Abstract
Objective
To investigate sex-specific vascular mechanisms for mental stress-induced myocardial ischemia (MSIMI).
Approach and Results
Baseline data from a prospective cohort study of 678 patients with coronary artery disease underwent myocardial perfusion imaging before and during a public speaking stressor. The rate-pressure product (RPP response) was calculated as the difference between the maximum value during the speech minus the minimum value during rest. Peripheral vasoconstriction by peripheral arterial tonometry (PAT) was calculated as the ratio of pulse wave amplitude during the speech over the resting baseline; ratios < 1 indicate a vasoconstrictive response. MSIMI was defined as percent of left ventricle (LV) that was ischemic and as a dichotomous variable. Men (but not women) with MSIMI had a higher RPP response than those without MSIMI (6,500 vs. 4,800 mmHg bpm), while women (but not men) with MSIMI had a significantly lower PAT ratio than those without MSIMI (0.5 vs. 0.8). In adjusted linear regression, each 1,000-unit increase in RPP response was associated with 0.32% (95% CI: 0.22, 0.42) increase in inducible ischemia among men, while each 0.10-unit decrease in PAT ratio was associated with 0.23% (95% CI: 0.11, 0.35) increase in inducible myocardial ischemia among women. Results were independent of conventional stress induced myocardial ischemia.
Conclusions
Women and men have distinct cardiovascular reactivity mechanisms for MSIMI. For women, stress-induced peripheral vasoconstriction with mental stress, and not increased hemodynamic workload, is associated with MSIMI, while for men it is the opposite. Future studies should examine these pathways on long-term outcomes.
Keywords: Peripheral vasculature, vasoconstriction, hemodynamics, myocardial ischemia
Subject codes: Myocardial Infarction, Hemodynamics, Vascular Biology
Graphical Abstract
Introduction
Acute emotional stress and stress-induced physiological perturbations have long been observed to adversely affect cardiometabolic risk and predict future cardiovascular events.1 Nonetheless, stress remains a relatively understudied and under-recognized risk factor.2 Of recent growing interest is the effect of acute mental stress on inducible ischemia in patients with coronary artery disease (CAD).3,4 Mental stress-induced myocardial ischemia (MSIMI) is associated with a twofold increased risk for adverse cardiac events and mortality, which is not explained by established cardiovascular risk factors or whether patients also have conventional (exercise or pharmacological) stress ischemia.5,6 Recently, interest has grown in MSIMI as a metric that may index an individual’s cardiovascular vulnerability to emotional stressors.7
Emerging data suggest that women may be more vulnerable than men to the adverse effects of emotional stress on the cardiovascular system.3,7 We and others have shown that women, and young women in particular, are more likely to develop MSIMI as compared to men and older patients.8–10 The increased risk of MSIMI among women is not accounted for by severity of disease, traditional cardiovascular risk factors, or even behavioral or psychological risk factors, suggesting that alternative mechanisms may be important in understanding disparities in MSIMI.8 Clarifying the mechanisms that may predispose women to MSIMI may help identify distinct physiological pathways for adverse long-term cardiovascular outcomes.
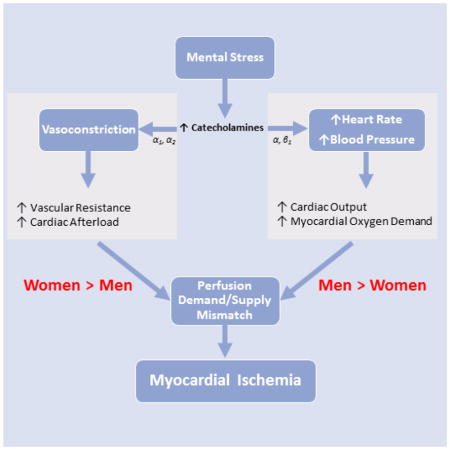
Postulated mechanisms of MSIMI involve the well-known cardiovascular effects of sympathetic nervous system stimulation, including increased hemodynamic workload and enhanced vasoconstriction.4,11–13 Stress-induced coronary vascular reactivity in the epicardial and microvascular circulation has been especially implicated in MSIMI.4,12–15 Coronary microvascular dysfunction is characterized by a failure of resistance arterioles to dilate in response to myocardial demand or abnormal vasoconstriction to stimuli.4,14,16 It is thought to be common in women, especially in the setting of non-obstructive epicardial CAD.17 Thus, it is possible that stress-induced vasoconstriction is a predominant mechanism of MSIMI among women, although this question has not been directly assessed.
