Abstract
Wrinkles can have a negative effect on quality of life and Botox is one of the most effective and common treatments. Argireline (Arg0), a mimetic of Botox, has been found to be safer than Botox and effective in reducing wrinkles, with efficacies up to 48% upon 4 weeks of twice daily treatment. However, the skin permeation of Arg0 is poor, due to its large molecular weight and hydrophilicity. Arg0 exists in zwitterionic form and this charged state hindered its skin permeation. Chemical modification of the peptide structure to reduce the formation of zwitterions may result in increased skin permeability. We investigated a total of 4 peptide analogues (Arg0, Arg1, Arg2, Arg3), in terms of skin permeation and wrinkle reduction. The 4 peptides were dissolved in various propylene glycol and water co-solvents. Enhanced human skin permeation was demonstrated by both Arg2 and Arg3 in vitro. On the other hand, the abilities of the 4 analogues to reduce wrinkle formation were also compared using primary human dental pulp stem cells derived neurons. By measuring the inhibition of glutamate release from the neurons in vitro, it was shown that Arg3 was the most effective, followed by Arg1, Arg0 and Arg2.
Introduction
Wrinkles are visible creases or folds in the skin1 and they are often the first sign of ageing2. Changes in physical appearance due to wrinkles can have a negative effect on the quality of life. In some cases, concerns over physical appearances can affect personal interactions, occupational functioning and self-esteem3. By far, Botox has been one of the most effective and commonly administered compounds to reduce wrinkles in the United States, with up to 2.6 million injections in 20114. However, Botox is often questioned for its safety in human, due to its high toxicity and therefore, its use must be under strict control5.
Argireline® (Arg0), developed as a topical mimetic of Botox, is a synthetic acetyl hexapeptide, patterned after the N-terminal end of the protein Synaptosomal-associated protein 25 (SNAP-25). Arg0 competes with SNAP-25 for the binding with vesicle-associated membrane protein (VAMP). This destabilises the formation of a ternary Soluble N-ethylmaleimide-sensitive factor Attachment protein REceptor (SNARE) complex resulting in the inhibition of neuronal exocytosis. As a result, Arg0 inhibits the release of acetylcholine6 and reduces repetitive contraction of the intrinsic muscles of facial expression, thereby decreases hyperkinetic facial lines or expression wrinkles7. However, unlike Botox, the acute toxicity is insignificant (≥2000 mgkg−1 for Arg0 versus 20 ngkg−1 for Botox)6. In addition, Arg0 has been found to be effective in reducing wrinkles, with efficacies up to 48% upon 4 weeks of twice daily treatment6,8–10.
While Arg0 has good efficacy and safety profiles, the permeation of Arg0 through skin is poor. With a high molecular weight of 889 Dalton and a low LogP value of −6.3, Arg0 remains mainly on the surface of the skin upon topical application11. In an in vitro skin permeation study using human cadaver skin samples, it was found that the majority of Arg0 remained on the surface of the skin and was washed away subsequently. Only 0.22% of the total amount permeated through the skin and retained within stratum corneum, the topmost layer skin; 0.01% of the peptide made it through to the epidermis12. The poor skin permeation can lead to a significant wastage of peptide, resulting in unwanted high cost of production and an ineffective final dosage form.
To overcome the problem, several research groups have attempted to enhance transdermal delivery of Arg0 by optimising the formulations. Hoppel et al. showed a clear superiority of water-rich water-in-oil-in-water (W/O/W) and oil-in-water (O/W) emulsions over oil-rich water-in-oil emulsion (W/O) emulsions, due to increased absorption of water-rich emulsions into the skin13. However, there was no comparison to the pure Arg0 solution and no comparison could be drawn to see the effect of emulsion. Kraeling et al. used a high concentration of Arg012 (at 10% w/w in an O/W emulsion), yet, the results were disappointing when only 0.23% of the total amount of Arg0 permeated through and stayed in the human epidermis. Also, this approach may result in costly products. Ruiz et al. attempted to incorporate Arg0 into either a cream or gel dosage form and demonstrated that a cream based dosage form is superior to a gel dosage form in transdermal delivery of Arg014. While these methods were relatively simple to perform, the increase of Arg0 delivery through skin was unsatisfactory.
Other than modifying the formulation, several techniques have been put forth to overcome the stratum corneum for other peptide drugs, for e.g., chemical enhancers, iontophoresis15, microneedles16–19, sonophoresis20, thermal ablation21, radiofrequency ablation22, jet injectors23 and electroporation24,25. A number of commercial iontophoretic devices have been available for transdermal delivery, although their market penetration has been limited because of cost and technical issues15. Other physical methods, such as electroporation or ablation, are generally inconvenient to users as they require special machineries and expertise to perform. In addition, microneedle is a physical enhancement method which creates micro-pores in the skin in a minimally invasive manner to deliver peptides. However, the idea of placing needles on the face may be of concern to users.
Another approach to enhance transdermal delivery of Arg0 is structural modification of the amino acid side chains. Arg0 exists in zwitterionic form and this charged state makes it very hydrophilic and poses a major obstacle for transdermal delivery, with the other being its high molecular weight. Chemical modification of the peptide structure to reduce the formation of zwitterions and/or to increase lipophilicity may result in increased permeability of Arg0 into the skin.
