Abstract
Aims
The external anal sphincter (EAS) is essential for maintaining fecal continence. Neurological disorders or traumatic injuries to muscle and nervous systems could lead to EAS denervation. Currently there are no techniques available to document global innervation changes in the EAS in vivo. The aim of this study was to develop a novel approach to non-invasively estimate the number of functioning motor units (MUs) in the EAS and validate with immunofluorescent techniques in rats.
Methods
Intra-rectal surface electromyography (EMG) signals of the EAS, induced by a series of intra-vaginally delivered pudendal nerve stimulations with different intensities, were recorded. Variation in EMG responses at different intensities was used to estimate the value of a single motor unit potential (SMUP) in order to perform the proposed EAS motor unit number estimation (MUNE) approach. The EAS MUNE was tested in 12 female Sprague-Dawley rats, and validated by comparing against the EAS myofiber counting results achieved by performing immunostaining of acetylcholine receptors in 7 of the 12 rats.
Results
The mean MU number was 35 ± 9, with an averaged SMUP size of 52.49 ± 20.39 μV. The mean number of successfully identified myofibers was 652.7 ± 130.6 myofiber/EAS. Significance of linear regression between the immunofluorescent results and the MUNE was confirmed (p<0.01).
Conclusions
Our study represents the first effort to non-invasively assess the innervation of the EAS in vivo using the rat as a pre-clinical model. This approach can potentially enable future clinical applications for advanced diagnosis and treatment of neurogenic EAS disorders.
Keywords: Motor Unit Number Estimation, External Anal Sphincter, Nerve Stimulation, Surface EMG, Myofiber, Fecal Incontinence
INTRODUCTION
Regulated colonic function is maintained by the coordinated myogenic work between the bowel, sphincters and other pelvic muscles. Reflexive and active control of the external anal sphincter (EAS) and rectal smooth muscles help with maintaining continence by sealing the rectal opening1. Aging, physical damage to the nervous system and/or degenerative disorders such as multiple system atrophy can result in loss of motor neurons and muscle denervation in the EAS, and consequently compromising the capacity of maintaining continence2.
Current clinical investigations of the EAS involve morphological evaluations, anorectal manometry studies, and electrophysiology techniques such as electromyography (EMG) and terminal motor latency (TML)3. Morphological evaluation employs imaging methods such as ultrasound or magnetic resonance imaging (MRI) to detect the structural defects in the EAS. However, neurological disorders do not necessarily lead to muscle atrophy that can be discerned by morphological evaluations. Anorectal manometry measures pressures of the anal sphincter muscles and neural reflexes that are needed for normal bowel movements. However, the pressure change does not specify the origin of the EAS weakness. Moreover, normal ranges of various parameters measured with anorectal manometry are highly variable and poorly reproducible4. Pudendal nerve TML is a neurologic examination that can be used for diagnosing nerve damage, where a prolonged latency often suggests neuropathy. The pudendal nerve TML has not been recommended for evaluation of fecal incontinence because of its limited sensitivity and specificity, and the results were highly operator dependent3. Intramuscular EMG (iEMG) can be used to document neurogenic sphincter injury as well as denervation-reinnervation processes that may indicate neuropathy. Its application is limited by its invasiveness nature, poor spatial coverage and reproducibility5. None of above described techniques is capable of documenting the actual number of motor units innervating the sphincter. Therefore, there is clearly an unmet need of a novel and reliable method for evaluating in vivo and non-invasively the global innervation of the EAS.
Motor unit number estimation (MUNE) is an EMG-based approach for assessing the motor unit (MU) number in specific target muscles6. MUNE can be implemented by calculating the ratio between electrically elicited maximal compound action potential (CMAP), and the estimated mean single motor unit potential (SMUP). As of today various MUNE methods have been developed to provide a reliable biomarker to track the progression of neurological dysfunctions. These methods, such as incremental stimulation, multipoint stimulation, decomposition7, spike triggered averaging (STA)8 and statistical MUNE9, mainly differ from one another in the way used for estimating the SMUP. Decomposition and STA extract action potential from voluntary contraction, while the incremental, multipoint stimulation and statistical MUNE employ multiple stimulations to determine the SMUP. In the aforementioned existing MUNE methods, the statistical MUNE stands out for its high reproducibility and low coefficient of variation because of the larger percent of MU pool it samples10.
