Abstract
The field of tissue engineering and regenerative medicine (TE/RM) involves regeneration of tissues and organs using implantable biomaterials. The term epigenetics refers to changes in gene expression that are not encoded in the DNA sequence, leading to remodeling of the chromatin and activation or inactivation of gene expression. Recently, studies have demonstrated that these modifications are influenced not only by biological cues but also by mechanical and topographical signals. This review highlights the current knowledge on emerging approaches in TE/RM with a focus on the effect of materials and topography on the epigenetic expression pattern in cells with potential impacts on modulating regenerative biology.
Keywords: epigenetics, tissue engineering, surface topography, material energy, titanium, regenerative medicine
INTRODUCTION
The field of tissue engineering and regenerative medicine (TE/RM) involves the process by which tissues and organs are regenerated by implantable biomaterials using in vivo or in vitro models.1 The term epigenetics refer to changes in gene expression that are independent of mutations or changes in the genetic coding. Epigenetic changes leads to remodeling of the chromatin resulting in activation or inactivation of genes. Histones are grouped in complexes called nucleosomes that are responsible for packing the DNA into the nucleus. Post-translation modifications of histones can be mediated by lysine acetylation, serine phosphorylation, lysine methylation, arginine methylation, and lysine ubiquitination. Acetylation of histones is associated with an activation of transcription of genes and is regulated by the histone acetyltransferases responsible for adding acetyl groups to histones and histone deacetylases (HDACs) that remove acetyl groups. In turn, DNA methylation involves the addition of methyl groups to specific cytosine bases in the DNA sequence, altering the configuration of the DNA and hence the transcription.2 Importantly, epigenetic mechanisms are reversible and have been considered potential treatment models for improving personalized drug therapy. This review highlights the present knowledge on current approaches in TE/RM with the focus on the influence of surface energy, mechanics, and topography of materials on the epigenetic pattern in cells and implications in affecting TE and RM (Table I).
TABLE I.
Overview of the Current Evidence on the Effect of Material Topography and Energy as well as Different Materials on Chromatin Configuration and Epigenetic Mechanisms in Cells
| Reference | Evidence | |
|---|---|---|
| Topography | Li et al., 2011 | Increase histone acetylation and decreased HDAC activity in MSCs grown on micropatterned substrates. |
| Lv et al., 2015 | hASCs grown on TiO2 nanogrooves showed increase in H3K4 trimethylation and inhibition of the demethylase RBP2. | |
| Kingham et al., 2013 | ESCs grown on nanotopographical substrates changed DNA methylation in parts of chromatin related to MSCs and early osteogenic progenitor cells. | |
| Material energy | Rabineau et al., 2015 | Stiff surface induced a transcriptionally active chromatin structure in kidney epithelial cells. Decreasing the substrate softness shifted the chromatin into a transcriptionally inactive structure. |
| Li et al., 2011 | Increase histone acetylation and decreased HDAC activity in MSCs grown on elastic PDMS substrate. | |
| Materialsz | ||
| Titanium | Setyawati et al., 2013 | Induced DNA damage in fibroblasts. |
| Toyooka et al., 2012 | TiO2 Induced DNA damage by phosphorylation of histone H2AX in adenocarcinoma epithelial cell line. | |
| Lv et al., 2015 | TiO2 enhanced methylation of histone H3K4 in hASCs. | |
| Silica | Morez et al., 2015 | Silicon microgrooves induced histone acetylation. |
| PLGA | Setyawati et al., 2013 | Did not induced DNA damage in fibroblasts. |
| Bioglass ceramic | Moorthi et al., 2013 | Reduced the level of HDACs in human osteoblastic cells. |
| Graphene | Yoo et al., 2014 | Mouse fibroblasts grown on graphene showed increased histone methylation in genes associated with mesenchymal-to-epithelial changes. |
HDAC, histone deacetylase; MSCs, mesenchymal stem cells; hASCs, human adipose stem cells; ESCs, embryonic stem cells; PDMS, poly(dimethyl siloxane).