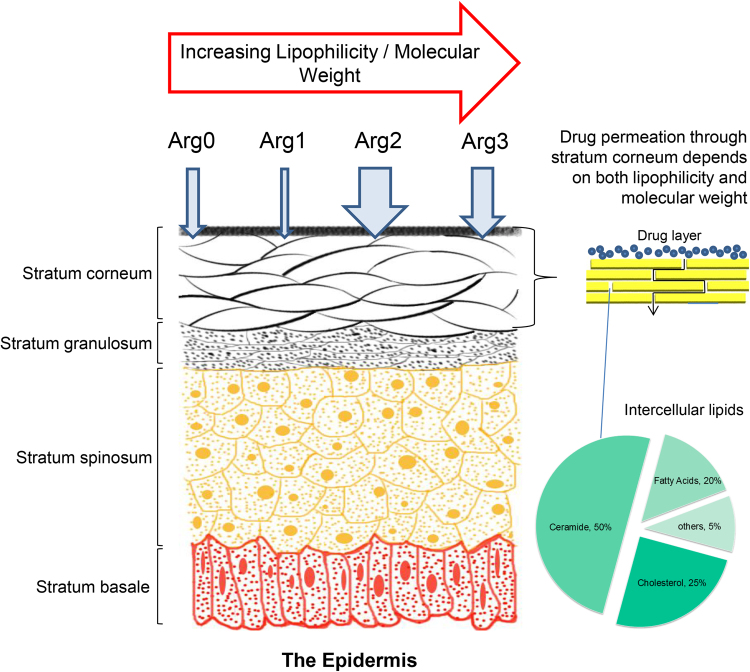
In this study, we investigated a total of 4 peptide analogues (Arg0, Arg1, Arg2, Arg3), on their abilities to permeate through skin and their in vitro efficacies on neuronal cell cultures. Each of these analogues has different amino side chain functional groups, with increasing lipophilicity and molecular weight (Fig. 1). The 4 peptide analogues were dissolved in various propylene glycol (PG) and water (H2O) co-solvent systems, as PG is widely used as co-solvent and permeation enhancer26 in the cosmetics. Further, PG is generally regarded as safe and does not cause irritation or side effects even at high concentrations27.
Figure 1.
Overall schematic for the enhancement of transdermal delivery of small peptide through chemical structure modification.
Results
Structural Modification of Amino Acid Side Chains
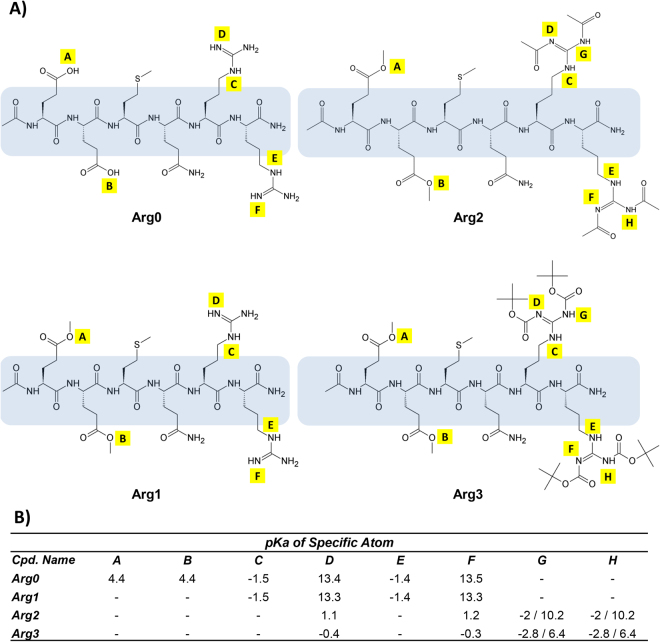
Figure 2A shows the chemical structures of the 4 peptide analogues, Arg0, Arg1, Arg2 and Arg3. The 3 peptide analogues were modified from their parent compound, namely, Arg0, to reduce the formation of zwitterions and increase the lipophilicity. In all 3 analogues (Arg1, Arg2, Arg3), the carboxylic acid functional groups (marked as position A & B, Fig. 2A) were esterified. In addition to esterification, Arg2 and Arg3 had 2 more structural modifications each. In Arg2, the guanidine functional group was modified to become an acetamide functional group for each end of the guanidine’s end nitrogen. In Arg3, the guanidine functional group was modified into a bis-carbamate functional group ending with a tertiary butyl group for each carbamate functional group.
Figure 2.
The 4 synthesised peptide analogues. (A) Chemical structures of parent compound (Arg0) and its chemical analogues. For all 3 compounds, Arg1, Arg2, Arg3, 2 esters functional groups have been added to the chemical structure to replace the 2 carboxylic acids at position A&B. (B) A table summary of the purity of synthesised peptide analogues and the various pKa changes to the specific atoms of the amino acid side chains. In general, the pKa favours that of deprotonation, thus reducing the formation of charged ions. In silico prediction of pKa is performed via ACD/Labs Percepta platform.
Physicochemical Properties of Peptide Analogues
A substantial decrease in pKa of ionisable atoms in the amino acid side chains was observed after structural modification (Fig. 2B). After esterification of the side chain, as seen in Arg1, Arg2 and Arg3, the originally ionisable atom A & B no longer form charged ions. In addition, after a second structural modification as seen in Arg2 and Arg3, comparing atom D & F, the pKa dropped from ~13 to ~0.5. A smaller pKa implied that the atom will less likely protonate and therefore less likely form charged ions. For atoms G & H for both Arg2 and Arg3, they have very low pKa and hence, were also unlikely to protonate to form charged ions. Table 1 shows the basic physicochemical properties of the 4 peptide analogues. From Arg0 to Arg3, there was an increasing trend of lipophilicity and molecular weight. Arg3 had the most optimal LogP of 1.75, while both Arg1 and Arg2 had LogP values less hydrophilic than that of Arg0 (−4.67 and −4.18 VS −6.37). However, all 4 analogues were high in molecular weight due to the nature of the peptide, with Arg3 being the highest (MW: 1317.51), followed by Arg2 (MW: 1085.19), Arg1 (MW: 917.05) and Arg0 (888.99).
Table 1.