Recent evidence also links MSIMI to peripheral vasoconstriction measured directly during mental stress using peripheral arterial tonometry (PAT).4,18,19 These data suggest that an increase in afterload caused by stress-induced peripheral microvascular resistance can affect the risk of MSIMI, or, alternatively, that peripheral vasoconstriction is a proxy for a similar response occurring in the coronary circulation. Using PAT, we sought to investigate sex differences in the role that stress-induced peripheral microvascular reactivity plays in MSIMI, and to contrast these results with those of stress-induced hemodynamic workload (blood pressure and heart rate). We hypothesized that in women, in contrast to men, vasoconstrictive responses to stress play a larger role than hemodynamic workload in predicting MSIMI.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement. Briefly, The Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) is a prospective study designed to investigate mechanisms and prognosis of MSIMI among patients with stable CAD. More detailed information on the MIPS objectives and study design has been described elsewhere.9,20
Results
Of the 678 participants included in the dataset, 186 were women (Table 1). The mean age was 63 years in both women and men. Women and men had similar clinical and lifestyle risk profiles, but women were less likely to be white, and more likely to be poor, to have more depressive symptoms, angina during the past month, lower functional capacity, and to be taking anti-depressants. CAD severity indicators (obstructive CAD with ≥ 50% stenosis, summed rest score, and Gensini score) were lower in women than men, indicating less CAD plaque burden in women, but there were no sex differences in inducible ischemia. Only five women were currently taking post-menopausal hormones, while 34 indicated that they used post-menopausal hormones in the past. At rest, women had higher systolic blood pressure (SBP), higher heart rate (HR), and higher rate pressure product (RPP). In bivariate analyses, without adjusting for body surface area (BSA) or other covariates, there were no significant sex differences in RPP response during stress, while men had significantly lower PAT ratio (indicating greater vasoconstriction)(Table 1).
Table 1.
Characteristics of Study Population by Sex.
| Women | Men | P-Value | |
|---|---|---|---|
| Total Number | 186 | 492 | |
| Demographic Factors | |||
| Age, years, mean (SD) | 62.6 (9.4) | 63.0 (0.1) | 0.65 |
| White, n (%) | 98 (52.7) | 332 (67.5) | 0.0004 |
| Income below poverty status (≤ $20,000), n (%) | 43 (24.4) | 63 (13.6) | 0.001 |
| Greater than high school education, n (%) | 128 (71.1) | 362 (74.6) | 0.36 |
| Clinical and Lifestyle Factors | |||