SURFACE ENERGY
Surface energy is a critical component of cell response to a biomaterial and is dependent on both surface chemistry and topography. Multiple studies have shown that cell adhesion is mediated by surface hydrophobicity, with surfaces that have low hydrophobicity being more favorable to integrate with bodily fluids and protein adhesion. Protein adhesion to a surface changes the surface energy and influence cell response. For example, the rate of osteoblast cell spreading and proliferation on fibronectin-coated glass slides (i.e., a high energy surface) was found to increase with enhancing surface hydrophilicity and was linearly dependent on surface energy.3 Glass is a high energy surface with a high affinity for water, making it hydrophilic and promoting the spread of water to lower the surface energy. Likewise, changes in surface topography through the incorporation of roughness results in highest surface energies if the existing surface is hydrophilic. Rough titanium implants have improved osseointegration and mechanical stability due to a higher surface energy compared to a polished surface as a result of higher wettability, which increases surface contact area.4 Surface energy changes affect cell-specific responses such as cell growth, with high surface energy materials promoting cellular differentiation, mineral deposition, and osteogenic maturation.5,6
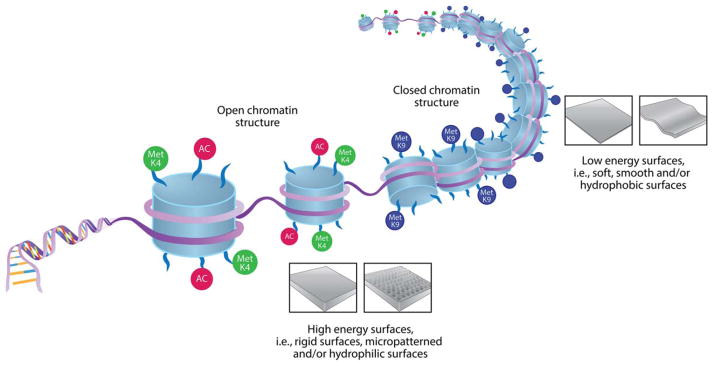
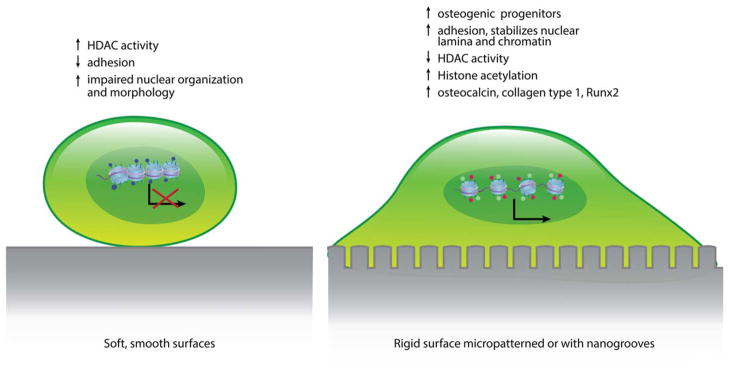
In TE, vital factors to ensure positive outcomes include adherence of cells to a scaffold and cell migration. Matrix characteristics influence the initial cell spreading, the 3D cell migration and the structure of cellular networks.1 The influence of substrate characteristics on form and size of cells and nuclei has been known for several years as well as being linked to chromatin alterations.7 A central factor inducing epigenetic changes in cells is the stiffness and structure of the surface matrix onto which cells adhere (Figure 1). Cells adhering to a rigid substrate display a transcriptionally active chromatin (euchromatin), while soft substrates result in a chromatin shift to a transcriptionally inactive state (heterochromatin).8 Using cell culture models, studies have shown that, not only do cells acquire specific matrix stiffness to maintain proper morphology and organization, but the matrix stiffness can also influence the differentiation of cells, for example, stem cells grown on substrate stiffness that mimics bone develop into an osteoblastic lineage.9 In line with these findings human umbilical vein endothelial cells were shown to acquire a 3D matrix with the stiffness of >15 kPa to maintain their proper morphology and organization.10 These findings show not only that mechanical signals are important in forming the chromatin structure and hence, cell responses and gene expression, but also presents a link between ECM and cytoskeleton that controls both cell shape and chromatin structure.11 Therefore, adjusting the matrix properties of putative target cells and tissues may improve regeneration as well as induce the development of specific cell lineages (Figure 2). A recent study using a 3D cell culture model mimicking soft tissue showed that a stiffness gradient affected both the migration and organization of different cell types.12
FIGURE 1.