Physicochemical properties of the 4 chemical analogues, based on in silico prediction by ACD/Labs Percepta platform. In general, all 4 analogues do not have significant potential for skin/eye irritation and mutagenecity, except for Arg1. Arg3 has the most optimal LogP but also with the highest molecular weight.
| Physicochemical Properties of Various Chemical Analogues | ||||
|---|---|---|---|---|
| Properties | Arg0 | Arg1 | Arg2 | Arg3 |
| LogP | −6.37 | −4.67 | −4.18 | 1.75 |
| MW | 888.99 | 917.05 | 1085.19 | 1317.51 |
| H-donors | 20 | 18 | 14 | 14 |
| H-acceptors | 26 | 26 | 30 | 34 |
| Rot. Bonds | 34 | 36 | 40 | 48 |
| Rings | 0 | 0 | 0 | 0 |
| Solubility | 57.9 mg/mL | 1000 mg/mL | 0.008 mg/mL | 0.0002 mg/mL |
| AMES | 0.27 | 0.40 | 0.38 | 0.37 |
| Skin irritation | 0.09 | 0.52 | 0.13 | 0.09 |
| Eye irritation | 0.01 | 0.06 | 0.01 | 0.00 |
In Vitro Skin Permeation of Peptide Analogues
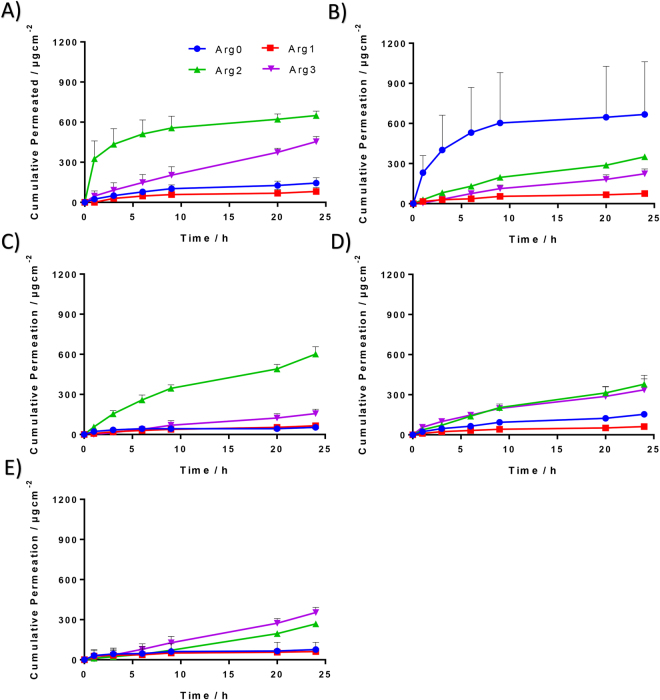
PG is commonly used in cosmetics and is well known for its co-solvent and permeation enhancing effect. Hence, pure PG was also used to prepare the donor peptide solution for each of the 4 analogues (Fig. 3A, Table 2). In pure PG, comparing to Arg0, a significantly higher amount of peptide permeated through skin by the end of 24 hrs (p < 0.01) was demonstrated by both Arg2 (~4.5 times) and Arg3 (~3.1 times). A lower amount of peptide permeated through the skin was observed in Arg1 as compared to Arg0, however, it was not significantly different. The transdermal delivery of all 4 peptide analogues was generally enhanced with the use of pure PG, compared to when the same analogue was dissolved in pure water. Significant difference was observed only for Arg2 (p < 0.01) (Fig. 4C).
Figure 3.
Cumulative release profiles of the 4 different peptide analogues over a time period of 24 hrs, in varying concentrations of PG:H2O co-solvent systems. (A) 100%PG, (B) 70%PG, (C) 50% PG, (D) 30%PG, (E) 0%PG. Each coloured curve represents a different cumulative release profile of a different chemical analogue, at the specified propylene glycol concentration.
Table 2.
A summary of the cumulative amount of chemical analogues, permeated through the skin after 24 hrs. Statistical comparison (one-way ANOVA, with post-hoc Tukey analysis) of the cumulative amount were compared between different chemical analogues within the same concentration of PG:H2O co-solvent system. In general, both Arg2 and Arg3 have greater permeation at 24 hrs as compared to Arg0, except in 70% PG co-solvent system where Arg0 performed significantly better than the other chemical analogues.
| Cumulative Amount Permeated Through Skin after 24 hrs | ||||
|---|---|---|---|---|
| Concentration of PG (%) | Compound No. | Average (µg) | SD | P-value (Compared to Arg0) |
| 0 | Arg0 | 76.4 | 7.6 | 1 |
| Arg1 | 61.9 | 69.0 | 0.969 | |
| Arg2 | 268.5 | 8.1 | **< 0.01 | |
| Arg3 | 353.6 | 38.3 | **< 0.01 | |
| 30 | Arg0 | 153.2 | 21.4 | 1 |
| Arg1 | 61.6 | 4.5 | 0.239 | |
| Arg2 | 378.2 | 67.6 | **< 0.01 | |
| Arg3 | 337.0 | 81.6 | * 0.013 | |
| 50 | Arg0 | 53.5 | 16.6 | 1 |
| Arg1 | 65.1 | 3.9 | 0.972 | |
| Arg2 | 601.7 | 54.4 | **< 0.01 | |
| Arg3 | 156.1 | 33.7 | * 0.022 | |
| 70 | Arg0 | 667.4 | 393.9 | 1 |
| Arg1 | 75.5 | 4.5 | 0.026 | |
| Arg2 | 350.8 | 6.9 | 0.277 | |
| Arg3 | 223.5 | 35.5 | 0.095 | |
| 100 | Arg0 | 144.4 | 40.5 | 1 |
| Arg1 | 81.6 | 29.0 | 0.218 | |
| Arg2 | 649.1 | 33.8 | **< 0.01 | |
| Arg3 | 454.1 | 38.4 | **< 0.01 | |
*p < 0.05.
**p < 0.01.
Figure 4.
