Abstract
A small number of signaling pathways are used repeatedly during organogenesis, and they can have drastically different effects on the same population of cells depending on the embryonic stage. How cellular competence changes over developmental time is not well understood. Here we used Xenopus, mouse, and human pluripotent stem cells to investigate how the temporal sequence of Wnt, BMP, and retinoic acid (RA) signals regulates endoderm developmental competence and organ induction, focusing on respiratory fate. While Nkx2-1+ lung fate is not induced until late somitogenesis stages, here we show that lung competence is restricted by the gastrula stage as a result of Wnt and BMP-dependent anterior-posterior (A-P) patterning. These early Wnt and BMP signals make posterior endoderm refractory to subsequent RA/Wnt/BMP-dependent lung induction. We further mapped how RA modulates the response to Wnt and BMP in a temporal specific manner. In the gastrula RA promotes posterior identity, however in early somite stages of development RA regulates respiratory versus pharyngeal potential in anterior endoderm and midgut versus hindgut potential in posterior endoderm. Together our data suggest a dynamic and conserved response of vertebrate endoderm during organogenesis, wherein early Wnt/BMP/RA impacts how cells respond to later Wnt/BMP/RA signals, illustrating how reiterative combinatorial signaling can regulate both developmental competence and subsequent fate specification.
Keywords: Endoderm, Patterning, Foregut, Midgut, Hindgut, Lung, Pharynx, Intestine, Retinoic Acid, Wnt, Bmp, Nkx2-1, Xenopus, mouse, hPSC
Introduction
The formation of organs from naïve progenitor cells is controlled by combinatorial signaling of a handful of pathways including Wnt, BMP, FGF, Notch, Hedgehog, and retinoic acid (RA) (Kraus and Grapin-Botton, 2012; Zorn and Wells, 2009). These signals are required in distinct temporal windows to progressively direct embryonic cells through a series of fate decisions into specific tissue lineages. In many cases these same signals can have dramatically different effects at different stages of development - promoting a given lineage at one time and then inhibiting at a subsequent time. This dynamic response is a commonly observed but poorly understood phenomenon in the development of many lineages, including cardiogenesis (Gessert and Kuhl, 2010; Loh et al., 2016), neurogenesis (Sasai et al., 2014) and endoderm organogenesis within the respiratory and digestive systems (Loh et al., 2014; Zorn and Wells, 2009). In most organ systems it is still poorly understood how the ability or “competence” of a tissue to respond to signals changes over time, and how early signaling events impact subsequent responses to the same signals. A detailed understanding of these reiterative signaling dynamics is required for a comprehensive roadmap of organogenesis, which is likely necessary to facilitate the generation of multi-lineage organ tissues from human pluripotent stem cells (hPSCs) (McCauley and Wells, 2017).
Development of the respiratory and digestive systems by dynamic paracrine Wnt, BMP, and RA signaling between the embryonic endoderm and the adjacent mesoderm is a good example of reiterative, combinatorial signaling in organogenesis. In vertebrate embryos and hPSCs, the endoderm is patterned during gastrulation and early neural stages of embryogenesis wherein high levels of RA, Wnt, and BMP from the posterior mesoderm promote hindgut fate in posterior endoderm (Bayha et al., 2009; Deimling and Drysdale, 2009; Spence et al., 2011; Stevens, 2017), whereas low Wnt and BMP activity, due to antagonists such as Dkk1, Sfrps, Noggin, and Chordin expressed in anterior mesendoderm, permit the development of foregut progenitors (McLin et al., 2007; Rankin et al., 2011). Within the next 24–48 hours of development, RA, Wnt, and BMP are produced by the anterior lateral plate mesoderm, have the opposite effect, and no longer suppress anterior identity but rather promote distinct foregut organ lineages including thyroid (Kurmann et al., 2015; Wang et al., 2011), lungs (Desai et al., 2004; Domyan et al., 2011; Goss et al., 2009; Harris-Johnson et al., 2009; Rankin et al., 2012), liver (Wandzioch and Zaret, 2009), stomach (McCracken et al., 2017; McCracken et al., 2014) and pancreas (Kraus and Grapin-Botton, 2012). How these dynamic Wnt/BMP/RA signals and changing developmental competence are coordinated to control lineage specification in the primitive gut tube is still poorly understood.
We recently demonstrated that RA signaling is critical for foregut progenitors to activate respiratory fate in response to Wnt and BMP signals (Rankin et al., 2016). During early somitogenesis stages of development, RA produced by anterior lateral plate mesoderm is required to make the foregut endoderm competent to adopt respiratory fate a day later in response to lung-inducing Wnt2/2b and Bmp2/4/7 signals from the adjacent splanchnic mesoderm. The molecular basis for this RA-dependent respiratory competence is unknown. Moreover it remains untested if RA can instructively change the developmental competence of non-foregut endoderm or if RA acts in strictly a permissive fashion within the foregut.
In this study we used Xenopus, mouse, and hPSCs to interrogate if and how early Wnt/BMP/RA-mediated patterning impacts the competence and response of developing endoderm to subsequent Wnt/BMP/RA-mediated organ induction, with a focus on respiratory fate. We show that respiratory competence is restricted to anterior endoderm by the gastrula stage, much earlier that previously appreciated. Early Wnt and BMP signals make the posterior endoderm refractory to subsequent RA/Wnt/BMP-mediated lung induction and instead make the endoderm competent to adopt an intestinal fate in response to these same signals. We show that RA has a temporally dynamic, and permissive role in modulating how cells respond to Wnt and BMP. During gastrulation RA promotes posterior identity, but during early somite stages RA regulates respiratory versus pharyngeal fate in the anterior endoderm and midgut versus hindgut fate within posterior endoderm. These data illustrate that evolutionarily conserved, reiterative Wnt/BMP/RA signaling impacts dynamic competence and lineage decisions in the developing gut tube.
Results
Respiratory competence is restricted to anterior endoderm by the early gastrula stage in Xenopus
We previously demonstrated that induction of the respiratory lineage in Xenopus, mouse, and human foregut endoderm requires the sequential activity of RA followed by Wnt+BMP signaling (Rankin et al., 2016). However, in these Xenopus experiments where endoderm was isolated at the early somite-stages of development (stage NF14), only a subset of the explants expressed the respiratory markers nkx2-1 and sftpc in response to RA/Wnt/BMP signals (Supplemental Fig.S1A), suggesting that a ‘pre-pattern’ or restricted domain of respiratory competence already existed in the foregut. To test if a restricted domain of lung-competent cells already existed by the gastrula stage, we micro-dissected gastrula stage Xenopus embryos (stage NF10.25) and prepared three types of explants (Fig.1A): 1) the entire definitive endoderm (DE) region; 2) the anterior endoderm (hhex-expressing AE, which normally contributes to foregut endoderm including Nkx2-1+ respiratory endoderm (Supplemental Fig.S1C-J); and 3) the posterior endoderm (ventx1/2-expressing PE). We then subjected the DE, AE, and PE explants to a respiratory fate induction protocol (Fig.1A), which consists of RA treatment during early somitogenesis (stages NF14–25) followed by culture in the GSK3β antagonist Bio (which stabilizes β catenin to mimic endogenous Wnt2/2b signals) + recombinant BMP4 protein from stages NF25–NF38. When sibling embryos reached stage NF38, the explants were analyzed by in-situ hybridization for nkx2-1 and sftpc.
Figure 1. Respiratory competence of Xenopus endoderm is restricted by the early gastrula stage.