A central factor inducing epigenetic changes in cells are the stiffness and structure of the biomaterial or tissue surface matrix. Cells that adhere to high energy surfaces have a more open and transcriptionally active chromatin structure (that is, euchromatin) while low energy surfaces induce a shift into a heterochromatin structure (i.e., transcriptionally inactive and more dense chromatin).
FIGURE 2.
A schematic drawing and summary on the structural and functional changes in cells grown on surfaces with different surface structure, stiffness, and energy.
Harvesting cells for TE requires trypsinization or scraping of the cells from the cell culture plate, methods that may influence cell functions. A recent study showed that, compared to cells grown on a rigid, cell culture plate, cells grown on thermoresponsive hydrogels with different stiffness induced a sustainable mechanical memory as well as a stable chromatin thereby maintaining the cells functions and phenotype induced by the material. The stiffness of the hydrogel influenced the DNA methylation pattern and the thermal harvesting method was found to stabilize the chromatin with the global methylation pattern and histone configuration intact. Furthermore, using an in vivo murine skin wound model it was shown that the cells obtained using the thermoresponsive hydrogels improved wound healing, thus indicating a new tool within the field of mechanobiology (Fan et al., 2017).
SURFACE TOPOGRAPHY
In addition to surface stiffness, nanotopographical structures, geometry, and surface energy of material interfaces influence cell function and differentiation. Both micro and nanoscaled topography of a substrate can induce epigenetic changes in cells. Culturing cells on microscaled substrates improved cellular reprogramming of cells into induced pluripotent stem cells (iPSCs) for application in gene therapy. A substrate with microgrooves of 10 μm width and spacing enhanced reprogramming of cells into iPSCs. A similar result was also obtained by using aligned nanofibers.13 Interestingly, it has been reported that cells were more affected by changes in surface topography at the nanometer scale as compared to microscales or macroscales.14 It was suggested that scaffolds with nanostructured topography may, therefore, be a tool to improve periodontal TE.14
A key mechanism involved in cellular responses to matrix topography and structure is the alteration in the level of histone acetylation. MSCs grown on micropatterned, flexible substrates showed a decrease in HDAC activity and an increase in histone acetylation indicating an increase in gene expression in these cells. Furthermore, mechanical compression and stretching of the substrate alters the nuclear shape and the histone acetylation pattern based on the directionality of the strain.15 Matrix elasticity was suggested to be able to influence cells in direct contact with the biomaterial.16 MSCs cultured on microgrooved poly(dimethyl siloxane) (PDMS) membranes (10 μm width, 3 μm height, 10 μm space between grooves) display an elongated nuclear shape as well as a decrease in HDAC activity with corresponding increase in histone acetylation and gene transcription compared to cells cultured on unpatterned surfaces.15 Mammary epithelial cells grown on a flat plastic substrate formed stress fibers and assembled as a monolayer on the surface, while cells cultured in a 3D laminin rich extracellular matrix (ECM) model and grown on 2D patterned substrate displayed a rounded morphology similar to their native in vivo phenotype. A correlation between cellular shape and histone acetylation has been reported.11 Adipose-tissue-derived mesenchymal stromal/stem cells (AMSCs) grown on 3D porous titanium discs in vitro presented a different expression of epigenetic regulators of histone methylation, as well as expression of genes related to bone extracellular proteins, compared to cells grown in a 2 D tissue culture polystyrene dish.17 A similar effect on cancer stem cells has been shown, with high levels of histone H3 observed in cancer cells growing in monolayer, but when cultured in low-adhesion conditions (holospheres), the levels of acetylated H3 decreased.18
In addition to histone acetylation, the methylation pattern of histones is also affected by surface structures. In a recent study, human adipose stem cells (hASCs) were grown on titanium dioxide (TiO2) nanotubes with sand-blasted, large grit, acid-etched (SLA) or smooth surfaces. TiO2 with 70 nm grooves influenced the histone methylation pattern to promote an osteogenic differentiation of hASC.19 Besides exposure to titanium particles, changes in DNA methylation pattern can be observed upon changes on the nanotopographical architecture.20 Embryonic stem cells (ESCs) grown on nanotopographical polycarbonate substrates changed the DNA methylation level in parts correlated to MSCs and early osteogenic progenitor cells phenotypes. In contrast