| BMI, kg/m2, mean (SD) | 30.2 (6.1) | 29.4 (4.9) | 0.11 |
| Smoking, n (%) | 30 (16.1) | 69 (14.1) | 0.05 |
| Depression (BDI ≥ 10), n (%) | 80 (44.4) | 135 (28.4) | <.0001 |
| Diabetes, n (%) | 71 (38.2) | 151 (30.7) | 0.06 |
| Hypertension, n (%) | 146 (78.5) | 370 (75.2) | 0.37 |
| Dyslipidemia, n (%) | 144 (77.4) | 412 (83.7) | 0.06 |
| Myocardial Infarction, n (%) | 69 (37.1) | 183 (37.2) | 0.98 |
| Heart Failure, n (%) | 29 (15.6) | 67 (13.6) | 0.51 |
| Angina past month, n (%) | 78 (42.2) | 118 (24.2) | <.0001 |
| Functional capacity, mean (SD) | 33.5 (14.7) | 43.6 (13.8) | <.0001 |
| Medications | |||
| Aspirin, n (%) | 156 (83.9) | 428 (87.2) | 0.27 |
| Beta Blocker, n (%) | 143 (76.9) | 361 (73.5) | 0.37 |
| ACE Inhibitors, n (%) | 65 (35.0) | 245 (50.0) | 0.001 |
| Anti-Depressant, n (%) | 60 (32.3) | 94 (19.1) | 0.0003 |
| Statins, n (%) | 154 (82.8) | 422 (86.1) | 0.28 |
| Imaging Data and CAD Severity Indicators | |||
| Obstructive CAD (≥ 50% stenosis), n (%) | 138 (86.3) | 394 (92.3) | 0.03 |
| Previous revascularization, n (%) | 145 (78.0) | 377 (76.6) | 0.71 |
| Abnormal exercise or nuclear test, n (%) | 38 (23.0) | 102 (22.3) | 0.84 |
| Gensini Score, mean (SD) | 2.9 (1.4) | 3.2 (1.3) | 0.01 |
| Summed rest score, mean (SD) | 3.5 (7.1) | 5.8 (9.3) | 0.001 |
| Percent LV with Inducible Mental Stress Ischemia, mean (SD) | 1.3 (3.6) | 1.1 (2.7) | 0.34 |
| Percent LV with Inducible Conventional Stress Ischemia, mean (SD) | 3.3 (6.4) | 3.9 (6.6) | 0.33 |
| Mental Stress Myocardial Ischemia, n (%) | 27 (14.2) | 81 (16.5) | 0.54 |
| Conventional Stress Myocardial Ischemia, n (%) | 56 (31.3) | 173 (36.1) | 0.25 |
| Resting Hemodynamics | |||
| SBP, mmHg, mean (SD) | 140.0 (20.5) | 133.9 (16.6) | 0.0003 |
| DBP, mmHg, mean (SD) | 78.1 (10.6) | 78.8 (10.2) | 0.45 |
| HR, beat/min, mean (SD) | 65.3 (11.3) | 62.6 (10.8) | 0.01 |
| RPP, beat × mmHg/min, mean (SD) | 9141.7 (2110.9) | 8400.0 (1850.1) | <.0001 |
| Response to Stress | |||
| Subjective Units of distress, mean (SD)* | 11.9 (24.3) | 9.7 (13.7) | 0.20 |
| PAT Ratio† | 0.82 (0.43) | 0.68 (0.30) | 0.002 |
| RPP Response‡ | 5087.6 (2639.3) | 4909.7 (2732.7) | 0.45 |
Difference between posttest and pretest values. A positive value indicates higher distress with mental stress.
The PAT ratio is calculated as the ratio of pulse wave amplitude during the speaking task over the resting baseline, with lower values indicating greater vasoconstriction.
RPP Response is calculated as the difference between maximum value during stress and minimum value during rest.
Abbreviations: BDI: Beck depression inventory; BMI: Body mass index; DBP: Diastolic blood pressure; HR: Heart rate; LV: Left ventricular; PAT: Pulsatile arterial tonometry; RPP: Rate pressure product; SBP: Systolic blood pressure.