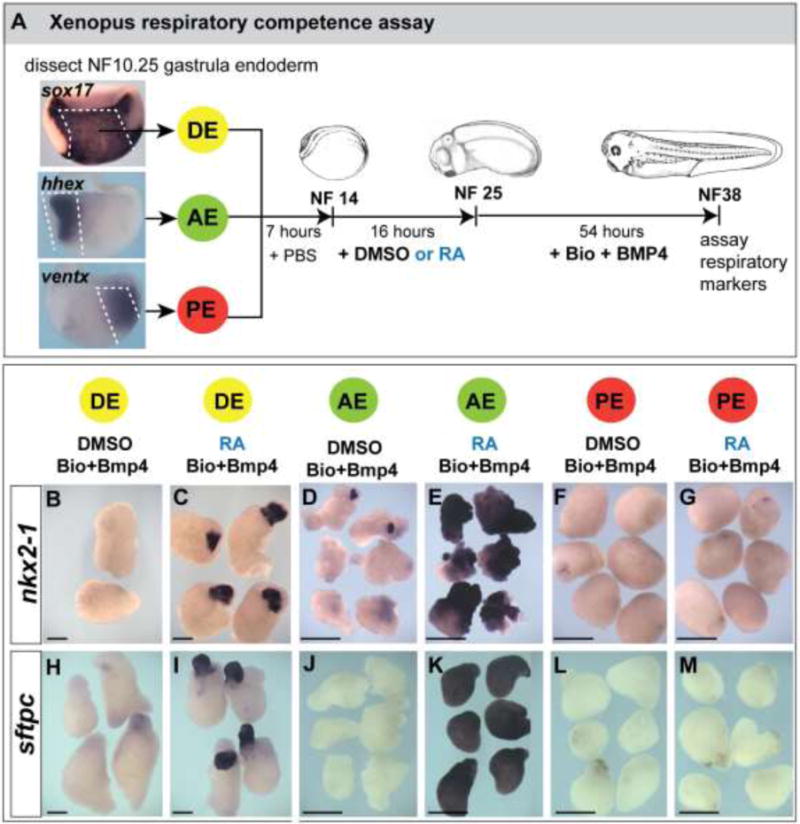
(A) Experimental diagram of the Xenopus respiratory competence assay. Bisected early gastrula stage (NF10.25) Xenopus embryos assayed by in-situ hybridization show the expression of sox17α throughout the definitive endoderm (DE), hhex in anterior endoderm (AE) and ventx2 in posterior endoderm (PE). DE, AE, or PE explants were dissected at NF10.25, cultured in isolation until NF14, treated +/− 50nM RA from NF14–25, and then in 3.5μM Bio + 50ng/mL BMP4 from NF25–38, at which time they were fixed and analyzed by in-situ hybridization for the respiratory endoderm markers nkx2-1 (B–G) and sftpc (H–M).
The DE explants treated with RA followed by Bio+BMP expressed respiratory markers only in a subset of the endoderm (Fig.1C, I), indicating that a restricted domain of lung competent cells existed in the gastrula DE. This was confirmed in the AE and PE explants: AE explants robustly expressed nkx2-1 and sftpc throughout the tissue (Fig.1E, K) whereas PE explants failed to express the respiratory markers (Fig.1G, M). RA prior to Bio+BMP was necessary for the gastrula DE and AE to express nkx2-1 and sftpc, as omission of RA resulted in no induction of the lung markers by Bio+BMP (Fig.1B, H, D, J) and RA alone was not sufficient to induce lung fate (data not shown and Supplemental Fig.S1A). These results indicate RA has a permissive role in enabling the endoderm to activate lung markers in response to Wnt/BMP, and we conclude that gastrula-stage DE already contains a restricted domain of respiratory competent cells, earlier than previously appreciated.
Stage-dependent effects of RA on Xenopus lung induction
One possible explanation for the restricted domain of lung competence in the gastrula DE was due to the timing of RA exposure. RA-synthesizing enzymes (rdh10 and raldh2) are expressed in gastrula stage mesoderm, suggesting that RA acts early in development (Supplemental Fig.S2A). We tested if earlier RA treatment, starting immediately after dissection at the gastrula stage, might expand the domain of lung competence in DE explants (experiment diagrammed in Supplemental Fig.S2B). However we found that this early RA treatment of DE not only failed to expand the nkx2-1 and sftpc domains in response to Bio+BMP, but also completely suppressed the activation of lung markers (Supplemental Fig.S2B). In contrast, this early RA treatment resulted in the up-regulation of pdx1 and cdx2 (Supplemental Fig.S2B), indicating that during this developmental time, RA promoted pancreatic and intestinal fate, consistent with previous reports (Bayha et al., 2009; Chen et al., 2004). We conclude that the timing of RA exposure elicits completely different responses in the developing endoderm: RA during gastrulation has a posteriorizing effect on the endoderm promoting pancreatic and intestinal fate, while RA starting at NF14 promotes respiratory fate in the AE.
RA regulates respiratory competence in mouse endoderm
We next sought to test whether RA also promotes respiratory competence in developing mouse endoderm. RA-dependent reporter activity in transgenic Tg(RARE:LacZ) mice (Rossant et al., 1991) was not detected in the mouse anterior endoderm at E8.0 (~3–5 somite stage, ss), however at E8.5 (8–10 ss) robust reporter activity was evident (Fig. 2A); this is prior to Wnt2/2b-dependent lung induction at ~E9.0 (16ss) when Nkx2-1 protein is first detectable in the ventral foregut (Havrilak et al., 2017). In Raldh2−/− embryos, which have a severe deficiency of RA production, Nkx2-1 protein is nearly absent from the respiratory domain but still present in the forebrain and thyroid domains (Fig.2B), confirming that RA is required for normal lung but not thyroid induction (Desai et al., 2006; Wang et al., 2011).
Figure 2. Analysis of RARE:lacZ RA signaling reporter and Raldh2−/− mouse embryos.
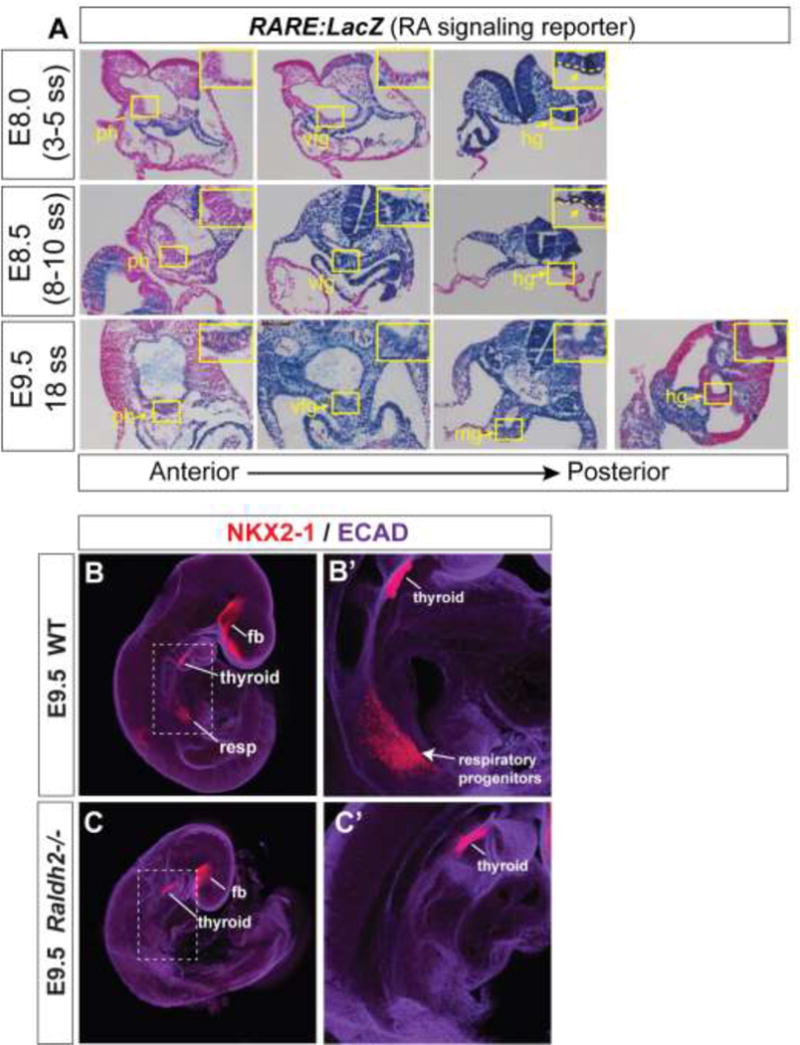
(A) Sections along the anterior-posterior axis of RARE:LacZ embryos stained for β-galactosidase (blue) and counterstained with fast red (pink) at the indicated developmental stages. Abbreviations: ph, pharynx; vfg, ventral foregut; hg, hindgut; mg, midgut. (B–C) Whole mount immunostaining of WT and Raldh2−/− embryos at E9.5 for Ecadherin (purple) and Nkx2-1 (red). Abbreviations: fb, forebrain; resp, respiratory domain. Thryoid and forebrain expression of Nkx2-1 is maintained in Raldh2−/− embryos, whereas there is a near complete loss of Nkx2-1 in the respiratory domain.