no difference was found between surface topography regarding global methylation compared to that of normal hESCs.20 Cellular and nuclear morphology along with histone modifications (acetylation) can also be observed upon cell culturing on microtopography and nanotopography surfaces. Interestingly, seeding cells over high grooves (10 μm width and 3 μm height) increases global histone acetylation of fibroblasts when compared to cell culture over flat surfaces. Of note, increased histone acetylation correlates with reduced HDAC activity. Besides enhanced histone acetylation, fibroblasts seeded over microgroove structure undergo a mesenchymal-to-epithelial transition with the result in transcription of epithelial-associated genes and the development of more polarized cells.13 The process of mesenchymal-to-epithelial transition is often observed during embryogenesis suggesting the potential ability of modified surfaces to favor cellular reprogramming as previously described.11
BIOMATERIAL INFLUENCES
At present, there exist a limited number of studies that have investigated how different biomaterials alter cellular epigenetic patterns, with most studies focusing on titanium and titanium dioxide (TiO2), and a few on silica, glass, and graphene. The most prominent readout between different materials and epigenetic modification has been the DNA damage pathway and the phosphorylation of Histone H2A.X (γH2AX), which is an early marker of DNA damage. γH2AX play a crucial role in the DNA damage response of cells. Upon double strand breaks, γH2AX is one of the first responders to a DNA insult. Interestingly, however, the efficacy of γH2AX in response to an injury can be epigenetically controlled by histone acetylation. Enhanced histone H3 acetylation at lysine 56 (H3K56ac) enhances the DNA damage response in stem cells.21 Therefore, γH2AX/H3K56ac interaction is suggested to play a major role in the control of hypersensibility of cells to DNA damage repair. Along these lines, exposure of cells to TiO2 particles may directly influence histone acetylation leading to enhanced DNA double strand breaks.
TiO2-induced γH2AX is observed at a low concentration of 10 μg/mL, compared with terbium-doped-gadolinium oxide (Tb-Gd2O3) that required a concentration of 1000 μg/mL to induce damage to the DNA or poly(lactic-co-glycolic acid) (PLGA) nanoparticles that did not induce any DNA damage.22 Interestingly, TiO2 particles induce γH2AX independent of reactive oxygen species produced by inflammatory cells in an innate immune response. This indicates that TiO2 itself can cause DNA damage in cells that come in contact with the particles.23 Particles of nanosize induced γH2AX damage DNA more efficiently than larger TiO2 particles.23 The structure of the titanium can further induce changes in the epigenetic pattern. Analysis of TiO2 nanotubes showed that an increase in TiO2 diameter resulted in a corresponding increase in the binding of histone IIA, with an 8 fold higher binding to the 100 nM nanotubes compared to titanium foil.24 Also, stem cells cultured on titanium nanotubes showed enhanced adhesion and collagen secretion, improving periodontal tissue regeneration and formation of collagen bundles. Furthermore, using a titanium/cell sheet/HA construct not only promotes periodontal regeneration but also induces blood vessels forming among the collagen fibers and a cementum-like tissue. Several sizes of titanium tubes were tested with 10 and 5 μm showing the optimal results with a structure most closely resembling the natural periodontal ligament structure.25
Other materials used in TE/RM that influence epigenetic patterns are silica, bioglass, and graphene. The use of silica substrate induces cell alignment and nuclear elongation related to induction of histone acetylation.26 Silicon wafers with microgrooves (10 μm wide, 3 μm deep) induce histone acetylation and was also shown to make the cells more responsive to treatment with HDAC inhibitors (HDACi).26 Gene expression in osteoblasts exposed to hydroxylapatite (HAp) nanoparticles, silica nanoparticles, calcium oxide, and phosphate, showed a unique expression in the cells exposed to nano-HAp. A decrease in alkaline phosphatase expression and an increase in DNA methylation of the ALP promoter region were reported.27 Nano bioglass ceramic particles reduced the levels of HDAC enzyme increasing osteoblast differentiation through induction of the microRNA miR-30c.28 Graphene, a substrate made of 2D-structures of carbon atoms enhanced cell growth and differentiation.29 By comparing reprogramming of mouse fibroblasts grown on graphene with cells grown on glass, it was shown that graphene induces an increase in histone methylation at the transcription start site for genes associated with inducing mesenchymal-to-epithelial changes in cells.29 Graphene also improved alkaline phosphatase staining in iPSCs, presenting a potential tool in bone tissue regeneration.