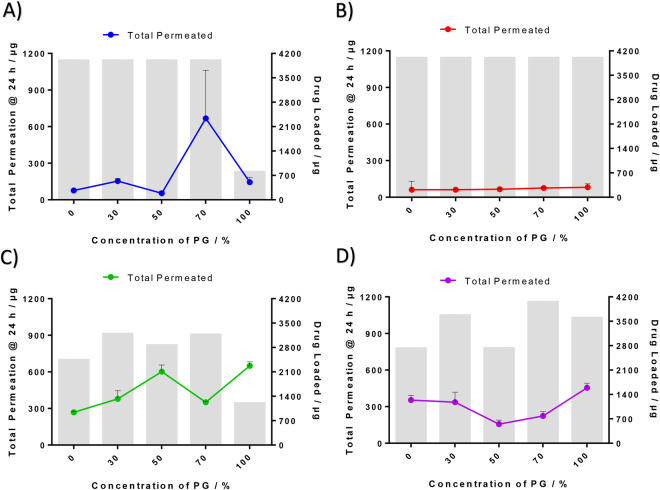
Effects of propylene glycol concentration on the cumulative permeation in 24 hrs and amount of drug in donor cell for different peptide analogues. Line graph: Amount of peptide analogue permeated through the skin after 24 hrs. Bar graph: Amount of drug loaded for each analogue at varying propylene glycol concentrations.
Varying concentrations of PG in water were also used as solvent to prepare the donor solution for different peptide analogues (Fig. 3B–D, Table 2). A total of 3 other concentrations of PG in water were 30%, 50% and 70%. In 30% PG, a higher cumulative amounts of peptide that permeated through the skin after 24 hrs, as compared to Arg0, were demonstrated by both Arg2 (p < 0.01, ~2.5 times) and Arg3 (p = 0.013, ~2.2 times). On the other hand, compared to Arg0, less than half the cumulative amount of peptide that permeated through the skin after 24 hrs was observed in the Arg1. In 50% PG, significantly higher cumulative amount of peptide that permeated through the skin after 24 hrs, as compared to Arg0, was demonstrated in Arg2 (p < 0.01, 11.2 times) and Arg3 (p = 0.022, ~2.9 times). In 70% PG, the highest cumulative amount of peptide permeated through the skin after 24 hrs amongst the 4 peptide analogues was observed in Arg0. Significantly less cumulative amount of peptide that permeated through the skin by 24 hrs, as compared to Arg0, was observed in Arg1 (p = 0.026). Across all 5 concentrations of PG investigated, a lowest cumulative amount of peptide that permeated through the skin by 24 hrs was consistently observed in Arg1. On the other hand, across all 5 concentrations of PG, higher cumulative amount of peptide that permeated through the skin after 24 hrs, as compared to Arg0, was consistently observed in Arg2 (p < 0.01) and Arg3), with the exception of 70% PG. The effect of PG in enhancing skin permeation of these peptide analogues is not uniform throughout the PG concentrations and the trend varies amongst the 4 peptide analogues. For Arg0, the highest amount of peptide that permeated through the skin at 24 hrs was demonstrated by 70% PG. For Arg1, Arg2 and Arg3 that concentration was 100% PG (Fig. 4).
In Vitro Inhibition of Glutamate Release
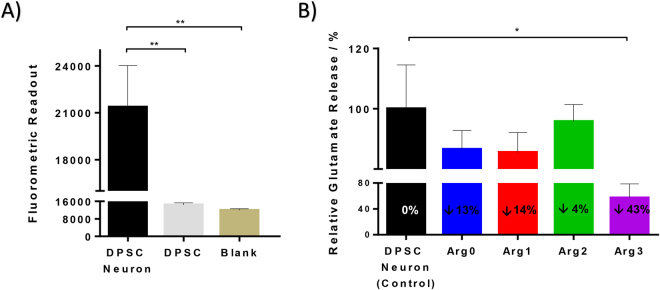
Arg0 inhibits the formation of a ternary SNARE complex and in turn inhibits neuronal exocytosis and decreases wrinkle formation. In this study, the degree of glutamate release is used as an estimate for acetylcholine release, as glutamate is the most prevalent excitatory neurotransmitter in the nervous system28 and can be detected more easily than acetylcholine which is present in less quantity. Inhibition of glutamate release has also been used previously as a validated cell assay for measuring the potential activity of Arg0 on the inhibition of neuronal exocytosis29. Primary human dental pulp stem cells derived neurons (DPSC neurons) were chosen due to its ability to differentiate into functionally active neurons and reduced problems associated with epigenetic changes and re-programming, as compared to induced pluripotent stem cells30. Comparing to the undifferentiated dental pulp stem cells (DPSC), a significantly higher fluorometric readout (indicative of the amount of glutamate released) was observed in the neuronally differentiated DPSC neuron group, as compared to blank and DPSC group (Fig. 5A). There was no significant difference between the blank and DPSC group (Fig. 5A). This indicated a successful differentiation of DPSC into functional DPSC neurons with exocytotic functions.
Figure 5.
In vitro inhibition of glutamate release using dental pulp stem cell derived neurons. (A) Comparison of glutamate release between DPSC and DPSC neurons after 12 weeks of differentiation. A significantly higher fluorometric readout (indicative of the amount of glutamate released) was observed in the neuronally differentiated DPSC neuron group, as compared to blank and DPSC group. No significant difference between blank and DPSC cultures was observed. (B) Comparison of glutamate release between DPSC neuron without any drug (control) and those with the 4 different peptides (Arg0, 1, 2, 3). Arg3 demonstrated the greatest anti-wrinkle efficacy with a reduction of 43% in the release of glutamate. (*p < 0.05; **p < 0.01).
Subsequently, the DPSC neuron cells were treated with the 4 different peptide analogues. A decrease of ~13% in the release of glutamate as compared to control was demonstrated by Arg0. A similar efficacy was demonstrated in Arg1, with a decrease of ~14% in the release of glutamate as compared to control. While Arg2 has good skin permeation as demonstrated above, a relatively small efficacy with a decrease of only ~4% in the release of glutamate as compared to negative control was demonstrated. The highest efficacy for in vitro inhibition of the release of glutamate as compared to negative control, was demonstrated by Arg3 (~43%). The greater the inhibition of glutamate release, the higher the efficacy in reducing wrinkles. Therefore, the in vitro efficacy is as such, in a descending order of Arg3, Arg1, Arg0, Arg2 (Fig. 5B).