To test if RA regulates lung competence of mouse endoderm, we performed a series of culture experiments using isolated endoderm tissue (Fig.3). DE from the node to the headfold was isolated from E7.5 embryos (Fig.3A; the posterior-most DE cannot be cleanly removed from the primitive streak at this stage) and cultured with or without RA for 24 hours, followed by 5 days in the GSK3 antagonist CHIR99021 (which stabilizes β-catenin to mimic endogenous Wnt2/2b signals) + recombinant FGF7+ FGF10, which are required for survival of the mouse endoderm explants (we refer to this combination of factors hereafter as C/F7/10). Explants cultured without RA expressed little if any Nkx2-1 (Fig. 3B), however 24 hours of RA exposure made the endoderm broadly competent to express Nkx2-1 in response to C/F7/F10 (Fig. 3C,C′). Portions of each explant expressed E-cadherin and Sox2 but not Nkx2-1, indicating that not all endoderm cells had activated respiratory fate (Fig. 3C′). Exogenous BMP was dispensable in these cultures for Nkx2-1 expression; however we note that Bmp2/4/7 transcripts are present in the E7.5 cardiac crescent and phospho-Smad1/5/8 is already detectable in the underlying anterior endoderm (Danesh et al., 2009; Wandzioch and Zaret, 2009), suggesting endogenous BMP signaling has occurred in E7.5 AE.
Figure 3. RA imparts respiratory competence to mouse definitive endoderm.
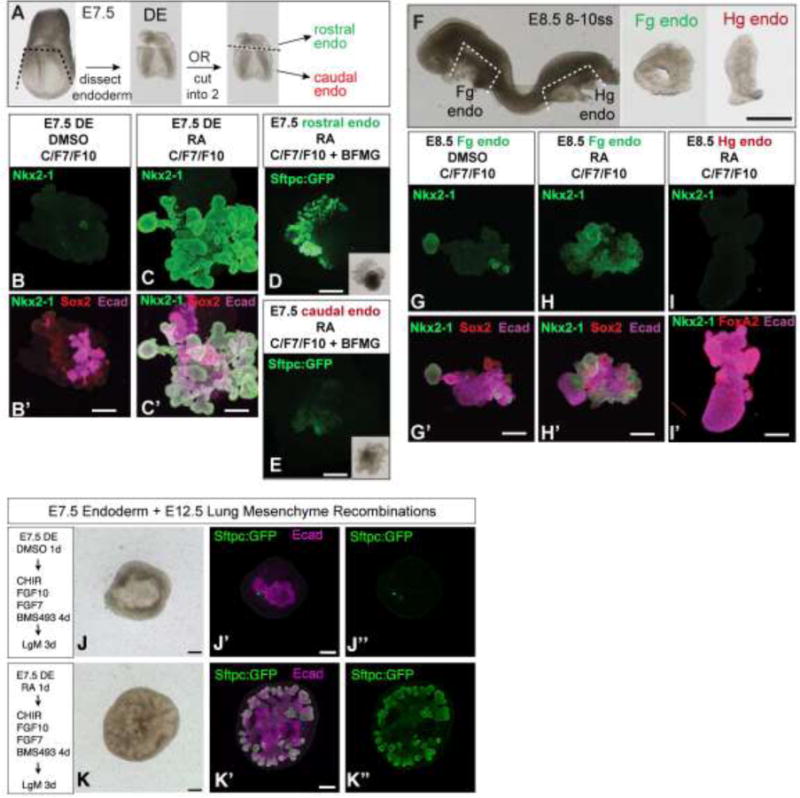
(A-E) Analysis of respiratory competence of E7.5 mouse endoderm. (A) Experimental dissection diagram. (B-C) E7.5 DE was isolated and cultured +/− RA for 24 hours followed by CHIR99021+FGF7+FGF10 (C/F7/F10) and analyzed by immunofluorescence for NKX2-1 (green), SOX2 (red), and E-CADHERIN (purple). (D-E) GFP fluorescence observed after E7.5 endoderm from Tg(Sftpc:GFP) transgenic embryos was isolated, separated into rostral AE underlying the cardiac crescent (D) or more caudal endoderm located posterior to the cardiac crescent (E), cultured 24 hours in RA, followed by C/F7/F10 + BFGM outgrowth media. (F-L) Analysis of respiratory competence of E8.5 endoderm. Foregut (Fg endo; G,H) or hindgut endoderm (Hg endo; I) was isolated from E8.5 embryos (F) and cultured +/− RA for 24 hours followed by C/F7/F10 and analyzed by immunofluorescence for NKX2-1 (green), FOXA2 (red) or SOX2 (red), and ECADHERIN (purple). (J–K) RA-dependent specified respiratory endoderm is competent to undergo early branching morphogenesis when recombined with distal lung mesenchyme. E7.5 DE from Tg(Sftpc:GFP) embryos was isolated, cultured without RA (J) or with RA (K) for 24 hours followed by C/F7/10+ the pan RAR antagonist BMS493 (in order to disrupt any RA signaling after the 24-hour pulse), and then recombined and cultured for 3 days with E12.5 distal lung mesenchyme (LgM) and analyzed by immunofluorescence for GFP (green) and ECADHERIN (purple). J and K are brightfield images of the recombinants.
The observation that Nkx2-1 is only expressed in some but not all of the cells in each RA-treated explant suggested that, like in Xenopus, mouse DE contained restricted regions of lung-competent cells by the end of gastrulation. To test this we subdivided the E7.5 DE into rostral anterior endoderm (underlying the cardiac crescent) and more caudal endoderm (posterior to the cardiac crescent) (Fig 3A). Moreover, since Nkx2-1 is expressed in both lung and thyroid, we performed these experiments with Tg(Sftpc:GFP) transgenic mouse embryos (Lo et al., 2008), enabling unambiguous identification of respiratory fate. Explants were treated with RA for 24 hours followed by 5 days in C/F7/10 followed by 1 day in a cocktail of factors (BFGM) optimized for growth of fetal lung tissue (Shannon et al., 1999). We found that the rostral anterior endoderm was much more competent than the more caudal endoderm to express Sftpc:GFP in response to the lung inducing signals (Fig. 3D,E); we note there was weak GFP expression in a small portion of the caudal explants, suggesting they contained a few anterior endoderm cells that can respond to RA.
Since it was not possible to cleanly isolate the PE from E7.5 mouse embryos we tested whether RA could impart respiratory competence to mouse hindgut endoderm (Hg endo) or foregut endoderm (Fg endo) explants from E8.5 (8–10 ss) embryos, which are roughly equivalent to Xenopus NF20 (Fig. 3F). Tg(RARE:lacZ) embryos indicated that both the foregut and hindgut endoderm had already experienced RA signaling by this time (Fig. 2A). Fg endo explants not exposed to RA and then cultured in C/F7/10 expressed small discrete small patches of Nkx2-1+ cells (Fig.3G,G′), indicating a limited amount of respiratory induction. RA treatment of E8.5 Fg endo resulted in a dramatic increase in Nkx2-1+ respiratory tissue in response to C/F7/10. In contrast, E8.5 Hg endo explants never had detectable Nkx2-1 in response to RA/C/F7/10 (Fig. 3I,I′). Thus at E8.5 mouse foregut but not hindgut endoderm is competent to activate respiratory fate.
An important hall mark of mammalian lung development is the stereotypical branching morphogenesis (Herriges and Morrisey, 2014; McCulley et al., 2015). We therefore set out to determine whether early RA treatment also imparted the ability to execute this lung morphogenesis program. To test this we performed tissue recombination experiments with isolated E12.5 distal lung mesenchyme (LgM), which is known to provide signaling cues sufficient to induce branching morphogenesis in competent respiratory epithelium (Shannon, 1994). E7.5 DE from Tg(Sftpc:GFP) embryos was isolated, cultured +/− RA for 24 hours followed by C/F7/10 and then recombined for 3 days with E12.5 LgM. To ensure that RA was only acting during the initial 24-hour period, and that there was not any residual RA activity during induction of the respiratory lineage, we included the pan-RAR inhibitor BMS493 to the media containing C/F7/10. We found that DE cultured without RA had few GFP+ cells and the endoderm did not branch (Fig.3J-J″); in contrast RA treated explants exhibited robust GFP+ respiratory epithelium that underwent extensive early branching morphogenesis at the distal tips of the growing lung buds (Fig.3K-K″). Together these data suggest that, similar to Xenopus, RA acts permissively to impart respiratory competence to a restricted region of mouse AE and that this restricted domain of lung competence exists at E7.5, much earlier than previously appreciated.