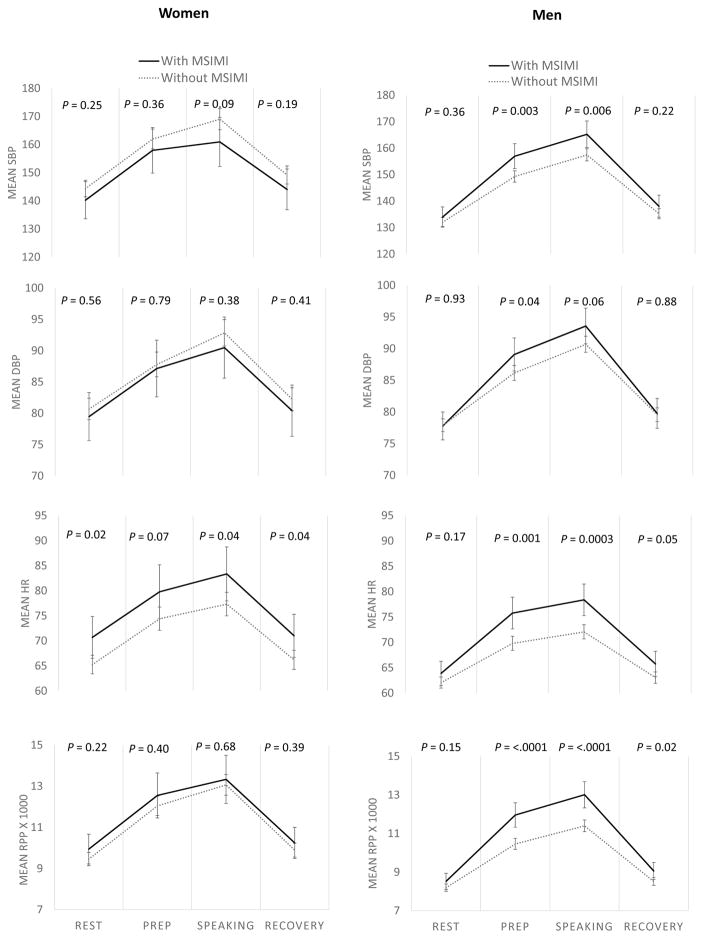
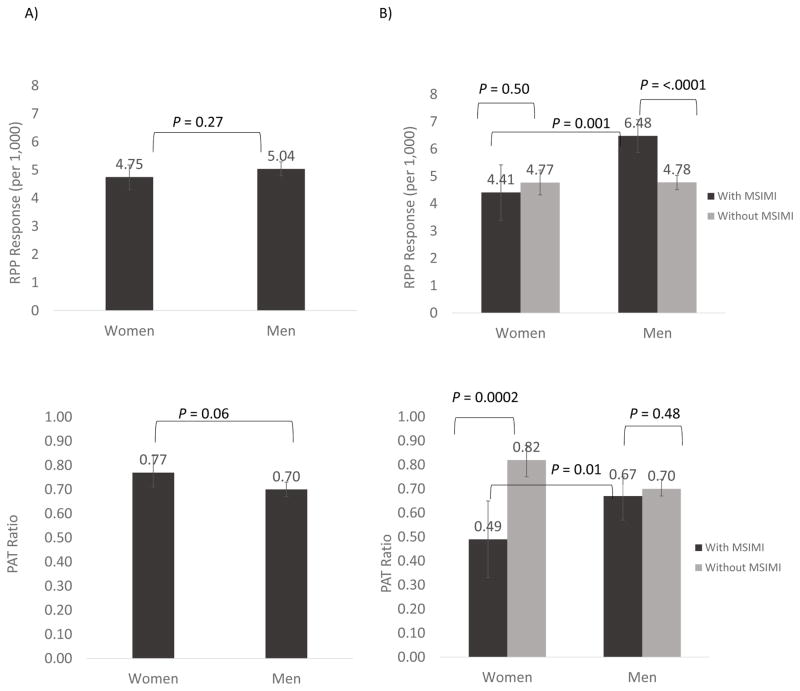
Sex differences in hemodynamic workload throughout mental stress testing are shown in Figure 1. For ease of interpretation, MSIMI was dichotomized using accepted clinical criteria as described under Materials and Methods. All comparisons were adjusted for BSA, an important confounding factor for vascular parameters given women’s smaller body habitus. Compared to women without MSIMI, women who developed MSIMI had significantly higher HR throughout (at rest, during the speaking task, and recovery), but showed no significant differences in hemodynamic workload to stress and the RPP did not differ. In contrast, men with MSIMI, compared to men without MSIMI, showed larger increases in all hemodynamic parameters during mental stress testing. When RPP response was examined as the difference between maximum value during stress and minimum value during rest, women and men had similar RPP responses overall (Figure 2, A). However, when data were analyzed by MSIMI status (Figure 2, B), men with MSIMI had significantly higher RPP response compared to men without MSIMI (6.5 vs. 4.8), as well as women with MSIMI (6.5 vs. 4.4), while there was no significant difference in RPP response between women with MSIMI and women without MSIMI (4.4 vs. 4.8). Results remained virtually unchanged after adjusting for resting RPP.
Figure 1.
Comparison of hemodynamic changes in women and men with and without MSIMI during mental stress testing. Hemodynamic changes measured as systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and rate pressure product (RPP). All measurements are adjusted for body surface area (BSA). Error bars represent 95% confidence intervals.
Figure 2.
Comparison of PAT ratio and RPP response in women and men (A), and by MSIMI status (B) during mental stress testing. RPP response is calculated as the difference between maximum value during stress and minimum value during rest. The PAT ratio is calculated as the ratio of pulse wave amplitude during the speaking task over the resting baseline, with lower values indicating greater vasoconstriction. All measurements are adjusted for body surface area (BSA). Error bars represent 95% confidence intervals.
A similar analysis for PAT provided opposite results. In the entire sample (Figure 2, A), there was little difference in PAT ratio between women and men (0.8 vs. 0.7) after adjusting for BSA. However, women with MSIMI had a significantly lower PAT ratio (denoting greater vasoconstriction) than women without MSIMI (0.5 vs. 0.8), as well as men with MSIMI (0.5 vs. 0.7), while there were no significant differences in PAT ratio for men by MSIMI status (0.7 vs. 0.7) (Figure 2, B).