SCAFFOLD DESIGN AND COMBINING SCAFFOLDS WITH EPI-DRUGS FOR GENE THERAPY
RM includes the use of biochemical molecules to induce changes in cells to improve the outcome of tissue regeneration by targeting specific biological mechanisms. The combined use of scaffolds with small molecules like novel epigenetic drugs (epi-drugs), may improve differentiation of cells and TE by enhancing and regulating epigenetic mechanisms.30 Silica has been approved by the U.S. Food and Drug Administration (FDA) and used as a delivery model for DNA methylation inhibitor 5-aza. Scaffolds present a good delivery system in RM, due to their ability to immobilize a drug (e.g., small molecules, nucleases, or viruses), thereby locally distribute the molecule to the specific site of tissue damage.31 Not only do scaffolds present an attractive method for efficient local delivery, but the structure of the scaffold itself can also induce epigenetic changes that influence cell behavior, gene expression, and hence, tissue regeneration. Small molecules, such as HDACis have been delivered extracellularly, and embedded in 3D-scaffolds, with microparticles and genetically modified cells.31 A local delivery approach could also reduce the potential side effects of the HDACi.32 Histones have nonchromatin related function that facilitates permeability of membranes thereby mediating transport of substances. Histones immobilized on microspheres facilitates adhesion, proliferation, and network formation.33 This presents an additional tool for using epigenetic molecules to improve surface modifications of scaffold design and tissue regeneration.34
Several small epigenetic molecules are available and approved by the FDA that are currently being used in clinical trials for the treatment of various cancers.35 HDACi are small compounds able to inhibit HDACS thereby inducing transcription of genes. HDACi, such as TSA, Valproate and MS-275, have been investigated for the potential use in regulating bone formation and it has been suggested that HDACi are suitable agents for both local and systemic treatment of bone loss.36 Interestingly, a positive effect of HDACi on both bone and inflammation in animal models of rheumatoid arthritis, provide a treatment option for simultaneously targeting both the inflammation, bone, and tissue destruction thereby presenting a new treatment option in bone TE.32
By treating human bone marrow stromal cells with either an HDACi (TSA) or with a DNA methylation inhibitor (5-aza-dC) stimulated cells to differentiate into osteogenic and chondrogenic, populations respectively.37 This indicates a use for epigenetic regulatory compounds to induce different cells lineage from one line of stem cells. Coating of surfaces with keratin and 5-Azacytidine induced differentiation of hMSCs into a cardiomyocyte lineage.38
Valproic acid (VPA) is considered safe for use in clinical settings and promotes regeneration of nerves in vitro using a silicon tube connecting two nerve ends. VPA applied in the silicon tube created a microenvironment resulting in nerve bundles properly oriented and shaped.39 Collagen sponges and macroporous biphasic calcium phosphate scaffolds mixed with HDACi induced woven bone formation and newly formed bone at the contact with the scaffold.40
To address a critical first step of initiating and controlling stem cell differentiation, a vital component in TE/RM, the use of the histone H3K4 demethylase LSD1 on hASCs-scaffolds containing an inhibiting molecule for LSD1 was investigated. Compared with anorganic bovine bone-collagen scaffold the prototype scaffold resulted in an increase in H3K4me in osteogenic associated genes along with higher osteogenic differentiation.41 Furthermore, when adding an HDACi to a soft substrate, it preserved the euchromatin structure, compensating for the unfavorable effect of the soft substrate.8 An additional use for HDACi is as a tool to improve ex vivo genome engineering by restoring gene expression of gene delivery by lentiviral vectors.42