Gastrula Wnt/BMP-dependent patterning impacts endoderm competence and the response to subsequent RA/Wnt/BMP inductive cues in Xenopus
Our data suggest that a restricted domain of lung competence exists in frog and mouse endoderm by the end of gastrulation. Given the well-established role of Wnt and BMP in gastrula stage A-P patterning, we hypothesized that these signals might predispose how different regions of the endoderm respond to later RA/Wnt/BMP-dependent organogenesis cues. To test this we disrupted early A-P pattern in Xenopus via Wnt and BMP loss-of-function and determined how this would impact the ability of the endoderm to respond to subsequent RA/Wnt/BMP signals and activate different endoderm lineage markers (Fig.4). To inhibit endogenous Wnt and BMP signaling, we microinjected synthetic RNAs encoding either the antagonists Dkk1 or Noggin (along with GFP RNA lineage tracer) into ventral-vegetal cells of 16-cell stage embryos (Moody, 1987), which targets the ventx1/2-expressing PE (Fig.4A). In situ hybridization at NF10.5 confirmed that both Dkk1 and Noggin suppressed ventx1/2 and expanded the hhex domain (Fig. 4C), indicating a loss of posterior and an expansion of anterior identity. AE and PE explants were micro-dissected and subjected to the respiratory fate induction protocol, consisting of RA from stages NF14–25 followed by Bio+BMP4 from stages NF25–38 (Fig.4B). We then assayed explants for respiratory fate as well as a panel of markers reflecting different endoderm lineages along the A-P axis (Fig.4N-W). In all conditions expression of the pan-endoderm transcription factor foxa1 was robustly expressed, with a modest fold-change increase in response to RA (Fig.4M).
Figure 4. Retinoic acid treatment changes the response of both anterior and posterior Xenopus endoderm to Bio+Bmp4.
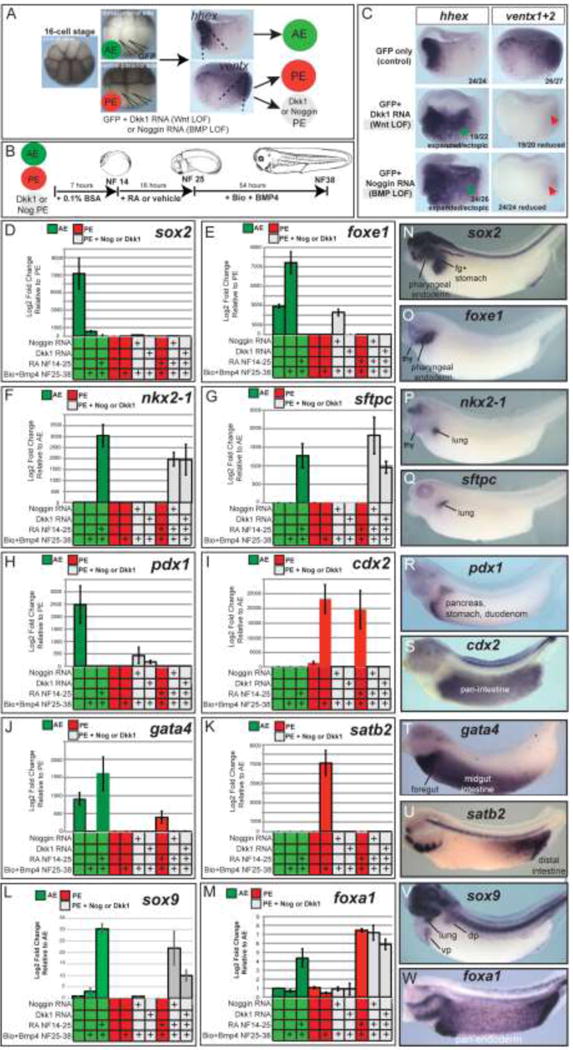
(A,B) Experimental diagram. Xenopus embryos at the 16-cell stage with clear pigment differences (animal pole, dorsal-anterior, and ventral-posterior views of such embryos are shown) were injected with 25pg of Noggin or 100pg of Dkk1 RNAs (to inhibit BMP and Wnt/β catenin signaling, respectively) into each ventral-vegetal V2.1 blastomere along with 25pg eGFP RNA as a lineage tracer, which targets the ventx1/2-expressing posterior mesendoderm. Hhex+ AE tissue was targeted by injection of each dorsal-vegetal D1.1 blastomere with 25pg eGFP RNA. At gastrula stage NF10.25, GFP fluorescence was monitored and used to dissect AE, PE, and BMP- or Wnt-inhibited PE explants, which were then treated as indicated in panel B (50nM RA from NF14–25 followed by 3.5μM Bio + 50ng/mL BMP4 from NF25–38). (C) In-situ hybridization analysis of hhex and ventx1+2 (both probes mixed together) in bisected gastrula NF10.25 embryos confirms effective inhibition of the posteriorizing Wnt and BMP pathways by dkk1 and noggin RNA injection. Numbers in the lower left corner indicate numbers of embryos assayed with the gene expression pattern shown. (D–M) Relative gene expression analysis (RT-qPCR) of different anterior-posterior endoderm lineages assayed in explants as prepared/treated in panels A,B. Graphs display the average 2−ΔΔCt value +/− SEM of 3 biological replicates. (N-W) In-situ hybridization of control embryos showing the endogenous spatial domains of expression along the anterior-posterior axis.
As expected control PE explants failed to express lung markers or any of the other anterior lineage markers analyzed (sox2, foxe1, pdx1; Fig.4D-H) in response to any of the treatments of +/−RA, +/− Bio+BMP4, confirming that gastrula stage PE is not competent to active foregut gene expression. PE explants did express cdx2 as predicted; the addition of Bio+BMP4 dramatically increased cdx2 and induced satb2 (Fig.4I,K), indicating distal intestine fate (Munera et al., 2017). Exposing PE to RA followed by Bio+BMP resulted in satb2 suppression (Fig.4K) and up-regulation of cdx2 and gata4 (Fig.4J), indicating a shift to midgut fate. Strikingly, both the Noggin and Dkk1-injected PE explants expressed nkx2-1, sox9, and sftpc in response to RA followed by Bio+BMP4 (Fig.4F,G, L); these explants failed to express cdx2, satb2 or gata4 (Fig.4I,J,K), confirming they had lost their posterior identity. RA was essential for the respiratory markers to be induced, since Bio+BMP treatment alone of Noggin or Dkk1-injected PE was not sufficient to induce nkx2-1 or sftpc.
Although AE explants cultured without RA/Wnt/BMP did not express the lung markers (Fig. 1, Fig.4F,G); they did express foxe1, sox2, gata4, and pdx1 (Fig.4D,E,H,J), suggesting that in the absence of any exogenous or mesodermal derived signals AE adopted a mixed foregut fate including pharyngeal, pancreatic, and stomach character. If RA was omitted, the Bio+BMP treated AE cultures expressed foxe1 (Fig.4E) but not gata4, pdx1, nkx2-1 or sftpc; this is indicative of pharyngeal/thyroid identity. However when AE explants were treated with the full lung-inducing regime of RA followed Bio+BMP4 to induced nkx2-1, sox9, and sftpc, we observed a complete suppression of foxe1 (Fig.4 E,F,G,L), suggesting that RA promotes lung competence, at least in part, by suppression of pharyngeal/thyroid fate in agreement with previous studies (Wang et al., 2011).
Together these data indicate that early Wnt/BMP-mediated patterning, present by the gastrula stage, impacts the competence of endoderm to activate gene expression reflective of different organ lineages in response to subsequent RA/Wnt/BMP signals. Moreover our data suggest that somite-stage RA signaling acts along the A-P axis, promoting respiratory while suppressing pharynx potential in the AE, and midgut over hindgut potential in the PE.
Reiterative Wnt/BMP/RA impacts developmental competence of human definitive endoderm derived from pluripotent stem cells
We next set out to determine whether the reiterative Wnt and temporal RA signaling that we observed in Xenopus are conserved in hPSC cultures. To assess how early endoderm pattering impacts subsequent Wnt/BMP/RA-mediated lineage induction, we adapted a stepwise protocol to induce respiratory fate in hPSCs (Green et al., 2011; Rankin et al., 2016). Following the differentiation of hPSCs into DE by treatment with Activin A for 3 days (d), we generated foregut endoderm by inhibiting BMP activity in the cultures via Noggin treatment from d3–6 (72 hours), which mimics gastrula stage A-P patterning. Finally to induce NKX2-1+ respiratory progenitors, cultures were treated with the GSK3 antagonist CHIR99021 and BMP4 from d6–9 (72 hours).