MUNE has been employed as a reliable index reflecting progressive alterations in pathological conditions such as amyotrophic lateral sclerosis (ALS) or stroke11. Although high sensitivity and reliability for neuromuscular alterations in limb muscles has been reported in many MUNE studies, no effort has been taken to apply this approach on anorectal muscles. The absence of reports on anorectal MUNE may be related to the complexity of the pelvic anatomy, which significantly hampers an efficient access to the innervating nerve axons of pelvic muscles, including the EAS. In this study, a novel non-invasive EAS MUNE technique was successfully developed by combining intra-vaginal pudendal nerve stimulation, intra-rectal surface EMG recording, and statistical MUNE techniques. The feasibility of our EAS MUNE approach was tested in 12 female rats and its performance evaluated by comparing against corresponding immunostaining of EAS myofibers in 7 of the 12 animals tested.
MATERIALS AND METHODS
Animals
The experimental protocol was approved by the IACUC from Houston Methodist Research Institute and performed in accordance with the guidelines established in the Guide for the care and use of laboratory animals (National Research Council of the National Academies). The EAS MUNE technique was tested in 12 female virgin Sprague-Dawley rats (200–250g). Animals were housed in a pathogen free environment, with 12h light/dark cycles, controlled room temperature of 25 ºC, and had ad-libitum access to food and water in plastic cages containing corn cob bedding.
Electrophysiological recordings
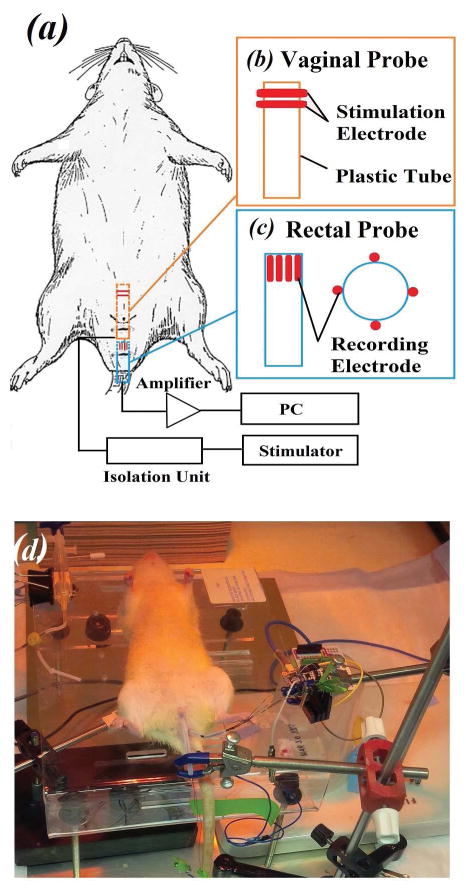
After reaching complete anesthesia following a subcutaneous injection of urethane (1.2 g/kg, diluted in normal saline solution) animals were immobilized on a surgical platform placed inside of an isolated Faraday’s cage. The skin hair close to the vaginal and rectal openings was removed using a hair clipper. The experiment configuration is shown in Fig 1a–c. Briefly, two ring-shaped copper electrodes were fixed onto the vaginal probe (~3.3 mm outer diameter) to trans-vaginally deliver electrical stimulus to the pudendal nerve (Fig 1b). On the other hand, four evenly-spaced bar-shaped copper electrodes were mounted on a rectal probe (~3.3 mm outer diameter; Fig. 1c) for intra-rectal acquisition of surface EMG signals. The design of the rectal probe was inspired by previous studies, where similar probes with high-density EMG recording were used for investigating EAS pathologies in humans12,13. The rectal probe was inserted and immobilized, with recording sites located around the neck of anal canal14. The EAS of the rat directly encircles the anus at the rectal opening, ensuring a close contact of the muscle with the recording electrodes. Recording ground was set at the proximal end of the tail using a 30G needle electrode. An appropriate amount of conductive gel was applied on the probe to facilitate insertion and to guarantee reliable electrode-tissue contact.
Figure 1.