MECHANICAL STIMULATION AND MICROSPHERES
Interestingly, not only does the material and structure of a scaffold that cells grow on/come in contact with induce epigenetic changes, recently mechanical stimulation was found to be an additional factor. Mechanical stimulation with fluid flow experiments reduced DNA methylation and increased gene expression, thus indicating that the mechanical microenvironment can induce epigenetic changes that not only can control the present cells but also pass this change on to the daughter cells.43 Induction of cells into iPSC may also be regulated by biomechanics and biophysical signals inducing changes in the nucleus and the chromatin, suggesting the use of surface topography as a tool for improving TE/RM and replacing the use of small-molecules.13
FUTURE CONSIDERATIONS
In summary, this review presents novel insights on the delivery of epigenetic modifications that are affected by biomaterial surfaces and scaffolds affecting TE/RM. At present, there is limited knowledge about the epigenetic effects of substrate biomaterials and topography on cellular activities. Greater investigation in these domains is important for the better understanding of stem cell differentiation and for the improvement of bone and soft tissue regeneration. Using the surface topography to induce a specific epigenetic pattern is an advantage since it is highly local and specific to cells in direct contact with biomaterials. Such approaches could enhance the function of local Epi-drug delivery. Finding the critical epigenetic mechanisms involved in stem cell differentiation may be imperative in the control of stem cell differentiation for clinical translation in TE/RM.
Acknowledgments
Contract grant sponsor: NIH/NIDCR; contract grant number: DE13397
Contract grant sponsor: Royal Swedish Academy of Sciences Stiftelsen PE Lindahls stipendiefond
Contract grant sponsor: National Institute of Dental and Craniofacial Research; contract grant number: DE13397
The authors thank Mr. Ken Rieger and Ms. Victoria Zakrzewski for their assistance with the figures.
References
- 1.Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, Giannobile WV. Regenerative Medicine for Periodontal and Peri-implant Diseases. J Dent Res. 2016;95:255–266. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson L, Castilho RM, Giannobile WV. Epigenetics and its role in periodontal diseases: A state-of-the-art review. J Periodontol. 2015;86:556–568. doi: 10.1902/jop.2014.140559. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy SB, Washburn NR, Simon CG, Jr, Amis EJ. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials. 2006;27(20):3817–3824. doi: 10.1016/j.biomaterials.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2010;93:289–296. doi: 10.1002/jbm.a.32542. [DOI] [PubMed] [Google Scholar]
- 5.Lim JY, Shaughnessy MC, Zhou Z, Noh H, Vogler EA, Donahue HJ. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials. 2008;29:1776–1784. doi: 10.1016/j.biomaterials.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Feller L, Jadwat Y, Khammissa RA, Meyerov R, Schechter I, Lemmer J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. Biomed Res Int. 2015;2015:171945. doi: 10.1155/2015/171945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergani L, Grattarola M, Nicolini C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. Int J Biochem Cell Biol. 2004;36:1447–1461. doi: 10.1016/j.biocel.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Rabineau M, Flick F, Mathieu E, Tu A, Senger B, Voegel JC, Lavalle P, Schaaf P, Freund JN, Haikel Y, Vautier D. Cell guidance into quiescent state through chromatin remodeling induced by elastic modulus of substrate. Biomaterials. 2015;37:144–155. doi: 10.1016/j.biomaterials.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 10.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28:677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joaquin D, Grigola M, Kwon G, Blasius C, Han Y, Perlitz D, Jiang J, Ziegler Y, Nardulli A, Hsia KJ. Cell migration and organization in three-dimensional in vitro culture driven by stiffness gradient. Biotechnol Bioeng. 2016;113:2496–2506. doi: 10.1002/bit.26010. [DOI] [PubMed] [Google Scholar]