We first set out to assess the temporal role of RA, and compared two different RA treatment periods: RA treatment for 72 hours (d3–6) versus only a later 24-hour RA treatment (d5–6), both in the presence or absence of Noggin (Supplemental Fig. S3). All conditions were followed by exposure to CHIR+BMP4 for 72 hours (d6–9). RT-qPCR analysis of d9 cultures revealed that robust NKX2-1 induction required d3–6 Noggin treatment and RA on d5–6 followed by CHIR+BMP4 (Supplemental Fig.S3C), consistent with previous reports (Huang et al., 2014; Rankin et al., 2016). Without RA the Noggin treated cultures exposed to CHIR+BMP4 expressed the pharyngeal marker TBX1 but not NKX2-1 (Supplemental Fig.S3B,C). Interestingly the 24-hour RA treatment on d5–6 resulted in suppression of TBX1 as early as d6 (data not shown), whereas NKX2-1 was not robustly expressed until d9, supporting the idea that suppression of pharyngeal gene expression was a prerequisite for lung induction. In contrast to d5–6 RA treatment, earlier addition of RA from d3–6 was inhibitory to NKX2-1 (Supplemental Fig.S3C) and resulted in the expression of mid-hindgut markers GATA4 and CDX2 (Supplemental Fig.S3E,F), indicating a posteriorizing effect of early RA. Overall these data are similar to our Xenopus results, as early RA treatment right after DE formation had a posteriorizing effect, suppressing anterior foregut lineages (pharynx and lung) and promoting mid-hindgut fates. RA treatment during a later time window makes the endoderm competent to activate respiratory gene expression in response to Wnt+BMP.
We next tested if early Wnt-dependent patterning also regulates the ability of human DE to respond to subsequent RA/Wnt/BMP signals (Fig.5). In these experiments we kept the timing of RA exposure consistent on d5–6, and manipulated Wnt/β-catenin activity (via addition of CHIR99021) for 72 hours during d3–6 when the DE is patterned into foregut by Noggin (experiment diagrammed in Fig.5A). We then examined how the culture responded to subsequent CHIR+BMP4 on d6–9, assaying a range of endoderm lineage markers (Fig.5B-J). Strikingly, we found that early CHIR treatment from d3–6 suppressed the induction of NKX2-1 by RA+CHIR/BMP4 (Fig.5F); instead the cultures expressed GATA4 and PDX1 consistent with a midgut/pancreatic fate (Fig. 5G,I). These results suggest that early Wnt treatment makes human endoderm refractory to respiratory induction. Interestingly we also found that when RA was omitted, the cultures treated with d3–6 Noggin (or CHIR+Noggin) followed by d6–9 CHIR+BMP expressed FOXE1 and PAX8, reflecting pharyngeal/thyroid fate (Fig.5C,D); RA treatment suppressed expression of these genes, which further suggests RA suppresses pharyngeal/thyroid potential in the foregut. Finally if both Noggin and RA were omitted, d3–6 CHIR followed by d6–9 CHIR+BMP treatment resulted in robust expression of CDX2 and SATB2 (Fig.5H,J) but no other foregut or midgut markers, consistent with a distal hindgut identity (Munera et al., 2017). We conclude that, similar to Xenopus, early Wnt patterning impacts how hPSC-derived endoderm can respond to subsequent Wnt-mediated organ induction.
Figure 5. Early CHIR treatment of human DE changes the ability of subsequent RA/CHIR/BMP4 to induce respiratory fate.
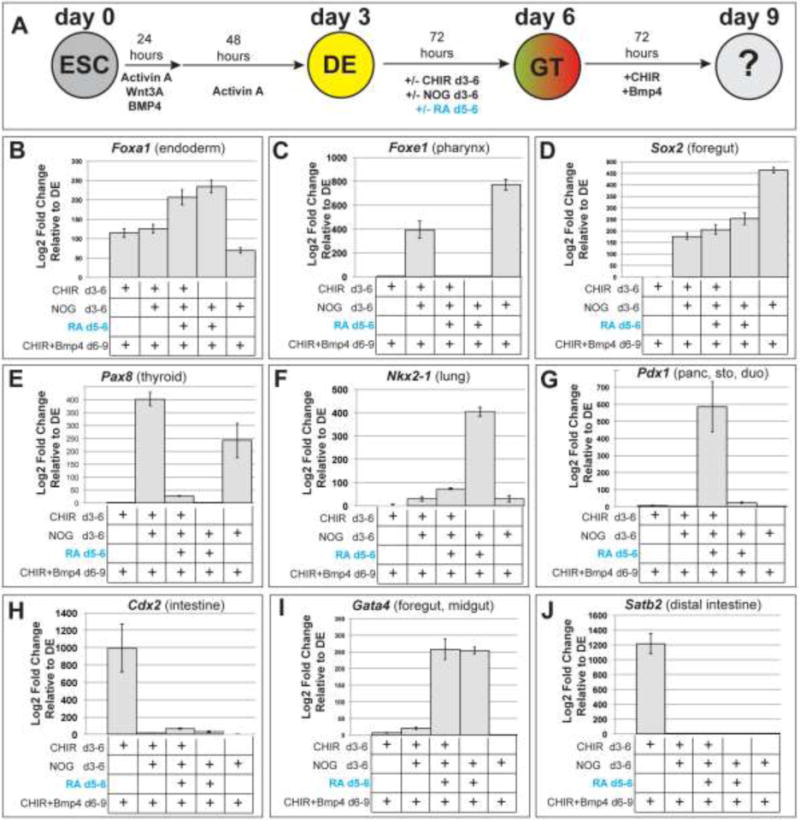
(A) Experimental diagram. Definitive endoderm (DE) was induced from human embryonic stem cells (ESC) and then patterned into different types of gut tube (GT) endoderm by exposure to one or more of the following: 2μM CHIR99021 (72 hours, days 3–6 to posteriorize DE), 200ng/mL Noggin (72 hours, days 3–6, to anteriorize DE), and/or 2μM RA (24 hours, day5–6). Subsequently all types of GT endoderm was then exposed to 3μM CHIR99021 + 50ng/mL Bmp4 for 72 hours (days 6–9). Cultures were then assayed by RT-qPCR for expression of the indicated genes (B-J). All culture conditions were performed in biological triplicate. Gene expression was normalized to the housekeeping gene PBGD and then log2 fold changes determined using the 2−ΔΔCt method relative to expression in DE. Graphs display the average 2−ΔΔCt value +/− SEM of the 3 biological replicates.
Discussion
In this study we investigated how reiterative signaling impacts developmental competence and lineage allocation in the developing endoderm. Although previous studies have examined individual roles of RA, Wnt, or BMP in the endoderm (Kraus and Grapin-Botton, 2012; Zorn and Wells, 2009), here provide an integrated view of how combinatorial Wnt/BMP/RA signaling orchestrates endoderm organogenesis, with remarkably similar temporal and spatial dynamics in Xenopus, mouse, and hPSCs (summarized in Fig.6). We draw three main conclusions: 1) Wnt/BMP-mediated pattern present in the gastrula restricts domains of competence in the endoderm and impacts how cells respond to the same signals during lineage induction. Specifically early Wnt/BMP makes PE tissue refractory to subsequent RA/Wnt/BMP-dependent lung induction. 2) RA has permissive role in AE respiratory competence and cannot instructively impart respiratory competence to the PE. 3) RA has distinct spatial and temporal functions. During early gastrula stages RA inhibits foregut and promotes pancreatic and hindgut fate. Then during early somitogenesis stages RA promotes respiratory and suppresses pharyngeal fate within the AE, and promotes midgut over hindgut fate within posterior endoderm.
Figure 6. Model depicting the reiterative use of Wnt, BMP, and RA signals during endoderm development.