Experiment setup for MUNE determination. (a) Diagram of the experimental system configuration. (b) Vaginal probe for trans-vaginal electrical stimulation (orange box) and (c) rectal probe for trans-rectal EMG recording (blue box). The red symbols represent the electrode contact sites for either electrical stimulation or EMG recording. (d) Photograph of a real experiment procedure after placement of both probes in a rat.
Positive square stimuli (pulse width 0.1 ms) were applied through a SIU5 stimulus isolation unit (Grass Instrument, Warwick, RI) and a Grass-88 dual channel stimulator (Grass Instrument). Surface EMG signals were amplified and recorded using a RHD2000 amplifier (Intan Technologies, Los Angeles, CA), with a gain of 200 and sampling frequency of 5 kHz. Optimal placement of the vaginal probe was considered when the largest CMAP response was achieved at specific stimulation intensity. Position of the vaginal probe was then fixed using a magnetic stand holder.
Another set of incremental stimulations were applied to determine 0% (the maximum intensity that elicit no CMAP) and 100% (the minimum intensity that elicited supramaximal CMAP) intensities. Stimulations were set to fit the CMAP responses into three recording windows: 5–25%, 25–45% and 45–65% of CMAP generation. Then, electrical stimulations were repeatedly performed in sets of 30 at a frequency of 1–2 Hz with the intensity determined by the pre-set percentage windows. For each recording window, 4–5 sets (i.e. 120–150 stimulations) were applied and intra-rectal surface EMG signals of the EAS were recorded and stored for offline signal processing. A photo of our experimental EMG assemble is shown in Figure 1(d), also illustrating the two probes in place.
MUNE Calculation
Recorded EMG signals were processed using a second order, 1 Hz high-pass and 60 Hz notch Butterworth filters. Stimulation artifacts were detected automatically using a Salvasky-Golay filter, and an Otsu thresholding protocol15. The amplitude of the first negative peak following the stimulation onset was extracted as the CMAP value for further MUNE calculation. Recorded data were excluded if more than 50% of the CMAP responses fell out the pre-set CMAP window16. Briefly, the statistical MUNE employs the variance of CMAP value under the same stimulation intensity to estimate the value of SMUP. This variance is considered to follow a Poisson distribution9,17. The relationship between recorded CMAPs and variance can be expressed as:
| (1) |
Where x is the vector of extracted CMAP values, n is the number of trials (in this case 30), and ν̃ is the estimated variance. The SMUP value can be estimated as:
| (2) |
Where x̃ is the mean CMAP value, and min(x) represents the minimum CMAP value in this set. The effect of the MUs that were always active during this specific trial was excluded by subtracting the min(x) from the mean. Thus, the MU number can be estimated by dividing the maximal CMAP value by the averaged SMUP value across three recording windows, representing the global MU activity and averaged single MU activity, respectively. The statistical MUNE were calculated separately for each EMG recording channel, and the final SMUP and MUNE results are presented as the mean of all the 4 channels.
Immunofluorescence studies
After electrophysiological characterization, the EAS of the tested rats were overnight fixed in 10% formalin at 4°C, and cryoprotected in 30% sucrose for staining purposes. The immunostaining of motor end plates was based on a procedure described by Song et al for external urethral sphincter staining18. Briefly, anorectal tissue was dissected (approximately 1 cm length) and embedded in optimal cutting temperature compound (Fisher Scientific, Waltham, MA). Every 10th cross section (thickness = 10 μm) was saved until a 4 mm depth is reached (in relation to the rectal opening). Glass slides containing the tissue were washed and blocked overnight at 4°C with blocking solution (10% Goat serum, 2% Bovine serum albumin, 0.1% triton X-100 and 0.05% Tween®20). Samples were incubated overnight at 4°C in a 1:100 dilution of a primary antibody against neurofilaments (mouse anti-neurofilament 68 and 200 kDa antibody; Sigma, St. Louis, MO) and followed by secondary antibody incubation for 1 hr at room temperature (1:500, FIT-C donkey anti-mouse, Invitrogen, Eugene, OR). Acetylcholine receptors (AChRs) were labeled on the same slides using tetramethyldhordamine-conjugated α-bungarotoxin (α-BTX, 4μg/ml; Invitrogen) for 1 hr at room temperature. An Alexa Fluor conjugated phalloidin solution (0.1%, Invitrogen) was used to identify myofibers in the EAS. Finally, all slides were mounted with antifade reagent and examined under fluorescence microscopy, acquiring high definition images with a CCD camera controlled by the NIS-Advanced Research software (Nikon, Brighton, MI).