- 13.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du M, Duan X, Yang P. Induced Pluripotent Stem Cells and Periodontal Regeneration. Curr Oral Health Rep. 2015;2:257–265. doi: 10.1007/s40496-015-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Chu JS, Kurpinski K, Li X, Bautista DM, Yang L, Sung KL, Li S. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J. 2011;100:1902–1909. doi: 10.1016/j.bpj.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellenberg A, Joussen S, Moser K, Hampe N, Hersch N, Hemeda H, Schnitker J, Denecke B, Lin Q, Pallua N, Zenke M, Merkel R, Hoffmann B, Wanger W. Matrix elasticity, replicative senescence and DNA methylation patterns of mesenchymal stem cells. Biomaterials. 2014;35:6351–6358. doi: 10.1016/j.biomaterials.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 17.Lewallen EA, Jones DL, Dudakovic A, Thaler R, Paradise CR, Kremers HM, Abdel MP, Kakar S, Dietz AB, Cohen RC, Lewallen DG, van Wijnen AJ. Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene. 2016;581:95–106. doi: 10.1016/j.gene.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida LO, Guimaraes DM, Squarize CH, Castilho RM. Profiling the Behavior of Distinct Populations of Head and Neck Cancer Stem Cells. Cancers (Basel) 2016:8. doi: 10.3390/cancers8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv L, Liu Y, Zhang P, Zhang X, Liu J, Chen T, Su P, Li H, Zhou Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials. 2015;39:193–205. doi: 10.1016/j.biomaterials.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Kingham E, White K, Gadegaard N, Dalby MJ, Oreffo RO. Nanotopographical cues augment mesenchymal differentiation of human embryonic stem cells. Small. 2013;9:2140–2151. doi: 10.1002/smll.201202340. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs KM, Misri S, Meyer B, Raj S, Zobel CL, Sleckman BP, Hallahan DE, Sharma GG. Unique epigenetic influence of H2AX phosphorylation and H3K56 acetylation on normal stem cell radioresponses. Mol Biol Cell. 2016;27:1332–1345. doi: 10.1091/mbc.E16-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setyawati MI, Khoo PK, Eng BH, Xiong S, Zhao X, Das GK, Tan TT, Loo JS, Leong DT, Ng KW. Cytotoxic and genotoxic characterization of titanium dioxide, gadolinium oxide, and poly(lactic-co-glycolic acid) nanoparticles in human fibroblasts. J Biomed Mater Res A. 2013;101:633–640. doi: 10.1002/jbm.a.34363. [DOI] [PubMed] [Google Scholar]
- 23.Toyooka T, Amano T, Ibuki Y. Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutat Res. 2012;742:84–91. doi: 10.1016/j.mrgentox.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni M, Flasker A, Lokar M, Mrak-Poljsak K, Mazare A, Artenjak A, Cucnik S, Kralj S, Velikonja A, Schmuki P, Iglic A, Sodin-Semri S. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int J Nanomed. 2015;10:1359–1373. doi: 10.2147/IJN.S77492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao H, Li B, Zhao L, Jin Y. Influence of nanotopography on periodontal ligament stem cell functions and cell sheet based periodontal regeneration. Int J Nanomed. 2015;10:4009–4027. doi: 10.2147/IJN.S83357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morez C, Noseda M, Paiva MA, Belian E, Schneider MD, Stevens MM. Enhanced efficiency of genetic programming toward cardiomyocyte creation through topographical cues. Biomaterials. 2015;70:94–104. doi: 10.1016/j.biomaterials.2015.