherosclerotic plaque formation and found that upregulation of Sema7A by d-flow in large arteries contributes to endothelial dysfunction via endothelial integrin β1, whereas deficiency of Sema7A in ApoE−/− mice confers significant protection against atherosclerosis.
Members of semaphorin family are known to promote atherosclerosis by enhancing monocyte-endothelial adhesion, promoting macrophage retention and neovascularization,32–34 while studies indicate that Sema3A expression is reduced in response to proatherogenic conditions.35 To extend our knowledge on the role of semaphorin family members in atherosclerosis, we measured their expression at distinct aortic regions exposed to d-flow and s-flow. Compared with the descending aorta (s-flow), the aortic arch (d-flow) showed significantly increased Sema7A level but reduced expression of Sema3A, Sema3C, Sema3D, Sema3F and Sema6A (Figure VA in the online-only Data Supplement). Given the fact that the causative role of d-flow in atherosclerosis may not be directly estimated due to variations of leukocyte mediated inflammation, genetic background, and diverse embryonic origin of the aorta in the conventional HFD-fed dyslipidemic mouse model36, 37, we employed a PCL model that allows a more accurate delineation of d-flow mediated endothelial dysfunction during atherosclerosis.25, 38–40 In this modified model, increased Sema7A expression was found in mouse LCA subjected to PCL compared with the control RCA, suggesting that Sema7A expression is regulated by d-flow induced shear stress (Figure VA, B in the online-only Data Supplement). Using the atherosclerotic-onset mouse model, LDLR−/− mice fed a Western diet for 2 weeks, the expression of more semaphorins have been shown to be affected at sites of altered hemodynamic stress.35 In this analysis Sema7A mRNA expression did not show a significant difference between the lesser and greater curvature of the aortic arch, however whole aorta and not just endothelial cells were analysed. In addition, it will be interesting to examine if hyperlipidemic conditions alters Sema7A expression in endothelial cells.
D-flow antagonizes the protective effect of s-flow on blood vessel, leading to chronic inflammation, EC dysfunction, and atherosclerosis.41, 42 Interestingly, Sema7A is hardly detected in ECs cultured under static or s-flow conditions. In contrast, exposure to d-flow markedly increased Sema7A mRNA and protein levels in the endothelium. To determine how Sema7A transcription is elevated in response to d-flow we analyzed the Sema7A promoter sequence and identified conserved binding sites (CREs) for the CREB transcription factor. Activated by s-flow or laminar shear stress, PKA/CREB pathway reduces the expression of pro-inflammatory genes and maintains endothelial homeostasis.26 These findings collectively indicate Sema7A as a potential transcriptional target to the shear responsive transcription factor CREB. This notion is supported by our observation that EC Sema7A expression was increased by H89, an inhibitor of PKA/CREB signaling. Sema7A promoter analysis further indicated the presence of consensus binding sites for other shear responsive elements, including NFκB, IRF-1 and KLF4, implying that a comprehensive network of transcription factors may be involved in hemodynamic regulation of Sema7A. The regulation of Sema7A by d-flow may also be achieved through epigenetic DNA methylation in its promoter.40 Future studies are warranted to address the effect of these factors, and to provide more mechanistic insights on how Sema7A is regulated under proatherogenic hemodynamics.
Our findings reveal a key role of endothelial Sema7A/β1 integrin axis in d-flow induced EC dysfunction, inflammation and atherogenesis. As shown in Figure 5C, first, endothelial Sema7A was upregulated by d-flow, while Sema7A deletion attenuated leukocyte adhesion induced by d-flow and atherosclerotic plaque formation in ApoE−/− mice. Second, integrin β1 ligation by Sema7A activates FAK/MEK1/2/NFκB signaling in ECs. Third, overexpression of Sema7A in ECs promotes adhesion molecule expression and monocyte-endothelial interaction. Consistently, Sema7A has been shown to mediate acute inflammation in lung ECs via activating integrin β1. The other known receptor for Sema7A is Plexin C1, which has been reported to mediate EC cytoskeleton change and swelling under seawater stimulation.21 Whether plexin C1 is involved in atherosclerosis remains to be elucidated.