Calculation of myofibers
After staining we excluded from the analysis those EAS sections where an incomplete sphincter and/or a folding of the section were established. Consequently, only 7 of the 12 EAS remained fully intact and thus included in the analysis. The number of myofibers was counted according to the number of labeled AChRs on the myofibers. Multiple neighboring AChRs located on the same myofiber were considered as belonging to the same myofiber, unless obvious different innervating neurofilament axons were observed. In cases like these, AChRs were considered as belonging to a different neuromuscular junction (NMJ) therefore a different muscle fiber. The number of myofibers was counted twice by the same observer on two separate occasions (the second evaluation occurred 4–5 days after the first one). We managed to reduce bias in counting the number of endplates by blinding both the identification information of animals and the section set ID number to the observer during the second assessment. The mean values of two measurements were used for comparison with the corresponding MUNE results from each animal.
Statistical Analysis
The MUNE and SMUP are expressed as the mean ± standard deviation. Linear regression with zero intercept was performed to evaluate the relationship between MUNE results and corresponding myofiber counting. A significance level was set at p <0.05 using non-paired student’s t-tests.
RESULTS
Submaximal CMAP responses were successfully recorded in all 12 rats with our pudendal nerve stimulation and intra-rectal surface EMG recording techniques. Figure 2 shows an example of overlapping recorded CMAP responses in three different recording windows (shown as red, green, and blue, respectively). Most of the CMAP values varied within the pre-set recording window. The EAS MUNE was successfully calculated in all 12 rats based on their submaximal CMAP responses. Table 1 summarizes the EAS MUNE values across all animals. The averaged estimated value for SMUP was 52.49 ± 20.39 μV, and the average MU number was calculated as 35 ± 9 MUs/EAS.
Figure 2.
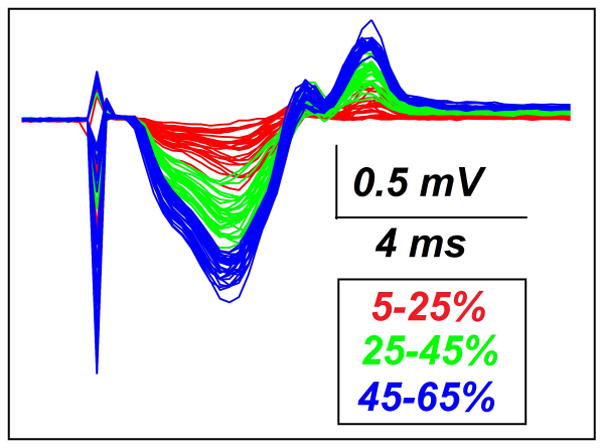
Example of overlapping CMAP responses. In this example three recording windows are shown: 5–25% (red), 25–45% (green), and 45–65% (blue). The sharp spikes on the left part of the traces correspond to the electrical stimulation artifact.
Table 1. Values for SMUP, MUNE and myofiber calculations.
A total of 12 animals were used to calculate the amplitude of SMUP, which were used for calculating MUNE. The number of myofibers (NMJ/EAS) was determined in 7 out of 12 rat tissue preparations that retained a complete EAS. Results are shown as mean ± standard deviation. N/D indicates not-determined due to an incomplete EAS.