Linear regression models adjusted for BSA showed similar findings. The RPP response was significantly associated with a higher percent of ischemic left ventricular myocardium with mental stress only among men (Table 2). For each 1,000-unit increase in RPP response, there was a 0.28% increase in inducible ischemia for men (95% CI: 0.19, 0.38). The RPP response was not significantly associated with mental stress-induced ischemia among women, and there was a significant sex-by-RPP response interaction (P<0.0001). Results were similar after adjusting for demographic and clinical risk factors selected a priori, including age, race, history of diabetes, hypertension, previous myocardial infarction, subjective ratings of distress, beta-blocker use, and resting RPP (model 2). We also tested whether additional factors might confound our results, including Gensini Score, depression, anti-depressant use, angina, and functional capacity, by comparing effect estimates from our main effects model (model 1), and a subsequent model that included each variable separately. However, since effect estimates did not change by more than 10% and our results were materially unchanged, these variables were not included in model 2. A final model added PAT ratio with mental stress and percent ischemia by conventional stress as adjustment factors, but results remained similar (model 3).
Table 2.
Sex-specific Associations of Hemodynamic and Vasoconstrictive Measures with Mental Stress-Induced Myocardial Ischemia.
| Outcome: Percent Increase in
Left Ventricular Inducible Ischemia by Mental Stress |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RPP Response* per 1,000 Units Increase | PAT Ratio† per 0.10 Unit Decrease | |||||||||
|
| ||||||||||
| Women | P-value | Men | P-value | P-value of sex* RPP interaction | Women | P-value | Men | P-value | P-value of sex* PAT interaction | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |||||||
| Model 1 | −0.10 (−0.26, 0.06) | 0.21 | 0.28 (0.19, 0.38) | <.0001 | <.0001 | 0.23 (0.11, 0.35) | 0.0001 | 0.05 (−0.04, 0.15) | 0.27 | 0.02 |
| Model 2 | −0.09 (−0.25, 0.07) | 0.27 | 0.32 (0.22, 0.42) | <.0001 | <.0001 | 0.23 (0.11, 0.35) | 0.0002 | 0.06 (−0.04, 0.16) | 0.24 | 0.03 |
| Model 3 | −0.12 (−0.33, 0.10) | 0.28 | 0.24 (0.13, 0.35) | <.0001 | 0.004 | 0.22 (0.10, 0.34) | 0.0003 | 0.05 (−0.05, 0.14) | 0.35 | 0.03 |
RPP response is calculated as the difference between maximum value during stress and minimum value during rest.
The PAT ratio is calculated as the ratio of pulse wave amplitude during the speaking task over the resting baseline, with lower values indicating greater vasoconstriction.
Model 1: RPP Response and PAT Ratio are in separate models. Adjusted for sex, BSA, sex interaction term.
Model 2: RPP Response and PAT Ratio are in separate models. Adjusted for Model 1 covariates + age, race, diabetes, hypertension, beta blocker, previous MI, subjective units of distress scale, and resting RPP.
Model 3: RPP Response and PAT Ratio are included in the same model. Adjusted for Model 2 covariates and % LV conventional stress induced myocardial ischemia.
Abbreviations: CI: Confidence interval; PAT: Pulsatile arterial tonometry; RPP: Rate pressure product; BSA: Body surface area.
In contrast, PAT ratio was significantly associated with a higher percent of ischemic left ventricle with mental stress only among women (Table 2). For each 10-unit decrease in PAT ratio, indicating greater vasoconstriction, there was a 0.23% increase in inducible ischemia for women (95% CI: 0.11, 0.35). The PAT ratio was not significantly associated with mental stress-induced ischemia among men (model 1) and the sex-by-PAT interaction term was significant (0.02). Results were similar after adjusting for the same factors as above in model 2 (age, race, history of diabetes, hypertension, previous myocardial infarction, subjective ratings of distress, beta-blocker use, and resting RPP). Again, Gensini Score, depression, anti-depressant use, angina, and functional capacity were considered as potential confounders in model 2, but were not included as the results did not change. After adding RPP response and percent ischemia with conventional stress, results remained similar, although slightly attenuated (model 3). Baseline PAT amplitude was available in a subgroup of 308 participants. Women had significantly lower baseline amplitude values compared to men (390.7 vs. 680.1; P<0.0001). After adjusting for baseline amplitude in this subsample, the main findings did not change and in fact, the results were further away from the null in women.