Discussion
In this study, we demonstrated the use of chemical structure modifications to improve the transdermal delivery of small peptides. Out of the 3 modified peptide analogues, Arg2 and Arg3 had consistently greater cumulative amount of peptides permeated through the skin after 24 hrs, while Arg1 had consistently less cumulative amount of peptide permeated through the skin after 24 hrs, as compared to Arg0. Also in this study, peptide analogues dissolved in PG demonstrated higher amount permeated through the skin.
Stratum corneum, the outermost barrier of skin, is made up of corneocytes and acts as a rate limiting barrier to transdermal drug delivery. This layer of skin is lipophilic and allows only small and moderately lipophilic molecules to permeate passively into the deeper layers of the skin. On the other hand, the stratum corneum restricts the transdermal delivery of peptides and proteins, which have high molecular weights and are hydrophilic or charged in nature24. Therefore, when the lipophilicity was increased from Arg0 to Arg1 to Arg2 to Arg3, there was in general, a corresponding higher amount of peptide permeating through the skin by 24 hrs. However, to increase the lipophilicity, there was an accompanying increase in molecular weight. A balance between the two components is required. This was illustrated in the case of Arg1, where it is more lipophilic than Arg0, but yet, had lower skin permeation as compared to Arg0 (most likely due to the increase in molecular weight). Similar findings was observed when we compared Arg3 to Arg2.
PG is an aliphatic alcohol commonly found in cosmetics. Recent data showed that PG has been used in over 9000 cosmetic formulations (out of a total of 34391 formulations reported) and its concentrations ranges from 0.0008% to 99%31. For leave-on formulations, the highest PG concentration is 73%31. PG has also been used as a co-solvent for poorly soluble materials and/or to enhance drug permeation through skin from topical preparations26. Unlike some penetration enhancers, PG does little to the skin as no evidence was found for an effect of PG pre-treatment on intercelluar lipid lamellar structure or for uptake of PG into corneocytes32. Its proposed mechanism of action is to partition into the stratum corneum and increase permeant solubility in and thus permeant flux through the stratum corneum33. Therefore, the higher the amount of PG, the greater the penetration enhancing effect.
However, this is different from what we expected as shown in Fig. 4. Based on the permeation profiles of the different peptide analogues at varying PG concentrations (Fig. 4), the amount permeated through the skin for each peptide analogue was not always positively correlated to the concentration of PG. In the case of Arg0, 70% PG was demonstrated to give the greatest cumulative amount permeated through the skin and this amount was significantly different (p < 0.05) from that of all other concentrations of PG. For Arg1, Arg2 and Arg3, the PG concentration that achieves the highest amount of peptide analogue permeated through the skin by 24 hrs was 100%. However, the amount permeated through the skin was not significantly different from all concentrations of PG.
One possible explanation for the varying effect of PG on skin permeation is the effect of PG on peptide solubility. In the event of Arg0, at all concentrations of PG from 70% w/w and below, the amount of Arg0 used in the donor compartment are all 1% w/w. Therefore, as the concentration of PG increases, the amount of Arg0 permeated through the skin increases as well since a greater proportion of PG can partition into the stratum corneum, bringing the peptide through to the stratum corneum and beyond. Using neat PG, the saturated solution in the donor compartment was only 0.2% w/w. Due to lower amount of dissolved peptide, regardless of how much PG partition into the stratum corneum, the total amount that permeated through will still be minimal.
From the results of Ostrenga et al.34, there was a dramatic decrease in permeability of fluocinolone acetonide for PG treated skin versus water based treated skin. This was later confirmed by other groups, that one likely interaction of concentrated PG solutions with skin is dehydration of corneocytes due to the hygroscopic nature of PG. When the skin becomes dehydrated, the permeation of peptide decreases. The interaction of three factors, (1) peptide solubility; (2) partitioning effect of PG; (3) dehydrating effect of concentrated PG could have resulted in the erratic permeation profiles seen in Fig. 4C and D for Arg2 and Arg3.
Structural modification has been successfully used to enhance peptide permeability across intestinal and rectal barriers35. However, this method remains largely unexplored for transdermal delivery of peptides and the knowledge gained from this study will be useful. Through the structural modifications, the pKa values of the amino acid side chains were reduced and the side chains were less likely to protonate to form charged ions. Although there is a corresponding increase in molecular weight from the structural modifications, it appeared that the benefits from reducing formation of charged ions and increased logP outweighed the disadvantages associated with increased molecular weight for the purpose of crossing the stratum corneum. Other research groups have attempted chemical structure modification to various peptide compounds to improve transdermal delivery of the peptide. The most common strategy is to synthesise lipophilic derivatives of peptides which can be made either to increase the encapsulation efficiency into liposomes or directly increase their delivery through skin36. Foldvari et al. demonstrated the use of liposomes and fatty acylation as ways to increase interferon alpha delivery into cutaneous layer for about five to six times greater than the parent protein, for the treatment of genital warts37,38. The coupling of a short chain lipoamino acid to a tetrapeptide has also been demonstrated to enhance its delivery across human epidermis, from almost 0% that permeated through the skin for the original peptide, to about 2% that permeated through the skin for the lipophilic derived peptide. A similar study by Namjoshi et al. in 2014 also used similar method to enhance a peptide permeation through human epidermis for management of psoriasis39. Other structural modifications that have been performed for oral dosing of peptide previously, includes cyclisation40 and PEGylation41, both of which confer benefits to the systemic stability of the peptide.
In terms of efficacy for potential wrinkle reduction, similar results were demonstrated by Arg1 as compared to Arg0, due to its largely similar chemical structure and physiochemical properties. On the other hand, the highest efficacy was demonstrated by Arg3. This may be attributed to an increased uptake of Arg3, due to a favourable logP of 1.75. The increased uptake was not seen in the case of Arg2. The poor efficacy of Arg2 may be attributed to a reduced cellular uptake of Arg2, due to an increased molecular weight of 1085 Da, while without a significant change in logP, as compared to Arg0. Yet, the relatively higher amount of Arg2 that permeated the skin may still provide an advantage in the use of Arg2 as an alternative to Arg0, alongside Arg3. The results of Arg2 has shown that the permeation through skin can be different from that through cell membranes.