| Rat ID Number | SMUP(μV) | MUNE | Myofiber/EAS |
|---|---|---|---|
| 1 | 67.95±4.04 | 49±2 | 766 |
| 2 | 33.80±2.77 | 33±1 | 528.5 |
| 3 | 39.85±11.71 | 42±2 | 608 |
| 4 | 57.36±8.21 | 36±3 | 564.5 |
| 5 | 68.91±4.50 | 28±1 | N/D |
| 6 | 37.18±4.06 | 31±8 | N/D |
| 7 | 31.60±2.69 | 41±2 | 604 |
| 8 | 99.14±24.66 | 20±1 | N/D |
| 9 | 41.87±1.77 | 37±3 | N/D |
| 10 | 46.08±15.56 | 22±3 | N/D |
| 11 | 36.73±4.81 | 48±5 | 837.5 |
| 12 | 69.36±6.40 | 29±1 | 471.5 |
| Mean Values: | 52.49+20.39 | 35±9 | 652.7±130.6 |
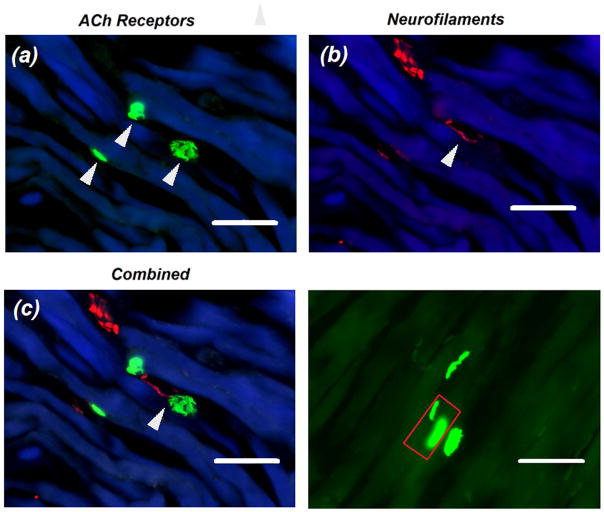
A typical example for a labeled NMJ is shown in Figure 3. The number of myofibers was counted based on the number of α-BTX-labeled AChRs regions. Multiple neighboring AChRs located on the same myofiber were considered from the same myofiber (Figure 3d), unless obvious different innervating neurofilament axons were observed19. The average number of myofibers per EAS is shown in Table 1, where the mean number was determined as 652.7 ± 130.6 myofiber/EAS.
Figure 3.
Immunofluorescent characterizations of motor endplates and neurofilaments in the rat EAS. (a) Example of EAS motor endplates characterized by AChRs-positive regions (green) on muscle myofibers (blue). (b) Corresponding innervating axons that were positive for neurofilaments antibody (red). (c) Combined images showing the location of neuromuscular junctions marked by white arrows. (d) Example showing the distribution for AChRs on EAS myofibers. The red rectangular indicates two endplates on a single myofiber. Calibration bar represents 50 μm.
Figure 4 shows a linear fitting between MUNE and the number of myofibers in the EAS of the rat. Only seven fully-intact EAS were successfully processed from the 12 animals tested. Significance for linear regression was achieved (p<0.01, R2=0.88), suggesting a good correlation between myofibers and the MUNE results.
Figure 4.
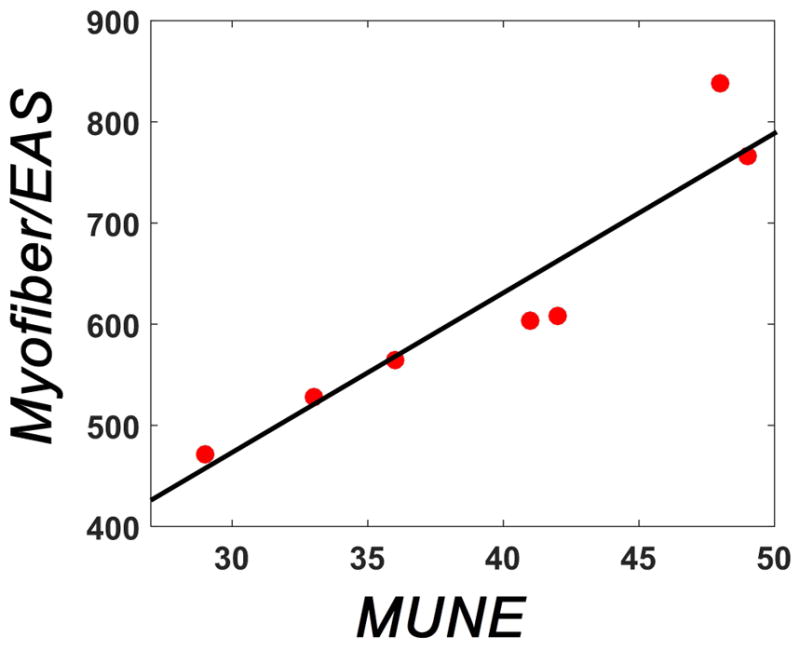
Linear correlation between MUNE and the number of EAS myofibers. Red dots show the pair-set for MUNE and corresponding NMJ counting. Using a linear correlation analysis between presented data we found a R2 = 0.88 and a p<0.01.