In summary, our data showed a significant role of Sema7A in d-flow mediated EC dysfunction and atherosclerotic plaque formation. Integrin β1 appears to play a crucial role in mediating the proatherogenic effect of Sema7A that activates a downstream FAK/MAPK/NFκB pathway, thus promoting leukocyte adhesion and infiltration, and the development of atherosclerosis. These findings suggest new strategies in the prevention and treatments for atherosclerosis.
Supplementary Material
Highlights.
Endothelial Sema7A is upregulated by disturbed blood flow potentially via PKA/CREB pathway.
Sema7A mediates disturbed blood flow induced vascular endothelial dysfunction and leukocyte infiltration in a β1 integrin-dependent manner.
Deletion of Sema7A ameliorates atherosclerotic plaque formation in mice.
Acknowledgments
Sources of Funding
This work was supported in part by grants from the Natural Science Foundation of China (81620108001, 81370373, and 91439112 to L.Z. and 31300781 and 81670134 to C.T.), the Netherlands Heart Foundation (2013T127 to H.Z. and J.M.G.), the National Institutes of Health (HL119798 and HL095070 to HJ) and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Non-standard Abbreviations and Acronyms
- PCL
Partial carotid artery ligation
- HUVECs
human umbilical venous endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule 1
- HFD
high-fat diet
- LPS
lipopolysaccharide
- AA
aortic arch
- DA
descending aorta
- LC
lesser curvature
- GC
greater curvature
- LCA
left common carotid artery
- RCA
right common carotid artery
- CREB
cAMP response element-binding protein
- FAK
focal adhesion kinase
- MAPK
mitogen-activated protein kinase
Footnotes
Disclosures
None.
References
- 1.Davies PF, Zilberberg J, Helmke BP. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92:359–70. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 2.Feaver RE, Gelfand BD, Blackman BR. Human haemodynamic frequency harmonics regulate the inflammatory phenotype of vascular endothelial cells. Nat Commun. 2013;4:1525. doi: 10.1038/ncomms2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–54. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Gimbrone MA. The Gordon Wilson lecture. Understanding vascular endothelium: a pilgrim’s progress. Endothelial dysfunction, biomechanical forces and the pathobiology of atherosclerosis. Trans Am Clin Climatol Assoc. 2010;121:115–27. discussion 127. [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–85. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarbell JM, Shi ZD, Dunn J, Jo H. Fluid Mechanics, Arterial Disease, and Gene Expression. Annu Rev Fluid Mech. 2014;46:591–614. doi: 10.1146/annurev-fluid-010313-141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S, Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal. 2014;20:899–913. doi: 10.1089/ars.2013.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons RD, Kumar S, Jo H. The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches. Arch Biochem Biophys. 2016;591:111–31. doi: 10.1016/j.abb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal. 2011;15:1405–14. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wannemacher KM, Wang L, Zhu L, Brass LF. The role of semaphorins and their receptors in platelets: Lessons learned from neuronal and immune synapses. Platelets. 2011;22:461–5. doi: 10.3109/09537104.2011.561891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Ng S, Wu ZL, Nguyen D, Homburger S, Seidel-Dugan C, Ebens A, Luo Y. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J Biol Chem. 1998;273:22428–34. doi: 10.1074/jbc.273.35.22428. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–61. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Areas R, Libreros S, Iragavarapu-Charyulu V. Semaphorin7A: branching beyond axonal guidance and into immunity. Immunol Res. 2013;57:81–5. doi: 10.1007/s12026-013-8460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma B, Herzog EL, Lee CG, Peng X, Lee CM, Chen X, Rockwell S, Koo JS, Kluger H, Herbst RS, Sznol M, Elias JA. Role of chitinase 3-like-1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Res. 2015;75:487–96. doi: 10.1158/0008-5472.CAN-13-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–4. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Yan X, Liu W, Sun R, Xie Y, Jin F. Endothelial semaphorin 7A promotes seawater aspiration-induced acute lung injury through plexin C1 and beta1 integrin. Mol Med Rep. 2017 doi: 10.3892/mmr.2017.7097. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Wang L, Dong M, Li Z, Jin F. Endothelial Semaphorin 7A promotes inflammation in seawater aspiration-induced acute lung injury. Int J Mol Sci. 2014;15:19650–61. doi: 10.3390/ijms151119650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morote-Garcia JC, Napiwotzky D, Kohler D, Rosenberger P. Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia. Proc Natl Acad Sci U S A. 2012;109:14146–51. doi: 10.1073/pnas.1202165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Stalker TJ, Fong KP, Jiang H, Tran A, Crichton I, Lee EK, Neeves KB, Maloney SF, Kikutani H, Kumanogoh A, Pure E, Diamond SL, Brass LF. Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1039–45. doi: 10.1161/ATVBAHA.109.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–43. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker EN, Jo H, Gardner J, Orosz Z, Ungvari Z. Downregulation of bone morphogenetic protein 4 expression in coronary arterial endothelial cells: role of shear stress and the cAMP/protein kinase A pathway. Arterioscler Thromb Vasc Biol. 2007;27:776–82. doi: 10.1161/01.ATV.0000259355.77388.13. [DOI] [PubMed] [Google Scholar]
- 27.Kim PG, Nakano H, Das PP, Chen MJ, Rowe RG, Chou SS, Ross SJ, Sakamoto KM, Zon LI, Schlaeger TM, Orkin SH, Nakano A, Daley GQ. Flow-induced protein kinase A-CREB pathway acts via BMP signaling to promote HSC emergence. J Exp Med. 2015;212:633–48. doi: 10.1084/jem.20141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albaugh G, Bellavance E, Strande L, Heinburger S, Hewitt CW, Alexander JB. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann Vasc Surg. 2004;18:302–7. doi: 10.1007/s10016-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 29.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavins FN, Chatterjee BE. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J Pharmacol Toxicol Methods. 2004;49:1–14. doi: 10.1016/S1056-8719(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 31.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 32.Luque MC, Gutierrez PS, Debbas V, Kalil J, Stolf BS. CD100 and plexins B2 and B1 mediate monocyte-endothelial cell adhesion and might take part in atherogenesis. Mol Immunol. 2015;67:559–67. doi: 10.1016/j.molimm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Yukawa K, Tanaka T, Kishino M, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med. 2010;26:39–44. doi: 10.3892/ijmm_00000432. [DOI] [PubMed] [Google Scholar]
- 34.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O’Brien ER, Fisher EA, Moore KJ. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013;33:886–93. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Gils JM, Ramkhelawon B, Fernandes L, et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013;33:911–9. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awgulewitsch A, Majesky MW. Interpreting inflammation: smooth muscle positional identity and nuclear factor-kappaB signaling. Arterioscler Thromb Vasc Biol. 2013;33:1113–5. doi: 10.1161/ATVBAHA.113.301407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trigueros-Motos L, Gonzalez-Granado JM, Cheung C, Fernandez P, Sanchez-Cabo F, Dopazo A, Sinha S, Andres V. Embryological-origin-dependent differences in homeobox expression in adult aorta: role in regional phenotypic variability and regulation of NF-kappaB activity. Arterioscler Thromb Vasc Biol. 2013;33:1248–56. doi: 10.1161/ATVBAHA.112.300539. [DOI] [PubMed] [Google Scholar]
- 38.Heo KS, Le NT, Cushman HJ, Giancursio CJ, Chang E, Woo CH, Sullivan MA, Taunton J, Yeh ET, Fujiwara K, Abe J. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest. 2015;125:1299–310. doi: 10.1172/JCI76453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merino H, Parthasarathy S, Singla DK. Partial ligation-induced carotid artery occlusion induces leukocyte recruitment and lipid accumulation–a shear stress model of atherosclerosis. Mol Cell Biochem. 2013;372:267–73. doi: 10.1007/s11010-012-1468-7. [DOI] [PubMed] [Google Scholar]
- 40.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–99. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–27. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–7. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seltsam A, Strigens S, Levene C, Yahalom V, Moulds M, Moulds JJ, Hustinx H, Weisbach V, Figueroa D, Bade-Doeding C, DeLuca DS, Blasczyk R. The molecular diversity of Sema7A, the semaphorin that carries the JMH blood group antigens. Transfusion. 2007;47:133–46. doi: 10.1111/j.1537-2995.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 44.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, Brass LF. Deciphering the human platelet sheddome. Blood. 2011;117:e15–26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