To our best knowledge, this is the first study to conduct multiple modifications on a single peptide compound to enhance transdermal delivery and it has been demonstrated that Arg 2 and Arg 3 were able to permeate through human cadaver skin consistently greater than Arg0 across all compositions of PG. However, molecular modification may affect the peptide’s pharmacological properties and hence must be carefully assessed to ensure that the enhanced delivery analogue has equally good safety and efficacy profiles as compared to the parent compound. Preliminary results from the in vitro inhibition of glutamate release study demonstrated the relative efficacy of the various analogues as compared to Arg0. Together with the in silico computed safety profile and in vitro skin permeation profiles, it appears that Arg2 and Arg3 may be viable alternatives to Arg0 in the topical treatment of wrinkles. However, while the application of molecular modification to enhance transdermal delivery of peptide is relatively new and has merits on a scientific basis, it may be less convenient from a regulatory viewpoint as chemical modification will imply that a new compound is generated. Nonetheless, the main molecular structures of the peptide analogues remain the same (hexapeptide), which may help to acquire regulatory clearance for cosmetic applications.
A candidate drug is expensive to develop (average cost being USD 1 billion per drug) and unlikely to make it to market, with high attrition rates42. With pharmacokinetics and pharmacodynamics being one of the major causes of drug development failure in Phase I clinical trials43, this study is focused on the skin permeation profiles of the 4 peptide analogues to identify any potential pitfalls in the pre-clinical drug development phase, before any full scale investigation on efficacy of the peptide analogue is conducted in the near future.
Methods
Materials
Arg0 and its peptide analogues were synthesised by Kaijie Peptide Company (Sichuan, China) and used as received. Propylene glycol was purchased from Sigma Aldrich (Singapore). All water used were deionised millipore water, employed from a Millipore DirectQ3® system (Massachusetts, USA). 10X phosphate buffered saline (PBS) was purchased from Vivantis Technologies Sdn Bhd (Selangor, Malaysia). Dulbecco’s Modified Eagle’s Medium, supplemented with fetal bovine serum and penicillin/streptomycin were purchased from Invitrogen (California, USA). Neural induction media consisting of Neurobasal®-A medium was purchased from Gibco (Life Technologies, USA) and supplemented with B-27® from Invitrogen (50 × B-27® supplements stock solution, Invitrogen, California, USA), penicillin/streptomycin (Gibco, Life Technologies, USA), EGF (Sigma Aldrich, Merck, Germany) and bFGF (Life Techologies, USA). L-glutamine was purchased from Sigma Aldrich (Merck, Germany). All other reagents used were of analytical grade.
Synthesis of Peptides
Briefly, the peptide analogues were synthesised through conventional methods for solid phase chemical peptide synthesis using FMOC based synthetic methodology on Rink-amide resin with modifications specific to each of the compounds. Purification of the final product were achieved by preparative HPLC and other common purification methods.
Physicochemical Properties of Peptide Analogues
In silico prediction of physicochemical and toxicity properties of all 4 peptide analogues were performed using Advanced Chemistry Development (ACD) Labs Percepta software (Advanced Chemistry Development Inc., Toronto, Ontario, Canada).
Preparation of Saturated Peptide Solution
The solubility of the various peptide analogues was determined for water and PG to prepare saturated peptide solution. Excess peptide was added into each solvent inside a 2 mL Eppendorf tube which was kept shaking in an incubation orbital shaker (Certomat S-1, Satorius, Germany) for 48 h. Entire set up was kept at room temperature. The samples were then centrifuged at 10000 rpm for 3 minutes. Subsequently, the supernatant was transferred into an amber coloured ampoule for assay.
Skin Preparation
Human dermatomed skin was obtained from Science Care (Arizona, USA). The skin tissues were excised from the thighs of Caucasian male cadaver, who died at the age of 43. The use of cadaver human skin for this study has been reviewed by the National University of Singapore Institutional Review Board (IRB) and subsequently exempted because the cadaveric tissues used in this study were without identifiable private information, in accordance to the NUS IRB-GUIDE-021 Guidelines on Research, using cadavers or cadaveric tissue specimens. Integrity of cadaver skin was investigated using visual inspection of the skin before use to ensure no visible pores or breaks in the skin surface. In addition, any compromise in skin integrity can be identified through a rapid and large increase in the amount of permeated peptide through the skin, at the first time point of sampling. The results of replicates with huge amount of peptide permeated through the skin by 1 hr time point will be discarded.
In Vitro Skin Permeation Experiment
Vertical Franz diffusion cells with an effective exposed area of 1 cm2 were used. The skin samples were mounted onto the Franz diffusion cells with epidermis facing up. The donor cell contained 400 µL of saturated solution, while the receptor cell contained 4.8 mL of 1X PBS. Compounds with solubility higher than 1% w/w were maintained at 1%w/w in the donor compartment in consideration of the cost of eventual product. The cells were placed inside a chamber with temperature controlled at 32 °C (comparable to the physiological temperature of the skin surface). Magnetic stirrers in the receptor cells stirred at a speed of 180 rpm. All the receptor solutions were withdrawn at pre-set time intervals and replaced with fresh ones. The receptor solutions were subjected to the measurement of peptide permeated by high performance liquid chromatography (HPLC). Each set of experiment were performed in triplicates.
HPLC for Drug Analysis
The amount of peptide permeated was determined by using Hitachi L2000 LaChrome Elite HPLC system with Agilent Zorbax Extend-C18 column (4.6 mm × 75 mm × 3.5 µm, 80 Å). The mobile phase consisted of mobile phase A (0.1%v/v trifluoroacetic acid in water) and mobile B (0.1%v/v trifluoroacetic acid in acetonitrile) with an isocratic elution program with ratios of solvents A and B as follows for the various peptide: Arg0 (9:1); Arg1 (17:3); Arg3 (39:1); Arg5 (39:1). The flow rate was set at 1 mL/min. The injection volume was 20 µL for each sampling and ultraviolet detection was performed at a wavelength of 215 nm. A calibration curve was conducted using the respective standard solutions from 1 ppm to 1000 ppm.