DISCUSSION
In this study, a statistical MUNE approach was employed to estimate the number of MUs in the EAS through intra-rectal signals elicited by intra-vaginal electrical stimulation in rats. The statistical MUNE does not require either multi-site stimulation or voluntary contraction of the EAS muscle, as such reduces the complexity of the animal experimental procedure in this validation study. The recording windows of 10–25%, 25–35%, 40–50%, and 55–65%, which are typically employed in the standard statistical MUNE technique, were not adopted in this study, because the EAS is a relatively weak skeletal muscle when compared with the limb musculature, and slight changes in MU recruitment can result in large variance (percentage to the maximal value) of the CMAP values. Therefore, the standard 10% window should be expanded to guarantee that the CMAP value falls into the recording window16. In this study, the recording window was set to 20% of the maximal CMAP amplitude. This is also the case in performing statistical MUNE in ALS or stroke patients16. In those patients, denervation and re-innervation progression of the skeletal muscle results in enlarged MU size, and the variation in the amplitude of CMAPs increases by recruiting those large MUs.
A direct counting of myofibers was difficult because of the discontinuity of the myofibers on the same EAS section. One possible reason is that the myofibers were not perfectly aligned in parallel with the section panel. In intact rats, it has been reported that most myofibers contained only one AChR-positive region or motor endplate19; therefore, the number of stained endplates can be considered as a marker reflecting the number of myofibers. As the endplate (marked by AChR-positive region) generally extends over 10 μm and can extend over 50 μm, particular attentions is needed to avoid over-estimating the number of endplates, which would likely be generated by counting a single endplate more than once over continuous sections (thickness = 10 μm). With the strategy of saving every 10th section as a representative of the 10 continuous sections, the impact of over-counting can be minimized and the results were considered proportional to the real number of the endplates. In this study, a batch of 60–80 sections were typically prepared from each animal, and the EAS myofibers were evaluated in 20–40 sections depending on the size of the intact EAS. Despite that the innervation ratio for single motor neurons varies greatly due to MU intrinsic characteristics, a relatively consistent average innervation ratio in limb muscle (number of myofiber per motor neuron) in EAS has been reported in the literature20. Therefore a lower but consistent average innervation ratio was assumed for validation purposes between the MUNE and the number of myofibers. The significance of the regression between immunofluorescent staining and MUNE results verifies the accuracy of the MU number estimated using the proposed EAS MUNE technique.
A different concern of our study may be related to the fact that transcutaneous stimulation could potentially activate other pelvic muscles such as the external urethra sphincter, or the levator ani (LA) muscle, which are also innervated by the pudendal nerve. Considering the anatomical proximity of these muscles, evoked potentials from these origins could be potentially picked up by the intra-rectal recording probe. The good linear fitting demonstrated that the CMAPs used for calculating MUNE is mostly coming from the EAS muscle. Although our study showed promising validation proof of EAS MUNE, the experiment sample size should be enlarged to further stablish our conclusions. Other staining methods, such as retrograde tracing, can label motor neurons in the lumbosacral spinal cord that specifically innervate EAS muscles. Previous reports using retrograde tracing have reported that the number of motor neurons innervating the EAS of the rat varies between 31.7 ± 8.5 21 and 51.7 ± 12.022 (for L6) to 73 ± 9.96 23. The estimated number (35 ± 9) fits into the lower portion of the previously reported range. A bilateral activation of the pudendal nerve should be expected with a ring-shape electrode employed in our study. However, there is still a possibility that the pudendal nerve was activated only on the side with better electrode-tissue contact in some cases in our study. This might explain the relatively low estimation.