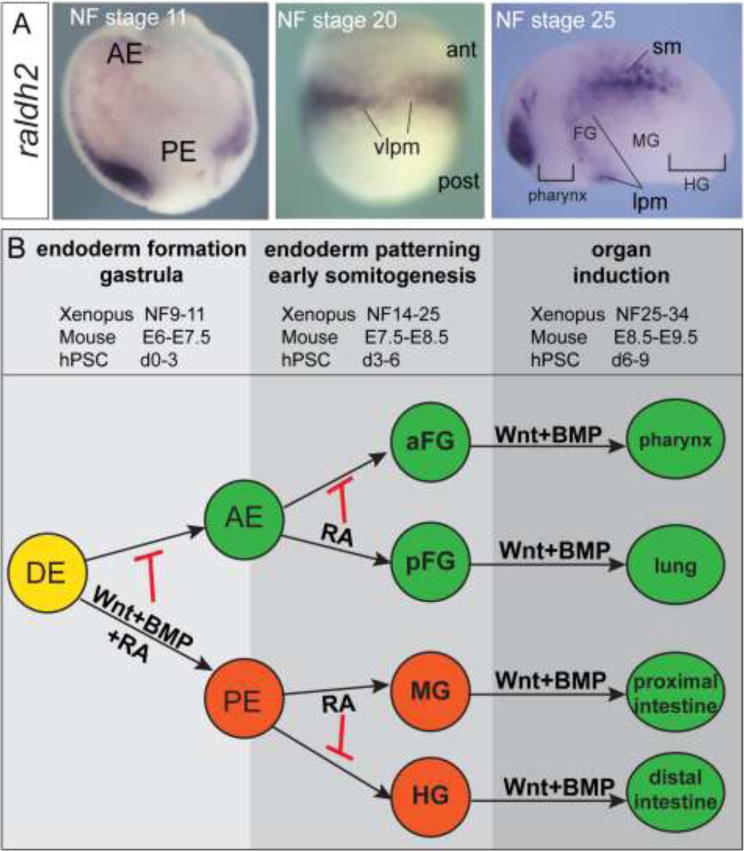
(A) In situ hybridization showing expression of the RA-synthesizing enzyme raldh2 (aldh1a2) during early Xenopus development. Abbreviations: AE, anterior endoderm; PE, posterior endoderm; lpm, lateral plate mesoderm; FG, foregut; MG, midgut; HG, hindgut; sm, somitic mesoderm; ant, anterior; post, posterior. (B) Schematic depicting the reiterative roles for Wnt, BMP and RA signals during endoderm vertebrate development.
We demonstrated that cellular responses to RA/Wnt/BMP-mediated organ induction are dictated in part by earlier Wnt/BMP patterning in the gastrula. Although the underlying molecular mechanisms remain to be resolved, one likely possibility is that each signaling event not only activates a given lineage program but also suppresses alternative fates (Loh et al., 2014). For example, the hindgut transcription factor CDX2, which is activated by gastrula stage Wnt and BMP in the posterior, might not only promoting intestinal gene expression but also actively repress lung transcription, thus making PE refractory to lung induction. Our data also suggest that RA permissively promotes lung competence in the anterior foregut endoderm in part by suppressing pharyngeal fate, and in the posterior endoderm RA promotes midgut while suppressing distal Satb2+ hindgut fate. These functions of RA could occur directly or indirectly. RA-bound retinoic acid receptors (RARs) can either activate or directly repress transcription depending on the recruitment of different proteins to target genes. For example, RA signaling is required for recruitment of the PRC2 repressive complex and NCOR1/2 nuclear co-repressors to the Fgf8 locus to directly repress Fgf8 transcription (Cunningham and Duester, 2015; Kumar et al., 2016). RARs may similarly repress key pharyngeal genes such as Tbx1 and FoxE1, thereby permitting lung genes to be activated by Wnt and BMP.
One caveat of our study is that we focused on the timing of RA rather than the concentration. In vivo bioavailability is tightly controlled by RA production and degradation (Cunningham and Duester, 2015), and this may play a key role in refining boundaries of competence. The concentrations of RA and other growth factors that we used in our ex vivo experiments were based on those from many previous publications (Bayha et al., 2009; Chen et al., 2004; Desai et al., 2006; Desai et al., 2004; Green et al., 2011; Loh et al., 2014; Wang et al., 2011; Wang et al., 2006) and on pilot experiments testing a range of concentrations (for RA; 5 μM-50 nM) to identify the lowest dose in each system that would give a robust response in the 24-hour period, which is the time frame that competence appears to be established in vivo (Rankin et al., 2016). In the future, it would be interesting to test whether a short pulse of high concentration RA has the same impact on as low RA concentrations over a longer period of time.
In addition to the role of RA, Wnt and BMP in endoderm patterning and lineage specification that we have examined here, RA, Wnt and BMP signaling are also required in mammals to regulate later fetal lung growth, branching morphogenesis and proximal-distal pattern, (Desai et al., 2004; Frank et al., 2016; Herriges and Morrisey, 2014; McCauley et al., 2017; McCulley et al., 2015; Wang et al., 2006). While we did not formally test the impact of early RA on subsequent proximal-distal patterning, our mouse explants experiments cultured with lung mesenchyme underwent extensive growth and branching morphogenesis suggesting that both distal and proximal fates were probably present.
It is unclear how Wnt and BMP activate different transcriptional programs in different contexts. Wnt and BMP they are typically thought to activate transcription by simulating the recruitment of β catenin and Smad1, along with co-activators, to transcription factor complexes bound to DNA cis-regulatory elements (Cadigan and Waterman, 2012; Gaarenstroom and Hill, 2014). However recent genome-wide studies in Xenopus have revealed a different picture where β catenin and Smad1 chromatin binding is also associated with genes that are repressed by Wnt and BMP signaling (Kjolby and Harland, 2017; Nakamura et al., 2016; Stevens, 2017). Interestingly, approximately 25% of the foregut-specific transcriptome at NF20 appears to be directly repressed by β catenin binding (Stevens, 2017); this supports the posteriorizing function of Wnt during endoderm patterning. These genomic analyses also found that 70–80% of the β catenin and Smad1 binding in the genome were not associated with actively expressed genes. This raises the possibility that early Wnt and BMP signaling may result in β catenin or Smad1 being recruited to cis-regulatory elements of lineage genes that will not be expressed until later in development and this may impact how these genes respond to later signaling. For example, gastrula stage Wnt-signaling may not only direct the activation of hindgut genes in PE but also actively repress lung genes in PE, and this inhibition may persists later in development, perhaps by modulating the epigenetic status of the chromatin.
Studies of endoderm differentiation in hPSCs indicate that ‘epigenetic priming’ of chromatin by histone modifications is a key step in the ability of developmental enhancers respond to inductive cues (Loh et al., 2014; Wang et al., 2015; Xie et al., 2013). But how cell signaling regulates epigenetic priming is still poorly understood. In one example, activated β catenin has been shown to recruit the histone arginine methyltransferase Prmt2 to target promoters in the Xenopus blastula, and the resulting H3R8 dimethylation is essential for transcription several hours later (Blythe et al., 2010). In the context of A-P patterning of the nervous system, RA and Wnt interaction are reported to dynamically remodel the chromatin landscape of several HOX clusters by selectively removing H3K27me3 repressive marks from distinct chromatin domains to allow expression (Mazzoni et al., 2013). RA could similarly control whether respiratory versus pharyngeal loci available for activation by Wnt and BMP in the gut tube.
Resolving how RAR, β catenin, and Smad effectors can bind cis-regulatory elements to activate or repress distinct genes in different contexts will require the genome wide identification of RA/Wnt/BMP-responsive cis-regulatory elements. For the most part such respiratory lineage enhancers are poorly characterized; even the cis-regulatory elements controlling Nkx2-1 transcription in respiratory progenitors are still unknown in any vertebrate. Our work has defined a temporal framework for how reiterative signaling impacts endoderm lineage allocation, and we are poised to apply chromatin profiling technologies, such as ATAC-seq, at different developmental times in multiple model systems to help identify conserved lineage specific enhancers and define the molecular basis of developmental competence and transcriptional specificity during endoderm organogenesis.
Materials and Methods
Xenopus Methods
Xenopus laevis were maintained according to CCHMC IACUC protocols. Ovulation, in-vitro fertilization, and de-jellying of embryos were performed as described (Sive et al., 2000). pBSRN3-Noggin, pBSRN3-eGFP (both linearized with Sfi1), and pCS107-Dkk1 (linearized with Not1) plasmids were used to synthesize mRNAs according to manufacturer’s instructions. 25 pg of Noggin or 100 pg of Dkk1 RNA was injected into each ventral-vegetal V2.1 blastomere of 16-cell Xenopus embryos along with 25pg eGFP RNA as a lineage tracer, which targets the ventx1/2-expressing posterior mesendoderm (Moody, 1987).