Bland-Altman plots showed good reproducibility of mental stress on cardiovascular responses and stress-induced myocardial ischemia with Tc99m sestamibi (Supplemental Material, Figure I). For SBP, 95.5% of data points were within the 95% limits of agreement (I-a). Results were similar for DBP (I-b), HR (I-c), and RPP (I-d). For PAT ratio (I-e), reproducibility results were similarly excellent, with only one data point falling outside the 95% limits of agreement. When we calculated reproducibility of mental-stress induced myocardial ischemia with Tc99m sestamibi of 19 patients who had repeated testing two weeks apart,21 only 2 data points were outside the 95% limits of agreement (I-f). Bland-Altman plots were also used to compare agreement between the two readers’ SPECT sum stress scores of perfusion defects at rest (n = 601), after mental stress (n = 600), and after conventional stress (n = 584). Of all data points, 94.5% were within the 95% limits of agreement for scores at rest (I-g), 94.3% for scores with mental stress (I-h), and 94.3% (I-i) for conventional stress. However it should be noted that, to minimize variability in the interpretation of the imaging results, discrepancies were resolved by consensus, or a third reader if needed.
Discussion
To our knowledge, this is the first study to empirically investigate sex-specific vascular mechanisms of MSIMI. Our findings suggest that MSIMI is primarily driven by microvascular vasoconstriction in women, while in men, it is driven by supply-demand mismatch due to increased hemodynamic workload.
Despite growing research on the mechanisms and outcome of MSIMI, this phenomenon remains understudied among women. Until recently, the majority of studies on MSIMI have included few or no women.6 Recently, we and others reported that women, especially younger women, develop MSIMI more often than men.8–10 We now show that women and men differ in vascular response patterns that predict MSIMI. These new findings complement previously observed sex differences in MSIMI and may help identify distinct stress-related risk pathways in women and men that could aid future interventions.
Overall, we found that women and men had similar RPP responses to mental stress. These results are similar to other studies that did not find hemodynamic differences to mental stress between men and women.8,22,23 However, other studies reported greater hemodynamic responses to mental stress among men.10,24 The relationship between hemodynamic responses to mental stress and MSIMI has also been inconsistent to date, with some studies showing no association,25–27 while others reported a positive association.11,28 However, little is known about whether the association between hemodynamic reactivity with stress and MSIMI differs in men and women. While York et al.22 did not directly address this question, they found no sex differences in hemodynamic responses overall and among patients who developed MSIMI. In contrast, we found that men who developed MSIMI had a larger increase in RPP than women who developed MSIMI, and that the stress-induced RPP change was associated with MSIMI only in men. These sex differences persisted after adjusting for differences in BSA, cardiovascular risk indicators, and even occurrence of ischemia with conventional stress testing. These inconsistent findings may be due to low statistical power since previous studies included only a small number of women.
When comparing PAT ratio in women and men overall, we found that men exhibited a marginally lower PAT ratio, which is consistent with results from a previous study by Hassan et al.23 To our knowledge, however, ours is the first study to empirically compare stress-induced vasoconstrictive responses between men and women in relation to MSIMI provocation. Our findings support a vulnerability of women towards vasoconstriction-induced MSIMI, suggesting that stress-induced vasomotor responses are more likely to induce MSIMI in women than in men. In the absence of MSIMI, however, women have similar or slightly less vasoconstriction with stress than men. Our observation of these sex-specific effects in relation to MSIMI could explain some of the inconsistencies in the previous literature.
While exact mechanisms for the observed sex differences are unknown, women’s proclivity to vasoconstriction-induced MSIMI may operate through an imbalance between vasodilating and vasoconstricting mediators regulating the endothelium, resulting in microvascular dysfunction. Increased sympathetic tone during mental stress induces vasoconstriction of peripheral arteries, especially in the microvascular bed, through α1 adrenergic receptor activation; an endothelin-1-dependent pathway may also be implicated.13,29,30 This differs from physical exertion, where a vasodilator response predominates through a β2 adrenoceptor mechanism. Research has suggested that MSIMI is partly mediated by microvascular dysfunction in response to mental stress and may be a potential mechanism by which young women have a higher risk of MSIMI.4,7,14,16,31,32 Microvascular dysfunction is commonly seen in women with chest pain, even in the absence of significant epicardial coronary obstruction.33,34 Women may also exhibit more microvascular dysregulation and endothelial dysfunction with stress. Research has suggested that repeated and cumulative exposure to stress can lead to microvascular dysfunction leading to myocardial diastolic dysfunction.35,36 These vascular effects could be accentuated in young women given their higher baseline levels of inflammation.37 It has also been hypothesized that women have greater vasomotor reactivity with stress than men9,31,38,39 in part because they have smaller coronary arteries.9,39,40 However, we observed sex differences in vasoconstriction-induced MSIMI even after adjusting for BSA.