The proposed non-invasive MUNE methods bring a novel perspective for evaluating EAS neuromuscular function, while also met the challenge of measuring EMG signals of pelvic muscles and sphincters. Because of the complicated anatomy of pelvic floor muscles, our approach can also be used for delivering electrical stimulations to their innervating nerves. The rationale for using vaginal stimulation is that the delivery of an intra-vaginal stimulus could, according to previous reports, better suppress the stimulation artifact, compared with simultaneous intra-rectal stimulation and recording3. Previous study has reported the feasibility of trans-vaginally delivery of stimuli to the pudendal nerve using St. Mark’s electrodes and simultaneous surface recording of CMAPs from the EAS24. Undoubtedly, an electrical stimulus applied transcutaneously requires higher voltage to generate the same current density, and can result in a broader spatial activation, while direct electrode contact with the pudendal nerve can have better stimulation accuracy but provide lower stimulation voltage. Nonetheless, the MUNE method developed in this study enables the possibility for long term tracking of disease progression, while greatly reducing the complexity, duration and cost of experiment procedure compared with applying traditional MUNE approaches to EAS. The alterations in MU number or SMUP will reveal the process of muscle denervation and reinnervation. With the intra-rectal surface EMG probe and intra-vaginal stimulating probe developed in this study, we should be able to perform multiple MUNE of the EAS in a non-invasively way on the same animal.
The system configuration in this MUNE technique also mimics the clinical setup, and the validation using immune-histochemical staining provided the potential feasibility to future clinical studies and diagnosis. Moreover, the established animal model described can be further extended to study the impact of aging, degenerative diseases or spinal cord injury (SCI) on the neuromuscular functionality of EAS muscles. For instance, previous studies have reported a post-SCI reduction on MU number in rat25. These results suggested that the gradual degradation of post-SCI neuromuscular function can be assessed using MUNE techniques. However, there remains a knowledge gap to find out whether similar degenerative changes stand remain true for the EAS. Applying the proposed technique to an SCI model, we will be able to track pathological alterations in MU number, SMUP size and myofiber number in the EAS. These valuable data will be helpful to reveal the post injury neurological process including motor neuron loss, muscle denervation and reinnervation, to advance our understanding of SCI-induced fecal incontinence.
CONCLUSIONS
A novel EAS MUNE technique was successfully developed to, for the first time, non-invasively evaluate the EAS innervation in vivo after assessing the number of MUs. The EAS MUNE approach was rigorously tested and validated, and the results demonstrate the high performance of this approach in estimating the number of MUs in the EAS. Our MUNE technique can potentially be applied to human studies for long-term tracking of alterations in the innervation of the EAS in patients with diabetes, spinal cord injury and other diseases which may lead to neurological dysfunction of the EAS.
Acknowledgments
Authors would like to thank Kristopher Hoffman and Hanna Tang for technical assistance, and Yun Peng for useful discussion of data and results. This work was supported by NIH DK082644, the University of Houston, the Brown foundation and the Houston Methodist foundation.
References
- 1.de Groat WC, Tai C. Impact of Bioelectronic Medicine on the Neural Regulation of Pelvic Visceral Function. Bioelectronic medicine. 2015;2015:25. [PMC free article] [PubMed] [Google Scholar]
- 2.Lefaucheur JP. Neurophysiological testing in anorectal disorders. Muscle & nerve. 2006;33(3):324–333. doi: 10.1002/mus.20387. [DOI] [PubMed] [Google Scholar]
- 3.Remes-Troche JM, Rao SS. Neurophysiological testing in anorectal disorders. Expert review of gastroenterology & hepatology. 2008;2(3):323–335. doi: 10.1586/17474124.2.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Sachdeva M, Jain P, Ranjan P, Arora A. Anorectal manometry: current techniques and indications. JIMSA. 2013;26:169–170. [Google Scholar]
- 5.Neill M, Swash M. Increased motor unit fibre density in the external anal sphincter muscle in ano-rectal incontinence: a single fibre EMG study. Journal of Neurology, Neurosurgery & Psychiatry. 1980;43(4):343–347. doi: 10.1136/jnnp.43.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooch CL, Doherty TJ, Chan KM, et al. Motor unit number estimation: a technology and literature review. Muscle & nerve. 2014;50(6):884–893. doi: 10.1002/mus.24442. [DOI] [PubMed] [Google Scholar]