For Xenopus explant studies, DE, AE, or PE tissue was micro-dissected in 1× MBS (Modified Barth’s Saline) + 50 ug/mL gentamycin sulfate (MP Biochemicals 1676045), and explants were then cultured in 0.5× MBS + 0.1% Fatty Acid Free BSA (Fisher BP9704) + 50 ug/mL gentamycin sulfate with the following concentrations of small molecules or recombinant proteins: 50 nM all-trans retinoic acid (Sigma R2625), 3.5uM Bio (TOCRIS 3194), 50ng/mL recombinant human BMP4 (R&D systems 314-BP), 10 uM DEAB (Sigma D86256). For Xenopus whole embryo treatments, embryos were cultured in 0.1× MBS + 50ug/mL gentamycin sulfate, with 10 uM DEAB from NF14–20 (Supplemental Fig.S1).
In-situ hybridization of Xenopus embryos and explants was performed mostly as described in Sive et al 2000. Briefly, embryos and explants were fixed overnight at 4°C in MEMFA (0.1 M MOPS, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde), dehydrated directly into 100% ethanol, and stored at −20°C. The following minor modifications to the in-situ protocol were used: proteinase K (ThermoFisher AM2548) on day 1 was used at 2ug/mL for 10 minutes on explants and gastrula stage embryos, and at 5ug/mL for 10 minutes on stage NF20–35 whole embryos; the RNAse A step was omitted on day 2; and finally the anti-DIG-alkaline phosphatase antibody (Sigma 11093274910) was used at a 1:5,000 dilution in MAB buffer (maleic acid buffer, 100 mM Maleic acid, 150 mM NaCl, pH7.5) + 10% heat-inactivated lamb serum (Gibco 16070096) + 2% blocking reagent (Sigma 11096176001) on day2/3. Anti-sense DIG labeled in-situ probes were generated using linearized plasmid full-length cDNA templates with the10X DIG RNA labeling mix (Sigma 11277073910) according to manufacturer’s instructions. Details on plasmids use to make in situ probes are available upon request.
For Xenopus immunofluorescence, embryos were fixed in 100mM NaCl, 100mM HEPES (pH7.5), 1.7% methanol-free paraformaldehyde for 1 to 1.5 hours at room temperature and then dehydrated directly into Dent’s post-fixative (80%Methanol/20% DMSO) and stored at −20°C. Primary antibodies were as follows: chicken anti-GFP (Aves GPF-1020; diluted 1:1,000), rabbit anti-Nkx2-1 (Santa Cruz Biotechnologies sc-13040X; diluted 1:500), mouse anti-Sox2 (Abcam ab79351; diluted 1:1,000), and rabbit anti-B-catenin (Santa Cruz Biotechnology sc-7199; red membrane staining of the gastrula embryo of Fig.S1C). Secondary antibodies were donkey anti-Chicken 488, donkey anti-rabbit Cy3, and donkey anti-mouse Cy5 (Jackson ImmunoResearch nos. 703-546-155, 711-166-152, and 715-175-151, respectively; all used at 1:1,000 dilution). Briefly, embryos in Dent’s fixative were serially rehydrated into PBS+0.1% TritonX-100 (PBSTr). Embryos, were bisect with a razor blade in PBSTr on an agarose-coated dishes and whole-mount staining was then performed on the gastrula halves or the tailbud foreguts. Both sample types were blocked for 2 hours in PBSTr + 5% normal donkey serum (Jackson Immunoresearch 017-000-001) + 0.1% DMSO at room temperature and then incubated overnight at 4°C in this blocking solution + primary antibodies (dilutions listed above). After extensive washing in PBSTr, samples were incubated overnight at 4°C in PBSTr + secondary antibodies (dilutions listed above). Samples were again extensively washed in PBSTr, dehydrated directly into 100% methanol, cleared and imaged in Murray’s Clear (2 parts benzyl benzoate, 1 part benzyl alcohol) using a metal slide with a glass coverslip bottom using a Nikon A1R confocal microscope.
RT-qPCR analysis of Xenopus explants was performed in biological triplicate with n=4 explants (DE, AE or PE) for each replicate. RNA was extracted using TRIzol (ThermoFisher 15596018) and purified using the Direct-zol RNA miniprep plus kit (ZymoResearch R2070); 500ng of RNA was used in cDNA synthesis reactions using Superscript Vilo Mastermix (ThermoFisher 11755050), all according to manufacturer’s instructions. Real-time PCR reactions were carried out using the Powerup Sybrgreen Mastermix (ThermoFisher A25742) on ABI StepOnePlus or QuantStudio3 qPCR machines. Ornithine decarboxylase (ocd) was used as a reference gene for Xenopus samples. All Ct values for the experimental genes were normalized to the ODC Ct value, and then Log2 fold changes in gene expression were determined using the 2−ΔΔCt method, relative to the experimental gene’s ODC-normalized expression in DE, AE, or PE cultured in isolation. Graphs display the average 2−ΔΔCt value +/− SEM of the 3 biological triplicate samples. Xenopus RT-qPCR primer sequences are listed in Table S1.
Mouse Methods
Wild-type CD1 mice were purchased from Charles River Laboratories (Wilmington, MA). Tg(Sftpc:GFP) mice were provided by Dr. Brigid Hogan (Duke University Medical Center, Durham, NC), and were originally generated by John Heath (Lo et al., 2008). Raldh2+/− heterozygous mice (Molotkov et al., 2005) were obtained from Dr. Gregg Duester (Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA), and also carried the RARE:lacZ reporter allele. All mice were housed according to CCHMC IACUC protocols. Timed pregnant mice were sacrificed on gestational days E7.5, E8–8.5, or E9–9.5 and individual embryos were dissected out and staged according to somite number.
Mouse embryos were micro-dissected Hank’s balanced salt solution (HBSS; ThermoFisher 14025092) using tungsten needles. To promote separation of germ layers, dissected embryo regions were incubated in HBSS + 1.25% pancreatin (Sigma P3292), 0.25% Difco trypsin 250 (ThermoFisher 27250018) for 20 minutes. The proteolytic enzymes were neutralized with HBSS containing 10% bovine serum albumin (Invitrogen 15561020) and 0.1 mg/ml DNase I (Sigma 11284932001) to reduce tissue stickiness. To isolate E8.5 foregut endoderm (FgE) from 8–10ss embryos, tungsten needles were used to cut at the anterior intestinal portal (AIP), and then as much of the neuro-epithelium as possible was removed without damaging the endoderm, and the foregut region was incubated for 20 minutes in the proteases listed above before endoderm isolation. To isolate E8.5 hindgut endoderm (HgE), the enzyme incubation was increased to 30 minutes. E7.5 and E8.5 endoderm explants were rinsed in complete serum-free differentiation medium (cSFDM) as formulated by Longmire et al. 2012, with the exception that B27 was used as a supplement instead of B27 + retinoic acid. Explants were enrobed in Cultrex Basement Membrane Matrix (Trevigen) and cultured in the matrix in cSFDM media with or without the following small molecules or recombinant proteins: 1μM all-trans RA (Sigma R2625), 2uM CHIR99021 (C; TOCRIS 4423), 100ng/mL human FGF10 (F10; R&D 345-FG), 25ng/ml mouse FGF7 (F7; R&D 5028-KG). RA treatment was for 24 hrs, followed by 4 to 6 days of culture in C/F7/F10 as described in the figure legends.
Whole-mount immunostaining of mouse embryos and detection of B-gal activity in Tg(RARE:lacZ) experiments was performed as described in Rankin et al., 2016. The following primary antibodies were used: rabbit anti-Nkx2-1 (Seven Hills BioReagents WRAB-1231, 1:500) and rat anti-Ecadherin (R&D systems MAB7481, 1:1,000). For immunostaining of mouse explants, they were carefully removed from the matrix, fixed overnight in PBS + 3.7% PFA at 4°C, dehydrated into 100% methanol, and stored at −20°C. Explants were rehydrated into PBSTr, blocked in PBSTr + 10% normal donkey serum (Jackson ImmunoResearch) for 1–2 hours at room temperature, and then incubated overnight at 4°C in blocking buffer + the following primary antibodies: rabbit anti-Nkx2-1 (Seven Hills BioReagents WRAB-1231, 1:500), rat anti-Ecadherin (R&D systems MAB7481, 1:1,000), goat anti-FoxA2 (Santa Cruz Biotechnology sc-6554, 1:500), mouse anti-Sox2 (Abcam ab79351, 1:1,000). After extensive washing, explants were incubated overnight in PBSTr + secondary antibodies (1:500, all raised in donkey; Jackson Immunoresearch) at 4°C. Explants were washed extensively in PBSTr, dehydrated in 100% methanol, and imaged in Murray’s Clear using a Nikon A1R confocal microscope.