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha SW, Jang HL, Nam KT, Beck GR., Jr Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials. 2015;65:32–42. doi: 10.1016/j.biomaterials.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorthi A, Vimalraj S, Avani C, He Z, Partridge NC, Selvamurugan N. Expression of microRNA-30c and its target genes in human osteoblastic cells by nano-bioglass ceramic-treatment. Int J Biol Macromol. 2013;56:181–185. doi: 10.1016/j.ijbiomac.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo J, Kim J, Baek S, Park Y, Im H, Kim J. Cell reprogramming into the pluripotent state using graphene based substrates. Biomaterials. 2014;35:8321–8329. doi: 10.1016/j.biomaterials.2014.05.096. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Ding Q, Wang J, Deng L, Yang L, Tao L, Lei H, Lu S. 5-Azacytidine delivered by mesoporous silica nanoparticles regulates the differentiation of P19 cells into cardiomyocytes. Nanoscale. 2016;8:2011–2021. doi: 10.1039/c5nr08560h. [DOI] [PubMed] [Google Scholar]
- 31.Lorden ER, Levinson HM, Leong KW. Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv Transl Res. 2015;5:168–186. doi: 10.1007/s13346-013-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantley MD, Bartold PM, Fairlie DP, Rainsford KD, Haynes DR. Histone deacetylase inhibitors as suppressors of bone destruction in inflammatory diseases. J Pharm Pharmacol. 2012;64:763–774. doi: 10.1111/j.2042-7158.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 33.Goryukhina OA, Martyushin SV, Bilinova MI, Polianskaia GG, Cherepanova OA, Pinaev GP. Cell cultivation on microspheres coupled with histones. Cell Tissue Biol. 2010;4:14–24. [Google Scholar]
- 34.Goryukhina OA, Martyushin SV, Pinaev GP. On the possible use of exogenous histones in cell technology. Cell Biol Int. 2011;35:1189–1193. doi: 10.1042/CBI20100741. [DOI] [PubMed] [Google Scholar]
- 35.McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene. 2011;474:1–11. doi: 10.1016/j.gene.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HN, Lee JH, Bae SC, Ryoo HM, Kim HH, Ha H, Lee ZH. Histone deacetylase inhibitor MS-275 stimulates bone formation in part by enhancing Dhx36-mediated TNAP transcription. J Bone Miner Res. 2011;26:2161–2173. doi: 10.1002/jbmr.426. [DOI] [PubMed] [Google Scholar]
- 37.El-Serafi AT, Oreffo RO, Roach HI. Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: Differential effects of 5-aza-deoxycytidine and trichostatin A. Differentiation. 2011;81:35–41. doi: 10.1016/j.diff.2010.09.183. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh LD, Ravi V, Sanpui P, Sundaresan NR, Chatterjee K. Keratin mediated attachment of stem cells to augment cardiomyogenic lineage commitment. Colloids Surf B Biointerfaces. 2017;151:178–188. doi: 10.1016/j.colsurfb.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Wu F, Xing D, Peng Z, Rao T. Enhanced rat sciatic nerve regeneration through silicon tubes implanted with valproic acid. J Reconstr Microsurg. 2008;24:267–276. doi: 10.1055/s-2008-1078696. [DOI] [PubMed] [Google Scholar]
- 40.Lee SU, Kwak HB, Pi SH, You HK, Byeon SR, Ying Y, Luesch H, Hong J, Kim SH. In Vitro and In Vivo Osteogenic Activity of Largazole. ACS Med Chem Lett. 2011;2:248–251. doi: 10.1021/ml1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge W, Liu Y, Chen T, Zhang X, Lv L, Jin C, Jiang Y, Shi L, Zhou Y. The epigenetic promotion of osteogenic differentiation of human adipose-derived stem cells by the genetic and chemical blockade of histone demethylase LSD1. Biomaterials. 2014;35:6015–6025. doi: 10.1016/j.biomaterials.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Chuah MK, VandenDriessche T. Optimizing delivery and expression of designer nucleases for genome engineering. Hum Gene Ther Methods. 2013;24:329–332. doi: 10.1089/hgtb.2013.166. [DOI] [PubMed] [Google Scholar]
- 43.Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech. 2010;43:2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]