DPSC Cell Culture
Primary human dental pulp stem cells (DPSC) from a single donor (DPF003, AllCells, USA) were cultured in a complete growth media containing Dulbecco’s Modified Eagle’s Medium, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cell cultures were kept at 37 °C in a humid incubator, supplied with 5% carbon dioxide. The cells used were between passage 4 and passage 9 for all experiments.
Differentiation of DPSC into DPSC Neurons
DPSC was seeded in 24 well plate with complete growth media as discussed above and left uninterrupted for 24 hours for cells to adhere to the well plate, in a humid incubator kept at 37 °C with 5% carbon dioxide throughout the entire duration of study. Subsequently, complete growth media was removed and cells were rinsed with Dulbecco’s phosphate-buffered saline. For all subsequent duration, the culture media was changed to a neural induction media that consists of Neurobasal®-A medium, 1 × B-27® supplements, 1% penicillin/streptomycin, 20 ng/mL EGF and 40 ng/mL bFGF for 12–14 weeks44. Three hundred and fifty µL of neural induction media was used per well. The media was changed every three days by completely removing old media and adding fresh media.
In Vitro Inhibition of Glutamate Release
DPSC neurons were used for the glutamate release study. 4 mM of Arg0 with 200 mM of L-glutamine in PBS was prepared and loaded into individual DPSC neuron cell culture with equal volume of culture media to obtain a final concentration of 2 mM Arg 0 and 100 mM of L-glutamine, for a total duration of 5 h. The same was replicated for Arg1, while saturated solution of Arg2, Arg3 in PBS were used due to its poor solubility in water. Saturated solution of Arg2 and Arg3 in PBS were prepared with the method as described above. Final concentration of 1.35 mM and 1.27 mM in culture media was obtained for Arg2 and Arg3 respectively. The results were subsequently normalised to 2 mM of drug loaded. Following drug loading of 5 h, culture media was removed and washed with PBS. Depolarising media was added to the individual DPSC neuron culture to trigger the exocytosis of glutamate. Depolarising media was prepared using 20 mM of HEPES, 6 mM of calcium chloride, 75 mM of potassium chloride and 66 mM of sodium chloride in deionised water. After 23 h of depolarisation, contents of the cell culture were withdrawn and assay reagents were added for subsequent detection via a fluorometric plate reader (Infinite m200, Tecan Trading AG, Switzerland). The content of glutamate was detected via the use of a commercial fluorometric glutamate assay kit, ab138883 (Abcam plc., Cambridge, UK). Briefly, the coupled enzyme system catalysed the reaction between glutamate and NADP+ to produce NADPH, which is specifically recognized by the NADPH sensor and recycled back to NADP+. During the reaction, a red fluorescence product is produced, which in turn was detected in a fluorescence microplate reader at Ex/Em = 540/590 nm.
Data Analysis
All data were collated and prepared using GraphPad Prism 6 (GraphPad Software Inc, CA, USA) for any graphical outputs. All results were presented as mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance followed by Tukey post hoc test using IBM SPSS Statistics 21.0 (IBM, New York, USA). A probability value of p < 0.05 was considered statistically significant.
Acknowledgements
The authors acknowledge funding support from the Singapore Ministry of Education (MOE) Academic Research Fund (AcRF) Tier 1 grant (R-148-000-207-112), the Singapore National Additive Manufacturing Innovation Cluster (NAMIC) grant (R148-000-236-592) and the grant (R-148-000-220-597) from GRF Biotechnology Inc.
Author Contributions
S.H. Lim wrote the main manuscript text and prepared Figures 2–5 and Tables 1–2, with the guidance of L. Kang. Y. Sun prepared Figures 1 with the guidance of L. Kang. T. Thiruvallar Madanagopal and V. Rosa cultured and differentiated the DPSC neurons and provided guidance for the conduct of the work in Figures 5. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-24806-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manriquez JJ, Majerson Gringberg D, Nicklas Diaz C. Wrinkles. BMJ Clin Evid. 2008;2008:1711. [PMC free article] [PubMed] [Google Scholar]
- 2.Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308–319. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M, Gupta A. Photodamaged skin and quality of life: Reasons for therapy. Journal of Dermatological Treatment. 1996;7:261–264. doi: 10.3109/09546639609089563. [DOI] [Google Scholar]
- 4.ASAPS. 15th Annual Cosmetic Surgery National Data Bank Statistics, http://www.surgery.org/sites/default/files/ASAPS-2011-Stats.pdf (2011).