- 7.Peng Y, He J, Khavari R, Boone TB, Zhang Y. Functional mapping of the pelvic floor and sphincter muscles from high-density surface EMG recordings. International urogynecology journal. 2016:1–8. doi: 10.1007/s00192-016-3026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boe SG, Stashuk DW, Doherty TJ. Motor unit number estimation by decomposition-enhanced spike-triggered averaging: Control data, test–retest reliability, and contractile level effects. Muscle & nerve. 2004;29(5):693–699. doi: 10.1002/mus.20031. [DOI] [PubMed] [Google Scholar]
- 9.Olney RK, Yuen EC, Engstrom JW. Statistical motor unit number estimation: reproducibility and sources of error in patients with amyotrophic lateral sclerosis. Muscle & nerve. 2000;23(2):193–197. doi: 10.1002/(sici)1097-4598(200002)23:2<193::aid-mus8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Lomen-Hoerth C, Slawnych MP. Statistical motor unit number estimation: from theory to practice. Muscle & nerve. 2003;28(3):263–272. doi: 10.1002/mus.10351. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg MB. Updating motor unit number estimation (MUNE) Clinical neurophysiology. 2007;118(1):1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- 12.Ullah K, Cescon C, Afsharipour B, Merletti R. Automatic detection of motor unit innervation zones of the external anal sphincter by multichannel surface EMG. Journal of Electromyography and Kinesiology. 2014;24(6):860–867. doi: 10.1016/j.jelekin.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, He J, Khavari R, Boone TB, Zhang Y. Functional mapping of the pelvic floor and sphincter muscles from high-density surface EMG recordings. International urogynecology journal. 2016;27(11):1689–1696. doi: 10.1007/s00192-016-3026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Z, Lui V, Tam P. Deficient motor innervation of the sphincter mechanism in fetal rats with anorectal malformation: a quantitative study by fluorogold retrograde tracing. Journal of pediatric surgery. 2003;38(9):1383–1388. doi: 10.1016/s0022-3468(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Li S, Li X, Klein C, Rymer WZ, Zhou P. Suppression of stimulus artifact contaminating electrically evoked electromyography. NeuroRehabilitation. 2014;34(2):381–389. doi: 10.3233/NRE-131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y-H, Sung J-J, Park KS, Kwon O, Min J-H, Lee K-W. Statistical MUNE: a comparison of two methods of setting recording windows in healthy subjects and ALS patients. Clinical Neurophysiology. 2007;118(12):2605–2611. doi: 10.1016/j.clinph.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 17.Daube JR. Motor unit number estimates—from A to Z. Journal of the neurological sciences. 2006;242(1):23–35. doi: 10.1016/j.jns.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Song QX, Balog BM, Kerns J, et al. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourology and urodynamics. 2015;34(4):381–386. doi: 10.1002/nau.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Liu Y, Sugiura Y, Allen PD, Gregg RG, Lin W. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nature neuroscience. 2011;14(5):570–577. doi: 10.1038/nn.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura A, Inokuchi S. Muscle Fibre Composition and Innervation Ratios of the Extensor Digitorum and Extensor Hallucis Brevis Muscles in the Crab-Eating Macaque (Macaca fascicularis) Folia Primatologica. 1996;66(1–4):44–54. doi: 10.1159/000157184. [DOI] [PubMed] [Google Scholar]
- 21.Kane DD, Shott S, Hughes WF, Kerns JM. Motor pudendal nerve characterization in the female rat. The Anatomical Record. 2002;266(1):21–29. doi: 10.1002/ar.10029. [DOI] [PubMed] [Google Scholar]
- 22.Kerns J, Shott S, Brubaker L, et al. Effects of IGF-I gene therapy on the injured rat pudendal nerve. International Urogynecology Journal. 2003;14(1):2–8. doi: 10.1007/s00192-002-0995-2. [DOI] [PubMed] [Google Scholar]
- 23.Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Hormones and behavior. 1997;32(1):1–10. doi: 10.1006/hbeh.1997.1397. [DOI] [PubMed] [Google Scholar]
- 24.Gregory WT, Lou J-S, Stuyvesant A, Clark AL. Quantitative electromyography of the anal sphincter after uncomplicated vaginal delivery. Obstetrics & Gynecology. 2004;104(2):327–335. doi: 10.1097/01.AOG.0000134527.07034.81. [DOI] [PubMed] [Google Scholar]
- 25.Xiong G, Guan Y, Hong Y, Zhang J, Guan H. Motor unit number estimation may be a useful method to evaluate motor function recovery after spinal cord transection in rats. Spinal cord. 2010;48(5):363–366. doi: 10.1038/sc.2009.141. [DOI] [PubMed] [Google Scholar]