Our empirical research findings validate previous reports postulating an important role of stress-induced vasoconstrictive responses in relation to MSIMI among women.4,14,16,31 Thus, coronary microvascular dysfunction and vasoconstriction may be important in understanding why women have a greater propensity for MSIMI compared to men. Understanding women’s increased risk of MSIMI through vasoconstriction identifies a high risk population which may benefit from stress mitigation techniques or other tailored interventions.7 With regard to pharmacological interventions, combined α- and β-blockers might be beneficial in these patients if microvascular dysfunction is the substrate for their ischemia.41
There are several strengths and limitations of our study worth noting. First, the MIPS study provides one of the largest and most comprehensive studies of MSIMI to date. The large and diverse sample size, including a sizable number of women, along with state-of-the art myocardial perfusion imaging, are important strengths. The experimental manipulation of the exposure (mental stress) allows a controlled assessment of the effects of hemodynamic and peripheral vascular changes on the risk of MSIMI. A limitation worth noting is the smaller number of women relatively to men which may have limited the study power for some analyses. However, our results point to a clear demarcation of associations between women and men, with statistically significant interactions which argue against a type II error. Moreover, the relatively modest incidence of MSIMI precluded examination of subgroups, especially young women, who have shown a high proclivity towards MSIMI in previous research.8–10 Although there was no difference in MSIMI between women and men overall, younger women did have more ischemia with mental stress than men of similar age, as reported in a separate paper based on the same cohort of participants.9 Also, even though our sample was well characterized and we have carefully examined potential confounding factors, we cannot rule out unmeasured confounders, such as perceptions of difficulty, anxiety, engagement, or lower effort. Also, the baseline amplitude was not available in all participants for analysis involving the PAT ratio. Another limitation is the lack of long-term follow-up data, which is currently underway. Finally, we relied on peripheral indicators of increased microvascular tone and were not able to assess vasomotion responses to stress in the coronary bed.
In conclusion, we found that women and men have distinct cardiovascular reactivity responses associated with MSIMI. Our results support a more prominent microvascular role in the development of MSIMI among women than men and, conversely, a more prominent hemodynamic role in the development of MSIMI among men. These data are consistent with the notion of an important role of microvascular constriction in ischemic heart disease among women, and its relation with emotional stress. Furthermore, these results provide motivation for further research to understand sex differences in the effects of mental stress on cardiovascular responses, ischemia, and long-term outcomes.
Supplementary Material
Highlights.
This is the first study that has empirically investigated sex-specific patterns of cardiovascular reactivity, including hemodynamic response and peripheral vasoconstriction, in relation to mental stress-induced myocardial ischemia (MSIMI).
Greater peripheral vasoconstriction was associated with mental stress ischemia only in women, while greater hemodynamic response was associated with mental stress ischemia only in men.
These associations remained significant even after adjustment for age, race, medical conditions, and body surface area (BSA), and conventional stress induced myocardial ischemia.
Women and men have distinct cardiovascular reactivity mechanisms for MSIMI.
Acknowledgments
Sources of Funding: This work was supported by the NIH (P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413-02S1, R01 HL125246, UL1TR000454, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, and THL130025A).