- 5.Benedetto AV. The environment and skin aging. Clinics in dermatology. 1998;16:129–139. doi: 10.1016/S0738-081X(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 6.Blanes-Mira C, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. International journal of cosmetic science. 2002;24:303–310. doi: 10.1046/j.1467-2494.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi PS, Lowe NJ. Botulinum toxin types A and B: comparison of efficacy, duration, and dose-ranging studies for the treatment of facial rhytides and hyperhidrosis. Clinics in dermatology. 2004;22:34–39. doi: 10.1016/j.clindermatol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Lupo MP, Cole AL. Cosmeceutical peptides. Dermatologic therapy. 2007;20:343–349. doi: 10.1111/j.1529-8019.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. The anti-wrinkle efficacy of argireline, a synthetic hexapeptide, in Chinese subjects: a randomized, placebo-controlled study. American journal of clinical dermatology. 2013;14:147–153. doi: 10.1007/s40257-013-0009-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang, Y. et al. The anti wrinkle efficacy of synthetic hexapeptide (Argireline) in Chinese Subjects. Journal of cosmetic and laser therapy: official publication of the European Society for Laser Dermatology, 10.3109/14764172.2012.759234 (2013). [DOI] [PubMed]
- 11.Chen, Y., Chen, B. Z., Wang, Q. L., Jin, X. & Guo, X. D. Fabrication of coated polymer microneedles for transdermal drug delivery. Journal of controlled release: official journal of the Controlled Release Society, 10.1016/j.jconrel.2017.03.383 (2017). [DOI] [PubMed]
- 12.Kraeling, M. E., Zhou, W., Wang, P. & Ogunsola, O. A. In vitro skin penetration of acetyl hexapeptide-8 from a cosmetic formulation. Cutaneous and ocular toxicology, 10.3109/15569527.2014.894521 (2014). [DOI] [PubMed]
- 13.Hoppel M, et al. Topical delivery of acetyl hexapeptide-8 from different emulsions: influence of emulsion composition and internal structure. Eur J Pharm Sci. 2015;68:27–35. doi: 10.1016/j.ejps.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz MA, Clares B, Morales ME, Cazalla S, Gallardo V. Preparation and stability of cosmetic formulations with an anti-aging peptide. Journal of cosmetic science. 2007;58:157–171. [PubMed] [Google Scholar]
- 15.Krishnan G, et al. Iontophoretic skin permeation of peptides: an investigation into the influence of molecular properties, iontophoretic conditions and formulation parameters. Drug Deliv. and Transl. Res. 2014;4:222–232. doi: 10.1007/s13346-013-0181-8. [DOI] [PubMed] [Google Scholar]
- 16.Ling MH, Chen MC. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta biomaterialia. 2013;9:8952–8961. doi: 10.1016/j.actbio.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi H, et al. Development of a novel transdermal delivery system of peptide and protein drugs using microneedle arrays. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan. 2014;134:63–67. doi: 10.1248/yakushi.13-00221-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. Journal of controlled release: official journal of the Controlled Release Society. 2012;161:933–941. doi: 10.1016/j.jconrel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed YH, et al. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PloS one. 2014;9:e101956. doi: 10.1371/journal.pone.0101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luis J, Park EJ, Meyer RJ, Smith NB. Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. The Journal of the Acoustical Society of America. 2007;122:2022–2030. doi: 10.1121/1.2769980. [DOI] [PubMed] [Google Scholar]
- 21.Banga AK. Microporation applications for enhancing drug delivery. Expert opinion on drug delivery. 2009;6:343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- 22.Levin G, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharmaceutical research. 2005;22:550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- 23.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nature reviews. Drug discovery. 2006;5:543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011;12:431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao YL, et al. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24:1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Lane ME. Skin penetration enhancers. International journal of pharmaceutics. 2013;447:12–21. doi: 10.1016/j.ijpharm.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 27.Fowles JR, Banton MI, Pottenger LH. A toxicological review of the propylene glycols. Crit Rev Toxicol. 2013;43:363–390. doi: 10.3109/10408444.2013.792328. [DOI] [PubMed] [Google Scholar]
- 28.Niciu MJ, Kelmendi B, Sanacora G. Overview of Glutamatergic Neurotransmission in the Nervous System. Pharmacology, Biochemistry, and Behavior. 2012;100:656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubrizol. Lipotec ARGIRELINE® peptide - Argireline Technical Report, https://www.lipotec.com/en/products/argireline-reg-peptide/ (2017).
- 30.Victor AK, Reiter LT. Dental pulp stem cells for the study of neurogenetic disorders. Human molecular genetics. 2017;26:R166–r171. doi: 10.1093/hmg/ddx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiume MM, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31:245S–260S. doi: 10.1177/1091581812461381. [DOI] [PubMed] [Google Scholar]
- 32.Kasting GB, Francis WR, Roberts GE. Skin penetration enhancement of triprolidine base by propylene glycol. J Pharm Sci. 1993;82:551–552. doi: 10.1002/jps.2600820525. [DOI] [PubMed] [Google Scholar]
- 33.Trottet L, Merly C, Mirza M, Hadgraft J, Davis AF. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. International journal of pharmaceutics. 2004;274:213–219. doi: 10.1016/j.ijpharm.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Ostrenga J, Steinmetz C, Poulsen B, Yett S. Significance of vehicle composition. II. Prediction of optimal vehicle composition. J Pharm Sci. 1971;60:1180–1183. doi: 10.1002/jps.2600600813. [DOI] [PubMed] [Google Scholar]
- 35.Blanchfield JT, Toth I. Modification of peptides and other drugs using lipoamino acids and sugars. Methods in molecular biology (Clifton, N. J.) 2005;298:45–61. doi: 10.1385/1-59259-877-3:045. [DOI] [PubMed] [Google Scholar]
- 36.Benson HA, Namjoshi S. Proteins and peptides: strategies for delivery to and across the skin. J Pharm Sci. 2008;97:3591–3610. doi: 10.1002/jps.21277. [DOI] [PubMed] [Google Scholar]
- 37.Foldvari M, et al. Palmitoyl derivatives of interferon alpha: potential for cutaneous delivery. J Pharm Sci. 1998;87:1203–1208. doi: 10.1021/js980146k. [DOI] [PubMed] [Google Scholar]
- 38.Foldvari M, et al. Dermal and transdermal delivery of protein pharmaceuticals: lipid-based delivery systems for interferon alpha. Biotechnology and applied biochemistry. 1999;30(Pt 2):129–137. [PubMed] [Google Scholar]
- 39.Namjoshi S, et al. Enhanced transdermal peptide delivery and stability by lipid conjugation: epidermal permeation, stereoselectivity and mechanistic insights. Pharmaceutical research. 2014;31:3304–3312. doi: 10.1007/s11095-014-1420-5. [DOI] [PubMed] [Google Scholar]
- 40.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chemical biology & drug design. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 41.Calceti P, Salmaso S, Walker G, Bernkop-Schnurch A. Development and in vivo evaluation of an oral insulin-PEG delivery system. Eur J Pharm Sci. 2004;22:315–323. doi: 10.1016/j.ejps.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Bunnage ME. Getting pharmaceutical R&D back on target. Nature chemical biology. 2011;7:335–339. doi: 10.1038/nchembio.581. [DOI] [PubMed] [Google Scholar]
- 43.Waring MJ, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nature reviews. Drug discovery. 2015;14:475–486. doi: 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- 44.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem cells (Dayton, Ohio) 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]