hPSC Methods
Human ESC line WA01 (H1; WiCell) was maintained in feeder-free conditions on Matrigel (BD Biosciences) in mTesR1 media (Stem Cell Technologies). 3-day induction of DE was performed as briefly follows: hESCs were dissociated into single cell suspension using accutase and plated into a 24-well plates in mTesR1 with ROCK inhibitor Y-27632 (10 μM; Stemgent 04-0012). On the following day, cells were exposed to Day1 DE induction media: RPMI 1640 (ThermoFisher 61870036) + 1× non-essential amino acids (NEAA; ThermoFisher 11140050) + 25ng/mL Wnt3a (R&D systems 5036-WN) + 10ng/mL BMP4 (R&D 314-BP) + 100ng/mL Activin A (R&D 338-AC). Day 2 DE induction media consisted of RPMI 1640 + 0.2% defined fetal bovine serum (dFBS; Gibco 16141079) + 1× NEAA + 100ng/mL Activin A. Day 3 DE induction media consisted of RPMI 1640 + 2% dFBS +100ng/mL Activin A. DE was then exposed to various patterning conditions over the next 72 hours, all of which used RPMI 1640 media + 2% dFBS +1× NEAA containing the following small molecules or protein concentrations: 200ng/mL human Noggin (R&D 6057-NG), 2uM CHIR99021 (TOCRIS 4423), or 2uM all trans retinoic acid (Sigma R2625). For the last 72 hours of the culture, patterned endoderm was exposed to RPMI 1640 media + 2% dFBS +1× NEAA + 3uM CHIR99021 + 50ng/mL BMP4.
All culture conditions were performed in biological triplicate. RNA was extracted from each condition and purified using the Nucleospin RNA II kit (Machery-Nagel 740955.250), and 500ng of RNA was used in cDNA synthesis reactions using Superscript Vilo Mastermix (ThermoFisher 11755050) according to manufacturer’s instructions. Real-time PCR reactions were carried out using the Powerup Sybrgreen Mastermix (ThermoFisher A25742) on ABI StepOnePlus or QuantStudio3 qPCR machines. Porphobilinogen deaminase (PBGD; Loh et al 2014) was used as a reference gene for hPSC samples. All Ct values for the experimental genes were normalized to the PBGD Ct value, and then Log2 fold changes in gene expression were determined using the 2−ΔΔCt method, relative to the experimental gene’s PBGDC-normalized expression in DE. Graphs display the average 2−ΔΔCt value +/− SEM of the 3 biological triplicate samples. Human RT-qPCR primer sequences are listed in Table S1.
Supplementary Material
Highlights.
Reiterative RA, Wnt and BMP signaling controls developmental competence and lineage specification in the developing gut tube.
Wnt/BMP-mediated gastrula patterning impacts how endoderm tissue responds to subsequent Wnt/BMP-mediated lineage induction.
RA signaling after gastrulation is required to impart respiratory competence to the foregut endoderm in Xenopus, mouse and human PSCs.
Acknowledgments
We thank members of the Endoderm Club and the Wells/Zorn lab for helpful discussion and critical feedback.
Funding
This work was supported by NIH grants DK070858 and HL114898 to AMZ
This work was supported by: NIH grants U01DK103117, U19AI116491, R01DK092456 to JMW, and grants R01HL114898 and R01DK070858 to AMZ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing or financial interests.
References
- Bayha E, Jorgensen MC, Serup P, Grapin-Botton A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS. beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell. 2010;19:220–231. doi: 10.1016/j.devcel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns. 2009;9:255–265. doi: 10.1016/j.gep.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimling SJ, Drysdale TA. Retinoic acid regulates anterior-posterior patterning within the lateral plate mesoderm of Xenopus. Mechanisms of development. 2009;126:913–923. doi: 10.1016/j.mod.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Chen F, Lu J, Qian J, Niederreither K, Dolle P, Chambon P, Cardoso WV. Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol. 2006;291:12–24. doi: 10.1016/j.ydbio.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Developmental biology. 2004;273:402–415. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, Cui Z, Herriges MJ, Morley MP, Zhou S, Lu MM, Morrisey EE. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarenstroom T, Hill CS. TGF-beta signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin Cell Dev Biol. 2014;32:107–118. doi: 10.1016/j.semcdb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature biotechnology. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrilak JA, Melton KR, Shannon JM. Endothelial cells are not required for specification of respiratory progenitors. Dev Biol. 2017;427:93–105. doi: 10.1016/j.ydbio.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjolby RAS, Harland RM. Genome-wide identification of Wnt/beta-catenin transcriptional targets during Xenopus gastrulation. Dev Biol. 2017;426:165–175. doi: 10.1016/j.ydbio.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MR, Grapin-Botton A. Patterning and shaping the endoderm in vivo and in culture. Curr Opin Genet Dev. 2012;22:347–353. doi: 10.1016/j.gde.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Cunningham TJ, Duester G. Nuclear receptor corepressors Ncor1 and Ncor2 (Smrt) are required for retinoic acid-dependent repression of Fgf8 during somitogenesis. Dev Biol. 2016;418:204–215. doi: 10.1016/j.ydbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, Jean JC, Ikonomou L, Deterding RR, Shannon JM, Zorn AM, Hollenberg AN, Kotton DN. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell. 2015;17:527–542. doi: 10.1016/j.stem.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CY, Nichane M, Tan J, Noghabi MS, Azzola L, Ng ES, Durruthy-Durruthy J, Sebastiano V, Poellinger L, Elefanty AG, Stanley EG, Chen Q, Prabhakar S, Weissman IL, Lim B. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon REA, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell. 2016;166:451–467. doi: 10.1016/j.cell.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Peljto M, Patel T, Thornton SR, McCuine S, Reeder C, Boyer LA, Young RA, Gifford DK, Wichterle H. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat Neurosci. 2013;16:1191–1198. doi: 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, Wells JM. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144:958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell. 2017;20:844–857 e846. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH, Wells JM. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Munera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell. 2017;21:51–64 e56. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, de Paiva Alves E, Veenstra GJ, Hoppler S. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to beta-catenin recruitment to cis-regulatory modules. Development. 2016;143:1914–1925. doi: 10.1242/dev.131664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Gallas AL, Neto A, Gomez-Skarmeta JL, Zorn AM. Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/beta-catenin-mediated lung specification in Xenopus. Development. 2012;139:3010–3020. doi: 10.1242/dev.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Han L, McCracken KW, Kenny AP, Anglin CT, Grigg EA, Crawford CM, Wells JM, Shannon JM, Zorn AM. A Retinoic Acid-Hedgehog Cascade Coordinates Mesoderm-Inducing Signals and Endoderm Competence during Lung Specification. Cell Rep. 2016;16:66–78. doi: 10.1016/j.celrep.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Kormish J, Kofron M, Jegga A, Zorn AM. A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Developmental biology. 2011;351:297–310. doi: 10.1016/j.ydbio.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sasai N, Kutejova E, Briscoe J. Integration of signals along orthogonal axes of the vertebrate neural tube controls progenitor competence and increases cell diversity. PLoS Biol. 2014;12:e1001907. doi: 10.1371/journal.pbio.1001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Gebb SA, Nielsen LD. Induction of alveolar type II cell differentiation in embryonic tracheal epithelium in mesenchyme-free culture. Development. 1999;126:1675–1688. doi: 10.1242/dev.126.8.1675. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor NY: 2000. [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MLCP, Rankin SA, Macdonald M, Jagannathan S, Yukawa M, Barski A, Zorn AM. Genomic integration of Wnt/ß-catenin and BMP/Smad1 signaling coordinates foregut and hindgut transcriptional program. Development. 2017 doi: 10.1242/dev.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Yue F, Li Y, Xie R, Harper T, Patel NA, Muth K, Palmer J, Qiu Y, Wang J, Lam DK, Raum JC, Stoffers DA, Ren B, Sander M. Epigenetic priming of enhancers predicts developmental competence of hESC-derived endodermal lineage intermediates. Cell Stem Cell. 2015;16:386–399. doi: 10.1016/j.stem.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Deimling SJ, D’Alessandro NE, Zhao L, Possmayer F, Drysdale TA. Retinoic acid is a key regulatory switch determining the difference between lung and thyroid fates in Xenopus laevis. BMC developmental biology. 2011;11:75. doi: 10.1186/1471-213X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Dolle P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol. 2006;297:433–445. doi: 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Xie R, Everett LJ, Lim HW, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D’Amour KA, Robins AJ, Won KJ, Kaestner KH, Sander M. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annual review of cell and developmental biology. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