Abbreviations
- BMI
Body mass index
- BSA
Body surface area
- BDI
Beck depression inventory
- CAD
Coronary artery disease
- DBP
Diastolic blood pressure
- HR
Heart rate
- LV
Left ventricular
- MSIMI
Mental stress induced myocardial ischemia
- MIPS
Mental stress ischemia mechanisms and prognosis study
- PAT
Peripheral arterial tonometry
- RPP
Rate pressure product
- SBP
Systolic blood pressure
- SDS
Summed difference score
- SSS
Summed stress score
- SRS
Summed rest score
Footnotes
Disclosures: Ernest V. Garcia receives royalties from the sale of the Emory Cardiac Toolbox, used for some analyses in this study. None of the other authors report conflict of interest relevant to this article. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 2.Shah AJ, Vaccarino V. Psychosocial Risk Factors and Coronary Artery Disease. In: Roncella A, Pristipino C, editors. Psychotherapy for Ischemic Heart Disease: An Evidence-based Clinical Approach. Cham: Springer International Publishing; 2016. pp. 29–44. [Google Scholar]
- 3.Vaccarino V. Cardiovascular Diseases and Depression. Springer; 2016. Mental Stress-Induced Myocardial Ischemia; pp. 105–121. [Google Scholar]
- 4.Ramadan R, Sheps D, Esteves F, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2:e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang W, Babyak M, Krantz DS, et al. Mental stress--induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114:187–92. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wokhlu A, Pepine CJ. Mental Stress and Myocardial Ischemia: Young Women at Risk. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76:171–80. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccarino V, Wilmot K, Al Mheid I, et al. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samad Z, Boyle S, Ersboll M, et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669–78. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 12.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., 3rd Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–30. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 13.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102:472–80. doi: 10.1136/heartjnl-2014-307306. [DOI] [PubMed] [Google Scholar]
- 14.Kop WJ, Krantz DS, Howell RH, et al. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359–66. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 15.Arrighi JA, Burg M, Cohen IS, et al. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–1. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 16.Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–8. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 17.Bairey Merz CN, Pepine CJ. Syndrome X and microvascular coronary dysfunction. Circulation. 2011;124:1477–80. doi: 10.1161/CIRCULATIONAHA.110.974212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M, York KM, Li H, et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin Cardiol. 2009;32:E1–6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burg MM, Graeber B, Vashist A, et al. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosom Med. 2009;71:14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammadah M, Al Mheid I, Wilmot K, et al. The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2016 doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, Sheps DS. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol. 2003;10:56–62. doi: 10.1067/mnc.2003.26. [DOI] [PubMed] [Google Scholar]
- 22.York KM, Hassan M, Li Q, et al. Do men and women differ on measures of mental stress-induced ischemia? Psychosom Med. 2007;69:918–22. doi: 10.1097/PSY.0b013e31815a9245. [DOI] [PubMed] [Google Scholar]
- 23.Hassan M, Li Q, Brumback B, et al. Comparison of peripheral arterial response to mental stress in men versus women with coronary artery disease. Am J Cardiol. 2008;102:970–4. doi: 10.1016/j.amjcard.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker LC, Pepine CJ, Bonsall R, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation. 1996;94:2768–77. doi: 10.1161/01.cir.94.11.2768. [DOI] [PubMed] [Google Scholar]
- 25.Holmes SD, Krantz DS, Kop WJ, Del Negro A, Karasik P, Gottdiener JS. Mental stress hemodynamic responses and myocardial ischemia: does left ventricular dysfunction alter these relationships? Psychosom Med. 2007;69:495–500. doi: 10.1097/PSY.0b013e3180cabc73. [DOI] [PubMed] [Google Scholar]
- 26.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–22. doi: 10.1016/s0735-1097(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61:714–22. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepanovic J, Ostojic M, Beleslin B, et al. Mental stress-induced ischemia in patients with coronary artery disease: echocardiographic characteristics and relation to exercise-induced ischemia. Psychosom Med. 2012;74:766–72. doi: 10.1097/PSY.0b013e3182689441. [DOI] [PubMed] [Google Scholar]
- 29.Soufer R, Jain H, Yoon AJ. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep. 2009;11:133–40. doi: 10.1007/s11886-009-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burg MM, Soufer R. Psychological Stress and Induced Ischemic Syndromes. Current cardiovascular risk reports. 2014;8:377. doi: 10.1007/s12170-014-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepine CJ, Petersen JW, Bairey Merz CN. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. JACC Cardiovasc Imaging. 2014;7:362–5. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 34.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 35.Ersboll M, Al Enezi F, Samad Z, et al. Impaired resting myocardial annular velocities are independently associated with mental stress-induced ischemia in coronary heart disease. JACC Cardiovasc Imaging. 2014;7:351–61. doi: 10.1016/j.jcmg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA, Liu K. Chronic Stress and Endothelial Dysfunction: The Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2017;30:75–80. doi: 10.1093/ajh/hpw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rooks CR, Ibeanu I, Shah A, et al. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain Behav Immun. 2016;51:92–8. doi: 10.1016/j.bbi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–9. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 40.Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18:339–45. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean J, Cruz SD, Mehta PK, Merz CN. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol. 2015;12:406–14. doi: 10.1038/nrcardio.2015.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.